Abstract
Astaxanthin and vitamin E are both effective antioxidants that are frequently used in cosmetics, as food additives, and in to prevent oxidative damage. A combination of astaxanthin and vitamin E would be expected to show an additive anntioxidative effect. In this study, liposomes co-encapsulating astaxanthin and the vitamin E derivatives α-tocopherol (α-T) or tocotrienols (T3) were prepared, and the antioxidative activity of these liposomes toward singlet oxygen and hydroxyl radical was evaluated in vitro. Liposomes co-encapsulating astaxanthin and α-T showed no additive anntioxidative effect, while the actual scavenging activity of liposomes co-encapsulating astaxanthin and T3 was higher than the calculated additive activity. To clarify why this synergistic effect occurs, the most stable structure of astaxanthin in the presence of α-T or α-T3 was calculated. Only α-T3 was predicted to form hydrogen bonding with astaxanthin, and the astaxanthin polyene chain would partially interact with the α-T3 triene chain, which could explain why there was a synergistic effect between astaxanthin and T3 but not α-T. In conclusion, co-encapsulation of astaxanthin and T3 induces synergistic scavenging activity by intermolecular interactions between the two antioxidants.
Keywords: astaxanthin, vitamin E, tocotrienol, intermolecular interaction, synergistic activity
Introduction
The carotenoid astaxanthin (Asx, 3,3'-Dihydroxy-β,β-carotene-4,4'-dione; Fig. 1) is a common pigment found in algae, fish, and birds.(1,2) Asx is reportedly more effective than a representative carotenoid, β-carotene, for preventing production of reactive oxygen species (ROS), especially singlet oxygen.(3) We previously reported that Asx prevents lipid peroxidation in solution and in various biomembrane systems, such as egg yolk phosphatidylcholine liposomes and rat liver microsomes.(4–8) An earlier report suggested that the potent antioxidative activity of Asx in biomembranes may be attributable to the conjugated polyene moiety and terminal ring moieties of Asx that trap radicals on membrane surfaces and in membranes, respectively.(7) By evaluating the scavenging activity of liposomes encapsulating Asx (Asx-lipo), we recently found that Asx can scavenge hydroxyl radicals,(9) which suggested that liposomal formulations of Asx could have potent scavenging effects against various ROS. Indeed, in an ealier study we demonstrated that ultraviolet-induced melanin production in the skin could be prevented by transdermal administration of Asx-lipo via iontophoresis.(10) This preventative effect of Asx-lipo on melanin production could have been due to the potent radical scavenging capability of Asx in the liposomal formulation.
Fig. 1.
Chemical structures of astaxanthin (Asx), α-tocopherol (α-T), α-tocotrienol (α-T3) and γ-tocotrienol (γ-T3).
Vitamin E, the generic name for tocotrienols and tocopherols, has long been known as a lipophilic antioxidant.(11) Several derivatives of vitamin E exist, and these designated as α-, β-, γ-, and δ-tocopherol and tocotrienol. Since the phenolic hydroxyl group of vitamin E chroman ring is the active site for radical scavenging, vitamin E likely prevents ROS attack of biomembranes through the localization of this hydroxyl group near the lipid membrane interface.(12) Moreover, vitamin E can be recycled by ascorbic acid after radical scavenging.(11,12) Thus, vitamin E is recognized as a useful antioxidant for preventing various ROS-related diseases such as atherosclerosis, and for food preservation.
Although Asx and vitamin E are each effective antioxidant compounds, a combination of these agents should additively enhance their effects. A previous study assessed the ability of an Asx and vitamin E combination to prevent oxidative damage in diabetic model rats and found that Asx might complement the antioxidative effect of vitamin E by preventing dcreases in tocopherol caused by oxidation.(13) Based on this in vivo study, the inclusion of Asx and vitamin E in the same lipid membranes should facilitate the functional complementarity because of the high lipophilicity shown by both antioxidants, although this possibility has not yet been explored. Thus, in the present study, we evaluated the effect of combining Asx and with vitamin E in liposomes that co-encapsulate Asx with the vitamin E derivatives α-tocopherol (α-T) or tocotrienol (T3) by measuring the antioxidative activities of these liposomes toward singlet oxygen and hydroxyl radicals. Additionally, intermolecular interactions between Asx and vitamin E were predicted by computer simulation.
Materials and Methods
Materials
Asx, α-T, α-tocotrienol (α-T3), and γ-tocotrienol (γ-T) were purchased from Sigma-Aldrich Co., LLC. (St. Louis, MO). Egg phosphatidylcholine (EPC) was obtained from the NOF Corporation (Tokyo, Japan). Luminol reagent, 2-methyl-6(4-methoxyphenyl)-3,7-dihydroimidazol[1,2-a]pyrazin-3-one (MCLA) and rose bengal were purchased from Nacalai Tesque (Kyoto, Japan). All other reagents were of the highest commercially available grade.
Preparation of liposomes encapsulating Asx and vitamin E
EPC liposomes encapsulating antioxidants were prepared using a lipid hydration method.(14) Chloroform solutions containing 1 µmol EPC, and various antioxidants, Asx, α-tocopherol, α-tocotrienol or γ-tocotrienol, were dried to a thin film under vacuum in a test tube. The dried lipid film was hydrated with 0.1 ml 10 mM Tris buffer (pH 7.4) at room temperature to obtain a liposomal suspension, and liposomes were produced by sonication in a bath-type sonicator (AU-25C, Aiwa, Tokyo, Japan). The diameter of liposomes encapsulating antioxidants was measured by dynamic light scattering using a Zetasizer nano (Malvern Instruments Ltd., UK).
Evaluation of quenching activity of liposomes against singlet oxygen
Levels of singlet oxygen produced by light-irradiation of a rose bengal solution in a test tube can be measured in terms of the chemiluminescent intensity of MCLA.(15) The liposomal suspension (0.1 ml) was diluted with 0.879 ml water, followed by the addition of 20 µl 0.5 mM rose bengal dissolved with water. The mixture was then placed 10 cm from a 50 W halogen lamp (E11JDR, Ohm Electric Inc., Tokyo, Japan) and exposed to the lamp light for 2 min at 40°C as maintained by a constant-temperature water bath (WB-11, SHIBATA Scientific Technology, Ltd., Saitama, Japan), followed by immediate addition of 1 µl 1 mM MCLA solution. Chemiluminescence intensity was measured by a Luminescencer-PSN (ATTO, Tokyo, Japan).
Evaluation of scavenging activity of liposomes against hydroxyl radical
Hydroxyl radical generation was evaluated by chemiluminescence intensity using a previously reported method.(9) To generate hydroxyl radicals, 0.1 ml 0.25 M H2O2 (final concentration: 48 mM), 50 µl 10 mM liposomal suspension (final lipid concentration: 0.96 mM), 0.17 ml 1 mM luminol solution (final concentration: 330 µM), and 0.2 ml 1 mM FeSO4 solution (final concentration: 385 µM) were mixed in a test tube. After mixing, the chemiluminescence intensity was measured within 5 s by a Luminescencer PSN (ATTO, Tokyo, Japan). Hydroxyl radicals are known to be generated by the Fenton reaction as follows:(16) Fe2+ + H2O2 → Fe3+ + OH– + •OH. Therefore, the theoretically generated amount of hydroxyl radicals under the study conditions is predicted to be 385 µM.
Optimization of molecular structures of Asx and vitamin E
All calculations were performed using the technical computing software SCIGRESS ver. 2.1.0 (build 3050; Fujitsu Co., Ltd.: Chiba, Japan). The minimum energy conformations of Asx, α-T and α-T3 were first calculated with molecular mechanics (CONFLEX-MM3). Starting from the calculated conformations, optimized structures were then predicted using semi-empirical molecular calculations. Energies of intermolecular interactions were estimated by the heat of formation difference between Asx-α-T and Asx-α-T3, which were calculated with PM6.
Statistical analysis
Statistical analysis was performed using one-way ANOVA followed by Turkey-Kramer HSD test. P values <0.05 were considered to be significant.
Results and Discussion
Singlet oxygen quenching activity of liposomes encapsulating Asx and vitamin E
Liposomes encapsulating Asx and/or vitamin E were prepared using a conventional lipid film hydration method (see Materials and Methods) and the liposome diameters were measured by dynamic light scattering (Table 1). The diameters of the liposomes were between 100 and 250 nm, although liposomes encapsulating 10 µM α-T3, 5 µM Asx or both 10 µM α-T3 and 5 µM Asx were larger relative to the other liposome types. Both Asx and the vitamin E derivatives have unsaturated bonds in their hydrocarbon chain, which may confer a bulkiness to the hydrophobic region that could affect the membrane structure of the liposomes, although liposomes containing γ-tocotrienol (γ-T3) were not large.
Table 1.
Particle size of liposomes prepared in this study
| Liposomes | Diameter (nm)a |
|---|---|
| Control-lipo | 213.9 ± 102.1 |
| Asx (1)b-lipo | 204.3 ± 146.0 |
| Asx (5)-lipo | 262.4 ± 167.2 |
| α-T (5)-lipo | 122.5 ± 15.0 |
| α-T3 (5)-lipo | 203.6 ± 57.8 |
| α-T3 (10)-lipo | 245.6 ± 61.8 |
| γ-T3 (5)-lipo | 115.7 ± 40.2 |
| Asx (5)/α-T (5)-lipo | 125.5 ± 7.8 |
| Asx (5)/α-T3 (5)-lipo | 191.7 ± 60.0 |
| Asx (5)/α-T3 (10)-lipo | 254.5 ± 112.0 |
| Asx (5)/γ-T3 (5)-lipo | 141.1 ± 12.3 |
aData are average ± SD of diameters from at least 3 different samples, bAntioxidant concentration (µM).
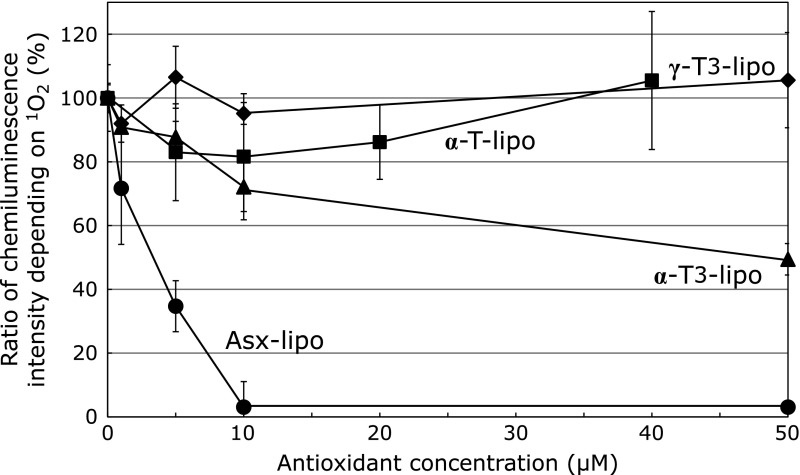
To evaluate the ROS quenching activity of the liposomes, levels of singlet oxygen in the presence of the different liposomes were measured using chemiluminescence intensity assay involving a light-irradiated rose bengal solution and MCLA. The chemiluminescent intensity of MCLA in solution was reduced in the presence of liposomes, although the effects differed for each antioxidant. Asx-lipo showed a dose-dependent potent decrease in chemiluminescence intensity (Fig. 2). In contrast, under the same conditions, the quenching effects of liposomes encapsulating the vitamin E derivatives, α-T, α-T3, and γ-T3, were less potent than Asx even at high concentration. These data are consistent with a previous report showing that in liposomal formulations the quenching activity of Asx toward singlet oxygen is more effective than that of vitamin E.(3)
Fig. 2.
Effect of various amounts of liposomal antioxidants (Asx, α-T, α-T3 or γ-T3) on singlet oxygen production. Singlet oxygen production was monitored by measuring the chemiluminescent intensity of MCLA in a rose bengal solution after light irradiation in the presence of liposomes encapsulating Asx (●), α-T (■), α-T3 (▲) or γ-T3 (◆).
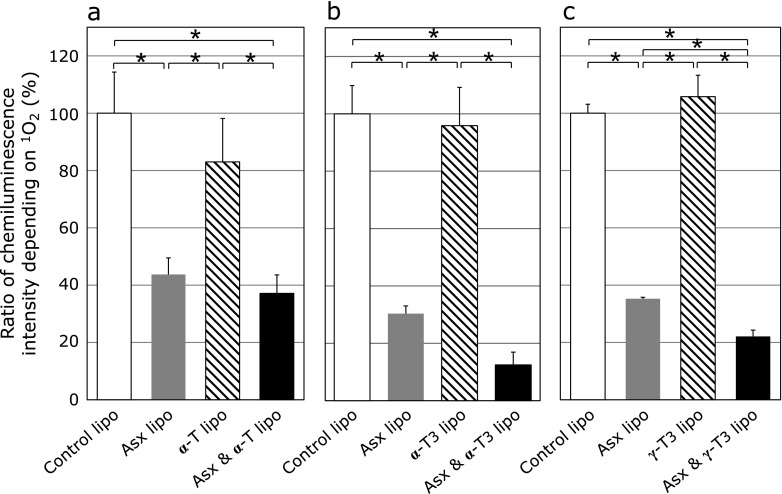
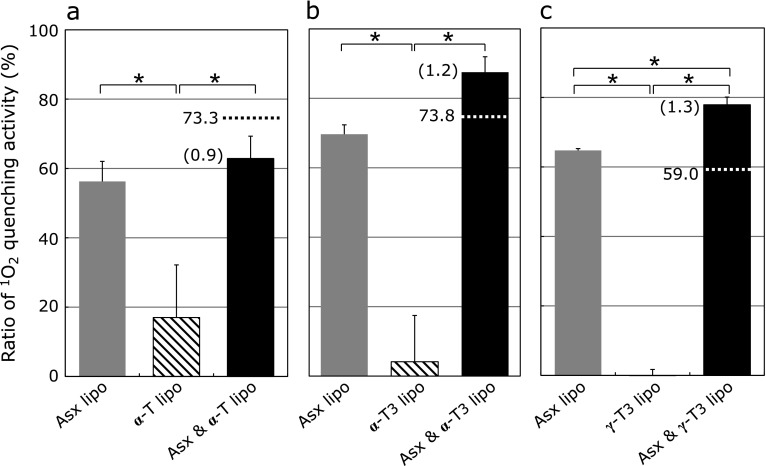
Next, the effects of liposomes co-encapsulating Asx and vitamin E on singlet oxygen production as measured by change in chemiluminescence intensity of MCLA in a light-irradiated rose Bengal solution were examined. Liposomes that co-encapsulated Asx/vitamin E reduced chemiluminescence intensity more effectively than did liposomes encapsulating either Asx or vitamin E alone (Fig. 3). To estimate the effect on quenching activity of co-encapsulating Asx and vitamin E in the same liposomes, the measured scavenging activities of liposomes co-encapsulating Asx/vitamin E were compared with the combined quenching activities of liposomes encapsulating either Asx or vitamin E alone (Fig. 4). For liposomes co-encapsulating Asx and α-T (Asx/α-T-lipo), the actual quenching activity was lower than the combined values of Asx-lipo and liposomes containing α-T (α-T-lipo). Moreover, an additive effect of both antioxidants was not observed in this combination. In contrast, for liposomes co-encapsulating Asx with either α-T3 or γ-T3, the actual measured values were higher than the calculated combined values. These results suggest that co-encapsulation of Asx and T3 produces synergistic effect on the singlet oxygen quenching activity of these compounds.
Fig. 3.
Effect of liposomes co-encapsulating Asx and vitamin E derivatives on singlet oxygen production. Singlet oxygen production was monitored by measuring relative chemiluminescence intensity of MCLA in a light-irradiated rose bengal solution in the presence of liposomes containing (a) 5 µM Asx (gray column), 5 µM α-T (hatched column) or both 5 µM Asx and 5 µM α-T (black column), (b) 5 µM Asx (gray column), 5 µM α-T3 (hatched column) or both 5 µM Asx and 5 µM α-T3 (black column), (c) 5 µM Asx (gray column), 5 µM γ-T3 (hatched column), or both 5 µM Asx and 5 µM γ-T3 (black column), respectively. Data are the average ± SD of values obtained from 3 different samples. *p<0.05.
Fig. 4.
Singlet oxygen quenching activity of liposomes co-encapsulating Asx and vitamin E derivatives. The ratios of singlet oxygen quenching activity of the liposomes were calculated from the chemiluminescence intensity of MCLA shown in Fig. 3. Relative scavenging activities of liposomes containing (a) 5 µM Asx (gray column), 5 µM α-T (hatched column) or both 5 µM Asx (gray column) and 5 µM α-T (black column), (b) 5 µM Asx (gray column), 5 µM α-T3 (hatched column) or both 5 µM Asx and 5 µM α-T3 (black column), (c) 5 µM Asx (gray column), 5 µM γ-T3 (hatched column), or both 5 µM Asx and 5 µM γ-T3 (black column), were calculated. The parenthetical number is the ratio of actual measured quenching activity of liposomes co-encapsulating Asx and vitamin E to the combined value of liposomes encapsulating either Asx or vitamin E alone and is indicated with a dotted line. Data are the average ± SD of values obtained from Fig. 3. *p<0.05.
Hydroxyl radical scavenging activity of liposomes encapsulating Asx and vitamin E
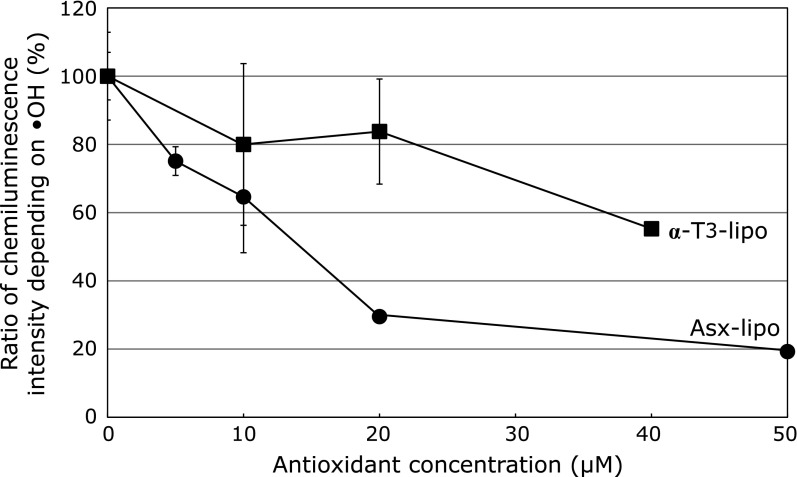
To evaluate the effect of liposomes toward the potent hydroxyl radical, we again prepared liposomes encapsulating Asx or α-T3 and performed a chemiluminescence intensity involving luminol and FeSO4, which allows the levels of hydroxyl radicals generated through the Fenton reaction to be determined. Since liposomes encapsulating α-T3 (α-T3-lipo) showed the most potent activity among the vitamin E derivatives examined in this study, for subsequent experiments we focused on α-T3. Although the prevention effect of Asx-lipo was more potent than that of α-T3-lipo, those liposomes showed significant dose-dependent preventative effects on chemiluminescence intensity depending on hydroxyl radical production (Fig. 5).
Fig. 5.
Effect of various amounts of liposomal antioxidants (Asx or α-T3) on hydroxyl radical production. Hydroxyl radical generated via the Fenton reaction in the presence of liposomes encapsulating Asx (●) or α-T3 (■) were measured using the chemiluminescence intensity method described in the Methods. Data are the average ± SD of values obtained from 3 different samples. *p<0.05.
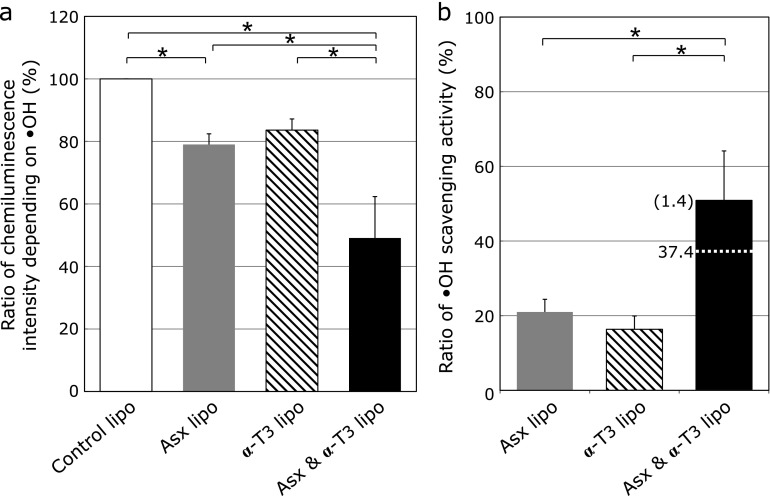
Then, the effect of liposomes co-encapsulating Asx and α-T3 (Asx/α-T3-lipo) on chemiluminescence intensity depending on hydroxyl radical production was examined. The Asx/α-T3-lipo showed a higher preventative effect than did Asx-lipo or α-T3-lipo (Fig. 6a). To evaluate the effect of co-encapsulation of Asx and α-T3 in the same lipid membranes, the actual measured scavenging activity of Asx/α-T3-lipo was compared with the combined value of Asx-lipo and α-T3-lipo (Fig. 6b). The actual measured value was higher than the calculated combined value, wherein the ratio of the actual value was 1.4-fold higher than the additive activity. This result indicated that, as with singlet oxygen, the Asx/α-T3-lipo showed a synergistic scavenging activity of the two antioxidants toward hydroxyl radicals. Thus, co-encapsulation of Asx and T3 could induce synergistic scavenging activities against different types of ROS.
Fig. 6.
Effect of liposomes co-encapsulating Asx and α-T3 on hydroxyl radical production. (a) Relative chemiluminescence intensity was measured after addition of liposomes containing 5 µM Asx (gray column), 10 µM α-T3 (hatched column) or both 5 µM Asx and 10 µM α-T3 (black column). (b) Hydroxyl radical scavenging activity of the liposomes co-encapsulating Asx and α-T3. Relative hydroxyl radical scavenging activities of liposomes containing Asx (gray column), α-T3 (hatched column) or both Asx and α-T3 (black column) were calculated from the chemiluminescence intensity shown in Fig. 6a. The parenthetical number is the ratio of actual measured scavenging activity of liposomes co-encapsulating Asx and α-T3 to the combined value of liposomes encapsulating Asx or α-T3 alone and is indicated with a dotted line. Data are the average ± SD of values obtained from Fig. 6a. *p<0.05.
Comparison of Asx/α-T and Asx/α-T3 conformational structures
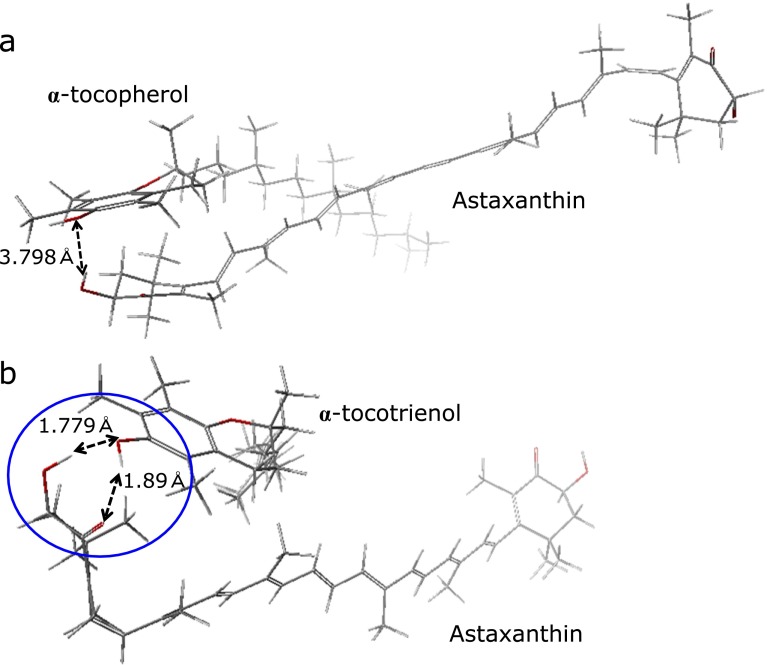
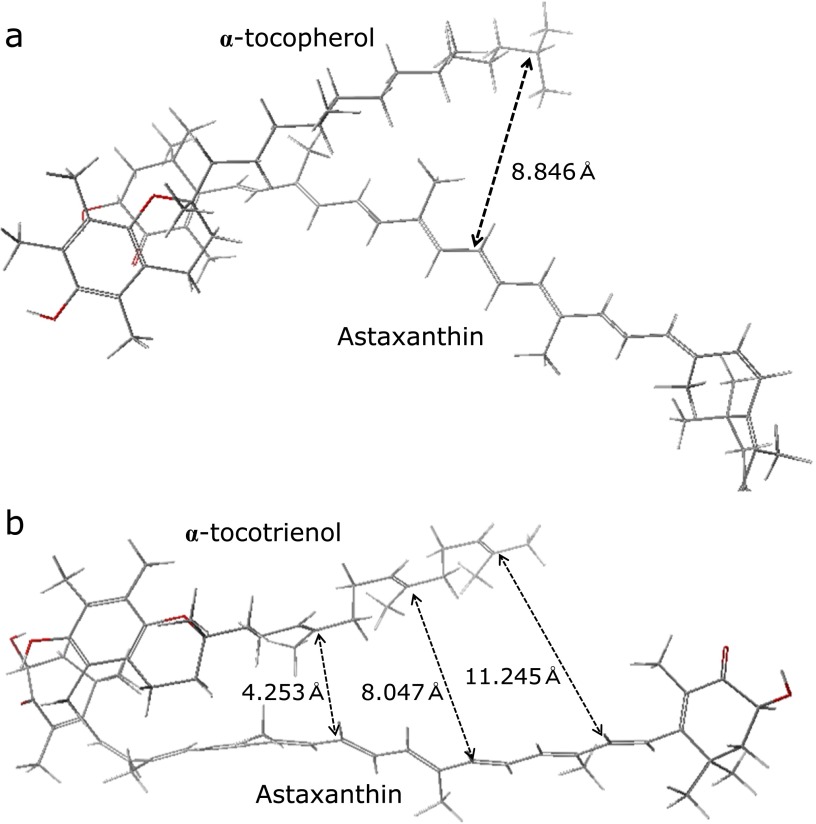
To clarify why the combination of Asx with T3 but not α-T might produce a synergistic effect on antioxidative activity, the minimum energy structure of Asx with α-T or α-T3 was calculated (see Materials and Methods). Based on the calculation, the predicted distance between the Asx terminal ring and the α-T chroman ring or the α-T3 chroman ring were approximately 3.8 Å and 1.8 Å, respectively. The predicted distances suggested that the hydroxyl moiety or carbonyl moiety in the Asx terminal ring can undergo hydrogen bonding with the hydroxyl moiety in the α-T3, not α-T, chroman ring (Fig. 7). In the intermolecular structures, although the distance between hydrophobic regions, such as the polyene chain of Asx and the hydrophobic chain of α-T3, would gradually spread for the other end of chain, small distance value (ca 4.2 Å) suggested that Asx polyene chain would partially interact with a T3 triene chain (Fig. 8). While the distance between the Asx polyene chain and the α-T phytyl chain was big (ca 8.8 Å). The trienol chain of T3 could affect the electron state of the Asx polyene chain through some kind of intermolecular interactions between π-conjugated systems, although no interaction could occur between the Asx polyene and the α-T phytyl moiety. Thus, the simulation results suggest that the synergistic effect of Asx and T3, and not α-T, on antioxidative activity is due to changes in the Asx polyene electron state resulting from intermolecular interactions with the T3 trienol chain. The hydrogen bonding at a terminal ring moiety of Asx with T3 would affect electron density of carbonyl-conjugated polyene structure. In Fig. 7 and 8, hydrogen bonding at only one side of Asx was shown. However, since Asx has two terminal ring, two molecules of T3 would interact at both terminal ring moiety of Asx. Thus, electron density of polyene moiety would be balanced by interaction at both terminal sides of Asx, and no or very little effect on scavenging activity at polyene moiety might be induced by hydrogen bonding. However, the effect of hydrogen bonding at terminal ring moieties of Asx with T3 on electron density of polyene moiety should be studied in the future.
Fig. 7.
Comparison of Asx/α-T and Asx/α-T3 optimized structures. Minimum energy conformation of Asx with (a) α-T or (b) α-T3 was calculated as described in the Materials and Methods. Hydrogen bonding between a hydroxyl moiety or a carbonyl moiety in the terminal ring of Asx and the hydroxyl moiety in chroman ring of α-T3. Blue circle indicates a hydrogen bonding region between Asx and α-T3. Oxygen atoms are shown in red.
Fig. 8.
Calculated intermolecular distances between Asx and α-T or α-T3. The calculated distances between double bonds of the Asx polyene chain and (a) α-T phytyl chain and (b) α-T3 triene chain are represented in the structures. Oxygen atoms are shown in red.
In conclusion, we found that co-encapsulation of Asx and T3 in the same liposomal membranes can produce the hydrogen bonding of T3 with Asx and the intermolecular interaction between the Asx polyen chainx and the T3 trien chain that promote synergistic antioxidative activity of these compounds toward singlet oxygen and hydroxyl radicals. Therefore, co-administration of Asx and T3 would be a useful approach for enhancing the functionality of each antioxidant in biomembranes.
Acknowledgments
This work was supported financially in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2013–2017 (S1311035).
Abbreviations
- α-T
α-tocopherol
- α-T-lipo
liposomes encapsulating α-tocopherol
- α-T3-lipo
liposomes encapsulating α-tocotrienol
- Asx
astaxanthin
- Asx-lipo
liposomes encapsulating astaxanthin
- Asx/α-T-lipo
liposomes co-encapsulating astaxanthin and α-tocopherol
- Asx/α-T3-lipo
liposomes co-encapsulating astaxanthin and α-tocotrienol
- EPC
egg phosphatidylcholine
- MCLA
2-methyl-6(4-methoxyphenyl)-3,7-dihydroimidazol [1,2-a]pyrazin-3-one
- ROS
reactive oxygen species
- T3
tocotrienol
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Boussiba S, Fan L, Vonshak A. Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. Method Enzymol. 1992;213:386–391. [Google Scholar]
- 2.Maoka T. Carotenoids in marine animals. Mar Drugs. 2011;9:278–293. doi: 10.3390/md9020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuzawa K. Singlet oxygen scavenging in phospholipid membranes. Methods Enzymol. 2000;319:101–110. doi: 10.1016/s0076-6879(00)19012-3. [DOI] [PubMed] [Google Scholar]
- 4.Terao J. Antioxidant activity of beta-carotene-related carotenoids in solution. Lipids. 1989;24:659–661. doi: 10.1007/BF02535085. [DOI] [PubMed] [Google Scholar]
- 5.Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992;1126:178–184. doi: 10.1016/0005-2760(92)90288-7. [DOI] [PubMed] [Google Scholar]
- 6.Palozza P, Krinsky NI. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch Biochem Biophys. 1992;297:291–295. doi: 10.1016/0003-9861(92)90675-m. [DOI] [PubMed] [Google Scholar]
- 7.Goto S, Kogure K, Abe K, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta. 2001;1512:251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 8.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740:101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Hama S, Uenishi S, Yamada A, et al. Scavenging of hydroxyl radicals in aqueous solution by astaxanthin encapsulated in liposomes. Biol Pharm Bull. 2012;35:2238–2242. doi: 10.1248/bpb.b12-00715. [DOI] [PubMed] [Google Scholar]
- 10.Hama S, Takahashi K, Inai Y, et al. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J Pharm Sci. 2012;101:2909–2916. doi: 10.1002/jps.23216. [DOI] [PubMed] [Google Scholar]
- 11.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med. 2014;66:3–12. doi: 10.1016/j.freeradbiomed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Fukuzawa K. Dynamics of lipid peroxidation and antioxidion of alpha-tocopherol in membranes. J Nutr Sci Vitaminol (Tokyo) 2008;54:273–285. doi: 10.3177/jnsv.54.273. [DOI] [PubMed] [Google Scholar]
- 13.Nakano M, Onodera A, Saito E, et al. Effect of astaxanthin in combination with alpha-tocopherol or ascorbic acid against oxidative damage in diabetic ODS rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:329–334. doi: 10.3177/jnsv.54.329. [DOI] [PubMed] [Google Scholar]
- 14.Kajimoto K, Yamamoto M, Watanabe M, et al. Noninvasive and persistent transfollicular drug delivery system using a combination of liposomes and iontophoresis. Int J Pharm. 2011;403:57–65. doi: 10.1016/j.ijpharm.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Hosaka S, Obuki M, Nakajima J, Suzuki M. Comparative study of antioxidants as quenchers or scavengers of reactive oxygen species based on quenching of MCLA-dependent chemiluminescence. Luminescence. 2005;20:419–427. doi: 10.1002/bio.867. [DOI] [PubMed] [Google Scholar]
- 16.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]