Abstract
Resveratrol is a natural polyphenol produced by plants in response to environmental stress. This compound has been shown to have pharmacological effects against a wide range of diseases including neurological, hepatic, cardiovascular and autoimmune conditions. The non-obese diabetic (NOD) mouse, in which loss of lacrimal and salivary gland function occurs, has been studied as an animal model for Sjögren’s syndrome. In this study, we confirmed that administration of resveratrol results in increased secretion of saliva in NOD mice. Although resveratrol enhanced Sirt1 activity, inflammatory cell infiltration was not affected. Moreover, expression of the anti-inflammatory cytokine IL-10 in salivary glands was enhanced in the resveratrol-administered group. Thus, we confirmed a novel therapeutic effect for resveratrol on salivary dysfunction in Sjögren’s syndrome.
Keywords: salivary gland, resveratrol, NOD mouse, salivary secretion, Sjögren’s syndrome
Introduction
Resveratrol (3,5,4-trihydroxystilbene), a nonflavonoid polyphenolic compound found in numerous plant products, was initially characterized as a phytoalexin, which is an antimicrobial substance synthesized by plants in response to infection.(1,2) Resveratrol also has potent anti-inflammatory, anti-tumor, immunomodulatory, cardioprotective, anti-oxidative and chemopreventive properties.(3–6) Several studies have shown that resveratrol supplementation directly suppresses the release of pro-inflammatory cytokines such as TNFα, IL-β, IL-6, IL-10, MCP-1, IFNα and IFNβ in a wide range of tissues.(7–9) Resveratrol also displays antioxidant activity in cell culture, which contributes to a reduction in inflammatory responses. A common link between the inhibitory effects of resveratrol mentioned above could be its ability to inhibit factors involved in gene transcription, such as MAPK, c-JNK, AP-1 and NF-κB.(10) It has been reported that resveratrol acts on NF-κB by inhibiting Iκ-B kinase, thus preventing the translocation of NF-κB into the nucleus.(11) Alternatively, the effects of resveratrol may involve its sirtuin-like activity, which results in the deacetylation of NF-κB.(12) Sirtuins are known to be protein deactylases involved in the regulation of metabolism and stress responses, which mediate the life-prolonging and stress-resistance effects of calorie restriction.(13–15) Furthermore, a recent report showed that mice lacking Sirtuin 1 (SIRT1) develop an autoimmune-like condition.(16) However, little is known about the effects of resveratrol on autoimmune diseases such as Sjögren’s syndrome (SS).
Non obese diabetic mice develop the corresponding clinical outcome of loss of secretory function,(17–19) and although they were first identified as a model for type I, insulin-dependent diabetes, they also develop an SS-like immunopathology of the exocrine glands. The first sign of SS-like disease is mononuclear cell infiltration in the salivary glands, which occurs at 8 weeks of age and is associated with a loss of salivary secretion later in life.(20,21) Lymphocytic infiltration of the salivary and lacrimal glands and functional decline in saliva flow and tear production are independent of the onset of diabetes.(18) Recently, epigallocatechin-3-gallate (EGCG), which increases the levels of the major antioxidant defense protein peroxiredoxin 6 (PRDX6) and catalase, has been identified as a therapeutic reagent for salivary dysfunction, thus suggesting the involvement of oxidative stress in NOD mice.(2) Moreover, oxidative stress has also been suggested to play a role in inducing the chronic inflammation in SS.(22) Oxidative biomarkers have been shown to be elevated in SS, thus suggesting that inflammation and oxidative stress contribute to its pathogenesis.
Salivary gland dysfunction leads to xerostomia (dry mouth syndrome) which causes various clinical conditions, including bacterial infection, dental decay, mastication dysfunction, swallowing dysfunction, dysgeusia and a general reduction in quality of life.(23,24) Therapies for xerostomia include administration of artificial saliva substitutes and sialogogues,(25) and/or medication with parasympathomimetic drugs.(25) Nevertheless, these treatments do not improve salivary gland dysfunction, and effective therapeutic treatments for salivary gland dysfunction remain to be developed. In the present study, we demonstrated that resveratrol administration ameliorated salivary dysfunction in NOD mice. This effect did not change the number of foci but increased IL-10 expression. Our results indicate that resveratrol can be used as a novel preventive and therapeutic approach against SS.
Materials and Methods
Ethics statement
All experimental protocols involving mice were approved by the Animal Welfare Committee at the Tsurumi University (Kanagawa, Japan).
Animals
Female NOD/shi mice were purchased from Clea Japan, Inc. (Tokyo, Japan). The animals were maintained under standard animal-housing conditions in animal facilities at Tsurumi University. The animals were maintained on a 12 h light-dark cycle and were provided water and food ad libitum.
Administration of resveratrol
The mice were orally administered the vehicle (Milli-Q) or resveratrol (Sigma-Aldrich, St. Louis, MO) at doses of 100 or 250 mg/kg using gastric intubation 6 days a week from 6 to 20 weeks of age (n = 6 mice/group).(26) Resveratrol was dissolved in 0.2 ml of H2O for administration.
Measurement of the stimulated salivary flow rate
Saliva secretion was measured prior to administration of the interventions and at 10, 14, 16 and 20 weeks of age. Mice were weighed and then anesthetized with an intraperitoneal injection of a mixture of xylazine (24 mg/kg) and ketamine (36 mg/kg). After 10 min, pilocalpine (0.1 mg/kg) was injected intraperioneally to stimulate salivation. The saliva secreted into the oral cavity during each 1-min period following injection of either of the above stimulants was carefully collected using capillaries (ringcaps; Hirschmann Laborgerate GmbH & Co. kG, Eberstadt, Germany) for 15 min. The total amount of saliva in the 15 min period was divided by the weight of the mouse, and the saliva secretion per 1 g in weight was calculated.
Histological analysis
NOD mice were anesthetized with diethyl ether (Wako Pure Chem. Ind., Osaka, Japan) and were killed. The salivary glands were then removed, fixed with 4% paraformaldehyde and embedded in paraffin. Sections (4 µm) were prepared and stained with hematoxylin and eosin (H&E) using standard methods. Histological grading of the inflammatory lesions in the salivary glands was performed as follows: the presence or absence of diffuse and focal chronic inflammatory cell infiltration was recorded, using a modification of the system originally introduced by Chisholm and Mason.
Deacetylase fluorometric assay for Sirt1
Animals were sacrificed with an overdose of anesthetic, and the salivary glands were immediately excised. The salivary gland complexes were carefully isolated and placed into 100 µl of lysis buffer and were then sonicated. The lysates were centrifuged at 15,000 rpm for 15 min at 4°C. The activity of Sirt1 in the supernatant was determined using SIRT1/Sir2 Deacetylase Fluorometric Assay kits (Cyclex, Ina, Japan) in accordance with the manufacturer’s protocols.
RNA extraction and real-time PCR from paraffin-fixed samples
Total RNA was isolated from the fixed 8 µm paraffin sections from each experimental group using the NucleoSpin® FFPE RNA kit (Macherey-Nagel, Düren, Germany), in accordance with the manufacturer’s protocol. Two micrograms of RNA was incubated with random primers at 70°C for 5 min, followed by reverse transcription with M-MLV reverse transcriptase (Promega Corp., Madison, WI) and 2.5 mM dNTPs at 42°C for 60 min. cDNA was assayed using quantitative RT-PCR with SYBR Premix Ex Taq II (Takara Bio Inc., Shiga, Japan). After an initial denaturation at 95°C for 30 s, PCR was carried out for 40 cycles consisting of 95°C for 5 s and 60°C for 30 s. The values for β-actin were used for normalization.
Statistical analyses
Statistical significance was determined using Student’s t test. P values of <0.05 were considered to be significant.
Results
Protective effects of resveratrol against hyposalivation in NOD mice
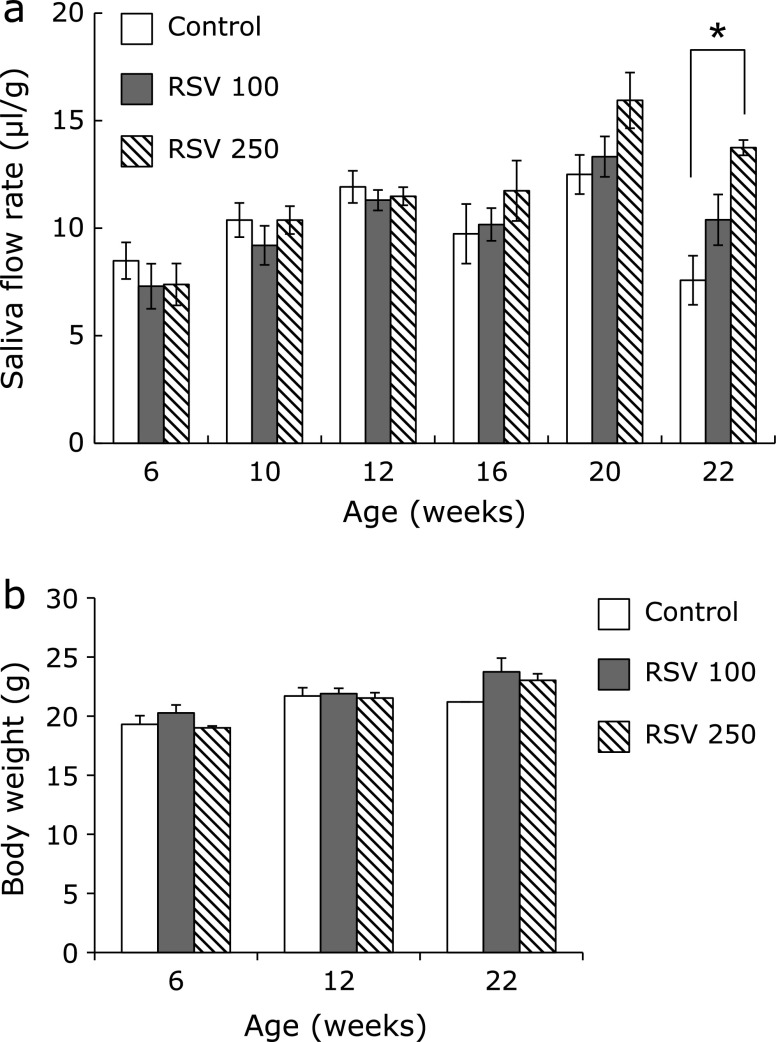
As reported previously,(18) the salivary flow rate in NOD mice was lower than in wild-type mice. A decreased flow rate was confirmed in the 22-week-old NOD mice in the present study. To investigate the effects of resveratrol on the secretion of saliva in NOD mice, 100 or 250 mg/kg resveratrol was administered beginning at 6 weeks of age, prior to the onset of hyposalivation. The salivary flow rate was not altered by resveratrol between 6 weeks and 20 weeks of age, whereas 250 mg/kg resveratrol showed protective effects on the hyposalivation observed in the NOD mice at age 22 weeks (Fig. 1a). The body weight of the mice in all groups increased in an age-dependent manner during the administration period, and no significant differences were observed (Fig. 1b), nor were any significant side effects observed.
Fig. 1.
Resveratrol treatment improves salivary dysfunction in NOD mice. (a) Saliva flow is expressed as total saliva output during the first 15 min after pilocarpine stimulation normalized to total body weight on the day of saliva collection. (b) Body weights for each administration group at age 6, 12 and 22 weeks. Error bars indicate the standard errors of the means; *p<0.5.
Relationship between saliva secretion and blood glucose levels
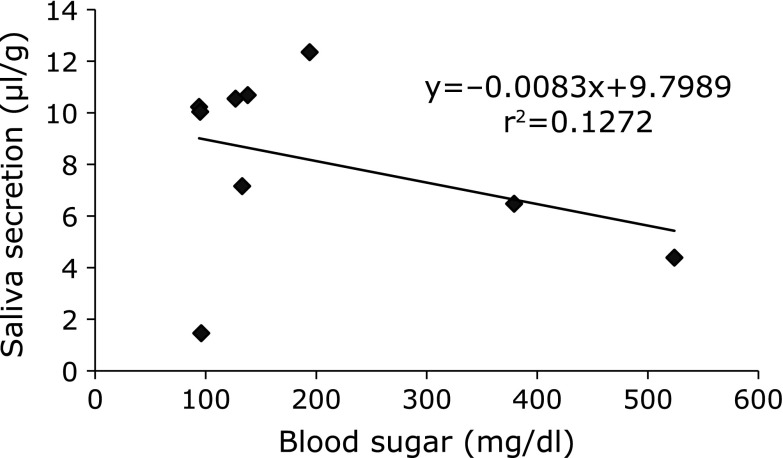
Saliva secretion in diabetic NOD mice was lower than that in BALB/c or prediabetic NOD mice.(18) To determine whether the salivary dysfunction was caused by diabetes in this study, the amount of saliva secreted was compared with the levels of blood glucose. Typically, BALB/c and nondiabetic NOD mice have blood glucose levels of 180–240 mg/dl, whereas diabetic NOD mice have blood glucose levels of 400–800 mg/dl.(18) Only 2 of 10 NOD mice showed diabetes in this study. Moreover, salivary dysfunction was not necessarily accompanied by diabetes (Fig. 2).
Fig. 2.
No correlations were observed between the saliva secretion and the blood glucose concentration. The saliva secretion and blood glucose concentrations were measured in 22-week-old NOD mice, the scores were plotted and the correlation coefficients were calculated.
Lymphocyte infiltration into the exocrine glands of NOD mice
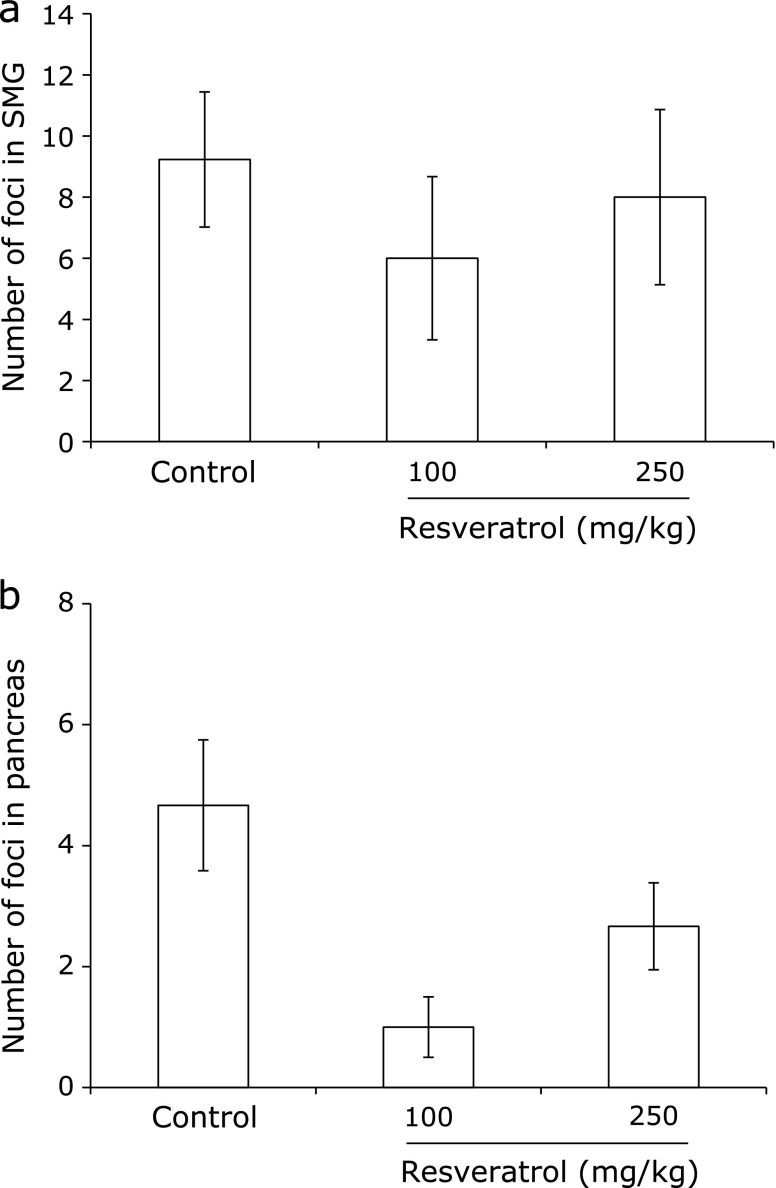
The NOD mouse is known to show hyposalivation accompanied by mononuclear cell infiltration of the salivary gland.(20,21) The effects of resveratrol on inflammatory cell infiltration in salivary glands were therefore studied. Histological examination of the submandibular, parotid and sublingual salivary glands in the NOD mice was performed to observe the anti-inflammatory effects of resveratrol at 22 weeks of age. In the parotid and sublingual salivary glands, no lymphocyte infiltration was observed in any groups of NOD mice (data not shown). The periductal inflammatory cell foci in submandibular glands were not affected by resveratrol administration (Fig. 3a), whereas the pancreas islets showed more sensitivity to resveratrol than the salivary glands (Fig. 3b).
Fig. 3.
Histochemical analysis of the submandibular glands and pancreata from 3 groups of NOD mice. At the end of the study, the salivary glands and pancreata from the control mice and the mice administered 100 or 250 mg/kg resveratrol were extracted. Inflammatory foci in hematoxylin and eosin-stained sections of the submandibular gland (a) and pancreas (b) were then counted.
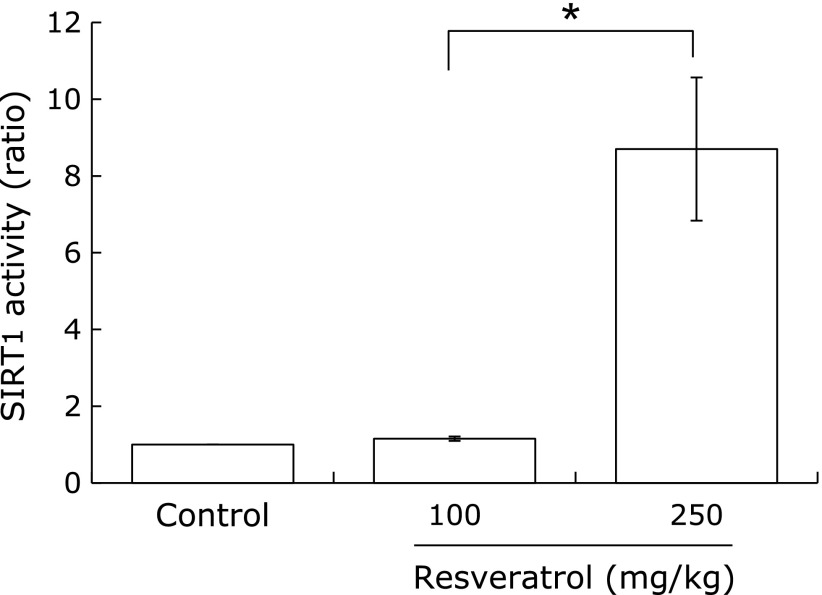
Sirt1 activity in the salivary glands
Resveratrol is known to be a pharmacological activator of Sirt1. We compared the Sirt1 activity in the salivary glands among the groups of mice. Although 100 mg/kg resveratrol did not change Sirt1 activity, 250 mg/kg resveratrol significantly induced Sirt1 activation (Fig. 4).
Fig. 4.
Resveratrol enhanced acetylase activity of Sirt1 in salivary glands. After the end of study, the salivary glands were extracted, and cell lysates were obtained. Sirt1 acetylase activities in cell lysates were calculated. Error bars indicate the standard errors of the means; *p<0.05.
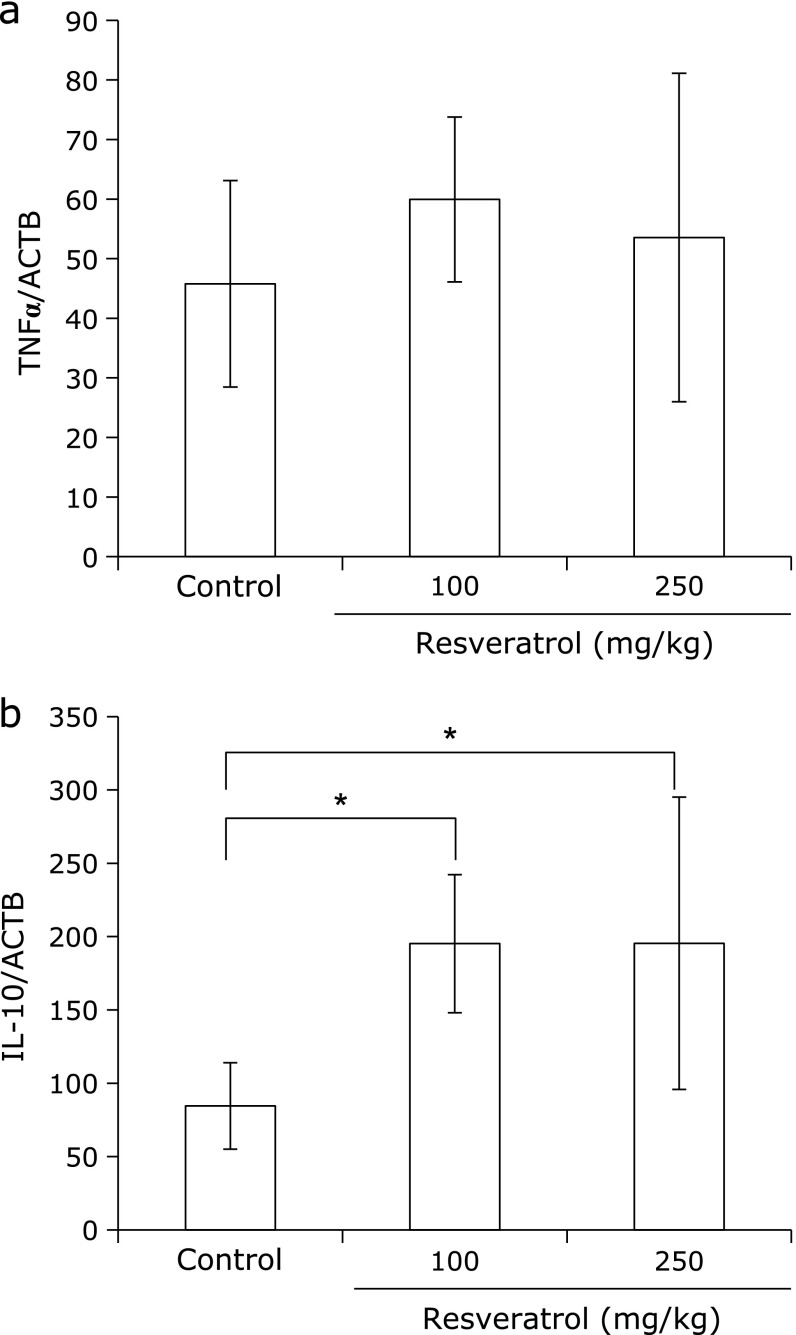
Resveratrol did not change TNFα, but increased IL-10 transcription in the salivary glands of the NOD mice
To investigate whether resveratrol affects the levels of expression of the cytokines IL-1, IL-6, TNFα and IL-10, the salivary glands from the mice were analyzed by real-time PCR. Resveratrol treatment did not change the expression of TNFα, but the expression of IL-10 significantly increased in the salivary glands of the resveratrol-treated mice when compared with those treated with saline (Fig. 5a and b). Expression of IL-1 and IL-6 was not detected in salivary glands of the NOD mice before or after resveratrol treatment (data not shown).
Fig. 5.
Resveratrol modified the cytokine expression in the salivary glands. RNA was extracted from paraffin-fixed sections, and cDNA was synthesized using specific primers. The results of real-time PCR for TNFα (a) and IL-10 (b) were normalized to the results for β-actin. Error bars indicate the standard errors of the means; *p<0.01.
Discussion
In the present study, we showed that resveratrol improved hyposalivation in NOD mice. Although there were no differences in the number of foci between the control and resveratrol-treated groups, the amelioration of hyposalivation in the 250 mg/kg group was significant. Correlation analysis revealed a negative association between salivary secretion and levels of inflammatory cytokines in the saliva obtained from the NOD mice, whereas the correlation with the inflammatory changes in the glands was consistently weak.(27) It has been suggested that the decrease in salivary flow follows the occurrence of focal lymphoid infiltration with a considerable delay in time, and that the sole destruction or replacement of glandular tissue by inflammatory cells is not sufficient to explain the severe impairment in salivary secretion.(28) The unclear interrelationship between glandular inflammation and hyposalivation has led to research initiatives to investigate the mechanisms of glandular dysfunction.(27) Autoantibodies that inhibit the receptors for neurotransmitters and defective water transport have been proposed.(29) Moreover, in the NOD mice, no augmentation of salivary flow rates has been observed after infusion of neuropeptides combined with muscarinic-cholinergic agonists,(30) indicating that the hyposalivation observed in NOD mice may, at least in part, be due to a general loss of neurotransmitter responsiveness in the salivary glands. Based on these observations, salivary secretion may be modified by autoantibody secretion and/or sensitivity to neurotransmitters rather than quantitative inflammatory changes.
Resveratrol has been shown to prevent or slow the progression of various diseases, including cancers, cardiovascular diseases and ischemic injuries, as well as to enhance stress resistance and prolong lifespan.(31–37) Multiple direct targets of resveratrol, including COX, PPAR, eNOS and Sirt1, have been identified.(5,38,39) We also showed that resveratrol administration increased Sirt1 activity by 8-fold when compared with the control group. Sirt1 deacetylates specific lysines on histone tails to induce transcriptional silencing. Sirt1 also deacetylates non-histone proteins such as p53, FOXOs, nuclear receptor corepressor (SMRT/NCOR) and PGC-1 alpha.(40–42) Oxidative stress-induced FOXO3 acetylation can lead to the formation of a Sirt1-FOXO3 complex, which is indispensable for cell cycle arrest and induction of DNA repair.(43) In turn, Sirt1 modulates the cellular stress response by directly deactylating specific proteins and regulating their expression.(44) In fact, Sirt1 modulates the threshold for cell death during exogenous stress including oxidative damage, interacts with p53, inhibits Bax-induced apoptosis by deacetylation of Ku70 and regulates other targets linked to cell death and cellular antioxidant activity (such as Mn-SOD and catalase).(45,46) Dickinson et al.(2) revealed that EGCG ameliorates the salivary dysfunction in NOD mice by increasing the major anti-oxidant defense protein PRDX6 and catalase. Thus, the anti-oxidative function of Sirt1 may play a role in improving salivary secretion in NOD mice.
NOD mice have a defect in the production of low-molecular-weight protein 2 (LMP2), leading to defective T cell selection and the presence of autoreactive T cells, thereby resulting in development of autoimmune diabetes and SS.(47) LMP2 is a catalytic subunit of the proteasomes, which are very large protein complexes inside all eukaryotic cells that are responsible for degrading proteins for which the cell has no more use. Proteasomes are also involved in the processing of NF-κB. Disruption of this process prevents the NF-κB-mediated protection from TNFα-induced apoptosis. Disruption of the function of proteosomes in antigen-presenting cells function in antigen-presenting cells results in the escape of autoreactive T cells from proper immune selection. NF-κB defects also increase the apoptosis of misselected T cells by TNFα-induced apoptosis.(48) Treatment of NOD mice with a TNFα inducer promotes the apoptosis of autoreactive T cells and eventually eliminates the autoimmunity.(49,50) After the autoimmunity is removed, the salivary gland function is restored. Sirt1 is known to play important roles in various aspects of the inhibition of T cell responses.(51–53) Sirt1 expression in T cells is regulated via TCR-mediated signaling. Increased levels of the Sirt1 protein compromise the T cell-mediated immune response by suppressing the activation of NF-κB and activated protein 1 (AP-1) transcription factors, both of which are required for production of IL-2, a cytokine that promotes T cell proliferation.(51,53,54) Genetic depletion of the Sirt1 gene also suppresses the innate immune response and the development of a lupus-like autoimmune syndrome.(51) In addition, Sirt1 suppresses the innate immune responses by opposing NF-κB-mediated inflammatory cytokine production by macrophages.(55)
IL-10 is a potent anti-inflammatory cytokine that plays a crucial role in preventing inflammatory and autoimmune pathologies.(56,57) Deficient or aberrant expression of IL-10 can enhance the inflammatory response to microbial challenge and may also lead to development of inflammatory bowel disease and a number of autoimmune diseases.(58,59) Thus, impaired IL-10 function can enhance the clearance of pathogens during acute infection and may also lead to exaggerated inflammatory responses resulting in immunopathology and tissue damage.(13,60) However, some pathogens can harness the immunosuppressive capacity of IL-10 to limit the host immune response, leading to persistent infection.(61,62) IL-10 plays a largely non-redundant role in mediating the host anti-inflammatory response; therefore, identifying the cellular sources of IL-10, as well as the molecular mechanisms that regulate IL-10 expression are critical to developing therapeutic strategies directed against pathology-associated impairments in IL-10 production. In the present study, although the anti-inflammatory phenomena have not been clarified, resveratrol-induced IL-10 expression in the salivary glands in NOD mice may play a role in the therapeutic effects.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B; 23390421) from the Japan Society for the Promotion of Science. We thank Drs. Junji Tokunaga and Yusuke Amano for kindly assaying the Sirt1 activity and the in vivo study.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Langcake P, Pryce RJ. Production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiological Plant Pathology. 1976;9:77–86. [Google Scholar]
- 2.Dickinson D, Derossi S, Yu H, et al. Epigallocatechin-3-gallate modulates anti-oxidant defense enzyme expression in murine submandibular and pancreatic exocrine gland cells and human HSG cells. Autoimmunity. 2014;47:177–184. doi: 10.3109/08916934.2013.879470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutati Res. 2003;523–524:145–150. doi: 10.1016/s0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 4.Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 5.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 6.Orihuela-Campos RC, Tamaki N, Mukai R, et al. Biological impacts of resveratrol, quercetin, and N-acetylcysteine on oxidative stress in human gingival fibroblasts. J Clin Biochem Nutr. 2015;56:220–227. doi: 10.3164/jcbn.14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X, Ma L, Ruan L, et al. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation. 2010;7:46. doi: 10.1186/1742-2094-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2011;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Yen GC, Chen YC, Chang WT, Hsu CL. Effects of polyphenolic compounds on tumor necrosis factor-α (TNF-α)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J Agric Food Chem. 2011;59:546–551. doi: 10.1021/jf1036992. [DOI] [PubMed] [Google Scholar]
- 10.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 11.Holmes-McNary M, Baldwin AS., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 12.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 13.Ejrnaes M, Filippi CM, Martinic MM, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarente L, Picard F. Calorie restriction--the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 16.Sequeira J, Boily G, Bazinet S, et al. Sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Kubota S, Kurihara T, Mochimaru H, et al. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 2009;50:3512–3519. doi: 10.1167/iovs.08-2666. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Nakagawa Y, Purushotham KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263 (4 Pt1):E607–E614. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys-Beher MG, Hu Y, Nakagawa Y, Wang PL, Purushotham KR. Utilization of the non-obese diabetic (NOD) mouse as an animal model for the study of secondary Sjögren’s syndrome. Adv Exp Med Biol. 1994;350:631–636. doi: 10.1007/978-1-4615-2417-5_105. [DOI] [PubMed] [Google Scholar]
- 20.Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92:265–275. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- 21.Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Pérez Leirós C. Defective signalling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin Exp Immunol. 2005;142:411–418. doi: 10.1111/j.1365-2249.2005.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S, Zhou M, Jiang J, et al. Systems biology analysis of Sjögren’s syndrome and mucosa-associated lymphoid tissue lymphoma in parotid glands. Arthritis Rheum. 2009;60:81–92. doi: 10.1002/art.24150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–543. doi: 10.1046/j.1532-5415.2002.50123.x. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49:309–326. doi: 10.1016/j.cden.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Fox PC. Salivary enhancement therapies. Caries Res. 2004;38:241–246. doi: 10.1159/000077761. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca-Kelly Z, Nassrallah M, Uribe J, et al. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson MV, Delaleu N, Brokstad KA, Berggreen E, Skarstein K. Impaired salivary gland function in NOD mice: association with changes in cytokine profile but not with histopathologic changes in the salivary gland. Arthritis Rheum. 2006;54:2300–2305. doi: 10.1002/art.21945. [DOI] [PubMed] [Google Scholar]
- 28.Dawson LJ, Fox PC, Smith PM. Sjögren’s syndrome--the non-apoptotic model of glandular hypofunction. Rheumatol (Oxford) 2006;45:792–798. doi: 10.1093/rheumatology/kel067. [DOI] [PubMed] [Google Scholar]
- 29.Delaleu N, Jonsson MV, Appel S, Jonsson R. New concepts in the pathogenesis of Sjögren’s syndrome. Rheum Dis Clin North Am. 2008;34:833–845. doi: 10.1016/j.rdc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto H, Ishibashi K, Nakagawa Y, et al. Detection of alterations in the levels of neuropeptides and salivary gland responses in the non-obese diabetic mouse model for autoimmune sialoadenitis. Scand J Immunol. 1997;45:55–61. doi: 10.1046/j.1365-3083.1997.d01-375.x. [DOI] [PubMed] [Google Scholar]
- 31.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 32.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur J Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 33.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 34.Bradamante S, Barenghi L, Villa A. Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev. 2004;22:169–188. doi: 10.1111/j.1527-3466.2004.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 36.Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 37.Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Nakata R, Takahashi S, Inoue H. Recent advances in the study on resveratrol. Biol Pharm Bull. 2012;35:273–279. doi: 10.1248/bpb.35.273. [DOI] [PubMed] [Google Scholar]
- 39.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 40.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Eng J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 43.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 44.Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005;26:94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 46.Corbi G, Conti V, Russomanno G, et al. Is physical activity able to modify oxidative damage in cardiovascular aging? . Oxid Med Cell Longev. 2012;2012:728547. doi: 10.1155/2012/728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi T, Faustman D. NOD mice are defective in proteasome production and activation of NF-kappaB. Mol Cell Biol. 1999;19:8646–8659. doi: 10.1128/mcb.19.12.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu S, Kodama S, Ryu K, Schoenfeld DA, Faustman DL. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J Clin Invest. 2001;108:63–72. doi: 10.1172/JCI12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 50.Tran SD, Kodama S, Lodde BM, et al. Reversal of Sjögren’s-like syndrome in non-obese diabetic mice. Ann Rheum Dis. 2007;66:812–814. doi: 10.1136/ard.2006.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Lee SM, Shannon S, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong S, Kim SJ, Sandal B, et al. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J Biol Chem. 2011;286:16967–16975. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon HS, Brent MM, Getachew R, et al. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe. 2008;3:158–167. doi: 10.1016/j.chom.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Zhou Y, Mueller-Steiner S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 55.Schug TT, Xu Q, Gao H, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 57.Kuhn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 58.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 60.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]