Abstract
Common cyclooxygenase (COX)-inhibiting drugs enhance resistance exercise induced muscle mass and strength gains in older individuals. The purpose of this investigation was to determine whether the underlying mechanism regulating this effect was specific to Type I or Type II muscle fibers, which have different contractile and metabolic profiles. Muscle biopsies (vastus lateralis) were obtained before and after 12 weeks of knee-extensor resistance exercise (3 days/week) from healthy older men who consumed either a placebo (n = 8; 64±2 years) or COX inhibitor (acetaminophen, 4 gram/day; n = 7; 64±1 years) in double-blind fashion. Muscle samples were examined for Type I and II fiber cross-sectional area, capillarization, and metabolic enzyme activities (glycogen phosphorylase, citrate synthase, β-hydroxyacyl-CoA-dehydrogenase). Type I fiber size did not change with training in the placebo group (304±590 μm2) but increased 28% in the COX inhibitor group (1,388±760 μm2, p < .1). Type II fiber size increased 26% in the placebo group (1,432±499 μm2, p < .05) and 37% in the COX inhibitor group (1,825±400 μm2, p < .05). Muscle capillarization and enzyme activity were generally maintained in the placebo group. However, capillary to fiber ratio increased 24% (p < .1) and citrate synthase activity increased 18% (p < .05) in the COX inhibitor group. COX inhibitor consumption during resistance exercise in older individuals enhances myocellular growth, and this effect is more pronounced in Type I muscle fibers.
Keywords: Cyclooxygenase, Prostaglandin, Fiber-type specific, Sarcopenia
Daily cyclooxygenase (COX) inhibitor consumption enhances the gains in muscle mass and strength obtained during 12 weeks of resistance exercise in older individuals (1). Subsequent studies have started to delineate the underlying mechanism for this effect, which appears to be related to the prostaglandin/COX pathway effects on cellular regulators of protein synthesis and degradation (2–4). However, it is unclear whether this mechanism regulates myocellular metabolism and adaptations differently between slow (Type I) and fast (Type II) muscle fibers. Indeed, slow and fast muscle-specific regulation of prostaglandin production, and the associated protein metabolism response, has been shown in animals (5,6). Recent evidence also suggests that human Type I and Type II muscle fibers have different levels of the PGE2/COX pathway enzymes and receptors (7). Thus, an orally consumed prostaglandin/COX inhibitor may differentially influence the exercise training adaptations in Type I and Type II muscle fibers in humans.
A muscle fiber type–specific COX inhibitor effect on exercise training adaptations could be important, considering the substantial differences in the contractile function and metabolic profile of Type I and Type II muscle fibers (8–11). Type I fibers have a greater mitochondrial capacity and capillary blood supply (8,9), and a COX inhibitor effect specific to this fiber type may be beneficial for the reduced skeletal muscle endurance and increased fatigability associated with sarcopenia (12). Conversely, Type II fibers produce substantially more power than Type I fibers (10,11), and a Type II fiber type–specific effect may be beneficial for targeting a key component of sarcopenia, the loss of skeletal muscle strength and power (12). Of course, enhancement of myocellular adaptations in a nonfiber type–specific manner could be beneficial for both skeletal muscle metabolism and contractile performance.
The purpose of the current investigation was to examine whether the previously reported COX inhibitor enhancement of whole muscle growth in response to resistance training in older individuals (1) was targeted to Type I or Type II muscle fibers. Based on the existing human and animal data related to the PGE2/COX pathway and the mixed fiber type distribution in human muscle, we hypothesized that the COX inhibitor would influence the hypertrophic adaptations to resistance training in both fiber types, with a preferential impact on the Type I muscle fibers. A secondary purpose was to determine whether the COX inhibitor effects on muscle also influenced the capillary or the metabolic enzyme adaptations to resistance exercise training.
Materials and Methods
Overall Study Design and Subject Characteristics
This study was a randomized, placebo-controlled, double-blind, 12-week investigation that examined a subset of male participants from a previous investigation (1). During the 12 weeks, participants completed a progressive resistance training program of the knee-extensor muscles three times per week and consumed a placebo (n = 8; 64±2 years, range: 60–73 years) or COX inhibitor (acetaminophen 4g/day; n = 7; 64±1 years, range: 60–70 years). After approval by the Institutional Review Board, all study procedures, risks, and benefits were explained to the participants before giving written consent to participate. Methodological details and related findings from the larger study have been presented previously (1), and relevant details are replicated later in this article. Other skeletal muscle and tendon related findings have also been published on this cohort (4,13).
Screening and Exclusion Criteria
Participants completed a medical screening exam, which included routine blood and urine clinical chemistries, a resting and exercise electrocardiogram, and a detailed health and exercise history questionnaire. Participants were excluded if they had any cardiac, orthopedic, or neuromuscular conditions that would preclude them from participating in a resistance exercise training program, abnormal blood or urine chemistries, arthritis, diabetes, uncontrolled hypertension, or any condition that would be a contraindication to taking acetaminophen for 3 months, if they were chronically consuming any prescription or nonprescription COX-inhibiting drugs, if they were involved in any formal aerobic or resistance exercise training program, if they smoked, or if they were younger than 60 or older than 85 years of age.
Resistance Exercise Training Protocol
All participants completed a progressive resistance exercise training program of bilateral knee extension that was designed to hypertrophy and strengthen the m. quadriceps femoris (14–16), using a protocol employed for several previous investigations in our laboratory (16). Each participant was scheduled for resistance training three times per week over the 12 weeks for a total of 36 sessions on an isotonic knee extension device (Cybex Eagle, Medway, MA). All sessions were supervised by a member of the research team. Each session was separated by at least 1 day and consisted of 5 minutes of light cycling (828E, Monark Exercise AB, Vansbro, Sweden), two sets of five knee extensions at a light weight, followed by three sets of 10 repetitions with 2 minutes of rest between sets. Training intensity was based on each individual’s one repetition maximum (1RM) and adjusted during the training based on each individual’s training session performance and biweekly 1RM. Compliance to the resistance training program was excellent, with all individuals completing 100% of their scheduled training sessions (1). In addition, training intensity (%1RM) and training load were similar between both groups (1).
COX Inhibitor Consumption
The COX inhibitor was administered in double-blind, placebo-controlled fashion, as we have previously described (17,18), in three doses/day (~8 AM, ~2 PM, ~8 PM) corresponding to the maximal over-the-counter daily dose (Acetaminophen: 1,500mg, 1,500mg, 1,000mg, 4,000mg total). The placebo group was given an identical number of pills/dose (three), which were indistinguishable from the drug doses. Each participant was given their doses in weekly batches (21 doses) in pillboxes labeled with the date and consumption time. At the end of each week, participants were asked to return all of the pillboxes. Subjects were instructed to not consume any other COX-inhibiting drugs outside of the study.
Compliance with the requested drug consumption was completed in two ways, direct and indirect. Direct compliance was determined by a member of the research team watching the participants consume their dose in person while at their scheduled training session (3 doses/week) or by personal digital video (18 doses/week), as previously described (17). Each participant was provided a small camera that allowed them to video record, by virtue of a rotating lens feature, the consumption of each dose. Each video was automatically time and date stamped, downloaded to a laboratory computer, and watched by a research team member to confirm dose consumption. Indirect compliance was monitored by counting the number of pills remaining in the pillboxes returned by the participants each week. Overall compliance was nearly 100% for both groups, as previously presented and discussed (1).
Potential side effects of drug consumption were monitored via monthly blood draws for renal (creatinine), hepatic (alanine aminotransferase), and hematologic (hematocrit) measures. There were no measured changes in these markers during the 12 weeks, as previously presented (1).
Muscle Volume and Muscle Strength
Knee extensor (m. quadriceps femoris) muscle volume was measured with MRI before and at the end of the 12-week period as we have previously described in detail for sarcopenia studies of aging and chronic bed rest (19,20). Subjects rested in the supine horizontal position for 1 hour prior to scanning to prevent the influence of fluid shifts on muscle volume (21). No exercise or strenuous activity was allowed within 24 hours of scanning, which was completed at the same time of day for each participant using a non-metallic foot restraint to control joint angle (muscle length) and leg compression. Imaging was completed in a 1.5T scanner (Genesis Signa, GE Medical Systems, Milwaukee, WI) using serial interleaved images 8-mm thick (TR: 2,000ms, TE: 9.0ms, 512×512 matrix, field of view: 480×480mm). MR images were transferred electronically from the scanner to a personal computer (iMac G5) at the Human Performance Laboratory and analyzed with NIH Image software (Image J, version 1.34h) using manual planimetry. A detailed description of the manual planimetry measurements and associated variability has been presented previously (19,20). The cross sectional area (CSA; cm2) of the muscle(s) of interest in a given slice was determined and the muscle volume (cm3) was calculated by multiplying the CSA by the slice thickness. The right limb of each participant was used for all measurements. Measurements were completed by the same investigator in blinded fashion.
Muscle strength was measured thrice prior to training and twice during the final week of the 12-week training period by determining the maximum amount of weight each participant could lift through a full range of motion one time (ie, 1RM) on the resistance exercise training device (16). The highest weight lifted before and at the end of the 12 weeks was considered the pre- and posttraining 1RM.
Muscle Biopsy
Subjects underwent a muscle biopsy (22) of the m. vastus lateralis before and at the end of the resistance training and drug intervention in the basal state (ie, no testing or training was completed for 3 days prior to each biopsy). Biopsies were obtained in the early morning (~7 AM) after at least 30 minutes of supine rest and after an overnight fast of ~12 hours, as previously described (1). Following the biopsy, a portion of the muscle to be used for muscle fiber size and capillarization measurements was oriented longitudinally in a mounting medium (tragacanth gum, Sigma, St. Louis, MO) atop a cork, frozen in isopentane cooled in liquid nitrogen, and subsequently stored in liquid nitrogen until analysis. A portion of the muscle to be used for enzyme analysis was immediately frozen and stored in liquid nitrogen until analysis. Analyses were completed on 14 individuals (minus one participant from the COX inhibitor group) for muscle fiber size and capillarization and on all 15 individuals for enzyme activities.
Muscle Fiber Size, Fiber Type, and Capillarity
For each biopsy sample, transverse sections (10 µm) for histochemical analysis were cut on a microtome-cryostat (HM 525, Microm, Walldorf, Germany) at −20°C. Separate serial sections were cut for adenosine triphosphatase (ATPase) (fiber type and size), reduced nicotinamide adenine dinucleotide (NADH) (fiber size), and periodic acid-Schiff (capillarity) staining. ATPase staining was completed with a 5-minute preincubation at pH 4.3 (~22°C), followed by a 45-minute incubation at pH 9.4 (37°C) (23). NADH staining was completed with a 30-minute incubation (37°C) in a tetrazolium salt (nitro-blue) and NADH (24). Capillary staining was completed with a 5-minute fixation in Carnoy’s, followed by a 30-minute incubation (37°C) in 1% amylase, and then periodic acid-Schiff staining (25). Section images were captured with a microscope (BX51, Olympus, Tokyo, Japan) and camera (DP30BW or DP26, Olympus) interfaced to a computer with specialized software (MicroSuite Biological Software Suite, Olympus). Images were calibrated with a micrometer calibration slide and analyzed on a Macintosh computer with NIH ImageJ (1.46) software. All images for a given section were stitched together to recreate the complete section, which allowed for muscle fibers from each stain to be annotated and crossreferenced for comparison as needed.
Muscle fiber CSA was determined on 50 Type I and 50 Type II fibers from an artifact-free region of both the ATPase and NADH stained sections. The ATPase and NADH staining methods were both used for fiber size determination to provide independent assessments of this primary outcome measure, as the chemistries associated with staining can differentially influence fiber size. Fiber type of the fibers analyzed for both stains was taken from the ATPase stain, based on the nomenclature of Brooke and Kaiser (26). Type II fibers were not categorized into subtypes. Fibers were circumscribed by the same investigator in triplicate, and the average coefficient of variation for the triplicate measurements of both fiber types on both stains was less than 1.8%. As expected, fiber areas with NADH staining were somewhat larger (~8%) than those obtained with ATPase staining. However, the absolute and relative fiber size changes with the exercise and drug intervention were similar between the two staining methods. Thus, the two methods confirmed each other, and the ATPase data were used to represent the responses of the groups.
Capillary density and capillary to fiber ratio were determined on four separate 300 µm2 artifact-free regions. Capillary density was calculated as the number of capillaries in this defined area (capillaries/mm2). Capillary to fiber ratio was calculated as the number of capillaries divided by the number of muscle fibers in this defined area. Muscle fiber type–specific capillarization was determined by counting the number of capillaries in contact with each fiber used for Type I and Type II muscle fiber size analysis, which also allowed for the normalization to CSA (capillaries/µm2 × 10−3, where 1.0 would represent 1 capillary per 1,000 µm2 of fiber area). All capillary measurements were completed by two independent investigators and averaged to represent each sample. Collectively, these different methodological approaches provide a comprehensive view of muscle capillarization that no one method can capture alone (9,27–30).
Muscle Enzyme Activity
Glycolytic and oxidative enzyme activities were determined from a ~10-mg portion of each muscle biopsy. Muscle samples were weighed at −25°C (Cahn C-35; Orion Research, Beverly, MA) and homogenized using a motorized ground-glass homogenizer (Duall; Kimble Chase, Vineland, NJ) in 100 volumes of cold buffer (20mM K2HPO4, 5mM 2-mercaptoethanol, 0.5mM ethylenediaminetetraacetic acid (EDTA), 0.02% bovine serum albumin (BSA), 50% glycerol, pH 7.4). Glycogen phosphorylase activity was determined fluorometrically from the production of reduced nicotinamide adenine dinucleotide phosphate (NADPH) (8). Citrate synthase activity was determined spectrophotometrically through the reduction of DTNB (5,5′-dithiobis-2-nitrobenzoate) by the release of CoA-SH in the cleaving of acetyl-CoA (31). β-hydroxyacyl-CoA dehydrogenase (β-HAD) was determined fluorometrically from the appearance of NAD+ (32). All assays were determined in triplicate for each sample.
Statistical Analysis
Pre- to postresistance training and drug intervention, as well as group comparisons, were analyzed with a t test and corrected for multiple comparisons using the Bonferroni correction, when appropriate. Data are presented as means ± SE.
Results
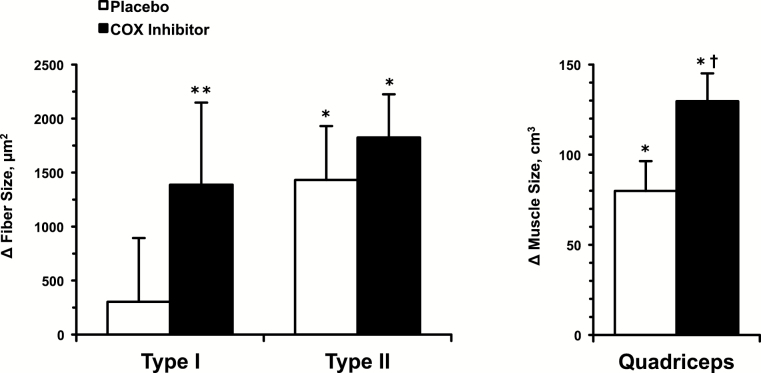
Quadriceps muscle size and strength were similar between the placebo (1,048±88cm3, 89.8±4.1kg) and COX inhibitor (1,033±51cm3, 86.3±5.8kg) groups at the beginning of the resistance training and drug interventions. These data and the response over the 12 weeks have been presented for the larger cohort in detail previously (1). Muscle fiber size changes in the two groups corresponded with the difference in quadriceps muscle size changes over the 12 weeks (Figure 1). In the placebo group, Type I muscle fiber size did not increase (Pre: 5,856±223, Post: 6,160±625 μm2), whereas Type II fiber size increased 26% (Pre: 5,567±525, Post: 6,999±735 μm2; p < .05) over the 12 weeks of training. In the COX inhibitor group, Type I (Pre: 5,128±318, Post: 6,517±788 μm2; p < .1) and Type II (Pre: 5,037±517, Post: 6,862±723 μm2; p < .05) muscle fiber size increased 28% and 37%, respectively (Figure 1).
Figure 1.
Change in Type I and Type II muscle fiber size (left) and quadriceps muscle size (MRI determined volume) (right) from the beginning to the end of the 12-week resistance exercise training and drug interventions. The quadriceps muscle size data have been presented for the larger cohort previously (1). *p < .05 vs pre. **p < .1 vs pre. † p < .05 vs placebo.
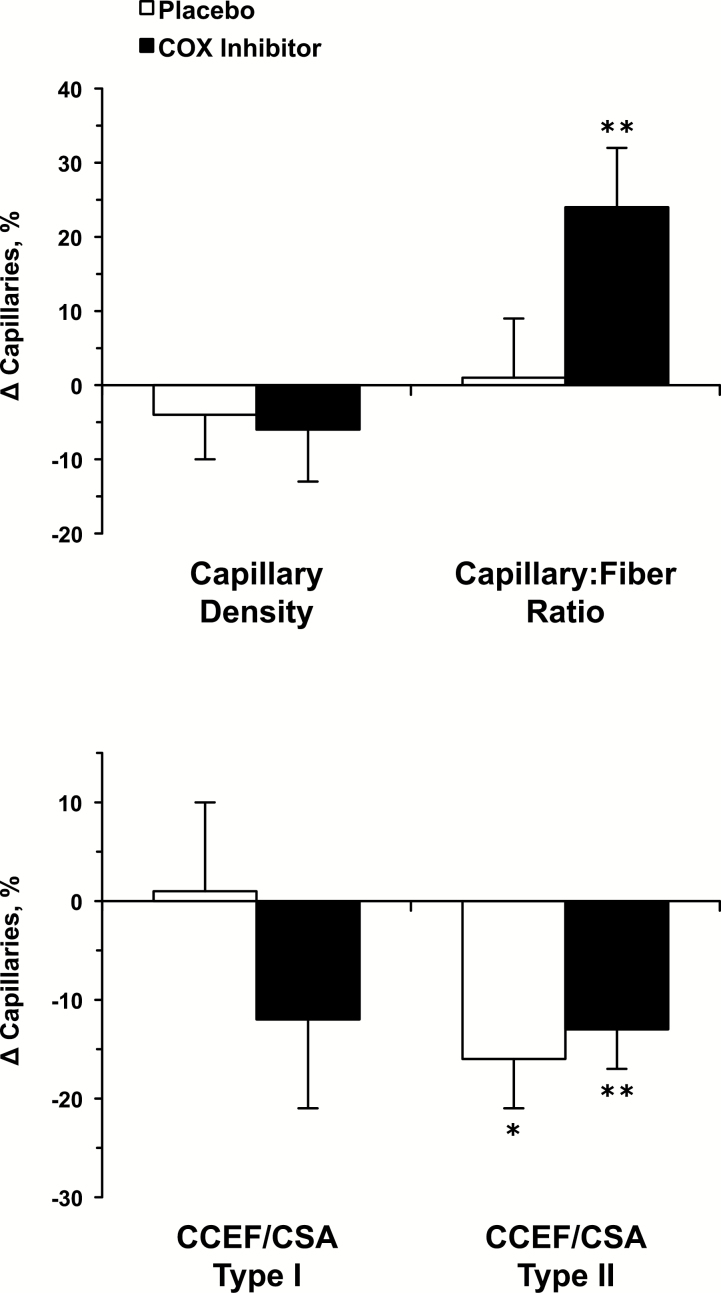
Muscle fiber type–independent and fiber type–specific capillary changes over the 12 weeks are presented in Figure 2. Capillary density was maintained over the 12 weeks in the placebo (Pre: 337±19, Post: 320±19 capillaries/mm2) and COX inhibitor (Pre: 330±17, Post: 305±14 capillaries/mm2) groups. Capillary to fiber ratio was also unchanged in the placebo group (Pre: 2.1±0.2, Post: 2.1±0.2), whereas it increased in the COX inhibitor group (Pre: 1.7±0.2, Post: 2.0±0.2; p < .1). The number of capillaries in contact with each fiber averaged 4.9±0.2 for Type I fibers and 4.1±0.2 for Type II fibers (p < .05) across both groups. Capillary changes generally reflected the change in fiber size over the 12 weeks when the number of capillaries in contact with each fiber was normalized to fiber CSA for the Type I (Pre: 0.90±0.05, Post: 0.89±0.06 capillaries/µm2 × 10−3) and Type II (Pre: 0.81±0.07, Post: 0.68±0.07 capillaries/µm2 × 10-3; p < .05) fibers in the placebo group. This response also held for the Type I (Pre: 0.91±0.06, Post: 0.78±0.06 capillaries/µm2 × 10−3) and Type II (0.76±0.06, Post: 0.65±0.03 capillaries/µm2 × 10−3; p < .1) fibers in the COX inhibitor group.
Figure 2.
Change in fiber type–independent (top) and fiber type–specific (bottom) muscle capillarization from the beginning to the end of the 12-week resistance exercise training and drug interventions. CCEF = capillaries in contact with each fiber; CSA = cross-sectional area. *p < .05 vs pre. **p < .1 vs pre.
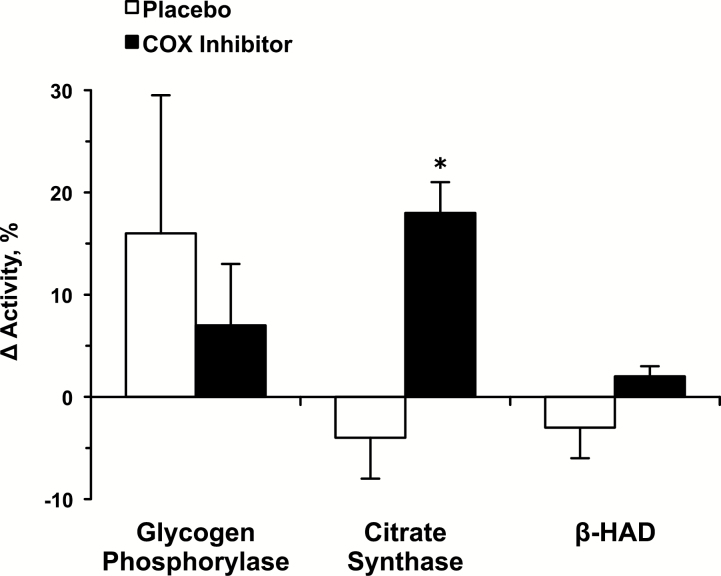
Glycogen phosphorylase (Pre: 29.8±3.5, Post: 31.9±1.5 µmol/min/g), citrate synthase (Pre: 22.6±1.9, Post: 21.3±1.0 µmol/min/g), and β-HAD (Pre: 26.9±0.9, Post: 26.2±1.0 µmol/min/g) enzyme activity levels were maintained over the 12 weeks in the placebo group (Figure 3). In the COX inhibitor group, glycogen phosphorylase (Pre: 32.4±2.4, Post: 33.9±1.7 µmol/min/g) and β-HAD (Pre: 22.7±1.3, Post: 23.3±1.5 µmol/min/g) were maintained, whereas citrate synthase (Pre: 15.9±0.9, Post: 18.6±0.8 µmol/min/g; p < .05) increased over the 12 weeks (Figure 3).
Figure 3.
Change in glycolytic and oxidative muscle enzyme activities from the beginning to the end of the 12-week resistance exercise training and drug interventions. β-HAD = β-hydroxyacyl-CoA dehydrogenase. *p < .05 vs pre.
Discussion
The goal of this investigation was to gain a better understanding of the COX inhibitor effects on human skeletal muscle adaptations to exercise at the muscle fiber level. In general, the COX inhibitor enhancement of whole muscle growth was reflected at the myocellular level. The Type I and Type II muscle fiber size responses in the placebo group suggest that their whole muscle growth of 8% was supported primarily by the 26% growth of the Type II fibers. This Type II hypertrophy response to resistance exercise is in agreement with previous studies (33–36), but other myofiber responses have been observed (15,37–39). In contrast to the placebo group, the 13% whole muscle growth in the COX inhibitor group was driven by the 37% hypertrophy of the Type II fibers, as well as the 28% hypertrophy of the Type I fibers. Evidence from the larger cohort suggests that the augmented muscle growth was primarily mediated by a reduction in intramuscular PGE2 and resultant PGE2 receptor downstream signaling effects (2–4). Specifically, the COX inhibitor appeared to reduce the negative effects of PGE2 on protein synthesis and degradation, working through established myokines and other cellular regulators of protein turnover (2–4). The myocellular findings from the current study suggest that these effects were more pronounced in the Type I fibers, possibly due to a more active PGE2/COX pathway in this fiber type.
Greater PGE2/COX pathway activity could be the result of increased arachidonic acid availability, increased PGE2-producing enzyme activity, increased PGE2 receptor density and downstream signaling, or a combination of these factors. A more active pathway in the Type I fibers from the current study is consistent with some animal data from prostaglandin/COX pathway studies of isolated slow and fast muscles (5,6). When incubated with the same dose of arachidonic acid, the lipid substrate for the COX enzyme, PGE2 production in mice (6) and PGE2 stimulation of protein degradation in rats (5) have been shown to be ~100% higher in the slow soleus compared with the fast extensor digitorum longus. In addition, recent studies of human skeletal muscle show that the predominately Type I soleus has an increased capacity to produce PGE2 due to higher levels of COX-1 and PGE2 synthase enzymes (7). PGE2 receptor levels also appear to be fiber type specific (7), although it is unclear which of the four PGE2 receptor subtypes are associated with the previously described PGE2 effects on skeletal muscle adaptations.
Prostaglandins produced in muscle fibers work locally in an autocrine and paracrine fashion. Given the interspersed Type I and II muscle fiber distribution and shared interstitial space in most human skeletal muscles, including the vastus lateralis studied in the current investigation, prostaglandin crosstalk between fiber types is a possibility. In support of this concept, the average Type II fiber growth in the COX inhibitor group was 27% more than that in the placebo group, albeit nonsignificant. This effect could have been the result of crosstalk from the Type I fibers or a direct COX inhibitor effect on these fibers, as Type II fibers in both animals and humans have an active prostaglandin/COX pathway (5–7). Nonetheless, it appears that the PGE2/COX pathway is more active and that the resultant effects of COX inhibition are greater in Type I fibers of humans.
Evidence from the larger cohort also suggested an additional mechanism for the COX inhibitor–induced supplemental growth, working through PGF2α receptor and protein synthesis upregulation (3,4). Although speculative, it is possible that this effect was more pronounced in the Type I fibers in the current investigation. However, animal data are equivocal on differences in slow and fast muscle regulation of protein synthesis by PGF2α (40). Unfortunately, to our knowledge, there is no other available information on potential fiber type–specific differences in the prostaglandin/COX pathway as it relates to PGF2α in humans.
The capillary responses of the placebo group (Figure 2) suggest that the addition of new capillaries to the muscle did not occur as a result of the resistance exercise program. Considering increases in muscle capillarization are typically associated with endurance exercise (9,28,29), the placebo group response is not surprising given the anaerobic and relatively brief nature of the exercise stimulus. However, depending on the parameter measured, some studies of resistance exercise in older individuals have shown an increase in muscle capillarization (30,35,41), whereas others have not (35,42). Collectively, these studies generally show that an increase in intramuscular capillarization is associated with myofiber hypertrophy. This response is consistent with the presumption that the diffusion distance for oxygen and nutrient supply to the muscle is linked with angiogenesis, although these factors likely play a much bigger role in endurance exercise capillary adaptations. The 24% increase in the capillary to fiber ratio and the additional increase in muscle fiber area support the idea that myofiber hypertrophy was involved in the increased capillarization in the COX inhibitor group. However, it cannot be ruled out that the COX inhibitor had a direct effect on capillary development. It is known that prostaglandins are involved in the regulation of skeletal muscle blood flow (43–45) and angiogenesis in other tissues (46). It is unclear what involvement, if any, the mechanisms related to these effects had in regulating the responses observed in the current study.
The metabolic enzyme activities also appeared to respond to the 12-week interventions based on the resistance exercise stimulus and the amount of myofiber growth. Although increases in glycogen phosphorylase might be expected given the glycolytic nature of the high-intensity resistance exercise (47–49), increases in oxidative enzymes have been observed with resistance exercise in older individuals (41). This adaptation may reflect the relatively untrained and sarcopenic condition of older muscle. Nonetheless, the placebo group maintained pretraining glycolytic and oxidative enzyme activity levels per unit muscle mass, suggesting a proportional change in these metabolic pathways equal to muscle fiber growth. This response was also seen for phosphorylase and β-HAD activities in the COX inhibitor group. The increase in citrate synthase activity in this group likely reflects the substantially greater growth of their Type I fibers (8,50). The maintenance or increase in the levels of enzymes found in the cytosol and mitochondria, along with the proportional increase in muscle strength and muscle mass (1), suggests that prostaglandins regulate protein turnover of the three main protein pools (sarcoplasmic, mitochondrial, and myofibrillar) in human skeletal muscle.
The current findings build on the previous data from this aging cohort examining the skeletal muscle effects of consuming common, over-the-counter COX inhibitors during resistance exercise. A potential mechanism and numerous cellular responses have been described to better understand the prostaglandin regulation of muscle mass and adaptations to exercise. In the context of aging, these data are important for our understanding and treatment of sarcopenia, which is associated with a myriad of negative health consequences (12). More data on different aged cohorts, different COX inhibitors and doses, different duration resistance training regimens, and other training modes are needed.
Funding
This work was supported by the National Institutes of Health (R01 AG020532 and R01 AG038576).
Acknowledgments
Thank you to all the volunteers who dedicated themselves to this project and to Bill Fink for his assistance with the analytical aspects of this project.
References
- 1. Trappe TA, Carroll CC, Dickinson JM, et al. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol. 2011;300:R655–R662. doi:10.1152/ajpregu.00611.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Standley RA, Liu SZ, Jemiolo B, Trappe SW, Trappe TA. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot Essent Fatty Acids. 2013;88:361–364. doi:10.1016/j.plefa.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol. 2013;115:909–919. doi:10.1152/japplphysiol.00061.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol. 2013;304:R198–R205. doi:10.1152/ajpregu.00245.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982;257:1632–1638. [PubMed] [Google Scholar]
- 6. Testa M, Rocca B, Spath L, et al. Expression and activity of cyclooxygenase isoforms in skeletal muscles and myocardium of humans and rodents. J Appl Physiol. 2007;103:1412–1418. doi:10.1152/japplphysiol.00288.2007 [DOI] [PubMed] [Google Scholar]
- 7. Liu SZ, Jemiolo B, Lavin KM, Lester BE, Trappe SW, Trappe TA. Prostaglandin E2 / Cyclooxygenase pathway in human skeletal muscle: Influence of muscle fiber type and age. J Appl Physiol. In press. doi:10.1152/japplphysiol.00396.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chi MM, Hintz CS, Coyle EF, et al. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983;244:C276–C287. [DOI] [PubMed] [Google Scholar]
- 9. Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey LD, Adrian RH, Geiger SR, eds. Handbook of Physiology-Skeletal Muscle. Bethesda, MD: American Physiological Society; 1983:555–631. [Google Scholar]
- 10. Trappe S, Luden N, Minchev K, Raue U, Jemiolo B, Trappe TA. Skeletal muscle signature of a champion sprint runner. J Appl Physiol. 2015;118:1460–1466. doi:10.1152/japplphysiol.00037.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi:10.1113/jphysiol.2003.044966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carroll CC, Dickinson JM, LeMoine JK, et al. Influence of acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. J Appl Physiol. 2011;111:508–515. doi:10.1152/japplphysiol.01348.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. doi:10.1001/jama.1990.03440220053029 [PubMed] [Google Scholar]
- 15. Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. [DOI] [PubMed] [Google Scholar]
- 16. Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol. 2008;295:R273–280. doi:10.1152/ajpregu.00093.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carroll CC, Trappe TA. Personal digital video: a method to monitor drug regimen adherence during human clinical investigations. Clin Exp Pharmacol Physiol. 2006;33:1125–1127. doi:10.1111/j.1440-1681.2006.04503.x [DOI] [PubMed] [Google Scholar]
- 18. Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol. 2002;282:E551–556. doi:10.1152/ajpendo.00352.2001 [DOI] [PubMed] [Google Scholar]
- 19. Trappe TA, Burd NA, Louis ES, Lee GA, Trappe SW. Influence of concurrent exercise or nutrition countermeasures on thigh and calf muscle size and function during 60 days of bed rest in women. Acta Physiol (Oxf). 2007;191:147–159. doi:10.1111/j.1748-1716.2007.01728.x [DOI] [PubMed] [Google Scholar]
- 20. Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol. 2001;90:2070–2074. [DOI] [PubMed] [Google Scholar]
- 21. Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. 1993;148:379–385. doi:10.1111/j.1748-1716.1993.tb09573.x [DOI] [PubMed] [Google Scholar]
- 22. Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;14:7–110.13862378 [Google Scholar]
- 23. Padykula HA, Herman E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955;3:170–195. doi:10.1177/3.3.170 [DOI] [PubMed] [Google Scholar]
- 24. Dubowitz V. Muscle Biopsy A practical approach. 2nd ed. London: Bailliere Tindall; 1985:34. [Google Scholar]
- 25. Anderson P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975;95:203–205. doi:10.1111/j.1748-1716.1975.tb10043.x [DOI] [PubMed] [Google Scholar]
- 26. Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi:10.1177/18.9.670 [DOI] [PubMed] [Google Scholar]
- 27. Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi:10.1113/jphysiol.1977.sp011975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coggan AR, Spina RJ, King DS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. [DOI] [PubMed] [Google Scholar]
- 29. Coggan AR, Spina RJ, Rogers MA, et al. Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J Appl Physiol. 1990;68:1896–1901. [DOI] [PubMed] [Google Scholar]
- 30. Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflugers Arch. 1997;433:238–244. doi:10.1007/s004240050273 [DOI] [PubMed] [Google Scholar]
- 31. Srere PA. Citrate synthase. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology. New York: Academic Press; 1969:5. [Google Scholar]
- 32. Passonneau JV, Lowry OH. Enzymatic Analysis A practical Guide. Totowa, NJ: Humana; 1993:274–275. [Google Scholar]
- 33. Charette SL, McEvoy L, Pyka G, et al. Muscle hypertrophy response to resistance training in older women. J Appl Physiol. 1991;70:1912–1916. [DOI] [PubMed] [Google Scholar]
- 34. Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi:10.1152/japplphysiol.01474.2005 [DOI] [PubMed] [Google Scholar]
- 35. Kryger AI, Andersen JL. Resistance training in the oldest old: consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand J Med Sci Sports. 2007;17:422–430. doi:10.1111/j.1600-0838.2006.00575.x [DOI] [PubMed] [Google Scholar]
- 36. Verdijk LB, Gleeson BG, Jonkers RA, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol. 2009;64:332–339. doi:10.1093/gerona/gln050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hikida RS, Staron RS, Hagerman FC, et al. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J Gerontol. 2000;55:B347–B354. doi:10.1093/gerona/55.7.B347 [DOI] [PubMed] [Google Scholar]
- 38. Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol. 2009;106:1611–1617. doi:10.1152/japplphysiol.91587.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol. 2000;89:143–152. [DOI] [PubMed] [Google Scholar]
- 40. Palmer RM. Prostaglandins and the control of muscle protein synthesis and degradation. Prostaglandins Leukot Essent Fatty Acids. 1990;39:95–104. [DOI] [PubMed] [Google Scholar]
- 41. Frontera WR, Meredith CN, O’Reilly KP, Evans WJ. Strength training and determinants of VO2max in older men. J Appl Physiol. 1990;68:329–333. [DOI] [PubMed] [Google Scholar]
- 42. Hagerman FC, Walsh SJ, Staron RS, et al. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol. 2000;55:B336–346. doi:10.1093/gerona/55.7. B336 [DOI] [PubMed] [Google Scholar]
- 43. Boushel R, Langberg H, Gemmer C, et al. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi:10.1113/jphysiol.2002.021477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nyberg M, Mortensen SP, Saltin B, Hellsten Y, Bangsbo J. Low blood flow at onset of moderate-intensity exercise does not limit muscle oxygen uptake. Am J Physiol. 2010;298:R843–848. doi:10.1152/ajpregu.00730.2009 [DOI] [PubMed] [Google Scholar]
- 45. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi:10.1113/jphysiol.2004. 061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi:10.1124/pr.56.3.3 [DOI] [PubMed] [Google Scholar]
- 47. Creer A, Gallagher P, Slivka D, Jemiolo B, Fink W, Trappe S. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol. 2005;99:950–956. doi:10.1152/japplphysiol.00110.2005 [DOI] [PubMed] [Google Scholar]
- 48. Robergs RA, Pearson DR, Costill DL, et al. Muscle glycogenolysis during differing intensities of weight-resistance exercise. J Appl Physiol. 1991;70:1700–1706. [DOI] [PubMed] [Google Scholar]
- 49. Tesch PA, Colliander EB, Kaiser P. Muscle metabolism during intense, heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. 1986;55:362–366. doi:10.1007/BF00422734 [DOI] [PubMed] [Google Scholar]
- 50. Galpin AJ, Raue U, Jemiolo B, et al. Human skeletal muscle fiber type specific protein content. Anal Biochem. 2012;425:175–182. doi:10.1016/j.ab.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]