Abstract
Oral dryness, a serious problem for the aging Japanese society, is induced by aging-related hyposalivation and causes dysphagia, dysgeusia, inadaptation of dentures, and growth of oral Candida albicans. Oxidative stress clearly plays a role in decreasing saliva secretion and treatment with antioxidants such astaxanthin supplements may be beneficial. Therefore, we evaluated the effects of astaxanthin on the oral saliva secretory function of aging mice. The saliva flow increased in astaxanthin-treated mice 72 weeks after administration while that of the control decreased by half. The plasma d-ROMs values of the control but not astaxanthin-treated group measured before and 72 weeks after treatment increased. The diacron-reactive oxygen metabolites (d-ROMs) value of astaxanthin-treated mice 72 weeks after treatment was significantly lower than that of the control group was. The plasma biological antioxidative potential (BAP) values of the control but not astaxanthin-treated mice before and 72 weeks after treatment decreased. Moreover, the BAP value of the astaxanthin-treated group 72 weeks after treatment was significantly higher than that of the control was. Furthermore, the submandibular glands of astaxanthin-treated mice had fewer inflammatory cells than the control did. Specifically, immunofluorescence revealed a significantly large aquaporin-5 positive cells in astaxanthin-treated mice. Our results suggest that astaxanthin treatment may prevent age-related decreased saliva secretion.
Keywords: astaxanthin, aquaporin-5, hyposalivation, oral dryness, inflammation
Introduction
Oral dryness, which is associated with reduced secretion of saliva with increasing age, is often a health concern for the elderly, who choose to seek medical treatment for this condition. While there is presently no effective treatment established for this condition, it is known that reduced salivary secretion is a constitutive symptom of several diseases including aspiration pneumonia.(1–3)
Several factors may cause reduced salivary secretion including various diseases, which increase with aging. In addition, in most cases, decreased saliva production in the elderly is often an associated side effect of drug therapy, except cases induced by radiation therapy and Sjögren syndrome.(4–8) Furthermore, the salivary glands atrophy with age, leading to reduced salivary secretion.(9–11) In particular, age-related changes in the submandibular gland have been shown to cause apoptosis of acinar cells owing to vacuolar degeneration and fatty degeneration. In addition, glandular parenchyma decreases while inflammatory cell infiltration also occurs in the submandibular gland, creating fibrotic tissue in the increasing interstitial tissue, which consequently causes the gland to atrophy.(12) Therefore, age-related structural changes in the salivary glands decrease the production and secretion of saliva. Aquaporin (AQP), a specific water channeling protein that is present on the surface of cell membranes, responds to the pressure gradient created by osmotic pressure and allows the movement of water molecules in and out of the cell.(13) AQP-5 is present in the salivary and lacrimal glands, the tracheal gills, the eyes, and the lungs and plays a role in the generation of saliva, tears, and pulmonary secretions.(14) AQP-5 has been reported to be downregulated in the lungs of aging mice, thereby decreasing their water transportation ability.(15) However, age-related changes in the production of AQP-5 in the salivary glands have not yet been elucidated.
In recent years, oxidative stress has been clearly shown to play a role in decreasing saliva secretion.(16) Reactive oxygen species (ROS) contribute to oxidative stress, promote aging, and are strongly associated with age-related diseases.(17) While the mitochondrial production of ROS increases with age, the antioxidative potential of the body decreases.(18) Consequently, it becomes challenging for the body to maintain the redox balance and ROS accumulate, causing oxidative stress. Furthermore, accumulated oxidative stress is deleterious to cell membrane proteins and phospholipids, and leads to cellular dysfunction.(19)
The oxidative damage caused by such ROS plays a role in several diseases, such as cancer, high blood pressure, diabetes, cardiovascular disease, cerebrovascular disease, myocardial infarction, Alzheimer’s disease, autoimmune disease, schizophrenia, and inflammatory disorders.(20–23)
Recent research reported inflammatory status improves antioxidant such as tempol.(24) The carotenoid 3, 3-dihydroxy-beta, beta-carotene-4, 4-dione (astaxanthin, AX) has shown strong antioxidative and anti-inflammatory effects(25) and is commonly used as a food supplement. AX is a red-orange pigment present in foods such as shrimp, crab, salmon, and sea bream; however, it cannot be biosynthesized by the human body. AX, which has been used clinically as an antioxidative supplement, is a naturally occurring substance originally extracted from the microalgae Haematococcus pluvialis, with no reported harmful side effects to date.(26) Several studies have reported the effects of AX, including singlet oxygen elimination,(26) lipid peroxidation control,(26) and anti-inflammation.(25) Furthermore, treatment with AX has led to improvements in metabolic syndrome, circulatory organ diseases, cerebral conditions, eye diseases, muscle fatigue, mitochondrial function, and blood flow, as well as the control of Helicobacter pylori.(25) When nematodes with gene sequences similar to those of humans were medicated with AX, their lifespan was found to increase 16–30% more than that of the experimental population.(27) Moreover, the oral administration of AX to a patient with Sjögren syndrome improved the decrease in salivary secretion.(28) Recently, Miyachi(29) reported that AX could be useful for improving chronic inflammation such as that associated with oral lichen planus. However, it remains unclear how the antioxidative effects of AX improve the structure of salivary glands that have been altered by aging, with the associated decreased production and secretion of saliva.
Therefore, the aim of this study was to first determine the structural changes induced in the submandibular glands by oxidative stress in an aging mouse model and then evaluate the effects of AX on the structural alterations as well as the age-related decrease in salivary secretion.
Materials and Methods
Animals
The animal study protocol received institutional approval from the Nippon Dental University (approval No. 08-02) and was conducted in strict accordance with the guidelines of the official Japanese Regulations for Research on Animals.
Female ICR mice (10-week-old, weighing approximately 36 g) were purchased from CLEA Japan Inc., (Tokyo, Japan). The mice were fed long-term with a powdered diet for mice, rats, and hamsters (CE-7, CLEA Japan Inc., Tokyo, Japan), provided water ad libitum, and maintained on a 12-h light-and-dark cycle at the Section of the Biological Sciences Research Center for Odontology, Nippon Dental University School of Life Dentistry, Tokyo, Japan.
AX administration
The mice were divided into an AX-treated group fed a powdered diet mixed with 0.02% AX powder (2% AX, AstaReal, Inc., Tokyo, Japan) from breeding week 48 to 72, and a control group fed the same diet but (no AX powder) from the start of breeding to week 72 week (n = 9 each). The final dose of AX was 1.7 mg/day per mouse and the powdered diet and AX powder were mixed on the clean research bench of the pathogen-free room every week. Both groups were provided food and water ad libitum during the study period, and we measured the powdered diets precisely using a covered feeder Roden CAFÉTM (Oriental Yeast Co., Ltd., Tokyo, Japan). The body weights of all the mice were measured once each month.
Collection of blood and saliva samples
The mice were anesthetized by isoflurane inhalation anesthesia (Mylan Pharmaceuticals, Pittsburgh, PA), blood samples were collected via the caudal vein, and then they were intraperitoneally injected with pilocarpine hydrochloride (0.5 mg/kg body weight, Wako Pure Chem. Ind., Ltd., Osaka, Japan) to stimulate saliva production, and allowed to rest for 5 min. Next, a surgical sponge (BD VisispearTM Eye Sponge 7 cm, Becton Dickinson and Company, Franklin Lakes, NJ) was placed sublingually in the mice for 5 min to absorb the saliva. Then, all the stimulated saliva was pooled, extracted from the inside of the mouths of the mice using syringes, and placed in microtubes stored at −80°C until further analyzed. The weight of the surgical sponge was measured before and after saliva was extracted using an electronic balance and the difference was considered the weight of the extracted saliva. Furthermore, we used pilocarpine hydrochloride to stimulate saliva production in the mice because it has shown highly reproducible results in the determination of salivary gland function in animal experiments. The collected blood samples were centrifuged (4°C, 10,000 × g for 20 min), and the resulting plasma was also stored at −80°C until further analyzed.
Collection of the submandibular gland
General anesthesia was induced in the mice by intraperitoneal injections of pentobarbital sodium (40 mg/kg body weight, Kyoritsu Seiyaku Corporation, Tokyo, Japan), followed by infiltration anesthesia with 0.3 ml lidocaine containing 2% epinephrine and sodium chloride (Xylocaine Cartridge for Dental Use, DENTSPLY International, Tokyo, Japan) subcutaneously administered to the median cervix, and then a skin incision was made.
Then, the both of submandibular glands were dissected from the surrounding tissue, and the both of submandibular glands were cutted in the shift portion of the main excretory duct.
Measurement of oxidative stress
Oxidative stress (natural peroxide concentration) was measured using an active oxygen analysis machine using the Diacron-reactive oxygen metabolites (d-ROMs) test (Diacron, Italy). This is a laboratory procedure that measures the blood concentration of hydroperoxide (ROOH), an active oxygen metabolic product using the exclusive Free Radical Elective Evaluator (FREE) analytical instrument equipped with an absorptiometer. The stored frozen plasma samples were moved to the ultrafiltration filter (Microcon YM-10, Millipore, MA), thawed at 25°C, and then centrifuged (10,000 × g) at 4°C for 50 min prior to the analysis.
Measurement of antioxidative levels
The saliva and plasma sample oxidative levels were analyzed using the same method used to measure oxidative stress. The degree of antioxidation (amount of ferric iron reduced) was measured using the FREE equipment, which is similar to the d-ROMs test using the biological antioxidative potential (BAP) test (Diacron, Italy). The BAP test measures the blood concentration of an antioxidant, by analyzing the conversion of a ferrous ion (Fe2+) to a ferric ion (Fe3+) using the same device used for the d-ROMs test.(30) The d-ROMs and BAP tests are used widely to assess oxidation/antioxidation in both basic research and clinical studies, and their usefulness has been widely reported.
Measurement of submandibular quotient
After removing the moisture from the surface of the submandibular glands extracted from each side, they were weighed using an electronic balance. An index of the submandibular gland atrophy was calculated from the weights of the right and left submandibular glands, and their ratio was also calculated. In addition, the average weight of the saliva samples, the ratio of the saliva flow of all mice, and the weight of submandibular glands were calculated.
Histopathological examination
The mandibular gland tissue was immersion fixed with 4% paraformaldehyde for 24 h, embedded in paraffin, cut into 4-µm-thick sections using a rotary microtome (HM335E, Microm International GmbH, Germany), and then hematoxylin and eosin (H&E) stained.
Immunofluorescence staining of AQP-5
After deparaffinizing the mandibular tissue sections, the AQP-5 antigen was activated with Histo VT One at 90°C for 20 min (Nacalai Tesque, Inc., Kyoto, Japan) and diluted 10-fold. Next, the specimens were incubated with a blocking solution of normal goat serum (Wako Pure Chem. Ind., Ltd.) at room temperature for 1 h. Then, the tissue was incubated with the primary anti-AQP-5 antibody (rabbit, 1:1,000, Abcam, Cambridge, MA) overnight at room temperature, followed by a secondary goat anti-rabbit IgG (H + L) antibody (1:800, Jackson ImmunoResearch Laboratories, Inc., West Grove). The tissue was visualized by nuclear dyeing using 4',6-diamidino-2-phenylindole (DAPI) staining at room temperature for 10 min. Fluorescence immunohistochemical analysis was performed using a confocal point laser scanning microscope (LSM 700, Carl Zeiss, Germany) and the area of the AQP-5 positivity domain was measured using the ImageJ software (National Institutes of Health, NIH, Bethesda, MD).
Statistical analysis
The results are presented as the mean ± SD. Differences between the 2 groups were analyzed using the Student’s t test with the Kaleida Graph software. A p<0.05 was considered statistically significant.
Results
Body weight determination
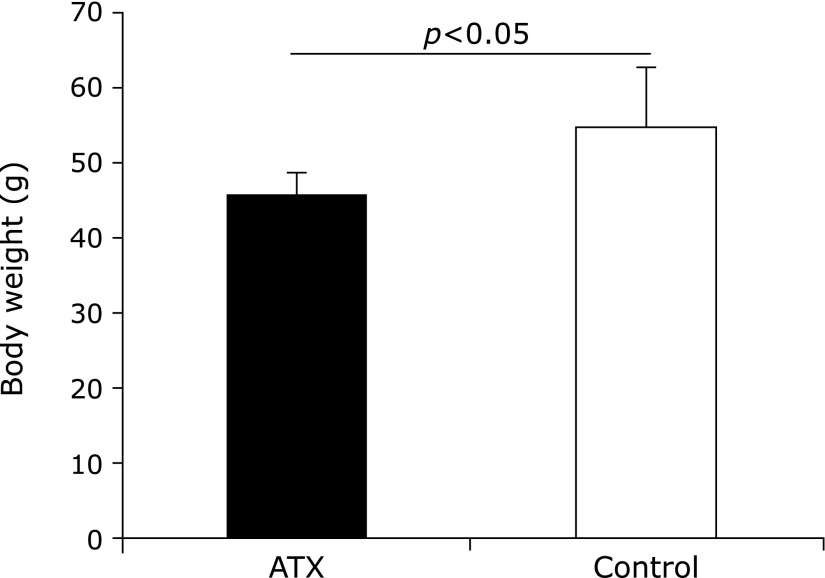
The body weight of the AX-treated group was compared before and 72 weeks after AX administration and the results showed no significant difference; however, that of the untreated control group exhibited an increase. Furthermore, the body weight of the AX-treated group 72 weeks after AX administration was lower than that of the control group was (Fig. 1).
Fig. 1.
Comparison of body weight of astaxanthin (AX) and control groups. Body weight of AX-treated group is significantly lower than that of control group 72 weeks after AX treatment (p<0.05).
Saliva measurement
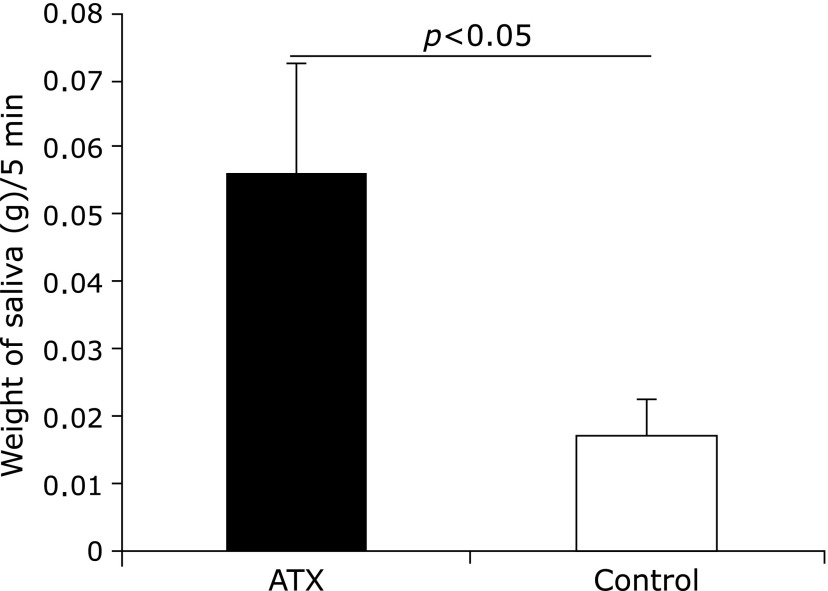
The saliva flow was found to increase in the AX-treated group 72 weeks after AX administration while that of the control group decreased by half (Fig. 2).
Fig. 2.
Comparison of saliva amounts between astaxanthin (AX) and control groups. Saliva flow of AX group was significantly higher than that of control group (p<0.05).
Measurement of plasma oxidative stress using D-ROMs test
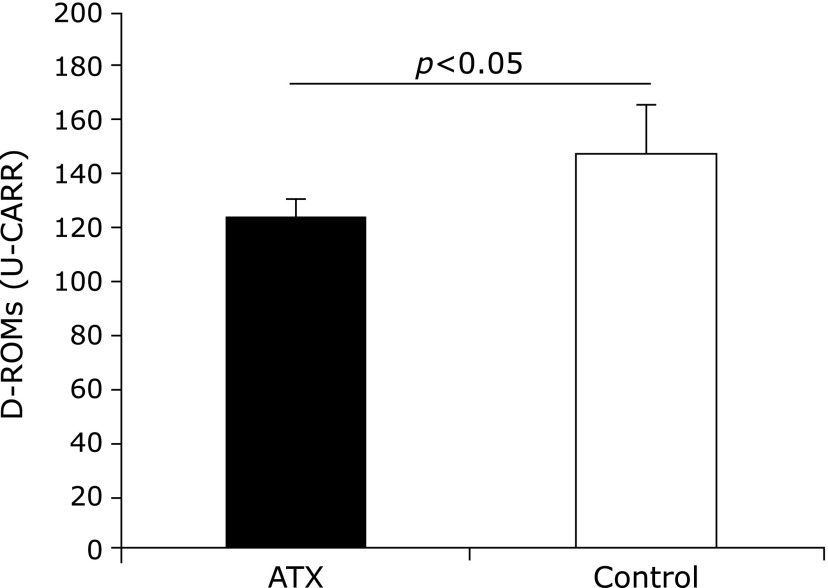
The plasma d-ROMs values of the AX-treated group measured before and 72 weeks after AX administration were not significantly different, however, the control group value was found to increase. Furthermore, the d-ROMs value of the AX-treated group 72 weeks after AX administration was significantly lower than that of the control group was (Fig. 3).
Fig. 3.
Comparison of Diacron-reactive oxygen metabolites (D-ROMs) value of astaxanthin (AX) and control groups. D-ROMs value of AX group 72 weeks after AX administration was significantly lower than that of control group (p<0.05).
Measurement of antioxidative levels using BAP test
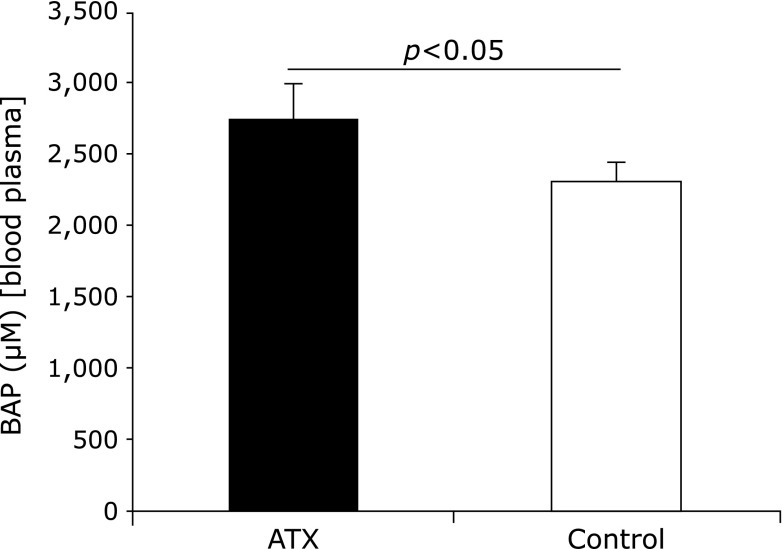
The plasma BAP values of the AX-treated group before and 72 weeks after AX administration were not significantly different; however, the value of the control group decreased. Moreover, the BAP value of the AX-treated group 72 weeks after AX administration was significantly higher than that of the control group was (Fig. 4).
Fig. 4.
Comparison of biological antioxidative potential (BAP) values of astaxanthin (AX) and control groups. BAP value of AX group 72 weeks after AX administration was significantly higher than that of control group.
Submandibular gland weight
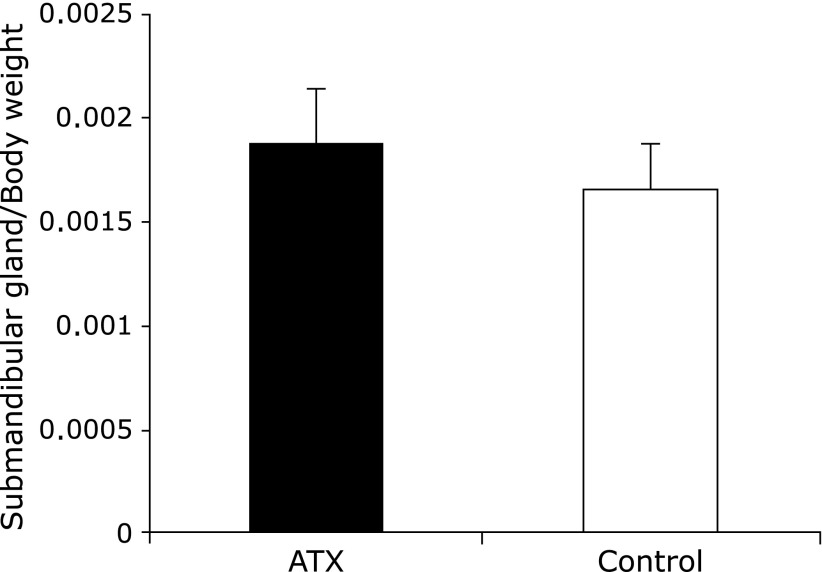
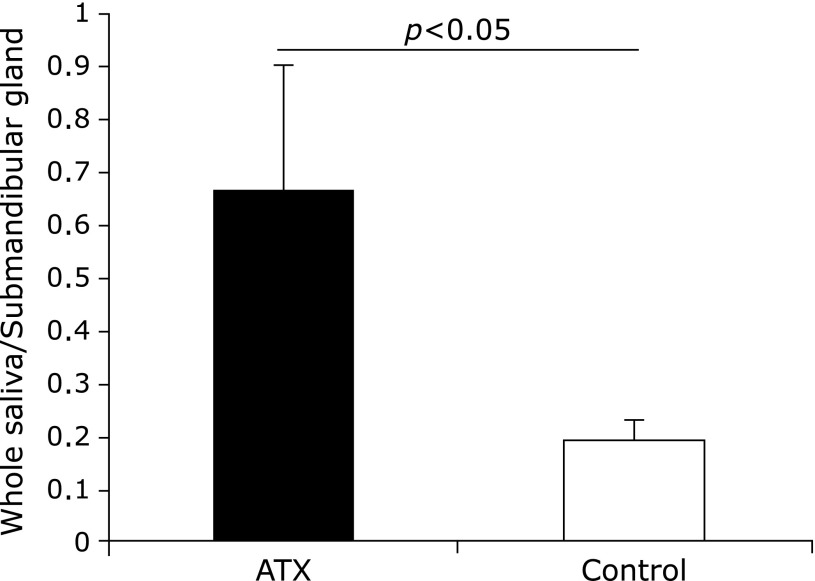
No significant differences were observed in the submandibular gland weight and submandibular gland weight/body weight ratio before and after AX administration in both groups (Fig. 5 and 6). However, the ratios of saliva flow and submandibular gland weight were significantly higher in the AX-treated group than they were in the control group.
Fig. 5.
Changes in ratio of mandibular gland weight to whole body weight. No significant differences were observed in mandibular gland weight/body weight ratio between the astaxanthin (AX) and control groups.
Fig. 6.
Changes in ratio of salivary flow to weight of mandibular gland. Ratio of saliva flow and mandibular gland weight was significantly higher in astaxanthin (AX) group than in control group.
Histopathological analysis
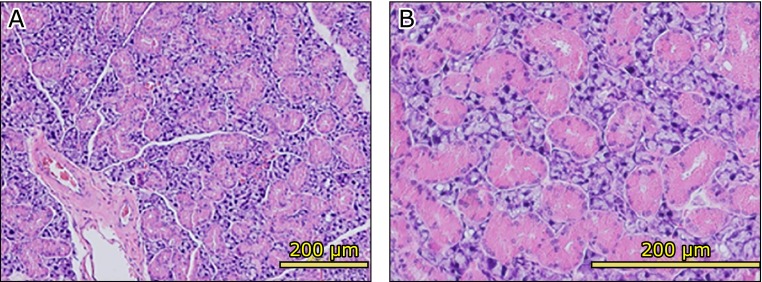
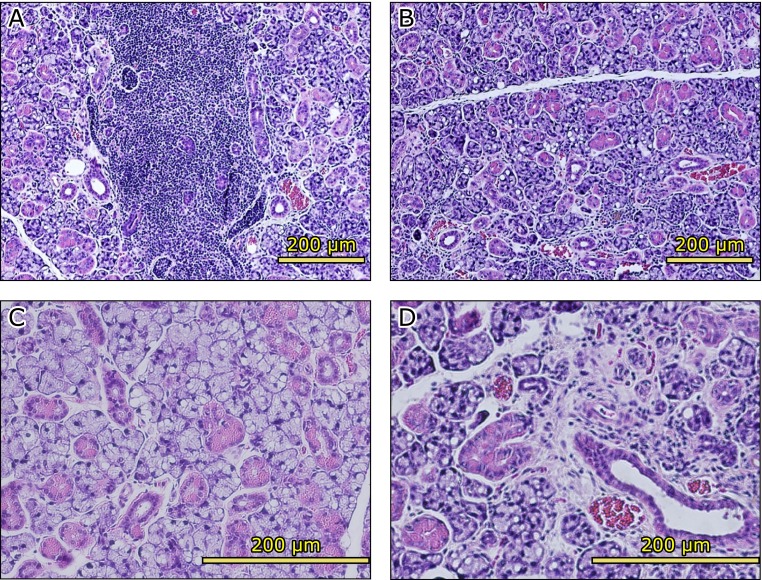
The submandibular glands of the AX-treated group showed numerous large striated ducts (Fig. 7A). The striated ducts were filled with serous granules present in the acinar cells (Fig. 7B). However, the submandibular gland of the control group showed vacuolar or fatty degeneration and inflammatory cells infiltration, which made lymphocytes, was observed (Fig. 8A and B). Moreover, the atrophy of acinar cells in the striated ducts and the disappearance of secretory granules were remarkable (Fig. 8C), while fibrillization was observed in part of the interstitial tissue (Fig. 8D).
Fig. 7.
Hematoxylin and eosin (H&E) staining of astaxanthin (AX) group submandibular glands. Submandibular glands of AX-treated group had several large striated ducts (A). Striated ducts were filled with secretory granules present in acinar cells (B).
Fig. 8.
Hematoxylin and eosin (H&E) staining of control group submandibular glands. Inflammatory cells infiltration (A) and atrophic acinar cells (B) were observed. Vacuolar or fatty degeneration (C) and fibrous connective tissue (D) were observed.
Immunohistochemical analysis of AQP-5
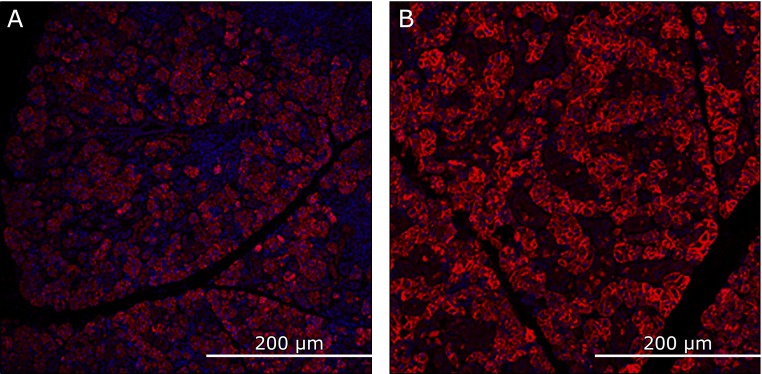
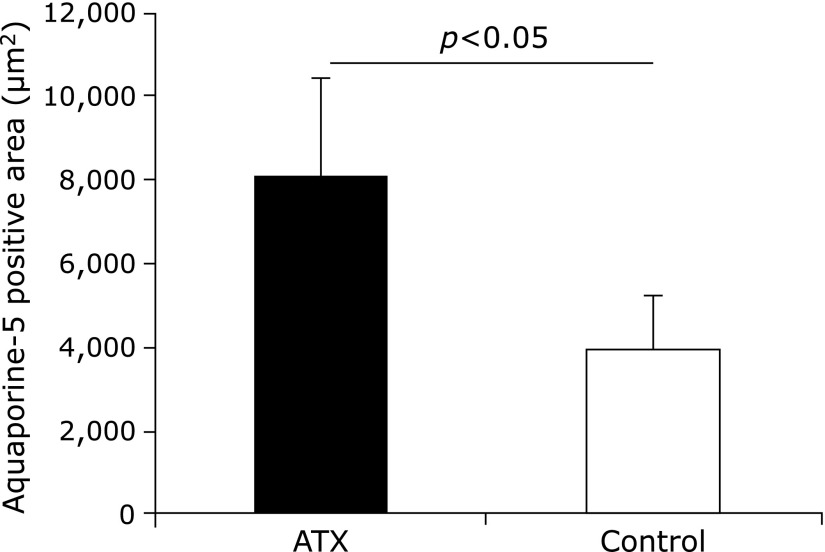
The mandibular glands of the AX-treated group showed several AQP-5-positive regions compared with the control group, which had fewer (Fig. 9). Furthermore, the positive domain of AQP-5 in the AX-treated group covered a larger area than that of the control group did (Fig. 10).
Fig. 9.
Immunohistochemically stained image of aquaporin (AQP)-5-positive domain in the mandibular gland (A) Control group. (B) Astaxanthin (AX) group. Mandibular gland of AX group shows numerous AQP-5-positive cells while that of control group shows few.
Fig. 10.
Positive area of aquaporin (AQP)-5. Positive domain of AQP-5 in astaxanthin (AX) group covered a larger area than that of control group.
Discussion
In recent years, oxidative stress has been suggested to be strongly associated with the development of the symptoms of aging as well as age- and lifestyle-related diseases.(17) ROS are the main cause of oxidative stress, which is deleterious to the proteins and phosphatides of the cell membrane and induces apoptosis. Furthermore, the process of aging itself decreases the mitochondrial function and induces the production of ROS. Consequently, the deterioration of various physiological processes leads to the dysfunction of several internal organs.(23) Moreover, it is now clear that oxidative stress affects the function of the salivary glands.(31) Therefore, in this study, we examined the antioxidative effects of AX on the salivary gland function of a naturally aging mouse model. The concentration of AX was adjusted to 0.02% of the feed in accordance with the study by Naito et al.(32) and, therefore, each mouse was administered 1.7 mg/day of AX (human equivalent dose would be 34 mg/day). Although the recommended daily intake of AX in an adult is 2–12 mg, no side effects were reported in a clinical study that administered 100 mg/day.(26) In addition, when breeding feed is supplemented with AX, the maximum concentration does not usually comply with the allowable daily intake (Health, Labour and Welfare Ministry No. 0825002). Compared with the control group, 72 weeks after AX administration, the AX-treated group had significantly lower and higher d-ROMs and BAP values, respectively. Therefore, our results show that AX administration increased the degree of antioxidation, suggesting that AX may play a role in controlling oxidative stress.
In this study, the survival rate 72 weeks after AX administration was 78 and 44% in the AX-treated and control groups, respectively. Although the survival rate was higher in the AX-treated group, the cumulative survival rate was not significantly different between both groups. It has been reported that AX increases the probability of survival concentration-dependently in nematodes.(27) This may be because AX is believed to increase the activity of superoxide dismutase (SOD), an antioxidant enzyme or catalase, thereby conferring a protective effect on mitochondria or the nuclear organelle film.
In this study, the AX-treated group showed higher salivary flow than the control group did for 24 weeks (week 48 to 72) after AX treatment. Moreover, the control group salivary flow was found to decrease, whereas that of the AX-treated group increased following treatment. In addition, the oxidative level of the AX group was lower than the control was, whereas the antioxidative potential was significantly higher following AX administration. In contrast, the control group showed an increase in the oxidative stress levels and the antioxidative potential decreased (both oxidant stress and antioxidative potential were hardly altered. Therefore, these results show the antioxidative effects of AX on the salivary glands and, therefore, it may control the age-related atrophy of the salivary glands.
While the control group showed an increase in bodyweight, the AX-treated group did not. By week 72, the AX-treated group showed a considerably lower weight than the control group did (Fig. 1). Therefore, we speculated that AX might have induced energy metabolism such as sugar and lipid metabolism and, thereby, maintained or raised the metabolic activity of the body. In contrast, no significant differences were observed in the submandibular gland weight and submandibular gland weight/weight ratio. While the submandibular gland weight increased in proportion to the age-related increase in the weight of the control group, there was almost no change in weight in the AX-treated group. This is likely because the AX-treated submandibular glands maintained their function without shrinking. The stimulated saliva flow/mandibular gland bulk density of the AX group showed a higher value than that of the control group. The flow of saliva and the weight of the salivary glands decreased in the control group, which suggest that the function and the weight of the salivary glands are not correlated. Therefore, since the weight of the salivary gland does not influence saliva flow, it is suggested that the accumulation of age-related oxidative stress results in deterioration and inflammation of the acinar cells. Furthermore, this may play a role in the production of saliva, thereby resulting in decreased salivary secretion.
The structural changes in the submandibular gland of the AX group included larger and more striated ducts than observed in the Control group. Submandibular gland of rodents is consisting of intercalated area with acinar cells and intercalated duct, striated duct area, excretory duct, and main excretory duct. Mouse granular cells have been shown to secrete compound cell growth factors such as the epidermal growth factor (EGF) and nerve growth factor (NGF),(33) from 3 weeks of age with the growth of striated ducts. In addition, after being stored in the secretory granules, the external secretion is carried out via the saliva. Although these striated ducts had shrunk in the control group, numerous secretory granules were present in the AX-treated group. Moreover, the control group exhibited an inflammatory response, which is observed Sjögren syndrome. In addition, vacuolar or fatty degeneration and fibrous connective tissue were widespread. The accumulation of ROS in the salivary glands activates the nuclear adduction copy of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which induces the production of various inflammatory cytokines, leading to inflammatory changes and apoptosis. Therefore, the secretary function of the salivary gland is inhibited, and the production of saliva is decreased by these actions. In contrast, the AX group did not exhibit these structural changes. These results suggest that the antioxidative activity of AX prevented the generation and accumulation of ROS, restored cells and tissues, and prevented or improved the organizational structure of the salivary glands. Furthermore, the AX-induced protective effects prevented the age-related functional decline of the salivary glands.
Fluorescence immunohistochemical analysis revealed a significantly large AQP-5-positive domain in the AX group. An increase in the electrolyte concentration and osmotic pressure of an acinar cell glandular cavity leads to a subsequent increase in the water transportation ability of AQP-5.(34) It has been reported that the production of saliva decreased in AQP-5 knockout mice, which emphasizes the importance of AQP-5 in saliva secretion.(35,36) Furthermore, AX extends perpendicularly so that it can attain the same length as the cell membrane, which it may then penetrate.(37) Therefore, AX can scavenge ROS that occur within the cell as well as on the cell membrane surface and, thereby protects the entire cell from oxidative stress. Furthermore, the oxidative stress that affects AQP-5 in the cell membrane is likely reduced by this action, which may be the underlying mechanism modulating the effects of AX on salivary secretion.
In conclusion, the results of this study suggest that the antioxidative action of AX maintains the function of salivary glands and controls the age-related decrease in salivary secretion. Therefore, the results of our study suggest that AX may be used as a new treatment for oral dryness.
Acknowledgments
We wish to thank Eiji Yamashita, the General Manager of AstaReal Co., Ltd., for providing us with AX and his generous technical support.
Abbreviations
- AQP
aquaporin
- AX
astaxanthin
- BAP
biological antioxidative potential
- D-ROMs
Diacron-reactive oxygen metabolites
- FREE
Free Radical Elective Evaluator
- ROS
reactive oxygen species
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ben-Aryeh H, Miron D, Berdicevsky I, Szargel R, Gutman D. Xerostomia in the elderly: prevalence, diagnosis, complications and treatment. Gerodontology. 1985;4:77–82. doi: 10.1111/j.1741-2358.1985.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 2.Vissink A, Spijkervet FK, Van Nieuw Amerongen A. Aging and saliva: a review of the literature. Spec Care Dentist. 1996;16:95–103. doi: 10.1111/j.1754-4505.1996.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 3.Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–543. doi: 10.1046/j.1532-5415.2002.50123.x. [DOI] [PubMed] [Google Scholar]
- 4.Baum BJ. Evaluation of stimulated parotid saliva flow rate in different age groups. J Dent Res. 1981;60:1292–1296. doi: 10.1177/00220345810600070101. [DOI] [PubMed] [Google Scholar]
- 5.Parvinen T, Larmas M. Age dependency of stimulated salivary flow rate, pH, and lactobacillus and yeast concentrations. J Dent Res. 1982;61:1052–1055. doi: 10.1177/00220345820610090501. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Aryeh H, Miron D, Szargel R, Gutman D. Whole-saliva secretion rates in old and young healthy subjects. J Dent Res. 1984;63:1147–1148. doi: 10.1177/00220345840630091001. [DOI] [PubMed] [Google Scholar]
- 7.Pajukoski H, Meurman JH, Halonen P, Sulkava P. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:641–649. doi: 10.1067/moe.2001.118478. [DOI] [PubMed] [Google Scholar]
- 8.van der Putten GJ, Brand HS, Bots CP, van Nieuw Amerongen A. Prevalence of xerostomia and hyposalivation in the nursing home and the relation with number of prescribed medication. Tijdschr Gerontol Geriatr. 2003;34:30–36. (in Dutch) [PubMed] [Google Scholar]
- 9.Pedersen W, Schubert M, Izutsu K, Mersai T, Truelove E. Age-dependent decreases in human submandibular gland flow rates as measured under resting and post-stimulation conditions. J Dent Res. 1985;64:822–825. doi: 10.1177/00220345850640050801. [DOI] [PubMed] [Google Scholar]
- 10.Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. J Dent. 2005;33:223–233. doi: 10.1016/j.jdent.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Villa A, Polimeni A, Strohmenger L, Cicciù D, Gherlone E, Abati S. Dental patients’ self-reports of xerostomia and associated risk factors. J Am Dent Assoc. 2011;142:811–816. doi: 10.14219/jada.archive.2011.0269. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa A, Tanaka M, Ogawa T, Takagi M. Histometrical study of age changes of the arteries in autopsied submandibular glands, and relation with age changes of the glands. Oral Med Pathol. 2003;8:105–115. [Google Scholar]
- 13.King LS, Yasui M. Aquaporins and disease: lessons from mice to humans. Trends Endocrinol Metab. 2002;13:355–360. doi: 10.1016/s1043-2760(02)00665-3. [DOI] [PubMed] [Google Scholar]
- 14.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 15.Zhang YW, Bi LT, Hou SP, Zhao XL, Song YL, Ma TH. Reduced lung water transport rate associated with downregulation of aquaporin-1 and aquaporin-5 in aged mice. Clin Exp Pharmacol Physiol. 2009;36:734–738. doi: 10.1111/j.1440-1681.2009.05156.x. [DOI] [PubMed] [Google Scholar]
- 16.Takeda I, Kizu Y, Yoshitaka O, Saito I, Yamane GY. Possible role of nitric oxide in radiation-induced salivary gland dysfunction. Radiat Res. 2003;159:465–470. doi: 10.1667/0033-7587(2003)159[0465:pronoi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Indo HP, Yen HC, Nakanishi I, et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J Clin Biochem Nutr. 2015;56:1–7. doi: 10.3164/jcbn.14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dukan S, Farewell A, Ballesteros M, Taddei F, Radman M, Nyström T. Protein oxidation in response to increased transcriptional or translational errors. Proc Natl Acad Sci USA. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- 21.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 22.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress Mol Psychiatry 2004;9:684–697, 643. [DOI] [PubMed] [Google Scholar]
- 23.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamato M, Ishimatsu A, Yamanaka Y, Mine T, Yamada K. Tempol intake improves inflammatory status in aged mice. J Clin Biochem Nutr. 2014;55:11–14. doi: 10.3164/jcbn.14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. 2011;16:355–364. [PubMed] [Google Scholar]
- 26.Fassett RG, Coombes JS. Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs. 2011;9:447–465. doi: 10.3390/md9030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazaki K, Yoshikoshi C, Oshiro S, Yanase S. Supplemental cellular protection by a carotenoid extends lifespan via Ins/IGF-1 signaling in Caenorhabditis elegans. Oxid Med Cell Longev. 2011;2011:596240. doi: 10.1155/2011/596240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada T, Ryo K, Tai Y, et al. Evaluation of therapeutic effects of astaxanthin on impairments in salivary secretion. J Clin Biochem Nutr. 2010;47:130–137. doi: 10.3164/jcbn.10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyachi M, Matsuno T, Asano K, Mataga I. Anti-inflammatory effects of astaxanthin in the human gingival keratinocyte line NDUSD-1. J Clin Biochem Nutr. 2015;56:171–178. doi: 10.3164/jcbn.14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez de Sotillo D, Velly AM, Hadley M, Fricton JR. Evidence of oxidative stress in temporomandibular disorders: a pilot study. J Oral Rehabi. 2011;38:722–728. doi: 10.1111/j.1365-2842.2011.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryo K, Yamada H, Nakagawa Y, et al. Possible involvement of oxidative stress in salivary gland of patients with Sjogren’s syndrome. Pathobiology. 2006;73:252–260. doi: 10.1159/000098211. [DOI] [PubMed] [Google Scholar]
- 32.Naito Y, Uchiyama K, Mizushima K, et al. Microarray profiling of gene expression patterns in glomerular cells of astaxanthin-treated diabetic mice: a nutrigenomic approach. Int J Mol Med. 2006;18:685–695. [PubMed] [Google Scholar]
- 33.Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980;28:836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- 34.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Iwata F, Muraguchi M, et al. Correlation between salivary secretion and salivary AQP5 levels in health and disease. J Med Invest. 2009;56 Suppl:350–353. doi: 10.2152/jmi.56.350. [DOI] [PubMed] [Google Scholar]
- 36.Krane CM, Melvin JE, Nguyen HV, et al. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 37.Goto S, Kogure K, Abe K, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta. 2001;1512:251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]