Abstract
l-Theanine (γ-glutamylethylamide), a component of green tea, is considered to have regulatory and neuroprotective roles in the brain. The present study was designed to determine the effect of l-theanine on excess dopamine-induced neurotoxicity in both cell culture and animal experiments. The primary cultured mesencephalic neurons or co-cultures of mesencephalic neurons and striatal astrocytes were pretreated with l-theanine for 72 h, and then treated with excess dopamine for further 24 h. The cell viability of dopamine neurons and levels of glutathione were evaluated. Excess dopamine-induced neurotoxicity was significantly attenuated by 72 h preincubation with l-theanine in neuron-astrocyte co-cultures but not in neuron-rich cultures. Exposure to l-theanine increased the levels of glutathione in both astrocytes and glial conditioned medium. The glial conditioned medium from l-theanine-pretreated striatal astrocytes attenuated dopamine-induced neurotoxicity and quinoprotein formation in mesencephalic neurons. In addition, replacement of l-glutamate with l-theanine in an in vitro cell-free glutathione-synthesis system produced glutathione-like thiol compounds. Furthermore, l-theanine administration (4 mg/kg, p.o.) for 14 days significantly increased glutathione levels in the striatum of mice. The results suggest that l-theanine provides neuroprotection against oxidative stress-induced neuronal damage by humoral molecules released from astrocytes, probably including glutathione.
Keywords: l-theanine, dopamine, astrocyte, glutathione, neuroprotection
Introduction
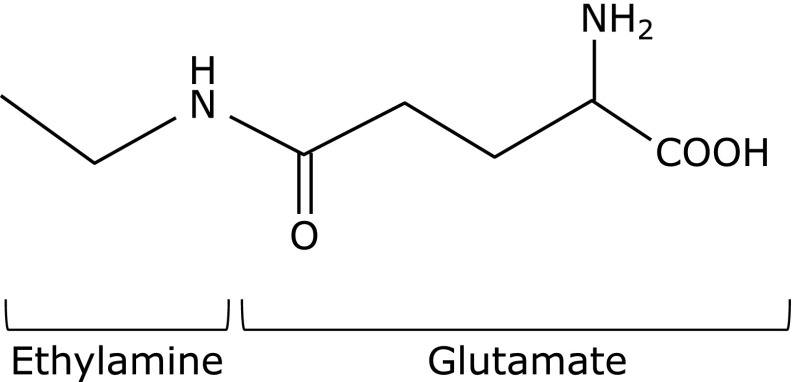
l-Theanine (γ-glutamylethylamide) is a major free amino acid component of green tea, and has a suppressive effect against the excitatory action of caffeine,(1) reducing effect on systemic blood pressure,(2) and anti-oxidative properties.(3,4) Because l-theanine has a chemical structure similar to glutamate (Fig. 1) and can cross the blood-brain barrier,(5) its effects on the central nervous system have received attention. Kakuda et al.(6) reported that l-theanine has neuroprotective effects against ischemic brain damage and glutamate-induced cell death in cortical neurons. However, l-theanine is a poor inhibitor of ligand binding to three different ionotropic receptor subtypes of l-glutamate in rat cortical neurons compared with that of glutamate itself.(7) Other studies confirmed that l-theanine inhibits the incorporation of extracellular glutamine into neurons, resulting in the suppression of exocytotic release of glutamate.(8) In addition, several studies have also demonstrated the neuroprotective effects of l-theanine against β-amyloid-induced cognitive dysfunction and neuronal cell death,(9) and against neurotoxicity induced by Parkinson’s disease-related environmental toxins, such as rotenone and dieldrin in cultured neuronal SH-SY5Y cells.(10) However, the mechanism(s) of the neuroprotective effects of l-theanine against dopaminergic neurotoxicity remains obscure.
Fig. 1.
Chemical structure of l-theanine.
Dopamine (DA) quinone formation as a dopaminergic neuron-specific oxidative stress plays an important role in dopaminergic neurodegeneration.(11,12) In damaged dopaminergic neurons, free excess DA in the cytosol outside synaptic vesicles is spontaneously oxidized to produce reactive oxygen species (ROS) and DA quinone. DA quinone covalently conjugates with the sulfhydryl group of cysteine on functional protein including tyrosine hydroxylase (TH), DA transporter, parkin protein in dopaminergic neurons, resulting in inhibition of their function.(13–15) Our previous studies demonstrated the importance of DA quinone formation in dopaminergic neuronal dysfunction using animal model of Parkinson’s disease(16) and methamphetamine-injected mice.(17) DA-induced formation of DA quinones and the consequent dopaminergic cell damage in both in vitro and in vivo experiments can be prevented by treatment with superoxide dismutase, glutathione (GSH), and certain thiol reagents based on their quinone-quenching activity.(18,19) We recently showed that astrocytes can protect dopaminergic neurons against DA quinone toxicity,(20) and that zonisamide, a novel antiparkinsonian agent, increases GSH levels in astrocytes and provides neuroprotection against dopaminergic neurodegeneration in the model of Parkinson’s disease.(21) The present study is an extension to the above studies and was designed to determine whether l-theanine increases GSH synthesis in astrocytes to act as a neuroprotectant against DA quinone toxicity.
Materials and Methods
Materials and animals
l-Theanine, DA hydrochloride, l-glutamate, l-cysteine and glycine were purchased from Wako Pure Chem. (Osaka, Japan). Pregnant Sprague–Dawley rats and male ICR mice were purchased from Charles River Japan Inc. (Yokohama, Japan). All animal procedures were in strict accordance with the Guideline for Animal Experiments of Okayama University Advanced Science Research Center and were approved by the Animal Care and Use Committee of Okayama University Advanced Science Research Center.
Cell culture
Primary neuronal cell cultures were prepared as described previously.(22) The mesencephalic area was dissected in Sprague–Dawley rat embryos at 15 days gestation. The tissue was incubated for 15 min in 0.125% trypsin at 37°C and then centrifuged (1,500 × g, 5 min). The cell pellet was treated with 0.004% DNase I for 7 min at 37°C, and recentrifuged at 1,500 × g for 5 min. The cell pellet was then gently resuspended in a small volume of Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and plated in the same medium at a density of 2.0 × 105 cells/cm2 in four-chamber culture slides or in 6-well plates coated with poly-d-lysine (Becton Dickinson, Franklin Lakes, NJ). The cells were maintained in this growth medium at 37°C under 5% CO2/95% air environment. Within 24 h of initial plating, the medium was replaced with fresh medium supplemented with 2 µM cytosine-β-d-arabinofuranoside to inhibit the replication of non-neuronal cells, and incubated for a further 5 days. In neuron-rich cultures, 95% of the cells were immunoreactive for the neuronal marker microtubule-associated protein 2. Furthermore, approximately half of the neurons were TH-positive dopaminergic neurons.
Cultures of primary astrocytes were prepared from the striata of Sprague-Dawley rat embryos at 15 days gestation using the method described previously.(22) The tissue was treated with 0.125% trypsin and then 0.004% DNase I in the same way as the mesencephalic neurons. After centrifugation (1,500 × g, 5 min), the cells were gently resuspended in DMEM containing 10% FBS and plated at a density of 2.0 × 105 cells/cm2 in 6-well plates coated with poly-d-lysine. The cells were cultured for 5 to 7 days in the same medium, and then subcultured to obtain astrocyte-rich cultures. Over 95% of these cultured glial cells showed immunoreactivity to glial fibrillary acidic protein (GFAP).
For neuron-astrocyte co-cultures, striatal astrocytes were seeded at a density of 2.0 × 104 cells/cm2 directly onto mesencephalic neuronal cell layers that had already been cultured in four-chamber culture slides for 4 days as described. The cell mixture was then co-cultured for another 2 days.
Mesencephalic neurons or neuron-astrocyte co-cultures of mesencephalic neurons and striatal astrocytes on four-chamber culture slides were pretreated with l-theanine (500 µM) for 72 h, then cultures were treated with DA (0, 50, 100, 150 µM) for further 24 h for the immunohistochemistry of dopaminergic neurons. For the GSH measurement and Western blot analysis, mesencephalic neuron-rich culture or striatal astrocyte-rich cultures in 6-well plates were treated with l-theanine (5, 50, 500 µM) or vehicle for 72 h.
Preparation of conditioned medium
The striatal astrocytes were plated onto 6-well plates (Becton Dickinson) and grown in DMEM containing 10% FBS at density of 3.6 × 104 cells/cm2 for 7 days. To prepare glia conditioned medium (GCM), astrocytes were treated with l-theanine (50, 500 µM) diluted in medium (l-theanine-GCM) or vehicle (control-GCM) for 72 h. The conditioned media were collected, centrifuged at 3,000 × g for 3 min to remove cellular debris, and the supernatants were stored at –80°C until use. For experiments, the thawed conditioned media were mixed with an equal volume of fresh culture medium (50% GCM of total medium).
To examine effects of GCM from l-theanine-treated astrocytes against DA-induced neurotoxicity, mesencephalic neurons were treated with normal medium or GCM from astrocytes that were pretreated with vehicle (control-GCM) or with l-theanine (50, 500 µM: l-theanine-GCM) for 24 h.
Animal experiments
Healthy male ICR mice weighing 32–34 g (8-week-old) were treated orally with l-theanine (4.0 mg/kg) dissolved in drinking water every day for 14 days. One day after the final administration of l-theanine, the mice were anesthetized with sodium pentobarbital (70 mg/kg, i.p.) and perfused transcardially with ice-cold saline, and the striatal or ventral midbrain tissue was dissected out immediately.
Fluorescent immunocytochemistry of primary cultures
The cells were fixed with 4% paraformaldehyde for 20 min at room temperature and washed in 10 mM phosphate-buffered saline (PBS, pH 7.4). After blocking with 2.5% normal goat serum for 20 min at room temperature, the cells were reacted with the primary antibodies for 18 h at 4°C: rabbit anti-TH (dilution, 1:1,000; Protos Biotech Corporation, New York, NY) diluted in 10 mM PBS containing 0.1% Triton X-100 (0.1% PBST). After washing in 10 mM PBS (pH 7.4) three times 10 min each, the cells were reacted for 2 h with goat anti-rabbit IgG conjugated to Alexa Fluor 488 (dilution, 1:1,000; Molecular Probes, Eugene, OR). The cells were counterstained with Hoechst nuclear stain (10 µg/ml) for 2 min and washed before mounting with Fluoromounting medium (Dako Cytomation, Glostrup, Denmark).
All slides were analyzed under a fluorescence microscope (Olympus BX50-FLA, Tokyo, Japan) using a mercury lamp through a 470–490 nm or 360–370 nm band-pass filter to excite Alexa Fluor 488 or Hoechst dye, respectively. The light emitted from Alexa Fluor 488 or Hoechst was collected through 515–550 nm band-pass filter or 420 nm long-pass filter, respectively. TH-immunopositive cells were counted under the microscope in all areas of each chamber slide. Counting was performed by an investigator blinded to the experiments.
Determination of total GSH
The GSH level was determined using the enzymatic recycling method of Tietze(23) with some modifications.(18) For preparation of the sample, the cells were homogenized in 0.1 M phosphate buffer (PB; pH 7.4) and then treated with equivalent volumes of 10% trochloroacetic acid. The extracts were mixed with 0.01 M PB (pH 7.4, 174 µl), NADPH (4 mM, 15 µl) and GSH reductase (6 U/ml, 30 µl) and 5,5'-dithiobis-2-nitrobenzoic acid (10 mM, 15 µl), and incubated at 37°C. The formation of 2-nitro-5-thiobenzoic acid was measured at 412 nm. The amount of total GSH was determined from a standard curve constructed using known amounts of GSH. The GSH level in GCM from l-theanine-treated striatal astrocytes was also determined as described above.
GSH assay in cell-free system
GSH production was determined in a cell-free system by mixing 0.05–5 mM l-glutamate or 0.05–5 mM l-theanine with 5 mM l-cysteine, 10 mM ATP, 0.6 ng/µl glutamate cysteine ligase (GCL) recombinant protein (Novus Biologicals), 100 mM Tris-HCl (pH 8.2) followed by the addition of 5 mM glycine and 0.05 ng/µl GSH synthase recombinant protein (Syd labs) over a period of 30 min under constant shaking at 37°C. GSH level was determined using the enzymatic recycling method as described above.
Measurement of quinoprotein levels
The levels of protein-bound quinones (quinoprotein) were determined by the nitrobluetetrazolium (NBT)/glycinate colorimetric assay, as described previously.(19) Total cell lysates were prepared by homogenization in ice cold-RIPA buffer [PBS (pH 7.4), 1% nonidet P-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS)] with 10 µg/ml phenylmethylsulfonyl fluoride (PMSF). The cell lysate was added to 100 µl of NBT reagent (0.24 mM NBT in 2 M potassium glycinate, pH 10.0) followed by incubation in the dark for 3 h under constant shaking. The absorbance of blue-purple color developed by the reaction mixture was measured at 530 nm.
Western blot analysis
Cytoplasmic lysate from cell cultures were extracted and prepared by using the PARIS protein and RNA isolation system (Ambion, Austin, TX) according to the protocol provided with the kit. Cells from the 6-well culture plates were lysed by incubation in ice-cold Cell Fractionation Buffer with 0.1 mg/ml PMSF for 10 min. The homogenates were centrifuged (500 × g for 3 min at 4°C), and the supernatant was collected as cytoplasmic protein lysate. The protein concentration in the lysate was determined by the DC protein assay kit (Bio-Rad, Richmond, CA), using bovine serum albumin as a standard.
Western blot analysis was performed as described previously.(24) Briefly, cytoplasmic lysate (10 µg) was loaded on 10% SDS-polyacrylamide gels and blotted onto polyvinylidene difluoride membranes (Hybond P; GE Healthcare UK, Buckinghamshire, UK). The blots were incubated at room temperature for 1 h with the following antibodies: rabbit polyclonal anti-GCL (dilution, 1:200, Lab Vision, Fremont, CA) and mouse monoclonal anti-α-tubulin (dilution, 1:500, Sigma-Aldrich, St. Louis, MO). After incubation with the corresponding peroxidase-conjugated secondary antibody, blots were washed with 20 mM Tris-buffered saline containing 0.1% Tween 20. Protein-specific signals were visualized by chemiluminescence using the ECL western blotting detection system (GE Healthcare UK). Images were obtained and quantified using a FUJIFILM Luminescent Image Analyzer LAS-3000 (FUJIFILM, Tokyo, Japan) and MultiGauge (ver. 3.0) software. For quantitative analysis, the ratio for specific signals of protein (relative chemiluminescence unit) to that of constitutively expressed α-tubulin protein was calculated to normalize for loading and transfer artifacts introduced in western blotting.
Statistical analysis
Data were expressed as mean ± SEM. Statistical significance was determined by one-way ANOVA followed by post hoc Fisher’s PLSD test. A two-tailed p value <0.05 denoted the presence of statistical significance.
Results
l-Theanine protects against excess DA-induced neuronal death in presence of astrocytes
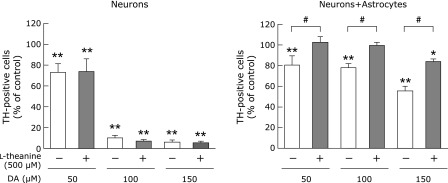
We first examined the neuroprotective effects of l-theanine against DA neurotoxicity using primary cultured cells. Pretreatment of mesencephalic neuron-rich cultures with l-theanine (500 µM) did not protect dopaminergic neurons against DA (50–150 µM)-induced neurotoxicity (Fig. 2). However, the addition of 500 µM l-theanine to cocultures of mesencephalic neurons and striatal astrocytes significantly lessened the percentage of TH-positive neurons damaged by 50–150 µM of DA (Fig. 2). These results indicate that the neuroprotective effects of l-theanine against DA neurotoxicity are mediated by astrocytes.
Fig. 2.
Effects of l-theanine on excess DA-induced neuronal death in neuron-rich cultures and neuron-astrocyte cocultures. Mesencephalic neuronal cultures or mesencephalic neurons and striatal astrocytes cocultures were pretreated with l-theanine (500 µM) for 72 h, followed by DA (0, 50, 100, 150 µM) for further 24 h. The viability of dopaminergic neurons was determined by immunostaining with anti-TH antibody. Data are mean ± SEM of 6 samples/experiment. *p<0.05, **p<0.01 compared with the corresponding control group, #p<0.01 compared between two indicated groups.
l-Theanine increases GSH level in astrocytes
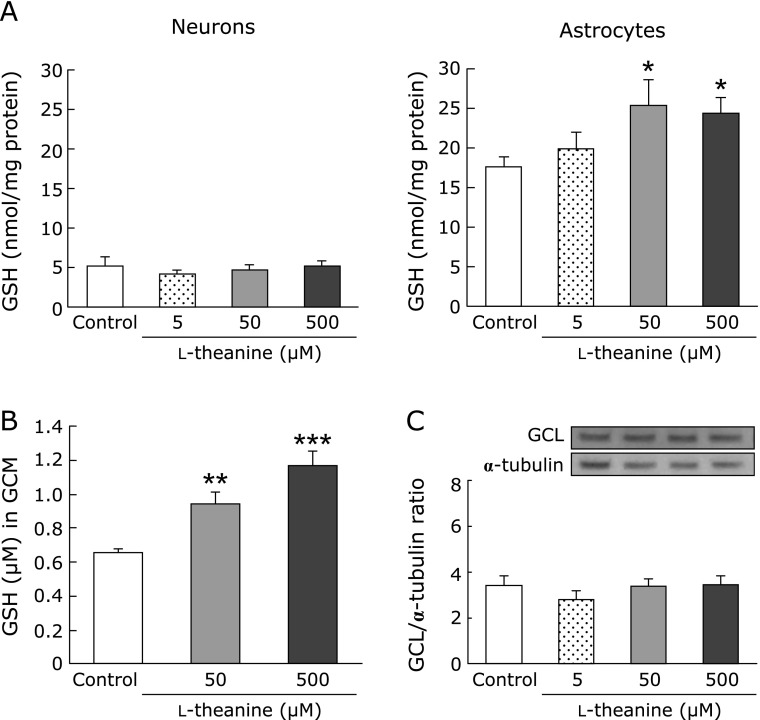
To clarify the mechanism of the neuroprotective effects of l-theanine, we compared its effect on GSH level in mesencephalic neuron-rich and striatal astrocyte-rich cultures. Incubation with l-theanine for 72 h significantly increased GSH level in astrocyte-rich cultures but not in neuron-rich cultures (Fig. 3A). Furthermore, the GSH level in the GCM from astrocyte cultures was significantly higher after l-theanine (50–500 µM) treatment for 72 h (Fig. 3B).
Fig. 3.
l-Theanine increased GSH levels in astrocytes. (A) Effects of l-theanine on GSH content in cultured neurons and astrocytes. GSH levels were measured in cultured neurons and astrocytes after treatment with theanine (5, 50, 500 µM) or vehicle for 72 h. Data are mean ± SEM of 6 samples/experiment. *p<0.05 compared with the vehicle-treated control group. (B) Changes in GSH levels in astroglial conditioned media (GCM) after treatment with l-theanine (50, 500 µM) or vehicle for 72 h. Data are mean ± SEM of 6 samples/experiment. **p<0.01, ***p<0.001 compared with the vehicle-treated control group. (C) Effects of treatment with l-theanine (0, 5, 50, 500 µM) for 72 h on GCL in cultured astrocytes. Protein levels were examined by western blot analysis. Data are mean ± SEM of 6 samples/experiment.
In the next step, we investigated the effects of l-theanine on GSH synthesis-related molecules in astrocyte cultures. Treatment with l-theanine for 72 h had no effect on the protein level of GCL, a rate-limiting enzyme of GSH synthesis, in astrocyte cultures (Fig. 3C).
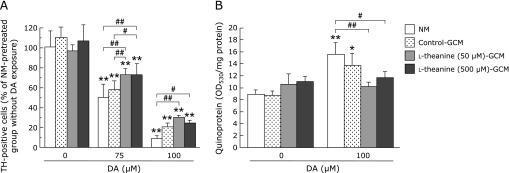
Glia conditioned medium from l-theanine-treated astrocytes protects dopaminergic neurons against DA-induced neurotoxicity
To further evaluate the role of astrocytes in the neuroprotective effects of l-theanine, mesencephalic neuron-rich cultures were incubated in conditioned media from striatal astrocytes treated with/without l-theanine. Preincubation with l-theanine-GCM attenuated the DA (75 or 100 µM)-induced cytotoxicity (Fig. 4A). Moreover, the marked increase in quinoprotein formation in mesencephalic neurons following DA exposure (100 µM) in NM-pretreated group was significantly prevented by the pretreatment with l-theanine-GCM (50 or 500 µM) (Fig. 4B). Furthermore, the pretreatment with l-theanine-GCM (50 µM) also tended to inhibit, but not significantly (p = 0.0585), DA-induced elevation of quinoprotein in control-GCM-pretreated neurons. These results suggest that secreted molecules from l-theanine-treated astrocytes exert neuroprotective effects against dopaminergic neurons by suppressing DA quinone formation.
Fig. 4.
Glia conditioned medium from l-theanine-treated astrocytes protects dopaminergic neurons against DA-induced neurotoxicity. (A) Mesencephalic neurons were treated with normal medium (NM), astroglial conditioned media (GCM) from astrocytes that were pretreated with l-theanine (50, 500 µM), or vehicle for 72 h. After the GCM treatment for 24 h, each culture medium was replaced with fresh normal medium, and then neurons ware exposed to DA (75, 100 µM) for further 24 h. The viability of TH-immunopositive dopaminergic neurons was counted. Data are mean ± SEM of 6 samples/experiment and expressed as percentage of NM-pretreated group without DA exposure. (B) Quinoprotein levels in mesencephalic neurons treated with NM, GCM, or l-theanine-GCM (50, 500 µM) for 24 h followed by exposure to DA (100 µM) for 24 h. Data are mean ± SEM of 6 samples/experiment. *p<0.05, **p<0.01, compared with the corresponding control group without DA exposure (DA 0), #p<0.05, ##p<0.01 compared between two indicated groups.
Involvement of l-theanine in GSH synthesis in vitro
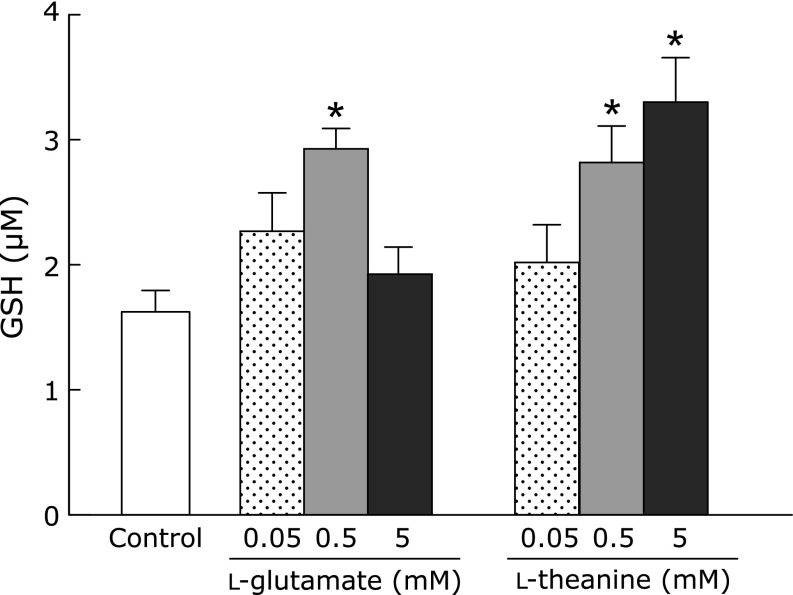
To examine whether l-theanine can be incorporated into GSH synthesis as a substrate, we performed an in vitro cell-free GSH synthesis experiment. GSH production as a positive control was detected by incubation of l-glutamate and cysteine with GCL and consequent reaction with glycine by GSH synthase. Replacement of l-glutamate with l-theanine in this GSH system also produced GSH-like thiol compounds (Fig. 5).
Fig. 5.
Involvement of l-theanine in GSH synthesis in vitro. GSH production was detected by incubation of l-glutamate or l-theanine and l-cysteine with GCL and consequent addition of glycine by GSH synthase in the cell-free system. Data are mean ± SEM of 6 samples/experiments. *p<0.01, compared with the corresponding control group.
l-Theanine upregulates GSH contents in striata of normal mice
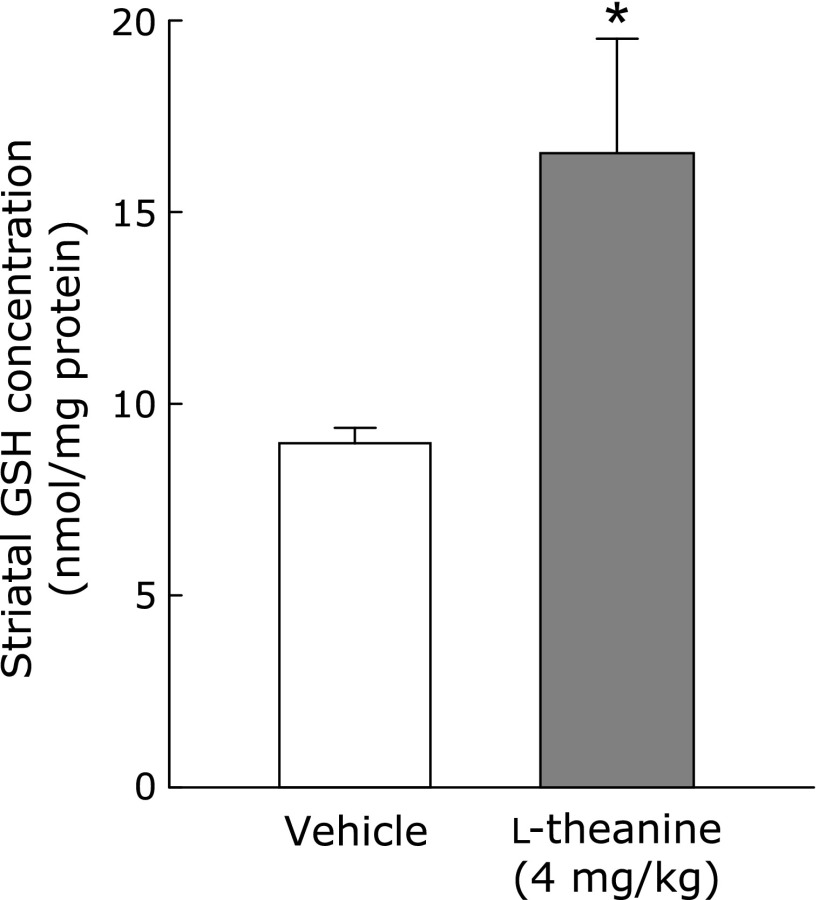
Finally, we examined the effects of l-theanine on GSH contents in the striatum and midbrain of normal mice (n = 5). Administration of l-theanine (4 mg/kg, p.o.) for 14 days (by mixing it in drinking water) significantly increased GSH content in the striatum (Fig. 6), but not in the midbrain (data not shown). The same treatment had no effect on body weight or water intake (data not shown).
Fig. 6.
l-Theanine administration increases GSH content in the striatum of mice. Changes in GSH levels in the striatum were measured at 24 h after administration of l-theanine at 4 mg/kg/day, p.o., for 14 days. Data are mean ± SEM of 5 samples/experiment. *p<0.01, compared with the vehicle-treated control group.
Discussion
The present study showed that l-theanine increased GSH supply in striatal astrocytes and exerted neuroprotective effects against excess DA-induced neurotoxicity.
First, we examined whether l-theanine protected dopaminergic neurons against DA-induced neurotoxicity using neuron-astrocyte cocultures. Because DA quinones are predominantly generated from excess cytosolic DA outside the synaptic vesicles in damaged DA neurons, we used astrocytes in the striatum in the present study. Although neuron-rich cultures were sensitive to excess DA-induced toxicity, the presence of astrocytes reduced dopaminergic neuronal loss (Fig. 2). These results indicate that astrocytes protect dopaminergic neurons against DA toxicity, consistent with our previous report.(20) Moreover, pretreatment with l-theanine for 72 h significantly attenuated DA-induced neuronal cell death in neuron-astrocyte cocultures, but not in cultures of neurons alone (Fig. 2). Cho et al.(10) reported that l-theanine (500 µM) attenuated neuronal cell death induced by neurotoxins such as rotenone and dieldrin, which are considered as potential environmental pathogens of Parkinson’s disease, in human dopaminergic SH-SY5Y cells, suggesting its direct detoxicating effects on neuronal cells. However, the present results indicate that l-theanine is able to indirectly protect dopaminergic neurons against excess DA-induced neurotoxicity through affecting astrocytes.
Second, we examined the effects of l-theanine on GSH synthesis in astrocytes. Astrocytes are abundant neuron-supporting glial cells that harbor a powerful arsenal of neurotrophic factors and antioxidants. Previous studies indicated that astrocyte-derived antioxidants, such as GSH and metallothionein, are important to the survival of neighboring neurons.(20,22) GSH is a major antioxidant and protects neurons against oxidative stress. Astrocytes but not neurons express cystine/glutamate exchange transporter (xCT), which takes up cystine, reduce it to cysteine, and release GSH and GSH disulfide through the multidrug resistance protein 1 (Mrp1) of ATP-driven exporters to consequently supply cysteine, the substrate for GSH synthesis in neurons.(25–29) We recently reported similar increases in GSH production in astrocytes and increase in GSH release to GCM from astrocytes by the treatment with an antiepileptic drug levetiracetum which exerts neuroprotective effects against dopaminergic neurotoxin 6-hydroxydopamine-induced mesencephalic neurodegeneration.(30) Therefore, GSH synthesis in neurons is dependent on astrocytes. Thus, GSH and its synthesis-related molecules in astrocytes seem to provide protection against dopaminergic neurodegeneration. In this study, we examined the effects of l-theanine on GSH levels in cultured neurons or astrocytes. The basal GSH level in astrocytes was greater than that in neurons. Treatment with l-theanine for 72 h significantly increased GSH levels in astrocytes, but not in neurons (Fig. 3A). Furthermore, treatment of cultured astrocytes with l-theanine also significantly increased GSH levels in the conditioned medium at dose-dependent manner (Fig. 3B). These results suggest that l-theanine up-regulates not only GSH synthesis in astrocytes but also GSH supply from astrocytes. In addition, GCM from l-theanine-treated astrocytes prevented excess DA-induced neuronal cell death (Fig. 4A) and quinoprotein formation (Fig. 4B). With regard to DA quinone neurotoxicity, DA quinones covalently conjugate with the sulfhydryl group of cysteine on various functional proteins including several key molecules involved in the pathogenesis of PD (e.g., TH, DA transporter and parkin) to form quinoproteins, and inhibit the protein function to cause DA neuron-specific cytotoxicity.(11,13–15,31) GSH prevents DA quinone from binding to the sulfhydryl groups of cysteine on functional proteins by their conjugation with DA quinone. Several reports indicated that DA quinone-induced dopaminergic neuronal cell damage can be prevented by GSH based on its quinone-quenching activity.(11,13,18,32) Taken together, the resulting data indicate that humoral factor(s) released from l-theanine-treated astrocytes is involved in the neuroprotective effects of l-theanine, and that GSH secreted from astrocytes is probably one of the neuroprotective humoral molecules against DA quinone neurotoxicity. In the present study, the increment of GSH content in GCM from the l-theanine (500 µM)-treated astrocytes was approx. 0.5 µM (Fig. 3B). In our recent study, the small increment of GSH content (0.5 µM) in GCM from astrocytes after the levetiracetam treatment, which is equivalent to the increment of GSH in l-theanine-GCM in the present study, exerted neuroprotective effects against 6-hydroxydopamine-induced dopaminergic neuronal death.(30) We also revealed that the pretreatment with the exogenous addition of reduced GSH (0.5 µM) in control-GCM significantly prevented 6-hydroxydopamine-induced dopaminergic neurodegeneration.(30) Therefore, even low increment of GSH (0.5 µM) in l-theanine-GCM would be enough to protect excess DA-induced neurotoxicity.
Recent studies reported that other food chemicals, such as flavonoids, protect against dopaminergic neuron-specific toxicity caused by psychostimulants or neurotoxins,(33,34) and increase GSH synthesis through the activation of nuclear factor erythroid-related factor-2 (Nrf2) transcription factor.(35,36) Therefore, we examined the effects of l-theanine on protein levels of GCL, a GSH-synthesizing enzyme regulated by Nrf2, in astrocytes. However, treatment with l-theanine had no effects on the protein levels of GCL in astrocyte cultures (Fig. 3C). GSH is a tripeptide of glutamate, cysteine and glycine. l-Theanine is also a derivative of glutamate (Fig. 1) and is metabolized to glutamate and ethylamine in the intestine and liver.(37) Kurihara et al.(38) reported the effects of oral administration of l-cysteine and/or l-theanine on GSH levels and immune responses, and reported that co-administration of l-cysteine and l-theanine for 11 days before immunization significantly increased the levels of total GSH in the liver 6 h after immunization compared with the levels in control mice. Considering together the above findings and the present results, we postulate that l-theanine is a substrate instead of glutamate in GSH synthesis in astrocytes. To examine this hypothesis, we constructed in vitro GSH-synthesis system by incubation with glutamate or l-theanine, cysteine and GCL followed by incubation with glycine and GSH synthase. Replacement of l-glutamate with l-theanine in the GSH-synthesis system could also generate GSH-like thiol compounds (Fig. 5). This suggests that l-theanine can be a substrate in GSH or related thiol compound synthesis instead of l-glutamate. Further experiments, that are mass spectrometry and tracing experiments testing incorporation of l-theanine compound labeled at alpha-carboxyl group or ethylamine group, would identify the resulting GSH-like thiol compound and clarify underlying detailed mechanism how l-theanine reacts with cysteine by GCL.
Finally, we confirmed the effects of l-theanine treatment on GSH levels in vivo. Administration of l-theanine for 14 days significantly increased striatal GSH levels in mice (Fig. 6). In general, DA-induced neurotoxicity with severe neuronal damage is observed in the striatum due to abundant dopaminergic neurons and high DA contents. l-Theanine is thought to cross the blood-brain barrier.(5) l-Theanine makes up 1–2% of the dry weight of tea leaves. Drinking two to four cups of green tea every day is equivalent to taking approximate 50–200 mg of l-theanine. It has been reported that oral administration of 50–200 mg of l-theanine in humans induces alpha-brain wave activity, which correlates with relaxation.(39,40) Taken together with these findings, our results suggest that l-theanine could be a useful and safe compound and it exerts neuroprotective effects against DA quinone-induced neuronal damage by released humoral molecules, in part, by glutathione from astrocytes.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (C) (KAKENHI #21591082, #22590934, #25461279) from Japan Society for the Promotion of Science, by Grant-in Aid for Scientific Research on Innovative Areas “Brain Environment” (KAKENHI #24111533) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by a Research Grant from the Okayama Medical Foundation.
Abbreviations
- DA
dopamine
- DMEM
Dulbecco’s modified Eagles’s medium
- FBS
fetal bovine serum
- GCL
glutamate cysteine ligase
- GCM
glia conditioned medium
- GFAP
glial fibrillary acidic protein
- GSH
glutathione
- NBT
nitrobluetetrazolium
- Nrf2
nuclear factor erythroid-related factor-2
- PBS
phosphate-buffered saline
- PBST
PBS containing Triton X-100
- PMSF
phenylmethylsulfonyl fluoride
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- TH
tyrosine hydroxylase
Author Contributions
M.T., I.M., T.K. and M.A. conceived and designed the study; M.T., I.M. and S.M. performed the experiments; M.T., I.M. and M.A. analyzed data; M.T., I.M. and M.A. wrote the manuscript.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kimura R, Murata T. Influence of alkylamides of glutamic acid and related compounds on the central nervous system. II. Syntheses of amides of gutamic acid and related compounds, and their effects on the central nervous system. Chem Pharm Bull (Tokyo) 1971;19:1301–1307. doi: 10.1248/cpb.19.1301. [DOI] [PubMed] [Google Scholar]
- 2.Yokogoshi H, Kato Y, Sagesaka YM, Takihara-Matsuura T, Kakuda T, Takeuchi N. Reduction effect of theanine on blood pressure and brain 5-hydroxyindoles in spontaneously hypertensive rats. Biosci Biotechnol Biochem. 1995;59:615–618. doi: 10.1271/bbb.59.615. [DOI] [PubMed] [Google Scholar]
- 3.Nishida K, Yasuda E, Nagasawa K, Fujimoto S. Altered levels of oxidation and phospholipase C isozyme expression in the brains of theanine-administered rats. Biol Pharm Bull. 2008;31:857–860. doi: 10.1248/bpb.31.857. [DOI] [PubMed] [Google Scholar]
- 4.Yokozawa T, Dong E. Influence of green tea and its three major components upon low-density lipoprotein oxidation. Exp Toxicol Pathol. 1997;49:329–335. doi: 10.1016/S0940-2993(97)80096-6. [DOI] [PubMed] [Google Scholar]
- 5.Terashima T, Takido J, Yokogoshi H. Time-dependent changes of amino acids in the serum, liver, brain and urine of rats administered with theanine. Biosci Biotechnol Biochem. 1999;63:615–618. doi: 10.1271/bbb.63.615. [DOI] [PubMed] [Google Scholar]
- 6.Kakuda T, Yanase H, Utsunomiya K, Nozawa A, Unno T, Kataoka K. Protective effect of gamma-glutamylethylamide (theanine) on ischemic delayed neuronal death in gerbils. Neurosci Lett. 2000;289:189–192. doi: 10.1016/s0304-3940(00)01286-6. [DOI] [PubMed] [Google Scholar]
- 7.Kakuda T, Nozawa A, Sugimoto A, Niino H. Inhibition by theanine of binding of [3H]AMPA, [3H]kainate, and [3H]MDL 105,519 to glutamate receptors. Biosci Biotechnol Biochem. 2002;66:2683–2686. doi: 10.1271/bbb.66.2683. [DOI] [PubMed] [Google Scholar]
- 8.Kakuda T, Hinoi E, Abe A, Nozawa A, Ogura M, Yoneda Y. Theanine, an ingredient of green tea, inhibits [3H]glutamine transport in neurons and astroglia in rat brain. J Neurosci Res. 2008;86:1846–1856. doi: 10.1002/jnr.21637. [DOI] [PubMed] [Google Scholar]
- 9.Kim TI, Lee YK, Park SG, et al. l-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radic Biol Med. 2009;47:1601–1610. doi: 10.1016/j.freeradbiomed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Cho HS, Kim S, Lee SY, Park JA, Kim SJ, Chun HS. Protective effect of the green tea component, l-theanine on environmental toxins-induced neuronal cell death. Neurotoxicology. 2008;29:656–662. doi: 10.1016/j.neuro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Asanuma M, Miyazaki I, Ogawa N. Dopamine- or l-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res. 2003;5:165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]
- 12.Lee SY, Moon Y, Hee Choi D, Jin Choi H, Hwang O. Particular vulnerability of rat mesencephalic dopaminergic neurons to tetrahydrobiopterin: Relevance to Parkinson’s disease. Neurobiol Dis. 2007;25:112–120. doi: 10.1016/j.nbd.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn DM, Arthur RE, Jr, Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson’s disease. J Neurochem. 1999;73:1309–1317. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- 14.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead RE, Ferrer JV, Javitch JA, Justice JB. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J Neurochem. 2001;76:1242–1251. doi: 10.1046/j.1471-4159.2001.00125.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Dopamine agonist pergolide prevents levodopa-induced quinoprotein formation in parkinsonian striatum and shows quenching effects on dopamine-semiquinone generated in vitro. Clin Neuropharmacol. 2005;28:155–160. doi: 10.1097/01.wnf.0000175523.33334.24. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki I, Asanuma M, Diaz-Corrales FJ, et al. Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone formation-related molecules. FASEB J. 2006;20:571–573. doi: 10.1096/fj.05-4996fje. [DOI] [PubMed] [Google Scholar]
- 18.Emdadul Haque M, Asanuma M, Higashi Y, Miyazaki I, Tanaka K, Ogawa N. Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim Biophys Acta. 2003;1619:39–52. doi: 10.1016/s0304-4165(02)00440-3. [DOI] [PubMed] [Google Scholar]
- 19.LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki I, Asanuma M, Kikkawa Y, et al. Astrocyte-derived metallothionein protects dopaminergic neurons from dopamine quinone toxicity. Glia. 2011;59:435–451. doi: 10.1002/glia.21112. [DOI] [PubMed] [Google Scholar]
- 21.Asanuma M, Miyazaki I, Diaz-Corrales FJ, et al. Neuroprotective effects of zonisamide target astrocyte. Ann Neurol. 2010;67:239–249. doi: 10.1002/ana.21885. [DOI] [PubMed] [Google Scholar]
- 22.Iwata-Ichikawa E, Kondo Y, Miyazaki I, Asanuma M, Ogawa N. Glial cells protect neurons against oxidative stress via transcriptional up-regulation of the glutathione synthesis. J Neurochem. 1999;72:2334–2344. doi: 10.1046/j.1471-4159.1999.0722334.x. [DOI] [PubMed] [Google Scholar]
- 23.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Corrales FJ, Asanuma M, Miyazaki I, Miyoshi K, Ogawa N. Rotenone induces aggregation of gamma-tubulin protein and subsequent disorganization of the centrosome: Relevance to formation of inclusion bodies and neurodegeneration. Neuroscience. 2005;133:117–135. doi: 10.1016/j.neuroscience.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XF, Cynader MS. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 2000;74:1434–1442. doi: 10.1046/j.1471-4159.2000.0741434.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirrlinger J, König J, Keppler D, Lindenau J, Schulz JB, Dringen R. The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J Neurochem. 2001;76:627–636. doi: 10.1046/j.1471-4159.2001.00101.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirrlinger J, Schulz JB, Dringen R. Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res. 2002;69:318–326. doi: 10.1002/jnr.10308. [DOI] [PubMed] [Google Scholar]
- 29.Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2006;97:373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki I, Murakami S, Torigoe N, Kitamura Y, Asanuma M. Neuroprotective effects of levetiracetam target xCT in astrocytes in parkinsonian mice. J Neurochem. 2016;136:194–204. doi: 10.1111/jnc.13405. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med Okayama. 2008;62:141–150. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- 32.Lai CT, Yu PH. Dopamine- and l-beta-3,4-dihydroxyphenylalanine hydrochloride (l-Dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Effects of oxidative stress and antioxidative factors. Biochem Pharmacol. 1997;53:363–372. doi: 10.1016/s0006-2952(96)00731-9. [DOI] [PubMed] [Google Scholar]
- 33.Kita T, Asanuma M, Miyazaki I, Takeshima M. Protective effects of phytochemical antioxidants against neurotoxin-induced degeneration of dopaminergic neurons. J Pharmacol Sci. 2014;124:313–319. doi: 10.1254/jphs.13r19cp. [DOI] [PubMed] [Google Scholar]
- 34.Takeshima M, Murata M, Urasoe N, et al. Protective effects of baicalein against excess l-DOPA-induced dopamine quinone neurotoxicity. Neurol Res. 2011;33:1050–1056. doi: 10.1179/1743132811Y.0000000032. [DOI] [PubMed] [Google Scholar]
- 35.Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myhrstad MC, Carlsen H, Nordström O, Blomhoff R, Moskaug JØ. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32:386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 37.Asatoor AM. Tea as a source of urinary ethylamine. Nature. 1966;210:1358–1360. doi: 10.1038/2101358b0. [DOI] [PubMed] [Google Scholar]
- 38.Kurihara S, Shibahara S, Arisaka H, Akiyama Y. Enhancement of antigen-specific immunoglobulin G production in mice by co-administration of l-cystine and l-theanine. J Vet Med Sci. 2007;69:1263–1270. doi: 10.1292/jvms.69.1263. [DOI] [PubMed] [Google Scholar]
- 39.Kimura K, Ozeki M, Juneja LR, Ohira H. l-Theanine reduces psychological and physiological stress responses. Biol Psychol. 2007;74:39–45. doi: 10.1016/j.biopsycho.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K, Nagato Y, Aoi N, et al. Effects of l-theanine on the release of α-brain waves in human volunteers. Nippon Nögeikagaku Kaishi. 1998;72:153–157. [Google Scholar]