Abstract
Studies in humans and animal models provide compelling evidence for age-related skeletal muscle denervation, which may contribute to muscle fiber atrophy and loss. Skeletal muscle denervation seems relentless; however, long-term, high-intensity physical activity appears to promote muscle reinnervation. Whether 5-month resistance training (RT) enhances skeletal muscle innervation in obese older adults is unknown. This study found that neural cell-adhesion molecule, NCAM+ muscle area decreased with RT and was inversely correlated with muscle strength. NCAM1 and RUNX1 gene transcripts significantly decreased with the intervention. Type I and type II fiber grouping in the vastus lateralis did not change significantly but increases in leg press and knee extensor strength inversely correlated with type I, but not with type II, fiber grouping. RT did not modify the total number of satellite cells, their number per area, or the number associated with specific fiber subtypes or innervated/denervated fibers. Our results suggest that RT has a beneficial impact on skeletal innervation, even when started late in life by sedentary obese older adults.
Keywords: Skeletal Muscle, Denervation, Exercise, Satellite Cells, Aging
Electrophysiological and histological measurements of skeletal muscle in older adults have detected fiber grouping and reductions in motor units (1–4) that are suggestive of denervation, which may contribute to aging-related muscle fiber atrophy and loss (5–8). However, characterizing the extent of human muscle denervation is difficult for two reasons. First, the neuromuscular junction rarely appears in muscle extracted using needle biopsies. Second, the suboptimal resolution of current imaging techniques limits our ability to quantify ventral spinal cord motor neurons and axons or to determine the integrity of axons and their myelin in vivo.
Studies in animal models provide compelling evidence for age-related skeletal muscle denervation (9). Muscle fiber-type phenotype is determined by interactions with subpopulations of ventral spinal cord motor neurons that activate contraction at different rates (10–12). Although denervation contributes to skeletal muscle atrophy and functional impairment (13), neither its time course, nor prevalence, in human and animal models of aging has been determined. Studies that combined contraction-force recordings with muscle immunostaining for neural cell-adhesion molecule (NCAM) protein, a marker of fiber denervation (14,15), show that old rats have significantly more denervated fibers than their young counterparts. Moreover, when we combined electrophysiological and immunohistochemical assays to detect the expression of tetrodotoxin-resistant Nav1.5 sodium channels in muscles from young-adult and old mice, we found a significantly larger percentage of fibers undergoing denervation than expected (16). When denervation outpaces reinnervation, a population of muscle fibers will atrophy and become excluded from functional recordings (16,17). Thus, the area of denervated fibers likely accounts for a significant fraction of the decline in specific force with aging (18).
Skeletal muscle denervation seems relentless, but, in mice, aerobic exercise in the form of wheel running can significantly reverse neuromuscular junction alterations with aging (19) and long-term, high-intensity physical activity appears to promote muscle reinnervation (20). Whether short-term resistance training (RT) enhances skeletal muscle innervation in obese older adults is unknown. To examine this question, we analyzed several muscle properties influenced by the status of muscle innervation at baseline and after 5 months of RT: fiber-type composition, myofiber cross-sectional area (CSA), fiber grouping, neural cell adhesion molecule (NCAM)–positive myofibers, and the number and density of satellite cells (SCs). We also examined the association between fiber grouping and NCAM+ myofibers with muscle strength.
Several studies demonstrate that SCs are needed in the regenerative response following skeletal muscle injury (21–24); however, their role in response to exercise is unclear. Because previous studies support an increase in muscle mass with RT in older adults (25–29) and fiber-type specific SC activation in response to RT (30,31), we examined whether this effect benefits obese older adults by quantifying the number of SCs and their relationship with innervated and denervated fast and slow fibers.
We hypothesized that RT enhances skeletal muscle innervation and muscle strength based on the decreased area of NCAM+ cells and the inverse relationship between muscle strength and changes in fiber grouping and NCAM+ cell area. Also, that this enhancement was not associated with significant changes in fiber type or size, the number of SCs or their association with a specific fiber subtype based on myosin heavy chain, or innervation status.
Materials and Methods
Study Participants
Participants included a subset of older men (n = 3) and women (n = 5) from the Piedmont Triad area of North Carolina who were enrolled in the RT arm of an NIH-funded study Improving Muscle for Functional Independence Trial (I’M FIT) (32). Inclusion criteria were as follows: (i) overweight/obese (body mass index = 27–35kg/m2); (ii) age = 65–79 years; (iii) nonsmoking; (iv) not on hormone replacement therapy; (v) sedentary (<15 minutes of exercise, 2 times/week) in the past 6 months; and (vi) weight stable (<5% weight change) for at least 6 months prior to enrollment. All had normal liver, kidney, pulmonary, and thyroid function; no history of excessive alcohol intake; and no major chronic illness, anemia, or orthopedic impairment. The study was approved by the Wake Forest Institutional Review Board for Human Research, and all participants signed informed consent to participate in the study.
Exercise Intervention
The exercise intervention consisted of 5 months of progressive RT designed to elicit adaptations in skeletal muscle and increase strength and power. To optimize the resistance stimulus for maximal functional gain, the exercise prescription was based on a relative intensity level and progressed with each individual’s strength gain. Participants exercised 3 days/week under the supervision of two exercise physiologists. They walked or cycled slowly for 5–10 minutes to warm-up prior to RT. Resistance exercises were performed on eight Nautilus (Vancouver, WA) resistance machines on which the load could be adjusted in small increments. The Nautilus machines included: (i) leg press; (ii) leg extension; (iii) seated leg curl; (iv) seated calf; (v) vertical chest; (vi) compound row; (vii) triceps press; and (viii) bicep curl. Two of the machines targeted the quadriceps muscle group. The program was based on American College of Sports Medicine guidelines (2009) for intensity, number of repetitions, number of sets, and number of days per week. The one-repetition maximum (1RM)—that is, the maximal weight that could be lifted with correct form in a single repetition—was used to prescribe intensity, which was increased gradually during the first month toward the goal of 3 sets of 10 repetitions at 70% 1RM for a given exercise. The 1RM strength testing was repeated every 4 weeks, and training loads were adjusted to achieve the 70% 1RM goal.
Maximal Isokinetic Knee Extensor Strength
RT procedures have been described in detail in (32). Maximal isokinetic knee extensor strength was measured using an isokinetic dynamometer (Biodex) at a speed of 60 degrees/second. Seat height and depth were recorded for consistency between tests. The best of three performances by the leg to be biopsied was used in analyses.
Body Composition
Fat and lean tissue mass were measured by dual-energy x-ray absorptiometry (Hologic Delphi QDR). All scans were performed and analyzed by a dual-energy x-ray absorptiometry technician certified by the International Society for Clinical Densitometry.
Skeletal Muscle Biopsy
Needle biopsies of the vastus lateralis were collected under local anesthesia with 1% lidocaine for RNA and single-fiber contraction studies (33). This muscle is commonly used for in vitro studies of older adults (34–36). All biopsies were performed in the early morning after overnight fast. Subjects were asked not to take aspirin, prescription and over-the-counter nonsteroidal anti-inflammatory drugs, or other compounds that might affect bleeding, platelets, or bruising for the week prior to the biopsy and to refrain from any strenuous activity (including RT) for 36 hours prior to the biopsy. Once the specimen was obtained, visible blood and connective tissue were removed. The sample was oriented so that the fibers ran longitudinally, mounted perpendicularly on a plastic dish in embedding medium (OCT compound, Miles Laboratory, Naperville, IL), thickened with baby powder, snap frozen in liquid nitrogen, and stored at −80 °C until analysis.
ATPase Staining, NCAM Immunohistochemistry, and Fiber Grouping
Because myosin ATPase activity correlates positively with muscle contraction speed, we used a histochemical assay for myofibrillar ATPase activity to distinguish type I and type II fibers. The first step was preincubation in a basic (pH 9.4) solution, which inhibits myosin ATPase activity in slow fiber types. Unfixed muscle sections used for fiber type characterization were postfixed and followed by immunofluorescence for NCAM with a primary polyclonal antibody (Millipore, Temecula, CA). Fiber grouping was quantified as the area occupied by at least two contiguous fibers expressing a predominantly similar myosin heavy chain isoform, fully surrounded by fibers of the same predominant myosin heavy chain.
SC Identification, Quantification, and Association With a Specific Fiber Subtype
We quantified SCs per muscle CSA and analyzed them by Pax7/NCAM immunostaining and Hoechst 33342 staining. Anti-NCAM (Cat AB5032, Millipore) and anti-Pax7 (deposited by Dr. Atsushi Kawakami, Iowa Developmental Studies Hybridoma Bank, Iowa, IA) together with secondary antibodies donkey anti-rabbit Alexa Fluor 488 (Cat# A21206) and donkey anti-mouse Alexa Fluor 568 (Cat# A10037) from Thermo Fisher Scientific (Grand Island, NY), respectively, were used. Complementary immunodetection of SCs, along with NCAM staining of the cytosol and Pax7 of the nucleus, greatly enhances the likelihood of accurately counting muscle progenitor cells (36). To ensure that all SCs were counted only once, alternate muscle sections were assessed for immunoreactivity to NCAM and the transcription factor Pax7 (36). This general histological procedure consists of skipping one every other section to avoid counting a SC twice when the muscle is cut through a SC. SC number per CSA is expressed in µm2.
Real-time PCR mRNA Analysis
RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH). The mRNA expression levels of target genes were quantified by quantitative real-time PCR with SensiFAST Probe Lo-ROX One-Step Kit (Bioline, Taunton, MA). Total RNA (20ng) was loaded for real-time PCR in 20 µl of total volume. The RT-PCR was carried out at 45 °C for 30 minutes, followed by 95 °C for 5 minutes, and then 40 cycles at 95 °C for 10 seconds and 60 °C for 1 minute. Results were normalized to glyceraldehyde 3-phosphate dehydrogenase RNA levels. Relative quantification was calculated using the comparative threshold (C T) method (37). All samples were run in duplicates. Primers and probes for NCAM, CHRNG, and GAPDH were purchased from Applied Biosystems (Foster City, CA).
Statistical Analysis
Data were analyzed using SigmaPlot 12.5 (Systat Software, San José, CA). All data are presented as means ± SEM, and reported p values are the result of two-sided t tests. The alpha level was set at p < .05. The Pearson Product Moment Correlation was used to measure the strength of the association between pairs of variables.
Results
Body Weight, Body Mass Index, and Leg Press and Extension Strength
RT did not change body weight and body mass index but significantly increased leg press and knee extension strength an average of 51±18% (pre: 118±22 Nm; post: 171±37 Nm, p = .039) and 18±5% (pre: 120±17 Nm; post: 140±19 Nm, p = .035), respectively.
Muscle Fiber Type and CSA
We examined whether RT modified the number of type I or type II fibers and their CSA. Figure 1A illustrates muscle sections stained with ATPase (pH: 9.4) to determine the relative number of fiber subtypes and their CSAs. Laminin staining (red) better defined the borders of individual myofibers. The analysis found, for type I and type II fibers, respectively: 1,191 and 2,199 pre-RT (total: 3390 fibers) and 1,856 and 2,908 post-RT (total: 4764 fibers). Figure 2A shows that, compared with baseline, RT did not modify the percent of type I or type II fibers, whereas the difference between type I and type II fibers was significant after, but not before, RT. Figure 2B shows that the CSA of type II fibers was smaller than that of type I fibers, and RT did not modify the CSA of either type I or type II fibers significantly.
Figure 1.
Effect of resistance training (RT) on type I and type II fiber grouping. Representative vastus lateralis muscle cross-sections stained for ATPase (pH: 9.2) and immunostained for laminin (red), showing type I fiber (A) and type II fiber (B) grouping. (C) Fiber grouping before and after RT. Relationship between type II fiber grouping and pre/post RT knee extensor strength percent change.
Figure 2.
Proportion of type I and type II fibers at baseline and after resistance training (RT). Percent of type I and type II fibers (A) and their cross-sectional area before and after RT (B).
Muscle Fiber Grouping
Fiber-type grouping indicates a denervation/reinnervation process. Although, normally, type I and type II fibers are disposed in a chessboard pattern, grouped fibers express a predominant myosin heavy chain isoform and are surrounded by fibers of the same predominant phenotype. Figure 1A and B show two representative examples of type I and type II fiber grouping in muscle cross-sections at baseline from study participants. Figure 1C shows that, at baseline, type I and type II fiber groupings did not differ significantly, but they did after RT; however, they had not changed significantly compared with baseline.
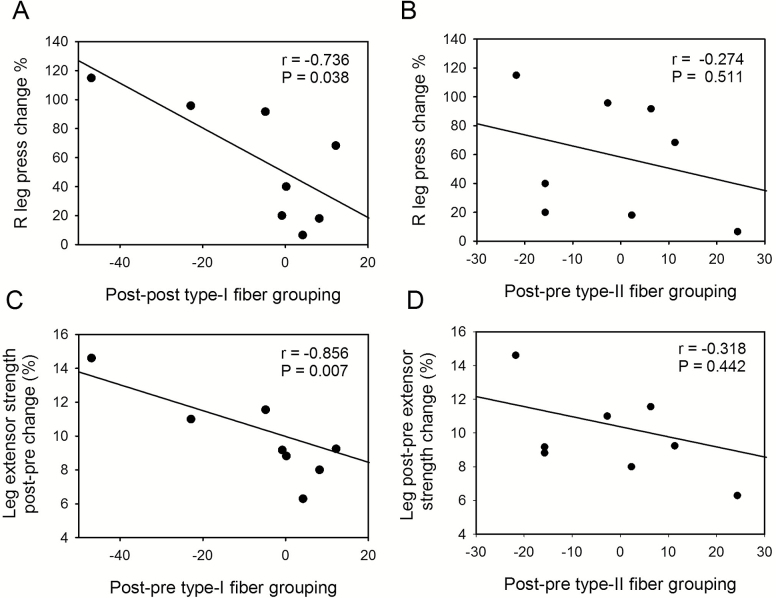
Increases in leg press and knee extensor strength inversely correlate with type I but not type II fiber grouping
Next, we analyzed whether change in type I and/or type II fiber grouping with RT correlated with change in leg press (Figure 3A, B) or knee extensor strength (Figure 3C, D). In contrast to type II (B, D), a decrease in type I fiber grouping was associated with larger increases in leg press (A) and knee extensor strength (C) after RT.
Figure 3.
Relationship between fiber grouping and leg strength. Type I (A, C) and type II (B, D) fiber grouping showing the relationship with change in leg press (A, B) or knee extensor strength (C, D) after resistance training.
NCAM+ muscle area declines with RT and inversely correlates with muscle strength
Figure 4A and C illustrate two typical NCAM myofiber staining patterns: contour (white arrows) and homogeneous (yellow arrow) (20,38). Denervated type I fibers show only the latter pattern, whereas denervated type II fibers show both (B, D). Figure 4E and F show a larger type II fiber (white arrow) and type I fibers with the homogenous NCAM+ pattern (yellow arrows). Figure 4G shows that, at baseline, the area of NCAM+ type I fibers exceeded the area of NCAM+ type II fibers almost fourfold. This relationship was less pronounced after RT due to a significant decrease in NCAM+ type I fibers.
Figure 4.
Denervated muscle fibers detected by NCAM immunostaining. NCAM+ denervated type I (yellow arrows) and type II (white arrows) fibers (A, B, E) and their overlap with ATPase staining (C, D, F). Panel G shows that the NCAM+ area is larger in type I than type II NCAM+ fibers at baseline and that NCAM+ type I fiber area decreases with resistance training.
Figure 5 shows that changes in the total NCAM+ muscle area of both type I plus type II fibers correlated inversely and significantly with changes in leg press (A) and knee extensor strength (B).
Figure 5.
Relationship between changes in leg press strength and type I plus type II NCAM+ fibers. Total NCAM+ muscle area and changes in leg press (A) and knee extensor strength (B) with resistance training were correlated significantly and inversely.
Real-time PCR ΔC t analysis showed a significant decrease in NCAM gene (NCAM1) transcript post-RT (0.54±0.12) compared with baseline (0.68±0.11; p = .029), which corresponds to ~21% decrease. RUNX1 (Runt-related transcription factor 1), a transcript highly expressed in aging (39) and denervation (40), was also decreased in the muscle of our participants after RT (pre: 1.24±0.26; post: 0.84±0.16; p = .01). Interestingly, the acetylcholine γ subunit (CHRNG), a gene that is expressed with muscle denervation, also showed a 15% decrease with RT (ΔC t pre-RT: 0.43±0.14; post-RT: 0.28±0.04).
RT does not modify the total number of SCs or those associated with type I or type II fibers or NCAM+ myofibers
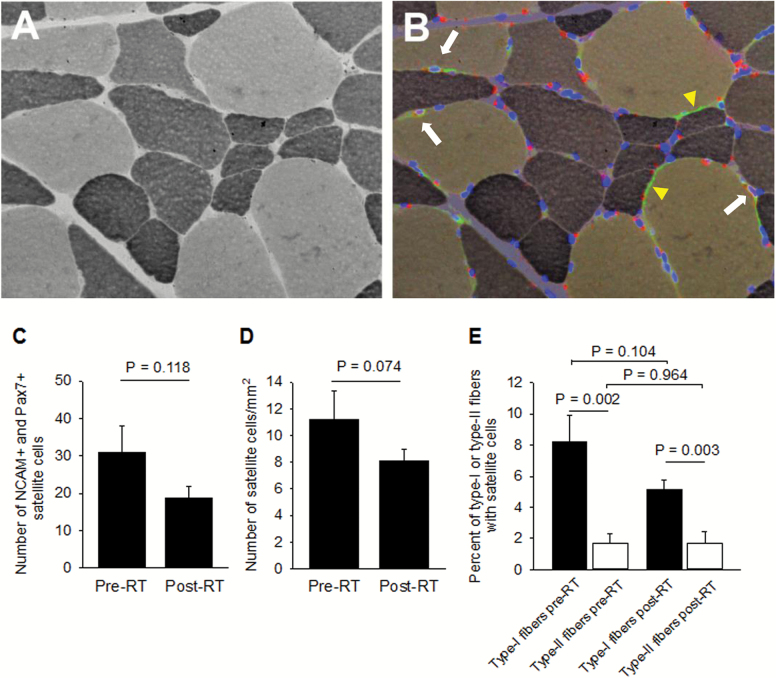
Figure 6 shows ATPase staining of a muscle cross-section (A) in which NCAM and Pax7 immunostaining and Hoechst 33342 staining were superimposed (B). The number of fibers analyzed is the same as for fiber type. Note three SCs (white arrows) and NCAM+ axons (yellow arrowheads). NCAM+ nerves have been previously reported (41–44). The red spots without nucleus are lipofuscin accumulations (45). Lindström and colleagues showed bright lipofuscin spots in the proximity of SCs (38). They are so often seen in aging skeletal muscle that some investigators proposed them as a marker of muscle decline with aging (46). SCs exhibit NCAM, Pax7, and Hoechst 33342 fluorescence overlap, while lipofuscin is intense in the red channel, is less intense in the green, and does not exhibit DNA staining. The number (C) and density (number/mm2) (D) of SCs did not change with RT. SCs do not seem to be incorporated into myofibers as the number of nuclei per fiber section did not change with RT (pre: 3.22±0.16; post: 3.5±0.19 nuclei/fiber). We also investigated whether RT modified the percent of SCs associated with type I or type II fibers. At baseline, more SCs were associated with type I than with type II fibers, and this difference remained significant after RT (E). Whether the number of SCs associated with innervated and denervated myofibers differs is unknown. Only three of 248 SCs (1.2%) associated with a type I NCAM+ myofiber at baseline and none of the total 150 associated with denervated myofibers after RT.
Figure 6.
Effect of resistance training (RT) on total number of satellite cells and those associated with type I or type II fibers. Representative ATPase-stained muscle cross-section (A) and its co-registration with NCAM and Pax7 immunostaining and Hoechst 33342 staining (B). White arrows indicate satellite cells, and yellow arrowheads indicate NCAM+ axons. Number (C) and density (number/mm2) (D) of satellite cells changed with RT. Panel E shows its effect on the percent of satellite cells associated with type I or type II fibers.
Discussion
This study found that type I and type II fiber grouping in the vastus lateralis of a small sample of older obese adults did not change significantly following 5 months of RT. Increases in leg press and knee extensor strength inversely correlated with type I, but not with type II, fiber grouping. NCAM+ muscle area decreased with RT and was inversely correlated with muscle strength. NCAM1 and CHRNGγ gene transcripts significantly decreased with the intervention. RT did not modify the total number of SCs, their number per area, or the number associated with specific fiber subtypes or innervated/denervated fibers.
RT and Fiber Grouping
It should be noticed that myofiber hypertrophy is impaired in aged mice regardless of SC content and that myonuclear accretion is insufficient to drive myofiber hypertrophy (47). The lack of hypertrophy could be that decreased SCs with aging increases extracellular matrix content that was exacerbated by overload, potentially limiting myofiber growth (47).
Participants at baseline showed a marked variability in fiber grouping that was less pronounced after RT. At baseline, type I and type II fiber groupings did not differ significantly, but they did after RT; however, they had not changed significantly compared with baseline, which is expected based on the short duration of the exercise intervention and the slow pace of the motor unit remodeling (13,48).
Indirect in vitro studies support the nervous system’s contribution to age-related structural and functional decline in skeletal muscle (9). In humans, a decrease in the number and size of spinal cord motor neurons with age has been reported (8). Morphological evidence of denervation/reinnervation can be found in the muscle itself (49). In animals, type I fibers reinnervate muscle faster compared with type IIB fibers, which leads to predominant type I fiber grouping (50,51). However, here, we found grouping of either type I or type II fibers in obese older adults.
Many factors may contribute to skeletal muscle fiber grouping. Decreased motor neuron firing rate resulting directly or indirectly from loss of supraspinal or segmental synaptic contacts (52); decreased motor neuron size and axon diameter, which slow nerve conduction; altered axonal signaling (53,54); or loss of axonal terminals at the neuromuscular junction may all lead to skeletal muscle denervation and, together with slow motor neuron sprouting (55), result in motor neuron remodeling or plasticity. Type II fiber synapses exhibit the highest vulnerability and fail to respond to any stimulus-induced sprouting, in contrast to type I fiber synapses, which are particularly disease resistant and plastic (55). Fiber denervation may be a terminal process, which is preceded by a prolonged period in which impaired motor neuron function leads to excitation–contraction uncoupling in aging muscle fibers (56–59) and decline in specific muscle force in both fiber subtypes (60,61). Whether denervation starts at the presynapse of the neuromuscular junction has been challenged recently in aging mice (62,63)
Quantifying muscle fiber grouping is essential to confirming the theory that alterations in spinal cord motor neurons lead to cycles of muscle denervation/reinnervation and that neuromuscular plasticity is maintained, allowing muscle innervation to improve with exercise, or other interventions into old age. Longer exercise periods than those used in this study may be required to modify fiber grouping (20).
The inverse relationship between the percent of type I fiber grouping and leg press and knee extensor strength indicates that although grouping may compensate for muscle denervation, it can also alter the muscle’s ability to contract smoothly and efficiently when stimulated, which may explain some age-associated changes in muscle function (64).
RT and NCAM
Our study shows that a percent of muscle fibers express NCAM, a marker of human muscle denervation with aging (20) and neurogenic diseases such as amyotrophic lateral sclerosis and postpolio syndrome (65). This marker of denervation is present in myoblasts and myotubes (14,15) but is undetectable in normally innervated adult muscles. It increases in old compared with young rodents (14,18,66), and its expression has been recently reported in aging human skeletal muscle (20). Here, we show that RT reduces NCAM1, RUNX1, and CHNRGγ transcripts at the whole muscle level and NCAM protein in type I fibers from obese older adults, indicating that its immunoreactivity with fiber membranes in muscle cross-sections provides a sensitive and dynamic way to track muscle fiber denervation (67,68). NCAM is not only expressed on the surface of denervated myofibers, it also appears on neural cells and projections of nerve terminals in our muscle cross-sections. The combination of contraction-force recordings and muscle immunostaining for NCAM has shown a significant number of denervated fibers in old rats, accounting for a significant fraction of the decline in muscle specific force (force normalized to muscle CSA) (18).
Our data show that a fraction of both type I and type II fibers are denervated in obese older adults. Type I denervated fibers rarely show the degree of atrophy found in type II fibers. Consistent with the literature, we infrequently see significant atrophy in type I myofibers with the typical angular shape (7,20). Denervated type I fibers show a homogenous NCAM+ staining pattern, whereas type II fibers show NCAM immunoreactivity either at their surface sarcolemma (contour) or across the whole myofiber section (homogenous). Note that, for no obvious reason, type II fibers exhibiting a contour pattern are smaller than those with a homogenous pattern. NCAM is a membrane glycoprotein, and perhaps denervation results in NCAM expression not only at the surface but in sarcolemmal T tubules. T-tubule immunoreaction may be reduced in atrophic type II fibers with advanced denervation. We do not know whether the progression of type I fiber denervation would show a NCAM contour pattern because we have not detected small, atrophic, denervated type I fibers.
Many approaches have been used to determine and to track the magnitude of human skeletal muscle denervation, focusing on angular/atrophic fibers, centrally nucleated myofibers, and fiber grouping. However, rapid responses to myofiber denervation and reinnervation are limited by slow changes in molecular markers or motor unit remodeling (13). Immunostaining for proteins that are upregulated with denervation, such as the tetrodotoxin-resistant sodium channel and immature myosin (MYH3), have worked successfully in rodent muscle (16) but not in human muscle, at least in our hands. Adding NCAM and genes activated by denervation to the classical battery can improve diagnosis and tracking of muscle denervation/reinnervation.
Future studies should examine whether a normal weight, age-matched cohort retains a “younger” muscle innervation pattern and determine the mechanism(s) that RT triggers to improve muscle innervation.
RT and SCs
NCAM immunostaining served a dual function in this investigation: it identified both denervated myofibers and SCs (69). To confirm the presence of SCs, we counterimmunostained the muscle sections with Pax7, a classical SC transcription factor (70). Exercise and trauma elicit skeletal muscle repair based strongly on the endogenous SC population (71). SCs are the resident stem cells in growing and adult skeletal muscle fibers, located between the plasmalemma and surrounding basal lamina (72). When new myonuclei are required, SCs proliferate and differentiate. They either fuse to existing myofibers or with each other to form new myotubes (73). Short-term denervation stimulates them to enter the mitotic cycle, whereas long-term denervation depletes them. Chronic denervation induces resistance to recruitment into the mitotic cycle, and their nonproliferative compartment increases robustly (74). Aging is associated with a decline in the number and function of muscle progenitor cells and their ability to self-repair (75,76). Therefore, analyzing the effects of RT on total number of SCs and their physical relationship with myofiber subtypes is critical.
We found that RT did not significantly modify the number of SCs. Also, more SCs associate with type I fibers than with type II fibers, and this difference remains significant after RT. Although the trend of decreasing SCs associated with type I fibers was obvious, it was not statistically significant. In summary, the RT intervention did not modify the number of SCs nor their relationship with myofiber subtypes. Whether obesity shuts down the response to the cellular and molecular program triggered by RT is unknown. SC proliferation with RT has been observed in younger people (77,78).
Determining the number of SCs associated with innervated and denervated myofibers will clarify the regenerative capacity of fibers and the nerve-dependent regulation of the myofiber/SC association. The fact that almost no SCs were associated with NCAM+ myofibers strongly suggests that fiber innervation regulates this association.
Limitations
The nature of the study design (eg, without a non-RT control group) cannot discern causation or the direction of the observed associations. Also, the study was not large enough to examine sex differences in the relationships among fiber grouping, NCAM+ fibers, SCs, and muscle strength. To determine whether obesity is a determining factor in muscle denervation requires examination of an age-matched, normal weight cohort.
The reported increase in single muscle fiber force and power with RT (35,36), despite no increase in CSA, as reported here and previously (36), indicates that RT improves muscle contraction efficiency, probably by enhancing fiber reinnervation and preventing denervation with aging. The present study is the first to examine whether a short-term RT intervention can modify the innervation status of skeletal muscle from obese older adults. Our data further elucidate the role of denervation in aging-related loss of muscle and muscle strength. We conclude that RT did improve myofiber innervation, although it had no effect on SCs. Results suggest that RT has a beneficial impact on factors that determine skeletal muscle structure, composition, and function, such as innervation, even when started late in life by sedentary obese older adults.
Funding
This work was supported by the National Institutes of Health grants R01AG020583 to B.N., AG13934 and AG15820 to O.D. and the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332)
Acknowledgments
The authors thank Dr. Mary Lyles, Heather Gregory and John Stones for performing, collecting, and distributing the muscle biopsies.
References
- 1. Stålberg E, Fawcett PR. Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiatry. 1982;45:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi:10.1152/japplphysiol.00347.2003 [DOI] [PubMed] [Google Scholar]
- 3. Lexell J, Downham DY. The occurrence of fibre-type grouping in healthy human muscle: a quantitative study of cross-sections of whole vastus lateralis from men between 15 and 83 years. Acta Neuropathol. 1991;81:377–381. [DOI] [PubMed] [Google Scholar]
- 4. Ling SM, Conwit RA, Ferrucci L, Metter EJ. Age-associated changes in motor unit physiology: observations from the Baltimore Longitudinal Study of Aging. Arch Phys Med Rehabil. 2009;90:1237–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lexell J, Taylor CC, Sjostrom M. What is the cause of ageing atrohy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 6. Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol (1985). 1993;74:868–874. [DOI] [PubMed] [Google Scholar]
- 7. Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. 1997;127:1011S–1013S. [DOI] [PubMed] [Google Scholar]
- 8. Payne AM, Delbono O. Neurogenesis of excitation-contraction uncoupling in aging skeletal muscle. Exerc Sport Sci Rev. 2004;32:36–40. [DOI] [PubMed] [Google Scholar]
- 9. Delbono O. Neural control of aging skeletal muscle. Aging Cell. 2003;2:21–29. [DOI] [PubMed] [Google Scholar]
- 10. Buller AJ, Eccles JC, Eccles RM. Differentiation of fast and slow muscles in the cat hind limb. J Physiol. 1960;150:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960;150:417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greensmith L, Vrbová G. Motoneurone survival: a functional approach. Trends Neurosci. 1996;19:450–455. [DOI] [PubMed] [Google Scholar]
- 13. Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45:397–458. [DOI] [PubMed] [Google Scholar]
- 14. Andersson AM, Olsen M, Zhernosekov D, et al. Age-related changes in expression of the neural cell adhesion molecule in skeletal muscle: a comparative study of newborn, adult and aged rats. Biochem J. 1993;290 (Pt 3):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gosztonyi G, Naschold U, Grozdanovic Z, Stoltenburg-Didinger G, Gossrau R. Expression of Leu-19 (CD56, N-CAM) and nitric oxide synthase (NOS) I in denervated and reinnervated human skeletal muscle. Microsc Res Tech. 2001;55:187–197. [DOI] [PubMed] [Google Scholar]
- 16. Wang ZM, Zheng Z, Messi ML, Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol. 2005;565(Pt 3):757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messi ML, Delbono O. Target-derived trophic effect on skeletal muscle innervation in senescent mice. J Neurosci. 2003;23:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Urbancheck MG, Picken EB, Kalliainen LK, Kuzon WM. Specific force deficit in skeletal muscles of old rats is partially explained by the existence of denervated muscle fibers. J Gerontol: Biol Sci. 2001;56A:B191–B198. [DOI] [PubMed] [Google Scholar]
- 19. Valdez G, Tapia JC, Kang H, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–14868. doi:10.1073/pnas.1002220107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mosole S, Carraro U, Kern H, et al. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol. 2014;73:284–294. doi:10.1097/NEN.0000000000000032 [DOI] [PubMed] [Google Scholar]
- 21. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi:10.1242/dev.067595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCarthy JJ, Mula J, Miyazaki M, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi:10.1242/dev.068858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi:10.1242/dev.064162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sambasivan R, Yao R, Kissenpfennig A, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi:10.1242/dev.067587 [DOI] [PubMed] [Google Scholar]
- 25. Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. [DOI] [PubMed] [Google Scholar]
- 26. Roth SM, Martel GF, Ivey FM, et al. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2001;56:B240–247. [DOI] [PubMed] [Google Scholar]
- 27. Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:E937–E946. [DOI] [PubMed] [Google Scholar]
- 28. Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985). 2008;104:1736–1742. doi:10.1152/japplphysiol. 01215.2007 [DOI] [PubMed] [Google Scholar]
- 29. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle: from birth to old age. Age. 2014;36:545–547. doi:10.1007/s11357-013-9583-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verdijk LB, Gleeson BG, Jonkers RA, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–339. doi:10.1093/gerona/gln050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joanisse S, Gillen JB, Bellamy LM, et al. Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J. 2013;27:4596–4605. doi:10.1096/fj.13-229799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101:991–999. doi:10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Rourke KS, Ike RW. Muscle biopsy. Curr Opin Rheumatol. 1995;7:462–468. [DOI] [PubMed] [Google Scholar]
- 34. Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol (1985). 2001;90:2070–2074. [DOI] [PubMed] [Google Scholar]
- 35. Zhang T, Choi SJ, Wang ZM, et al. Human slow troponin T (TNNT1) pre-mRNA alternative splicing is an indicator of skeletal muscle response to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:1437–1447. doi:10.1093/gerona/glt204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang T, Birbrair A, Wang ZM, et al. Improved knee extensor strength with resistance training associates with muscle specific miRNAs in older adults. Exp Gerontol. 2015;62:7–13. doi:10.1016/j.exger.2014.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 38. Lindström M, Thornell LE. New multiple labelling method for improved satellite cell identification in human muscle: application to a cohort of power-lifters and sedentary men. Histochem Cell Biol. 2009;132:141–157. doi:10.1007/s00418-009-0606-0 [DOI] [PubMed] [Google Scholar]
- 39. Ibebunjo C, Chick JM, Kendall T, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33:194–212. doi:10.1128/MCB.01036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, Blagden C, Fan J, et al. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev. 2005;19:1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daniloff JK, Levi G, Grumet M, Rieger F, Edelman GM. Altered expression of neuronal cell adhesion molecules induced by nerve injury and repair. J Cell Biol. 1986;103:929–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rieger F, Grumet M, Edelman GM. N-CAM at the vertebrate neuromuscular junction. J Cell Biol. 1985;101:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi:10.1146/annurev.cellbio.13.1.425 [DOI] [PubMed] [Google Scholar]
- 44. Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi:10.1038/nrn2285 [DOI] [PubMed] [Google Scholar]
- 45. Terman A, Brunk UT. On the degradability and exocytosis of ceroid/lipofuscin in cultured rat cardiac myocytes. Mech Ageing Dev. 1998;100:145–156. [DOI] [PubMed] [Google Scholar]
- 46. O’Leary MF, Vainshtein A, Iqbal S, Ostojic O, Hood DA. Adaptive plasticity of autophagic proteins to denervation in aging skeletal muscle. Am J Physiol Cell Physiol. 2013;304:C422–C430. doi:10.1152/ ajpcell.00240.2012 [DOI] [PubMed] [Google Scholar]
- 47. Lee JD, Fry CS, Mula J, et al. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J Gerontol A Biol Sci Med Sci. 2015. doi:10.1093/gerona/glv033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. [DOI] [PubMed] [Google Scholar]
- 49. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. [DOI] [PubMed] [Google Scholar]
- 50. Lowrie MB, Vrbová G. Different pattern of recovery of fast and slow muscles following nerve injury in the rat. J Physiol. 1984;349:397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi SJ, Harii K, Asato H, Ueda K. Aging effects in skeletal muscle recovery after reinnervation. Scand J Plast Reconstr Surg Hand Surg. 1996;30:89–98. [DOI] [PubMed] [Google Scholar]
- 52. Kullberg S, Ramirez-Leon V, Johnson H, Ulfhake B. Decreased axosomatic input to motoneurons and astrogliosis in the spinal cord of aged rats. J Gerontol A Biol Sci Med Sci. 1998;53:B369–B379. [DOI] [PubMed] [Google Scholar]
- 53. Bergman E, Kullberg S, Ming Y, Ulfhake B. Upregulation of GFRalpha-1 and c-ret in primary sensory neurons and spinal motoneurons of aged rats. J Neurosci Res. 1999;57:153–165. [DOI] [PubMed] [Google Scholar]
- 54. Caroni P, Aigner L, Schneider C. Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction. J Cell Biol. 1997;136:679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Delbono O, O’Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148:211–222. [DOI] [PubMed] [Google Scholar]
- 57. Wang ZM, Messi ML, Delbono O. L-Type Ca(2+) channel charge movement and intracellular Ca(2+) in skeletal muscle fibers from aging mice. Biophys J. 2000;78:1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiménez-Moreno R, Wang ZM, Gerring RC, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J. 2008;94:3178–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci. 2011;4:248–259. doi:10.2174/1874609811104030248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. González E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol. 2000;178:175–183. [DOI] [PubMed] [Google Scholar]
- 61. Choi SJ, Shively CA, Register TC, et al. Force generation capacity of single muscle fibers of vastus lateralis declines with age and correlates with physical function in African green vervet monkeys. J Gerontol Biol Sci. 2013;68:258–267. doi:10.1093/gerona/gls143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Y, Lee Yi, Thompson WJ. Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse. J Neurosci. 2011;31:14910–14919. doi:10.1523/JNEUROSCI.3590-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6:e28090. doi:10.1371/journal.pone.0028090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Larkin LM, Kuzon WM, Halter JB. Effects of age and nerve-repair grafts on reinnervation and fiber type distribution of rat medial gastrocnemius muscles. Mech Ageing Dev. 2003;124:653–661. doi:10.1016/s0047-6374(02)00190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Illa I, Leon-Monzon M, Dalakas MC. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992;31:46–52. doi:10.1002/ana.410310109 [DOI] [PubMed] [Google Scholar]
- 66. Kobayashi H, Robbins N, Rutishauser U. Neural cell adhesion molecule in aged mouse muscle. Neuroscience. 1992;48:237–248. doi:10.1016/0306-4522(92)90352-3 [DOI] [PubMed] [Google Scholar]
- 67. Covault J, Sanes JR. Neural cell adhesion molecule (NCAM) accumulates in denervated and paralysed skeletal muscles. Proc Natl Acad Sci U S A. 1985;82:4544–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gordon T, Ly V, Hegedus J, Tyreman N. Early detection of denervated muscle fibers in hindlimb muscles after sciatic nerve transection in wild type mice and in the G93A mouse model of amyotrophic lateral sclerosis. Neurol Res. 2009;31:28–42. [DOI] [PubMed] [Google Scholar]
- 69. Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM. Reparative myogenesis in long-term denervated skeletal muscles of adult rats results in a reduction of the satellite cell population. Anat Rec. 2001;263:139–154. doi:10.1002/ar.1087 [DOI] [PubMed] [Google Scholar]
- 70. Kuang S, Chargé SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi:10.1083/jcb.200508001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi:10.1634/stemcells.2006-0372 [DOI] [PubMed] [Google Scholar]
- 72. Di Mauro A. Satellite cells of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pallafacchina G, Blaauw B, Schiaffino S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis. 2013;23(suppl 1):S12–S18. doi:1016/j.numecd.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 74. Kuschel R, Yablonka-Reuveni Z, Bornemann A. Satellite cells on isolated myofibers from normal and denervated adult rat muscle. J Histochem Cytochem. 1999;47:1375–1384. [DOI] [PubMed] [Google Scholar]
- 75. Renault V, Thorne L-E, Eriksson P-O, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi:10.1046/j.1474-9728.2002.00017.x [DOI] [PubMed] [Google Scholar]
- 76. Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi:10.1016/j.ydbio.2006.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Crameri RM, Langberg H, Magnusson P, et al. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004;558:333–340. doi:10.1113/jphysiol.2004.061846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mackey AL, Kjaer M, Charifi N, et al. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve. 2009;40:455–465. doi:10.1002/mus.21369 [DOI] [PubMed] [Google Scholar]