Abstract
Background
The practice of swimming in "hypogravity" conditions has potential to decrease bone formation because it decreases the time engaged in weight-bearing activities usually observed in the daily activities of adolescents. Therefore, adolescents competing in national levels would be more exposed to these deleterious effects, because they are engaged in long routines of training during most part of the year. To analyze the effect of swimming on bone mineral density (BMD) gain among adolescents engaged in national level competitions during a 9-month period.
Methods
Fifty-five adolescents; the control group contained 29 adolescents and the swimming group was composed of 26 athletes. During the cohort study, BMD, body fat (BF) and fat free mass (FFM) were assessed using a dual-energy x-ray absorptiometry scanner. Body weight was measured with an electronic scale, and height was assessed using a stadiometer.
Results
During the follow-up, swimmers presented higher gains in FFM (Control 2.35 kg vs. Swimming 5.14 kg; large effect size [eta-squared (ES-r)=0.168]) and BMD-Spine (Swimming 0.087 g/cm2 vs. Control 0.049 g/cm2; large effect size [ES-r=0.167]) compared to control group. Male swimmers gained more FFM (Male 10.63% vs. Female 3.39%) and BMD-Spine (Male 8.47% vs. Female 4.32%) than females. Longer participation in swimming negatively affected gains in upper limbs among males (r=-0.438 [-0.693 to -0.085]), and in spine among females (r=-0.651 [-0.908 to -0.036]).
Conclusions
Over a 9-month follow-up, BMD and FFM gains were more evident in male swimmers, while longer engagement in swimming negatively affected BMD gains, independently of sex.
Keywords: Adolescent, Exercise, Sports, Stress mechanical
INTRODUCTION
Osteoporosis is a chronic disease with high prevalence worldwide,[1] which is responsible for great economic burden for individual and society as a whole.[2] The disease is characterized by deficiency of bone mass, bone strength and alterations in bone microstructure, which can lead to a higher risk of stress fractures.[3] Although we recognize the importance of bringing attention to the treatment of osteoporosis and its consequences among adults and elderlies, it is equally essential to focus on disease prevention during youth.[4]
Adolescence is a crucial juncture for bone mass acquisition,[5] and studies have shown a relationship between low bone mineral density (BMD) in adolescence and the occurrence of fracture [6] and osteoporosis in adulthood.[7,8] Along with biological factors, nutrition and having an active lifestyle [9] are influencing factors linked to peak bone mass gain reached in adolescence. Among adolescents, sports participation is a typical indicator of physical activity.[10] The osteogenic effect attributed to sports is mainly produced by muscle's mechanical load and strain on bones, affecting bone strength and geometry in sites specifically led by the form of activity.[11] However, not all sports have the same effects on bone; a minimum duration and intensity are required for this osteogenic stimulus to be produced.[12]
Studies have shown that high impact sports have greater osteogenic effect than non-impact sports, such as swimming or cycling, in children, young adults [13] or older adults. [14] Regarding swimming, the sport is widely performed around the world and its practice is recommended for all age groups.[15] However, one systematic review analyzed the effect of swimming on bone mass, and was not able to conclude if swimming negatively affects or is neutral to BMD accrual.[16] In fact, swimming in "hypogravity" conditions has potential to decrease the bone formation because it decreases the time engaged in weight-bearing activities usually observed in the daily activities of adolescents.[17,18] Therefore, adolescents engaged in organized sports who compete at the national levels would be more exposed to these deleterious effects, because they are engaged in long routines of training during most part of the year.
The objective of this longitudinal study was to analyze the effect of swimming on BMD gain among Brazilian adolescents engaged in national level competitions. We have hypothesized that BMD gains in swimmers are similar to those gains observed in the control group, as well as the time exposed to swimming practice is determinant on BMD gains independently of sex and biological maturation.
METHODS
1. Sample
This is a longitudinal study conducted from October 2013 to August 2014 and it was previously approved by the ethical board of the university. In the present study, the adolescents had to fulfill all inclusion criteria to be included in the follow-up group. As inclusion criteria we had: (i) age between 11 and 17 years old; (ii) prior authorization signed by coaches and parents; (iii) a minimum of six months of previous engagement for swimming group or absence of participation in any organized sport during the last six months (control); (iv) no use of medication that could affect bone metabolism; (v) a signed consent form.
The minimum number of adolescents per group (n=8) was previously estimated through an equation based in the parameters provided by the independent Student t-test. The parameter adopted were: mean difference between swimming and control groups (0.08 g/cm2), standard deviation for control group (0.06 g/cm2), standard deviation for swimming group (0.05 g/cm2), power of 80% and Z=1.96 [19]. Therefore, the minimum sample size of 32 adolescents was established (n=16 swimmers [8 boys and 8 girls] and n=16 controls [8 boys and 8 girls]).
The present follow-up study is part of a greater cohort study, which includes other sports. The realization of the study was divulgated to the Department of Sports (responsible by all public sport clubs), Department of Education (responsible by all public and private schools) and private sports clubs (located at metropolitan region and other cities around). Coaches (sport clubs) and principals (school units) were contacted after authorization of these administrative structures. Swimmers were contacted in sports clubs regularly registered in competitions at national level, while control group were contacted in three school units. At the end of the cohort period, the overall sample was composed of 55 adolescents (29 boys and 26 girls); the control group contained 29 adolescents (13 boys and 16 girls) and the swimming group was composed of 26 athletes (16 boys and 10 girls) participating in competitions at national level.
2. Data related to swimming and vitamin D score
Coaches reported training routines of athletes (mean= 1,051.9 min per week [95% confidence interval (CI) 968.4-1,135.3]; minimum 675 min per week and maximum 1,140 min week) and a minimum previous practice of six months was requested to consider the swimmer eligible to the cohort study (63.2 months [95% CI 46.9-79.5]; minimum 9 months maximum 155 months). The group of swimmers was engaged in a minimum of five days per week of training with a minimum of 130 min per session. Coaches also reported resistance training routines (n=14 swimmers [53.8%]; n=01 control group [3.4%]), which have been considered potential confounder in multivariate models.
A nutritionist created a questionnaire with foods rich in vitamin D commonly observed in Brazilian diet. The adolescents reported the frequency of consumption of vitamin D rich foods (Likert scale) during the week prior to evaluation (baseline [Swimmers vs. Controls with P-value=0.522] and end of follow-up [Swimmers vs. Controls with P-value=0.827]) and the sum of the generated score was considered proxy of vitamin D intake during the cohort period.
3. Bone mineral variables and body composition
In both moments of the cohort study, BMD (in g/cm2), body fat (BF; in percentage) and fat free mass (FFM; in kilograms) were assessed using a dual energy X-ray absorptiometry (DXA) scanner (Lunar DPX-NT; General Electric Healthcare, Little Chalfont, Buckinghamshire, UK) with GE Medical System Lunar software (version 4.7). A trained researcher tested the scanner quality prior to each day of measurement, following the manufacturer's recommendations. The precision of the device for measurements of BMD in terms of coefficient of variation was 0.66% (n=30 subjects assessed in two opportunities). The participants wore light clothing, without shoes and remained in the supine position on the machine for approximately 15 min. BMD was measured at: (i) upper limbs, (ii) lower limbs, (iii) spine and (iv) whole body.
4. Biological maturation
Body weight was measured using an electronic scale (Filizzola PL 150, Filizzola Ltda., Brazil), and height was assessed using a wall-mounted stadiometer (Sanny; American Medical do Brasil LTDA, São Bernardo do Campo, SP, Brazil). The leg length and sitting-height were measured using standardized techniques. These measurements were used to calculate the maturity offset, which denotes the time (years) from/to age peak of height velocity (PHV), an important maturational event.[19] Swimmers and control group were similar according to PHV in both baseline (P-value=0.077) and follow-up (P-value=0.141).
5. Statistical analysis
Descriptive statistics were presented as mean and standard error of the mean. Repeated measures analysis of variance (ANOVA) analyzed the effect of swimming and sex on body composition variables (adjusted by chronological age [baseline], vitamin D score [baseline], PHV [baseline and mean difference between baseline and follow-up], engagement in resistance training [baseline] and previous practice of swimming in months [baseline]). Measurements of the effect size were provided by Eta-Squared (ES-r; small effect size 0.010, medium effect size 0.060 and large effect size 0.140). Partial correlation analyzed the relationship between time of previous practice and BMD modifications among adolescents of both sexes, adjusted by confounders. All statistical procedures were conducted using BioEstat software, version 5.2 (Bioestat, Tefé, Brazil) and statistical significance set at 0.05.
RESULTS
Overall sample was composed of 55 adolescents (29 boys and 26 girls). At baseline, boys and girls were similar in age (boys 12.7±2.1 years, girls 13.1±2.1 years; P=0.526), age PHV (boys -2.17±1.6 years, girls: -1.77±1.48 years; P=0.363), BMD (boys 1.045±0.097 g/cm2, girls 1.042±0.107 g/cm2; P=0.903) and bone mineral content (BMC) (boys 2165.1±571.1 g, girls 2055.7±538.9 g; P=0.470).
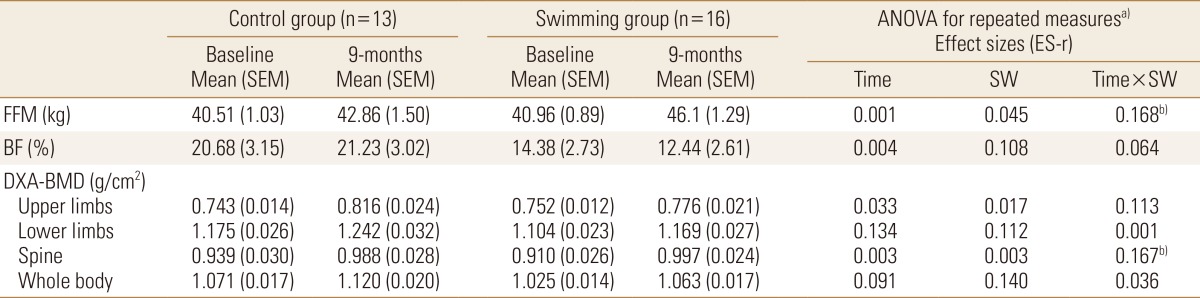
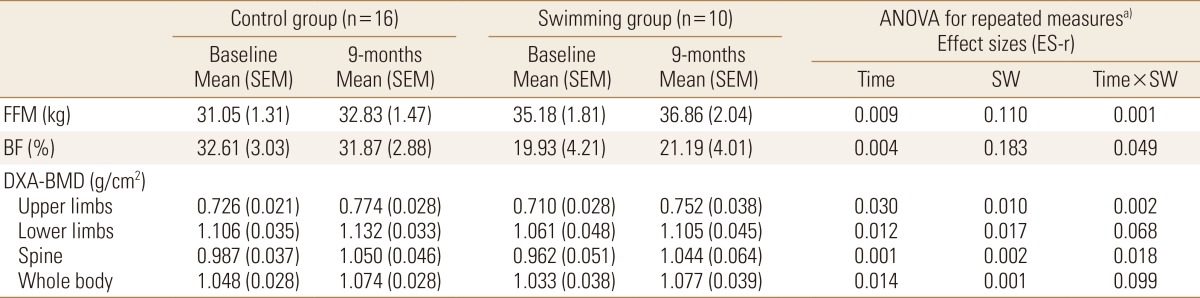
Among boys, FFM values were similar between control and swimming groups at baseline, while during the follow-up swimmers presented higher gains (Control 2.35 kg vs. Swimming 5.14 kg; large effect size [ES-r=0.168]) Body mass and height were similar between swimmers and controls, while swimmers were slightly older than control group at baseline (P=0.043). Similarly, gains in BMD-Spine were higher among swimmers compared to control adolescents (Control 0.049 g/cm2 vs. Swimming 0.087 g/cm2; large effect size [ES-r=0.167]) (Table 1). Girls, however, presented no differences when comparing the control and swimming groups. Body mass and height were similar between swimmers and controls, and different than observed among boys, chronological age was similar between swimmers and controls at baseline (P=0.344) (Table 2).
Table 1. Effect of swimming on body composition variables among male adolescents (n=29).
a)Model adjusted by chronological age (baseline), vitamin D score (baseline), peak height velocity (baseline and mean difference between baseline and follow-up), engagement in resistance training (baseline) and previous practice of the swimming in months (baseline). b)ANOVA with P<0.05.
ANOVA, analysis of variance; SEM, standard error mean; BMD, bone mineral density; FFM, fat free mass; BF, body fatness; DXA, dual energy X-ray absorptiometry; SW, swimming; ES-r, eta-squared.
Table 2. Effect of swimming on body composition variables among female adolescents (n=26).
a)Model adjusted by chronological age (baseline), vitamin D score (baseline), peak height velocity (baseline and mean difference between baseline and follow-up), engagement in resistance training (baseline) and previous practice of the swimming in months (baseline).
ANOVA, analysis of variance; SEM, standard error mean; BMD, bone mineral density; FFM, fat free mass; BF, body fatness; DXA, dual energy X-ray absorptiometry; SW, swimming; ES-r, eta-squared.
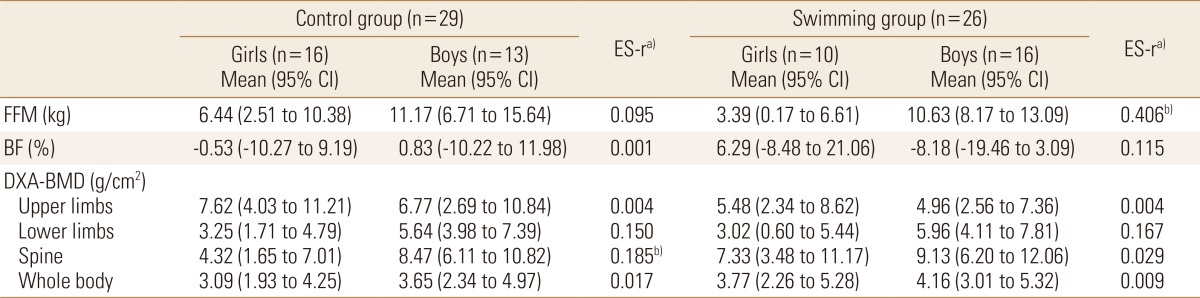
Taking into account the engagement in swimming, boys gained more FFM than girls (10.63% vs. 3.39%, respectively). In the multivariate model that compared swimmers boys and girls, there were no significant covariate, but even non-significant, the baseline values of PHV explained 15.1% of the variance observed in gains of FFM. Similarly, among control adolescents, boys gained more BMD in spine than girls (8.47% vs. 4.32%, respectively) (Table 3).
Table 3. Body composition changes (%) in adolescents according to swimming and sex (n=55).
a)Model adjusted by chronological age (baseline), vitamin D score (baseline and follow-up), peak height velocity (baseline and mean difference between baseline and follow-up), engagement in resistance training (baseline) and previous practice of the swimming in months (baseline). b)ANOVA with P<0.05.
CI, confidence interval; BMD, bone mineral density; FFM, fat free mass; BF, body fatness; DXA, dual energy X-ray absorptiometry; ES-r, eta-squared.
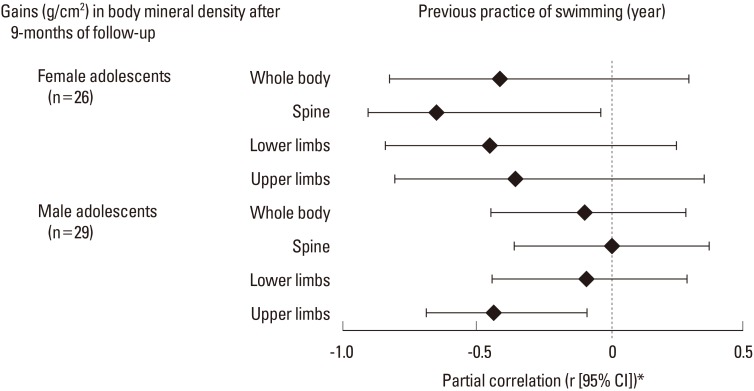
The relationship between previous time engaged in swimming and modifications in BMD identified that longer participation negatively affected gains in upper limbs among boys (r=-0.438 [-0.693 to -0.085]), and in spine among girls (r=-0.651 [-0.908 to -0.036]) (Fig. 1).
Fig. 1. Partial correlation between time of previous practice and bone mineral density modifications among adolescents of both sexes (n=55). *Partial correlation adjusted by chronological age (baseline), vitamin D score (baseline and follow-up), peak height velocity (baseline), fat free mass (baseline), engagement in resistance training (baseline) and height. CI, confidence interval.
DISCUSSION
This longitudinal study analyzed the effect of swimming on bone density gains among Brazilian adolescents and found that boys gained more whole body FFM and BMD in the spine than girls, despite participating in swimming. Additionally, we identified that longer participation in swimming negatively affected BMD gains in both sexes.
Since early age, sex seems to affect the effect of physical exercise on bone. In adolescence, there is a large increase in bone mass due to growth spurt and higher mineralization rate,[20,21,22] which is higher in boys and determines the stores observed in adult life.[23] The different gains observed between boys and girls are similar to other studies [17,18] and it can be explained at least in part by hormonal characteristics that affect the metabolic processes during this maturation period.[24]
Moreover, gains in FFM among male swimmers could be also used to explain these sex differences, because lean tissue mass is an independent predictor of regional and total BMD.[12,25,26,27] The relationship between BMD, total body weight and lean mass is supported by previous studies.[25,27] Therefore, bone could be considered a calcified tissue sensitive to loading and muscular contractions [25] and thus more sensitive in male adolescents due to the higher FFM gain.
Maturational aspects affect the differences in FFM gains between boys and girls. Among swimmers, baseline values of PHV explained 15% of the variance observed among boys and girls for FFM changes, while the same phenomenon was not observed in the control group, in which baseline values of PHV explained only 0.4% of the variance in FFM changes. Our findings identify that the effects of biological maturational on FFM gains can be boosted by sport participation, but additional studies are necessary to better understand the relationship between sports and maturation.[28]
Despite the beneficial effect of swimming on FFM, we identified that longer time engaged in swimming negatively affected BMD gains in upper limbs among boys and in spine among girls, consistent with the results found by Czeczelewski and colleagues.[29] This result could be explained due to the fact that swimming reduces the effect of gravitational forces on bone structure,[20,30] which is considered essential for shaping bone density.[31,32] Moreover, swimmers usually spend less time in weight-bearing daily activities.[17,18]
Another hypothesis to explain this finding is based on the fact that exercise performed at high intensity drives up levels of pro-inflammatory markers such as C-reactive protein and interleukin-6, reducing the action of the growth hormone (GH) / insulin-like growth factor (IGF-1) axis, thus resulting in a catabolic response in the bone tissue.[33] The exhaustive exercise routine, which athletes are expose to, can increase significantly inflammatory markers in bloodstream [33] leading to catabolic effects, mainly when maintained by long periods.
The negative relationship between time of practice and bone gains is a relevant concern, because its effects could be harmful later in life. For instance, in post-menopausal former athletes, BMD and BMC do not differ between swimmers and runners, while the same variables are significantly higher in athletes when compared to controls.[34] The increased muscle mass and strength in athletes reflects significant physical training they used to undergo, which positively affects bone health later in life, preventing the decline in muscle and bone mass, reducing the likelihood of falling, and delaying morbidity and mortality.[14] On the other hand, even with significant improvements of FFM in swimmers, swimmer's bone structure was not benefited, denoting that the osteogenic effect linked to muscle contraction seems more effective when performed in environments with normal gravity.
As limitations we recognize the lack of information regarding genetic predisposition, environmental factors, hormonal status and nutritional intake. While exercise plays a fundamental role on bone health and it was analyzed in the current investigation, studies have shown that bone catabolism and reduced bone formation may occur if energy intake is insufficient.[35] Moreover, insufficient calcium intake as well as inadequate calcium-to-phosphate and protein-to-calcium ratios could have played an important role on decreased BMD among swimmers.[29] Finally, sex hormones and balance between pro and anti-inflammatory markers are important in the maintenance of bone health among athletes performing high volumes of endurance training.[36,37]
In summary, over a 9-month follow-up, BMD and FFM gains were more evident in male swimmers, while longer engagement in swimming during childhood and adolescence seems negatively related to BMD gains, independently of sex.
Footnotes
This work was supported by the São Paulo Research Foundation (FAPESP). (Process: 2013/06963-5 and 2015/13543-8)
No potential conflict of interest relevant to this article was reported.
References
- 1.Winsloe C, Earl S, Dennison EM, et al. Early life factors in the pathogenesis of osteoporosis. Curr Osteoporos Rep. 2009;7:140–144. doi: 10.1007/s11914-009-0024-1. [DOI] [PubMed] [Google Scholar]
- 2.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis JA, Melton LJ, 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 4.Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40:13–21. doi: 10.1097/JES.0b013e318236e5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey DA, Faulkner RA, McKay HA. Growth, physical activity, and bone mineral acquisition. Exerc Sport Sci Rev. 1996;24:233–266. [PubMed] [Google Scholar]
- 6.Milgrom C, Simkin A, Eldad A, et al. Using bone's adaptation ability to lower the incidence of stress fractures. Am J Sports Med. 2000;28:245–251. doi: 10.1177/03635465000280021701. [DOI] [PubMed] [Google Scholar]
- 7.Nordström A, Karlsson C, Nyquist F, et al. Bone loss and fracture risk after reduced physical activity. J Bone Miner Res. 2005;20:202–207. doi: 10.1359/JBMR.041012. [DOI] [PubMed] [Google Scholar]
- 8.Saggese G, Baroncelli GI, Bertelloni S. Osteoporosis in children and adolescents: diagnosis, risk factors, and prevention. J Pediatr Endocrinol Metab. 2001;14:833–859. doi: 10.1515/jpem.2001.14.7.833. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Cabello A, Vicente-Rodríguez G, Albers U, et al. Harmonization process and reliability assessment of anthropometric measurements in the elderly EXERNET multi-centre study. PLoS One. 2012;7:e41752. doi: 10.1371/journal.pone.0041752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strong WB, Malina RM, Blimkie CJ, et al. Evidence based physical activity for school-age youth. J Pediatr. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 11.Frost HM, Schönau E. The "muscle-bone unit" in children and adolescents: a 2000 overview. J Pediatr Endocrinol Metab. 2000;13:571–590. doi: 10.1515/jpem.2000.13.6.571. [DOI] [PubMed] [Google Scholar]
- 12.Vicente-Rodríguez G. How does exercise affect bone development during growth? Sports Med. 2006;36:561–569. doi: 10.2165/00007256-200636070-00002. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson MK, Nordqvist A, Karlsson C. Physical activity increases bone mass during growth. Food Nutr Res. 2008;52 doi: 10.3402/fnr.v52i0.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guadalupe-Grau A, Fuentes T, Guerra B, et al. Exercise and bone mass in adults. Sports Med. 2009;39:439–468. doi: 10.2165/00007256-200939060-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kostka T, Furgal W, Gawronski W, et al. Recommendations of the Polish Society of Sports Medicine on age criteria while qualifying children and youth for participation in various sports. Br J Sports Med. 2012;46:159–162. doi: 10.1136/bjsports-2011-090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gómez-Bruton A, Gónzalez-Agüero A, Gómez-Cabello A, et al. Is bone tissue really affected by swimming? A systematic review. PLoS One. 2013;8:e70119. doi: 10.1371/journal.pone.0070119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Bruton A, González-Agüero A, Gómez-Cabello A, et al. Swimming and bone: is low bone mass due to hypogravity alone or does other physical activity influence it? Osteoporos Int. 2016;27:1785–1793. doi: 10.1007/s00198-015-3448-8. [DOI] [PubMed] [Google Scholar]
- 18.Gómez-Bruton A, González-Agüero A, Gómez-Cabello A, et al. The effects of swimming training on bone tissue in adolescence. Scand J Med Sci Sports. 2015;25:e589–e602. doi: 10.1111/sms.12378. [DOI] [PubMed] [Google Scholar]
- 19.Mirwald RL, Baxter-Jones AD, Bailey DA, et al. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34:689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Bellew JW, Gehrig L. A comparison of bone mineral density in adolescent female swimmers, soccer players, and weight lifters. Pediatr Phys Ther. 2006;18:19–22. doi: 10.1097/01.pep.0000200952.63544.16. [DOI] [PubMed] [Google Scholar]
- 21.Sanchis-Moysi J, Dorado C, Olmedillas H, et al. Bone mass in prepubertal tennis players. Int J Sports Med. 2010;31:416–420. doi: 10.1055/s-0030-1248331. [DOI] [PubMed] [Google Scholar]
- 22.Vicente-Rodriguez G, Dorado C, Perez-Gomez J, et al. Enhanced bone mass and physical fitness in young female handball players. Bone. 2004;35:1208–1215. doi: 10.1016/j.bone.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Davies JH, Evans BA, Gregory JW. Bone mass acquisition in healthy children. Arch Dis Child. 2005;90:373–378. doi: 10.1136/adc.2004.053553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 25.Andreoli A, Monteleone M, Van Loan M, et al. Effects of different sports on bone density and muscle mass in highly trained athletes. Med Sci Sports Exerc. 2001;33:507–511. doi: 10.1097/00005768-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Taaffe DR, Snow-Harter C, Connolly DA, et al. Differential effects of swimming versus weight-bearing activity on bone mineral status of eumenorrheic athletes. J Bone Miner Res. 1995;10:586–593. doi: 10.1002/jbmr.5650100411. [DOI] [PubMed] [Google Scholar]
- 27.Taaffe DR, Marcus R. Regional and total body bone mineral density in elite collegiate male swimmers. J Sports Med Phys Fitness. 1999;39:154–159. [PubMed] [Google Scholar]
- 28.Ferry B, Lespessailles E, Rochcongar P, et al. Bone health during late adolescence: effects of an 8-month training program on bone geometry in female athletes. Joint Bone Spine. 2013;80:57–63. doi: 10.1016/j.jbspin.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Czeczelewski J, Długołęcka B, Czeczelewska E, et al. Intakes of selected nutrients, bone mineralisation and density of adolescent female swimmers over a three-year period. Biol Sport. 2013;30:17–20. doi: 10.5604/20831862.1029816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taaffe DR, Robinson TL, Snow CM, et al. High-impact exercise promotes bone gain in well-trained female athletes. J Bone Miner Res. 1997;12:255–260. doi: 10.1359/jbmr.1997.12.2.255. [DOI] [PubMed] [Google Scholar]
- 31.Fehling PC, Alekel L, Clasey J, et al. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17:205–210. doi: 10.1016/8756-3282(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 32.Risser WL, Lee EJ, LeBlanc A, et al. Bone density in eumenorrheic female college athletes. Med Sci Sports Exerc. 1990;22:570–574. doi: 10.1249/00005768-199010000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Nemet D, Oh Y, Kim HS, et al. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002;110:681–689. doi: 10.1542/peds.110.4.681. [DOI] [PubMed] [Google Scholar]
- 34.Andreoli A, Celi M, Volpe SL, et al. Long-term effect of exercise on bone mineral density and body composition in post-menopausal ex-elite athletes: a retrospective study. Eur J Clin Nutr. 2012;66:69–74. doi: 10.1038/ejcn.2011.104. [DOI] [PubMed] [Google Scholar]
- 35.Hind K, Truscott JG, Evans JA. Low lumbar spine bone mineral density in both male and female endurance runners. Bone. 2006;39:880–885. doi: 10.1016/j.bone.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Ackerman KE, Misra M. Bone health and the female athlete triad in adolescent athletes. Phys Sportsmed. 2011;39:131–141. doi: 10.3810/psm.2011.02.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackerman KE, Skrinar GS, Medvedova E, et al. Estradiol levels predict bone mineral density in male collegiate athletes: a pilot study. Clin Endocrinol (Oxf) 2012;76:339–345. doi: 10.1111/j.1365-2265.2011.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]