Abstract
It is widely accepted that body weight and adipose mass are tightly regulated by homeostatic mechanisms, in which leptin plays a critical role through hypothalamic pathways, and obesity is a result of homeostatic disorder. However, in C57BL/6J mice, we found that Rcan2 increases food intake and plays an important role in the development of age-and diet-induced obesity through a leptin-independent mechanism. RCAN2 was initially identified as a thyroid hormone (T3)-responsive gene in human fibroblasts. Expression of RCAN2 is regulated by T3 through the PI3K-Akt/PKB-mTOR-Rps6kb1 signaling pathway. Intriguingly, both Rcan2 −/− and Rps6kb1 −/− mutations were reported to result in lean phenotypes in mice. In this study we compared the effects of these two mutations on growth and body weight in C57BL/6J mice. We observed reduced body weight and lower fat mass in both Rcan2 −/− and Rps6kb1 −/− mice compared to the wild-type mice, and we reported other differences unique to either the Rcan2 −/− or Rps6kb1 −/− mice. Firstly, loss of Rcan2 does not directly alter body length; however, Rcan2 −/− mice exhibit reduced food intake. In contrast, Rps6kb1 −/− mice exhibit abnormal embryonic development, which leads to smaller body size and reduced food intake in adulthood. Secondly, when fed a normal chow diet, Rcan2 −/− mice weigh significantly more than Rps6kb1 −/− mice, but both Rcan2 −/− and Rps6kb1−/− mice develop similar amounts of epididymal fat. On a high-fat diet, Rcan2 −/− mice gain body weight and fat mass at slower rates than Rps6kb1 −/− mice. Finally, using the double-knockout mice (Rcan2 −/− Rps6kb1 −/−), we demonstrate that concurrent loss of Rcan2 and Rps6kb1 has an additive effect on body weight reduction in C57BL/6J mice. Our data suggest that Rcan2 and Rps6kb1 mutations both affect growth and body weight of mice, though likely through different mechanisms.
Keywords: Rcan2 gene, Rps6kb1 gene, Growth, Body weight regulation, Obesity
1. Introduction
Obesity increases the risk for type 2 diabetes, cardiovascular disease, and early mortality, and over the past several decades, has reached epidemic proportions worldwide (Kahn and Flier, 2000; Zimmet et al., 2001; Eckel et al., 2005). The escalating prevalence of obesity puts tremendous pressure on public health and economic systems in both developed and developing countries. Understanding the physiological and molecular mechanisms that underlie the regulation of body weight is a significant challenge in current biomedical research. In recent years, many genes have been identified that function to regulate body weight, as evidenced through studies employing genetic mouse models. Mutations in these genes alter body weight of mice, often leading to lean or obese phenotypes (Reed et al., 2008). Some of these genes are linked to the leptin-melanocortin signaling system, which is believed to play a fundamental role in controlling energy balance (Garfield et al., 2009; de Jonghe et al., 2011; van der Klaauw and Farooqi, 2015), while other genes are associated with separate systems. Elucidating the complex interplay among these genes can increase our understanding of mechanisms of regulating body weight.
The Rcan2 gene is involved in the regulation of body weight (Sun et al., 2011). In mice, two splice variants of Rcan2 that display distinct tissue-specific expression patterns have been identified (Mizuno et al., 2004): Rcan2-3 is expressed predominately in the brain, whereas Rcan2-1 is also expressed in other tissues, such as the heart and skeletal muscle. Recently, it was reported that loss of Rcan2 function in mice did not affect their linear growth (Bassett et al., 2012), but significantly ameliorated age- and diet-induced obesity by causing a reduction in food intake (Sun et al., 2011). Using double-mutant (Lepob/ob Rcan2 −/−) mice, we demonstrated that Rcan2 and leptin regulate body weight through different pathways. Thus, we hypothesized that Rcan2 may increase food intake and promote weight gain through a leptin-independent pathway (Sun et al., 2011). RCAN2 was initially identified as a thyroid hormone (T3)-responsive gene in human fibroblasts (Miyazaki et al., 1996). Subsequent studies revealed that T3 regulates RCAN2 expression through the PI3K-Akt/PKB-mTOR-Rps6kb1 signaling pathway in vitro (Cao et al., 2005). The protein kinase mTOR (the mammalian target of rapamycin) functions as an evolutionarily conserved environmental sensor (Laplante and Sabatini, 2012). Recently, a large number of studies suggested a critical role for mTOR in the regulation of energy homeostasis (Cota et al., 2006; Mori et al., 2009; Roa and Tena-Sempere, 2010). Rps6kb1 (ribosomal protein S6 kinase 1, also known as S6K1) is a key downstream effector of mTOR that acts to integrate nutrient and insulin signals (Fingar et al., 2002), and is a ubiquitously expressed serine/threonine protein kinase that phosphorylates the 40S ribosomal protein S6 in response to mitogens (Dufner and Thomas, 1999). Rps6kb1 also phosphorylates a unique set of diverse targets, many of which promote protein production (Ma and Blenis, 2009). Rps6kb1 has been implicated in ribosomal biogenesis and translational regulation, as well as in the control of cell size, gene transcription, and feedback regulation of insulin signaling (Magnuson et al., 2012). Intriguingly, mice with whole-body knockout of Rps6kb1 were reported to be resistant to age- and diet-induced obesity (Um et al., 2004); however, Rps6kb1 −/− mice exhibited decreased fat accumulation, which was attributed to an enhanced metabolic rate. In addition, Rps6kb1 −/− mice exhibited small size and developmental delays (Shima et al., 1998). Thus, although Rcan2 was initially identified as an Rps6kb1-regulated gene in vitro, the interaction between their function of Rcan2 and Rps6kb1 on murine body weight regulation remains to be determined.
The present study was conducted to compare the effects of loss of Rcan2 and Rps6kb1 on growth and body weight in mice from the C57BL/6J (B6) genetic background. B6 mice develop an obese phenotype when allowed ad libitum access to a high-fat diet (HFD), whereas B6 body weight remains normal on a normal chow diet (NCD); this closely parallels the phenomenon of human obesity (Surwit et al., 1988; 1995; Fraulob et al., 2010). Therefore, the B6 mouse is a robust model used for both mechanistic studies and as a tool for developing novel therapeutic interventions in diet-induced obesity. Since the ovarian hormone estrogen plays a critical role in the regulation of body weight in females (Wade, 1972), this study only involved male mice to avoid any potential confounding impact of estrogen on our data and conclusions. We compared the effects of Rcan2 −/− and Rps6kb1 −/− mutations on newborn pups, and monitored changes in body weight and adipose mass in mice fed on NCD or HFD. To further determine the relationship between Rcan2 and Rps6kb1, we evaluated weight gain and growth in the double-mutant (Rcan2 −/− Rps6kb1 −/−) mice. Our results revealed that both Rcan2 −/− and Rps6kb1 −/− mutations have significant impacts on growth and body weight in B6 mice, but through independent mechanisms.
2. Materials and methods
2.1. Animals
Rcan2 +/− (RBRC04891) and Rps6kb1 +/− mice (RBRC02385) were generated as described previously (Kawasome et al., 1998; Sun et al., 2011), and imported from the RIKEN BioResource Center (Tokyo, Japan). Although both of these mouse strains were reported to have been backcrossed to B6 mouse for more than ten generations in the documentation of RIKEN BioResource Center, they were further backcrossed to B6 for more than six generations after arriving at our lab. Homozygous male Rcan2 −/− and Rps6kb1 −/− mice were obtained by mating the relevant heterozygous mice. Genotypes of pups were determined by polymerase chain reaction (PCR) (Kawasome et al., 1998; Sun et al., 2011).
To generate the double-knockout (Rcan2−/− Rps6kb1−/−) mice, Rcan2 +/− mice and Rps6kb1 +/− mice were crossed to produce the double heterozygote Rcan2 +/− Rps6kb1 +/−. These mice were then intercrossed to produce double homozygotes.
Mice were maintained in individually ventilated cage systems at Shandong University School of Life Science, in an environment maintained at 22–24 °C, with a 12-h light/dark cycle, and ad libitum access to NCD (4% fat, 3650 kcal/kg (1 kcal=4.1868 kJ), SLACOM® Mouse Breeder, Pu Lu Teng Biotechnology Co., Shanghai, China) and distilled water, unless noted otherwise.
2.2. Experimental design
The Rcan2 −/− mice and the Rps6kb1 −/− mice, which were obtained from mating the relevant heterozygous mice, were divided into two groups: NCD and HFD. At least 11 mice from each strain were weaned onto the NCD and remained on this diet for 16 weeks; at least 9 mice from each strain were fed HFD (60% fat, 5240 kcal/kg, D12492, Research Diets, New Brunswick, New Jersey) for 16 weeks. Wild-type (WT) littermates, which were obtained from the above mating, were used as controls.
Eight double-mutant (Rcan2−/− Rps6kb1−/−) mice were obtained from mating the double heterozygotes. Eight Rps6kb1 −/− littermates, which were obtained from the same mating, were used as controls. These mice were weaned onto NCD at 4 weeks of age, and remained on this diet for 16 weeks.
Two to four mice of the same age were housed in a single cage, regardless of mouse genotypes. Body weight was measured using a digital electronic balance once per week at a regular time (16:00–18:00).
The body weight of newborns was recorded at postnatal day zero, before the pups began nursing.
2.3. Food intake measurements
Food intake on the HFD was assessed by weighing food in the dispenser of each cage once every two days for a period of 16 weeks, to determine how much had been eaten. Referring to the method in our previous study (Sun et al., 2011), mice were housed individually in cages with a steel screen.
2.4. Body composition measurements
Mice were anesthetized with pentobarbital at 20 weeks of age, and white adipose tissue deposits (retroperitoneal and epididymal; RWAT and EWAT, respectively) and livers were dissected and weighed using a digital electronic balance. The left tibia from each animal was isolated and measured using a vernier caliper.
2.5. Histological examination
Epididymal adipose tissues and livers were dissected, fixed in 4% paraformaldehyde at 4 °C overnight, and dehydrated via an ethanol series. Tissues were embedded in paraffin, cut into 10-μm-thick sections, and stained with hematoxylin and eosin (H & E) for microscopic analysis. Digital images were acquired with a Nikon YS100 light microscope. Adipocyte volume was determined using ImageJ software (National Institutes of Health, Bethesda, USA).
2.6. Pair-feeding studies
From four weeks of age, four mice of the same genotypes were housed in single cages. Pair-feeding was accomplished by measuring the food intake of the ad libitum HFD-fed Rcan2 −/− male mice every 24 h and providing the same amount of food to the pair-fed WT mice. Body weights were recorded weekly. After 16 weeks of pair-feeding, mice were sacrificed, and fat pads and livers were removed and weighed.
2.7. Western-blot analysis
The hypothalami of 2-month-old mice were homogenized in ice cold cell lysis buffer (10 mmol/L Tris, pH 7.4, 1% Triton X-100, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.2 mmol/L phenylmethylsulfonyl fluoride (PMSF)). Aliquots of 40 μg of protein were separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore Corporation, Bedford, MA, USA). After blocking, the membranes were probed with anti-Rps6kb1 (ab32529, Epitomics) or anti-actin (P30002, Abmart) primary antibodies overnight at 4 °C, and with anti-rabbit horseradish peroxidase (HRP) conjugated IgG secondary antibody (ZB-2301; ZSGB-BIO) for 1 h at room temperature. The immunoreactive bands were visualized using enhanced chemiluminescence system (ECL) detection reagents.
2.8. Real-time PCR
Total RNA was extracted from mouse hypothalamus using TRIzol (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized from total RNA with a RevertAid first-strand cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA). Real-time quantitative PCR (qPCR) was carried out using the SYBR Green Premix Ex Taq (TaKaRa Biotechnology, Dalian, China) and performed using a Light Cycler 480 instrument (Roche Applied Science, Indianapolis, IN, USA). Relative gene expression levels were normalized against β-actin, then averaged and expressed as mean±standard error of the mean (SEM). Primers used for qPCR experiments were described previously (Sun et al., 2011).
2.9. Statistical analysis
Data were expressed as mean±SEM, and were considered significant at P<0.05. Statistical analysis of the data was performed using Student’s t-test and one-way analysis of variance (ANOVA), followed by Bonferroni’s multiple comparison correction, using GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA).
3. Materials and methods
3.1. Evaluation of Rcan2 and Rps6kb1 expressions in knockout mice
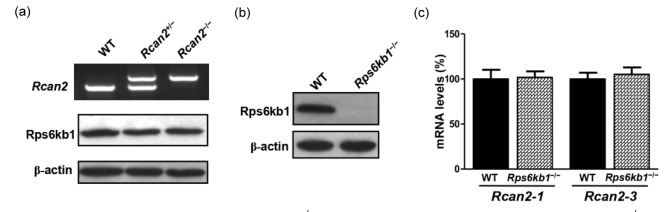
We initially determined if deletion of Rcan2 affected the expression of Rps6kb1 in vivo, or vice versa. Our results showed that loss of Rcan2 did not alter the protein expression of Rps6kb1 (Fig. 1a); likewise, absence of Rps6kb1 did not directly influence the expression of Rcan2 (Figs. 1b and 1c). We were unable to determine if loss of Rps6kb1 affected the protein expression of Rcan2, due to lack of an antibody to specifically detect murine Rcan2.
Fig. 1.
Expression of Rps6kb1 protein in Rcan2 −/− mice and expression of Rcan2 mRNA in Rps6kb1 −/− mice
(a) Expression of Rps6kb1 protein in Rcan2 −/− mice. Total protein was isolated from hypothalami of WT, Rcan2 +/−, and Rcan2 −/− mice and subjected to Western-blot probing for Rps6kb1 and β-actin (internal control). Genotyping of Rcan2 is shown for representative animals (top). (b) Expression of Rps6kb1 protein in Rps6kb1 −/− mice. (c) Expression levels of Rcan2-1 and Rcan2-3 mRNA in Rps6kb1 −/− mice. Total RNA was isolated from hypothalami of WT and Rps6kb1−/−mice and subjected to real-time quantitative PCR analysis using primers specific for Rcan2-1, Rcan2-3, and β-actin (internal standard). mRNA expression levels are reported as relative to expression from WT mice. n=4 in each group. All values are given as mean±SEM
3.2. Effect of loss of Rps6kb1 and Rcan2 on mouse birth weight
In order to determine the effect of these two mutations on embryonic development, we monitored the body weights of knockout pups through mating the heterozygous mice. The average body weights of the WT offspring in Rcan2 +/− mating or Rps6kb1 +/− mating were similar. Rps6kb1 −/− pups weighed about 17% less than WT littermates, while the weight of Rcan2 −/− pups was similar to that of WT pups (Table 1), indicating that loss of Rps6kb1, but not Rcan2, significantly affects mouse embryonic development.
Table 1.
Body weights of newborn pups at birth
| Genotype | Body weights of newborn pups (g) |
|
| Male | Female | |
| Rps6kb1 −/− Rcan2 −/− | 1.16±0.01a (n=21) | 1.15±0.02a (n=23) |
| Rps6kb1 −/− Rcan2+/+ | 1.16±0.02a (n=20) | 1.14±0.02a (n=19) |
| Rps6kb1+/+ Rcan2 −/− | 1.39±0.03b (n=24) | 1.41±0.03b (n=23) |
| Rps6kb1+/+ Rcan2+/+ (WT) | 1.43±0.02b (n=29) | 1.35±0.03b (n=14) |
Within the same sex, body weights not sharing the same superscript letter are significantly different (P<0.05). Values are given as mean±SEM
3.3. Effect of loss of Rcan2 and Rps6kb1 on growth and body weight in B6 mice on NCD
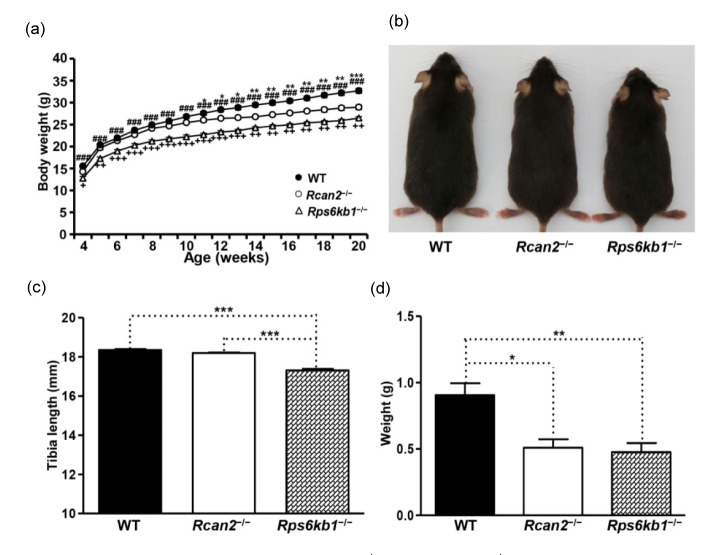
Smaller body size put the Rps6kb1 −/− pups at a disadvantage in their ability to compete for nursing, which led to a lower survival rate compared to WT littermates. In order to improve survival rate of these small pups, we random moved some bigger pups away, and retained single litters of 4–5 pups. This approach substantially improved the survival rate and increased the body weights of the Rps6kb1 −/− pups. Even so, Rps6kb1 −/− pups still weighed significantly less than WT mice at four weeks of age ((12.8±0.4) g in Rps6kb1 −/− mice vs. (15.5±0.3) g in WT mice; P<0.0001; Fig. 2a and Table S1), suggesting that early postnatal overnutrition cannot completely compensate for the negative impact of Rps6kb1 loss on prenatal growth and development. However, when we reduced the litter size of Rcan2 −/− pups, we saw that the weights of small litters of Rcan2 −/− pups were able to exceed the weights of WT mice in litters with more pups.
Fig. 2.
Phenotypes of WT, Rcan2 −/−, and Rps6kb1 −/− mice fed NCD
(a) Growth curves of WT, Rcan2 −/−, and Rps6kb1 −/− mice fed NCD. Body weights of 13 Rcan2 −/−, 11 Rps6kb1 −/−, and 21 WT male mice were analyzed from postnatal Week 4 to Week 20. * P<0.05, ** P<0.001, *** P<0.0001 (Rcan2 −/− vs. WT);### P<0.0001 (Rps6kb1 −/− vs. WT); + P<0.05, ++ P<0.001, +++ P<0.0001 (Rps6kb1 −/− vs. Rcan2 −/−). (b) Representative examples of 20-week-old WT, Rcan2 −/−, and Rps6kb1 −/− males. (c) Mean tibia lengths of 13 20-week-old WT, 7 Rcan2 −/− and 11 Rps6kb1 −/− males. *** P<0.0001. (d) Mean weight of epididymal white adipose tissue in 13 WT, 7 Rcan2 −/−, and 11 Rps6kb1 −/− males at 20 weeks of age. * P<0.05, ** P<0.001. All values are given as mean±SEM
Starting from four weeks of age, a cohort of mice was placed on the NCD and their growth was monitored. Rps6kb1 −/− mice were significantly lighter than WT and Rcan2 −/− mice at the initial measurement, and their body weight never caught up over the duration of the monitoring period (Fig. 2a). There were no significant differences in growth rates between WT and Rcan2 −/− mice before 10 weeks of age; however, after 10 weeks of age, WT mice gained weight more quickly (Fig. 2a). At 20 weeks of age, Rcan2 −/− mice weighed about 11.4% less than WT mice ((28.9±0.5) g in Rcan2 −/− mice vs. (32.6±0.6) g in WT mice; P<0.0001), but about 9.1% more than Rps6kb1 −/− mice ((28.9±0.5) g in Rcan2 −/− mice vs. (26.5±0.3) g in Rps6kb1 −/− mice; P<0.001; Fig. 2a, Fig. 2b, and Table S1). Tibia lengths of Rcan2 −/− mice were similar with those of WT mice ((18.22±0.02) mm in Rcan2 −/− mice vs. (18.35±0.06) mm in WT mice; P=0.12), while about 5.2% longer than those of the Rps6kb1 −/− mice ((18.22±0.02) mm in Rcan2 −/− mice vs. (17.32±0.07) mm in Rps6kb1 −/− mice; P<10−7; Fig. 2c and Table S1). The sizes of the epididymal fat pads, which are considered to be highly correlated with total amount of body fat (Rogers and Webb, 1980; Eisen and Leatherwood, 1981), were markedly reduced in Rcan2 −/− mice compared to those of the WT mice (about 44% reduction), but were similar in size to the epididymal fat pads of the Rps6kb1 −/− mice (Fig. 2d and Table S1). These data indicate that loss of Rcan2 only reduces body weight, but not body length, whereas loss of Rps6kb1 reduces not only body weight, but also body length.
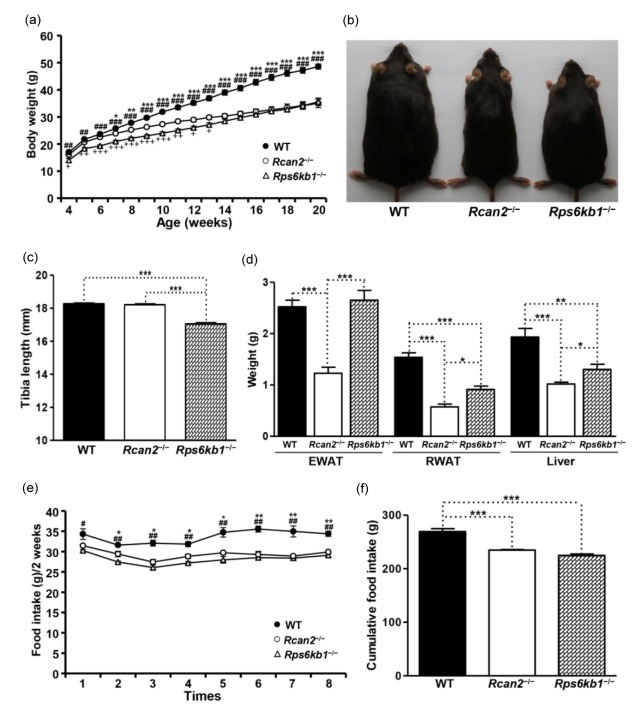
3.4. Effect of loss of Rcan2 and Rps6kb1 on growth and body weight in B6 mice fed HFD
Body growth is also influenced by diet composition, so a group of mice was placed on HFD and growth was monitored. At four weeks of age, the Rps6kb1 −/− mice were smaller than WT mice; the slower growth of Rps6kb1 −/− mice continued up to 20 weeks of age ((35.6±1.4) g in Rps6kb1 −/− mice vs. (48.6±0.9) g in WT mice; P<10−7; Fig. 3a, Fig. 3b, and Table S2). Interestingly, up to 13 weeks of age, Rps6kb1 −/− mice were also smaller than Rcan2 −/− mice. After 13 weeks of age, however, the body weights of Rps6kb1 −/− mice increased more quickly and, at 20 weeks of age, the weight of Rps6kb1 −/− mice was similar to that of Rcan2 −/− mice ((35.6±1.4) g in Rps6kb1 −/− mice vs. (35.0±1.5) g in Rcan2 −/− mice; P=0.77; Fig. 3a, Fig. 3b, and Table S2). At the beginning of these measurements, Rcan2 −/− mice had similar initial weights to WT mice (P=0.16), but the growth rate of Rcan2 −/− mice was slower than that of WT males. The weights of Rcan2 −/− mice were significantly different from weights of WT mice beginning at 7 weeks of age, the average weight difference reaching about 13.6 g at 20 weeks of age ((35.0±1.5) g in Rcan2 −/− mice vs. (48.6±0.9) g in WT mice; P<10−7; Fig. 3a, Fig. 3b, and Table S2). Consistent with the results from NCD (Fig. 2c), tibia lengths of Rcan2 −/− mice were similar to those of WT mice ((18.13±0.07) mm in Rcan2 −/− mice vs. (18.27±0.05) mm in WT mice; P=0.10), while about 6.4% longer than those of the Rps6kb1 −/− mice ((18.13±0.07) mm in Rcan2 −/− mice vs. (17.03±0.07) mm in Rps6kb1 −/− mice; P<10−8; Fig. 3c and Table S2). Although the weights of Rps6kb1 −/− mice were not different than those of the Rcan2 −/− mice at 20 weeks of age (Fig. 3a), anatomical analyses demonstrated that white fat deposits (including epididymal and retroperitoneal white adipose tissue) and livers of Rps6kb1 −/− mice were significantly heavier than those of Rcan2 −/− mice (Fig. 3d and Table S2). On the other hand, while the Rps6kb1 −/− mice were significantly lighter than WT mice, their epididymal fat deposits were of similar size (P=0.57; Fig. 3d and Table S2).
Fig. 3.
Phenotypes of WT, Rcan2 −/−, and Rps6kb1 −/− mice fed HFD
(a) Growth curves of WT, Rcan2 −/−, and Rps6kb1 −/− mice fed HFD. Body weights of 11 Rcan2 −/−, 9 Rps6kb1 −/−, and 12 WT males were analyzed from postnatal Week 4 to Week 20. * P<0.05, ** P<0.001, *** P<0.0001 (Rcan2 −/− vs. WT); ## P<0.001, ### P<0.0001 (Rps6kb1 −/− vs. WT); + P<0.05, ++ P<0.001, +++ P<0.0001 (Rps6kb1 −/− vs. Rcan2 −/−). (b) Representative examples of 20-week-old WT, Rcan2 −/− and Rps6kb1 −/− males. (c) Mean tibia lengths of 12 WT, 9 Rcan2 −/− and 8 Rps6kb1 −/− males at 20 weeks of age. *** P<0.0001. (d) Mean weight of epididymal and retroperitoneal white adipose tissue (EWAT and RWAT, respectively) and liver in 12 20-week-old WT, 9 Rcan2 −/−, and 8 Rps6kb1 −/− males. * P<0.05, ** P<0.001, *** P<0.0001. (e) Biweekly food intake measured from postnatal Week 5 to Week 20 in 4 WT, 4 Rcan2 −/−, and 6 Rps6kb1 −/− mice. * P<0.05, ** P<0.001 (Rcan2 −/− vs. WT); # P<0.05, ## P<0.001 (Rps6kb1 −/− vs. WT). (f) Cumulative food intake measured from postnatal Week 4 to Week 20 in 4 WT, 4 Rcan2 −/−, and 6 Rps6kb1 −/− mice. *** P<0.0001. All values are given as mean±SEM
All strains of mice gained more weight and had heavier fat pads on HFD compared to NCD. Relative gain in body weight of mice maintained on HFD compared to NCD was determined by dividing the body weights of mice fed on HFD by the weights of mice fed on NCD at 20 weeks of age (Black et al., 1998). The relative weight gain ratio was 1.49 in WT mice ((48.6±0.9) g on HFD vs. (32.6±0.6) g on NCD), 1.34 in Rps6kb1 −/− mice ((35.6±1.4) g on HFD vs. (26.5±0.3) g on NCD), and 1.21 in Rcan2 −/− mice ((35.0±1.5) g on HFD vs. (28.9±0.5) g on NCD). This indicates that, although Rps6kb1 −/− mice were shorter than Rcan2 −/− mice in body length (Figs. 2c and 3c), they gained weight more quickly on HFD.
To identify possible causes of differential body weight gain, we measured the food intake of WT, Rcan2 −/−, and Rps6kb1 −/− mice for a period of 16 weeks. WT mice ingested more food than Rps6kb1 −/− mice and Rcan2 −/− mice from the start of the measurement (Fig. 3e). Rcan2 −/− mice tended to consume more food than Rps6kb1 −/− mice during the first 10 weeks of the measurement, but this difference was not statistically significant (Fig. 3e). Cumulative food intake results showed that Rcan2 −/− mice ingested similar amounts of food compared to Rps6kb1 −/− mice; these were significantly lower compared to the cumulative food intake of WT mice (Fig. 3f).
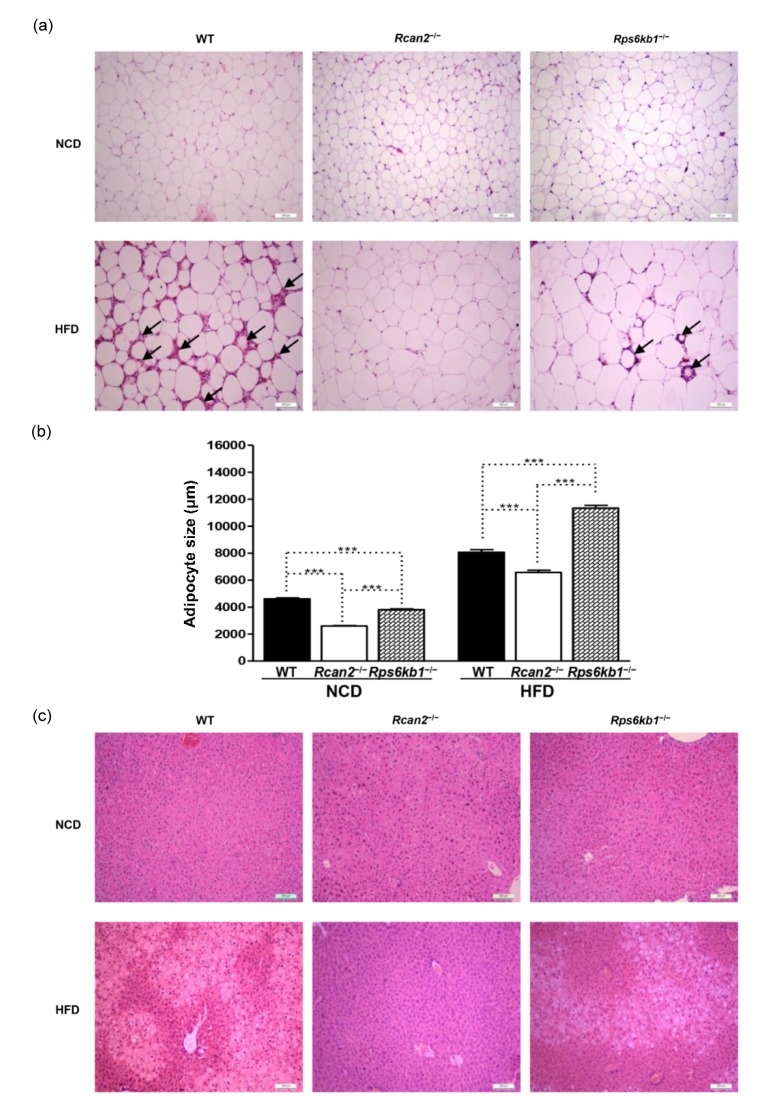
3.5. Histological analysis of epididymal white adipose tissue and liver
Histological analysis was performed to characterize the morphologic differences in EWAT and liver between the three strains of mice at 20 weeks of age, on either NCD or HFD. In the NCD-fed groups, the mean size of adipocytes in EWAT was larger in Rps6kb1 −/− mice compared to Rcan2 −/− mice, and adipocytes from both knockouts were smaller than adipocytes from WT mice (Figs. 4a and 4b).
Fig. 4.
Histological analysis of epididymal white adipose tissue (EWAT) and liver
(a) EWAT morphology. Rcan2 −/−, Rps6kb1 −/−, and WT mice were fed either NCD or HFD from postnatal Week 4 to Week 20; animals were humanely sacrificed and histological analysis was performed on EWAT samples. Representative H & E stains of EWAT from each of the six treatment groups are presented. Arrows indicate the presence of crown-like structures in the HFD-fed WT and Rps6kb1 −/− mice. Scale bar: 100 μm. (b) Average size of adipocytes. Adipocyte size was determined using ImageJ software. Statistics were performed in each diet group using one-way ANOVA, and individual group differences were measured using Bonferroni correction. *** P<0.0001. All values are given as mean±SEM. (c) Liver histology. Rcan2 −/−, Rps6kb1 −/−, and WT mice were fed either NCD or HFD from postnatal Week 4 to Week 20; animals were humanely sacrificed and histological analysis was performed on liver samples. Representative micrographs of H & E-stained liver sections demonstrate much more severe steatosis in livers of HFD-fed WT mice than Rps6kb1 −/− mice, while Rcan2 −/− mice did not suffer hepatic steatosis, even on HFD. Scale bar: 100 μm
Consumption of the HFD led to an increase of adipocyte size in all three strains of mice (Figs. 4a and 4b); however, there were apparent histological differences. We found no differences in EWAT mass between WT and Rps6kb1 −/− mice (Fig. 3d), but the mean size of adipocytes in WT mice was a little smaller compared with Rps6kb1 −/− mice, and the EWAT adipose tissue of WT mice contained more crown-like structures (CLSs) than that of Rps6kb1 −/− mice (Figs. 4a and 4b). EWAT adipocytes of Rcan2 −/− mice were significantly smaller than those of WT mice or Rps6kb1 −/− mice, on either NCD or HFD, and we detected very few CLS in the EWAT tissue of Rcan2 −/− mice (Figs. 4a and 4b).
All the three strains of mice fed NCD had well-organized liver structure, and no apparently obvious features of steatosis (Fig. 4c). On HFD, the extent of liver steatosis was associated with the weight of liver; Rps6kb1 −/− mice displayed steatosis and WT mice displayed more severe steatosis than Rps6kb1 −/− mice, while there was no hepatic steatosis detected in Rcan2 −/− mice on HFD (Fig. 4c). Increased liver weight and steatosis were previously reported to be associated with the loss of EWAT during the course of HFD feeding (Kolak et al., 2007), and in our study the liver histological results in these mice were closely correlated with morphologic changes in the EWAT.
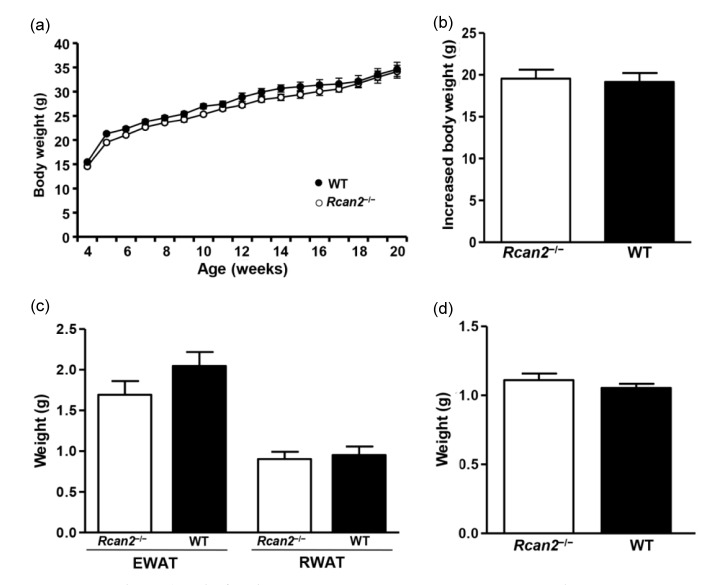
3.6. A pair-feeding between WT mice and Rcan2 −/− mice on HFD
According to the results on HFD (Fig. 3), WT mice ingested more food than Rcan2 −/− mice and gained more weight and fat deposits, but their body lengths were similar. To determine whether the weight differences between WT and Rcan2 −/− mice were solely due to differential food consumption, we performed pair-feeding experiments for a period of 16 weeks. Our results showed that if WT mice consumed the same amount of HFD as Rcan2 −/− mice, their body weight trajectories were nearly identical (Fig. 5a), and they gained similar amount of weight during the 16-week monitoring period ((19.2±1.2) g in WT mice vs. (19.6±1.2) g in Rcan2 −/− mice; P=0.80; Fig. 5b and Table S3). Dissection results revealed that, after long-term pair-feeding, WT mice and Rcan2 −/− mice contained similar amounts of adipose deposits, including EWAT, RWAT (P=0.19 and 0.73, respectively; Fig. 5c and Table S3), and liver (P=0.37; Fig. 5d and Table S3). These results showed that both weight gain and body composition of WT mice resembled those of Rcan2 −/− mice on HFD, if WT mice consumed similar amounts of food as Rcan2 −/− mice.
Fig. 5.
A pair-feeding study between WT and Rcan2 −/− mice on HFD
(a) Growth curves of the WT and Rcan2 −/− mice after a 16-week pair-feeding study. (b) Mean weight gain measured from postnatal Week 4 to Week 20 in WT and Rcan2 −/− mice. (c, d) Mean weights of epididymal and retroperitoneal white adipose tissue (EWAT and RWAT, respectively; c) and of liver (d) in WT and Rcan2 −/− mice after pair-feeding for 16 weeks. n=4 animals for each groups. All values are given as mean±SEM
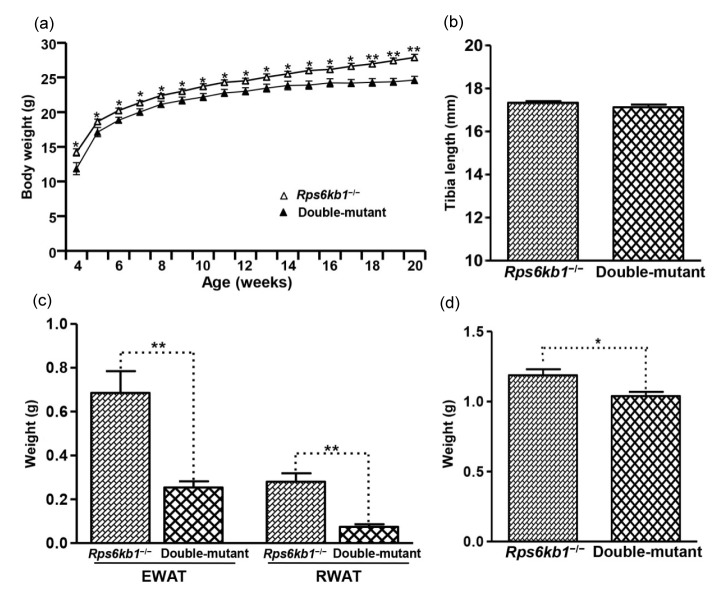
3.7. Growth of double-knockout (Rcan2 −/− Rps6kb1 −/−) mice fed NCD
The above results showed loss of Rcan2 or Rps6kb1 affects growth and body weight in mice, but the phenotypes were different, thus we hypothesized that these two mutations affect growth through different mechanisms. In order to confirm our hypothesis, we generated double-mutant (Rcan2 −/− Rps6kb1 −/−) mice. Double-mutant newborns weighed significantly less than Rcan2 −/− newborns, but had weights similar to the Rps6kb1 −/− newborns (Table 1). These results demonstrated that loss of Rps6kb1, but not Rcan2, affected embryonic development in mouse. The weights of animals fed NCD were monitored starting from 4 weeks of age. Double-mutant mice weighed significantly less than Rps6kb1 −/− mice from the beginning of the experiment, and never caught up during the whole monitoring period (Fig. 6a). Starting from about 13 weeks of age, double-mutants manifested slower weight gain, and at 20 weeks of age, double-mutant mice weighed 3.3 g less than Rps6kb1 −/− mice ((24.6±0.5) g in double-mutant mice vs. (27.9±0.5) g in Rps6kb1 −/− mice, P<0.0002; Fig. 6a and Table S4). Tibia length measurements showed that the bones of double-mutant mice were similar to those of the Rps6kb1 −/− mice ((17.13±0.14) mm in double-mutant mice vs. (17.33±0.08) mm in Rps6kb1 −/− mice; P=0.18; Fig. 6b and Table S4); however, the size of white adipose deposits (including epididymal and retroperitoneal fat pad) and livers were significantly smaller in double-mutant mice compared to those of Rps6kb1 −/− mice (Fig. 6c, Fig. 6d, and Table S4). These data showed in the Rps6kb1 −/− genetic background, loss of Rcan2 resulted in further reduction of body weight and adipose mass in mice, suggesting that Rcan2 and Rps6kb1 independently regulated murine growth and body weight.
Fig. 6.
Phenotypes of double-mutant (Rcan2 −/− Rps6kb1 −/−) mice
(a) Growth curves of Rps6kb1 −/− and double-mutant mice fed NCD. The body weights of 8 Rps6kb1 −/− and 8 double-mutant males were measured from postnatal Week 4 to Week 20. * P<0.05, ** P<0.001. (b) Mean tibia lengths from 8 Rps6kb1 −/− and 8 double-mutant males. (c, d) Weights of epididymal and retroperitoneal white adipose tissue (EWAT and RWAT, respectively; c) and of liver (d) from 8 Rps6kb1 −/− and 8 double-mutant males. * P<0.05, ** P<0.001. All values are given as mean±SEM
4. Discussion
In this report we describe the effects that loss of Rcan2 and Rps6kb1 have on growth and body weight in mice from the B6 genetic background. Consistent with results reported (Um et al., 2004; Sun et al., 2011), both Rcan2 −/− and Rps6kb1 −/− mutations reduced body weight and fat mass in mice. However, these two knockout strains displayed unique phenotypic traits. Firstly, while loss of Rcan2 did not lead to a significant change in body length, Rcan2 −/− mice exhibited reduced food intake. On the other hand, loss of Rps6kb1 greatly affected embryonic development, and led to smaller body size and reduced food intake in adulthood. Secondly, the manifestations of these two knockout mice were different in mice fed either HFD or NCD. On NCD, Rcan2 −/− mice weighed significantly more than Rps6kb1 −/− mice, but the epididymal fat pads of these mice were of similar sizes. On the HFD, Rps6kb1 −/− mice gained body weight and fat mass more quickly than Rcan2 −/− mice. Finally, using the double-mutant (Rcan2 −/− Rps6kb1 −/−) mice, we observed an additive effects of the Rcan2 −/− and Rps6kb1 −/− mutations on body weight of mice fed NCD. Therefore, our results suggest that Rcan2 and Rps6kb1 regulate growth and body weight through different mechanisms.
Growth, defined as an increase in size and body weight, is one of fundamental aspects of development (Baker et al., 1993). Mammals eventually reach a body size limit determined by the rate and duration of the growth process, which is mostly genetically controlled. However, body weight is not only controlled by genetic factors, but also by environmental factors, such as diet composition (Hill and Peters, 1998). Thus, we tried to control these environmental factors, especially diet composition, and then monitored body weight trajectory to determine the impact of loss of the target genes on growth. Additionally, we also used tibia length as an indicator of body size. When comparisons of body weight trajectories between WT and knockout mice are sought, plots of whole-body weight vs. developmental time, as well as records of tibia length, will provide adequate, although coarse, overall indexes of the effects of these mutations on mouse growth.
Although we had reported that inactivation of the Rcan2 gene ameliorated the age- and diet-induced obesity by causing a reduction in food intake, we simultaneously observed that Rcan2 −/− mice weighed significantly less than the WT mice starting at three weeks of age. Moreover, tibia lengths, which represent whole-body size, were about 2% shorter in Rcan2 −/− mice than in WT mice at 22 weeks of age (Sun et al., 2011). So, it is unclear whether reduced body size is the cause of altered food intake, or vice versa, in Rcan2 −/− mice. In the present study, we found that if we reduced the pup number in the mouse litters, the weight differences between Rcan2 −/− and WT mice at the time of weaning disappeared, and differences of tibia lengths also disappeared in adulthood. Recently, it was reported that early postnatal undernutrition leads to shorter body size in mice (Kappeler et al., 2009). Thus, we hypothesized that the reduced tibia lengths of Rcan2 −/− mice was possibly caused by the nutritional status, rather than the direct effect of Rcan2 −/− mutation on development. We further confirmed this hypothesis by a pair-feeding experiment on HFD. When Rcan2 −/− and WT mice were given ad libitum access to HFD for a period of 16 weeks, we observed tremendous differences in body weight and body composition. However, when WT mice were only allowed to ingest the same amount of food as Rcan2 −/− mice, the weight gain and body fat mass in WT mice were not different from those of the Rcan2 −/− mice. These data suggest that, even on HFD, the tremendous difference in body weight and body composition between WT mice and Rcan2 −/− mice may solely result from differential food intake. Therefore, our results further verify that there is an Rcan2-dependent mechanism that increases food intake and promotes weight gain in mice.
Consistent with the results reported (Shima et al., 1998), the body weights of Rps6kb1 −/− newborns were significantly lower than those of WT newborns, a phenotype that suggests intrauterine growth restriction (IUGR). The genetic backgrounds of the mice used in these two studies were different, which suggests that Rps6kb1 plays an essential role in murine embryonic development regardless of mouse genetic background, although it is unclear how loss of Rps6kb1 affects embryonic development. Recently, it was suggested that impaired placental development, as indicated by defects in the spongiotrophoblast and labyrinthine layers, may be responsible for IUGR of Rps6kb1 −/− mice (Um et al., 2015). On the other hand, deletion of Rps6kb1 resulted in reduced cell size in mice (Ohanna et al., 2005; Um et al., 2015), thus it may be reasonable to propose that body size is reduced in proportion to the altered cell size. In the subsequent postnatal developmental process, our results showed that Rps6kb1 −/− mice displayed shorter body size and reduced food intake. It is unclear whether reduction of food intake in Rps6kb1 −/− mice directly resulted from the deficiency of Rps6kb1 or was merely proportional to diminished body size. It has been suggested that hypothalamic Rps6kb1 signaling plays a key role in the control of food intake and energy homeostasis (Blouet et al., 2008; Cota et al., 2008; Dagon et al., 2012), although Smith et al. (2015) recently reported that Rps6kb1 signaling in pro-opiomelanocortin (POMC) and agouti-related protein (AgRP) neurons does not play a major role in feeding behavior or in the control of body weight. These conflicting studies suggest that the role of Rps6kb1 in regulation of body weight and food intake requires further investigation. The phenotypes of Rps6kb1 −/− mice described here were different from those described previously (Shima et al., 1998; Um et al., 2004), which reported that, in adulthood, the body size of Rps6kb1 −/− mice almost caught up with that of WT mice. The food intake of the Rps6kb1 −/− mice was similar to that of the WT mice, but energy expenditure was increased in the Rps6kb1 −/− mice, leading to a lean phenotype (Um et al., 2004). These discrepancies can possibly be attributed to the source of the mutant mice or to differences in the genetic background of the mice used in these studies. Our mice were generated on the uniform B6 background, and obtained from RIKEN BioResource Center (Tokyo, Japan); knockouts were prepared by targeting exon 9, which encodes a part of the catalytic domain (Kawasome et al., 1998), whereas the mutant mice in the study by Shima et al. (1998) were from the B6*129Ola hybrid background, and were prepared by replacing 1.2 kb of the genomic sequence encompassing the conserved Ser/Thr kinase catalytic subdomains VIII–X with a neomycin resistance cassette.
The histological analysis of EWAT in knockout and WT mice maintained on HFD indicated reduced CLSs in the Rcan2 −/− mice compared to either WT or Rps6kb1 −/− mice. CLSs, which are clusters of macrophages that have been recruited to the periphery of a dead or dying adipocyte, are an indicator of adipocyte death in models of diet-induced obesity (Weisberg et al., 2003; Cinti et al., 2005; Strissel et al., 2007). Adipose tissues are the main organs to store triglycerides, and their storage capacity depends on the expansion of the adipocytes (Monteiro et al., 2006; Smith et al., 2006). However, adipocyte death is significantly correlated with adipocyte expansion. It was reported that the frequency of adipocyte death in EWAT increased progressively in the duration of HFD feeding, and after 16 weeks of HFD feeding, the rate of adipocyte death reached a maximum level in B6 mice (Strissel et al., 2007). After 16 weeks, both adipocyte number and mean adipocyte size decreased, and fat deposit weight reduced by about 40%. Thus, at Week 16, the rate of adipocyte death exceeded the rate of tissue repair, resulting in net adipocyte and EWAT loss (Strissel et al., 2007). In our present study, the duration of HFD feeding was just 16 weeks. Thus, the reduced EWAT weight and increased CLS number in WT mice suggested that the frequency of adipocyte death was higher in WT mice than in Rcan2 −/− or Rps6kb1 −/− mice.
Body weights and adipose mass are widely accepted to be tightly regulated by homeostatic mechanisms, in which leptin, an adipocyte secreted hormone, plays a critical role through hypothalamic pathways (Flier, 2004; Schwartz and Porte, 2005; Morton et al., 2006). Even so, obesity has reached epidemic proportions globally over the past several decades, along with high circulating leptin levels. This phenomenon has been considered as a result of homeostatic disorder. However, in B6 mice, a golden animal model of human obesity (Surwit et al., 1988; 1995; Fraulob et al., 2010), we found that Rcan2 increases food intake and promotes weight gain through a leptin-independent mechanism (Sun et al., 2011). In this study, we demonstrated that if WT mice ingested the same amount of food as Rcan2 −/− mice, the weight gain and body fat mass in WT mice were equal to those of Rcan2 −/− mice. Additionally, HFD feeding alone also promotes weight gain, which is independent of Rcan2 gene. The interplay between the Rcan2 gene and HFD feeding leads to a rapid weight gain, which plays an important role in the development of diet-induced obesity. Thus, our findings coincide with the results of epidemiological studies that obesity is a result of the interaction of genetic and environmental factors (Lissner and Heitmann, 1995; Bray and Popkin, 1998) and, therefore, provide novel insights into the mechanisms of obesity epidemic. On the other hand, in the present study, loss of Rps6kb1 also resulted in a reduction of body weight and fat mass in B6 mice. However, according to the body weight trajectories of WT and Rps6kb1 −/− mice (Fig. 2a, Fig. 3a, Table S1, and Table S2), the reduced body weight and fat mass in Rps6kb1 −/− mice seem to be primarily due to a decrease of body size, rather than to an effect of Rps6kb1 mutation on energy homeostasis.
It has recently been reported that about 30% of the genetic manipulations in mice affect body weight, manifesting as lean or obese phenotypes (Reed et al., 2008). Body weight is affected by multiple factors, such as body size, energy expenditure, physical activity, food intake, and psychological stress. To carefully define the relevance of specific genes to these factors in the control of body weight regulation will facilitate our understanding of the mechanisms of obesity. Here we compared the effects of the Rcan2 −/− and Rps6kb1 −/− mutations on murine growth and body weight under carefully controlled conditions. Our data suggest that Rcan2 and Rps6kb1 regulate growth and body weight through different mechanisms. Our approach was not complicated, and can be applied in other genetic settings to provide useful data and information to deepen our understanding of the mechanisms of body weight regulation.
Acknowledgements
We are grateful to Prof. Yoshiharu MURATA (Nagoya University, Japan) for gifting Rcan2+/− mice.
Contributors
Jing ZHAO, Shi-wei LI, and Qian-qian GONG conducted the experimental studies, evaluated the data, and partially wrote the manuscript. Ling-cui DING and Ye-cheng JIN assisted in the conducting of the experimental studies. Jian ZHANG and Jian-gang GAO helped in the design of the study and revised the manuscript. Xiao-yang SUN designed the project, analyzed and interpreted the results, wrote the manuscript, and took full responsibility for the work as a whole.
Compliance with ethics guidelines
Jing ZHAO, Shi-wei LI, Qian-qian GONG, Ling-cui DING, Ye-cheng JIN, Jian ZHANG, Jian-gang GAO, and Xiao-yang SUN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
List of electronic supplementary materials
Table S1 Individual mouse data on NCD
Table S2 Individual mouse data on HFD
Table S3 Individual mouse data for pair-feeding
Table S4 Individual mouse data for double-mutants
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31140091 and 31371495) and Shandong Natural Science Foundation (No. ZR2013CM040), China
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1600276) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Jing ZHAO, Shi-wei LI, Qian-qian GONG, Ling-cui DING, Ye-cheng JIN, Jian ZHANG, Jian-gang GAO, and Xiao-yang SUN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Baker J, Liu JP, Robertson EJ, et al. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 2.Bassett JH, Logan JG, Boyde A, et al. Mice lacking the calcineurin inhibitor Rcan2 have an isolated defect of osteoblast function. Endocrinology. 2012;153(7):3537–3548. doi: 10.1210/en.2011-1814. (Available from: http://dx.doi.org/10.1210/en.2011-1814) [DOI] [PubMed] [Google Scholar]
- 3.Black BL, Croom J, Eisen EJ, et al. Differential effects of fat and sucrose on body composition in A/J and C57BL/6 mice. Metabolism. 1998;47(11):1354–1359. doi: 10.1016/s0026-0495(98)90304-3. [DOI] [PubMed] [Google Scholar]
- 4.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8(6):459–467. doi: 10.1016/j.cmet.2008.10.004. (Available from: http://dx.doi.org/10.1016/j.cmet.2008.10.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68(6):1157–1173. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 6.Cao X, Kambe F, Moeller LC, et al. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19(1):102–112. doi: 10.1210/me.2004-0093. (Available from: http://dx.doi.org/10.1210/me.2004-0093) [DOI] [PubMed] [Google Scholar]
- 7.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. (Available from: http://dx.doi.org/10.1194/jlr.M500294-JLR200) [DOI] [PubMed] [Google Scholar]
- 8.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. (Available from: http://dx.doi.org/10.1126/science.1124147) [DOI] [PubMed] [Google Scholar]
- 9.Cota D, Matter EK, Woods SC, et al. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28(28):7202–7208. doi: 10.1523/JNEUROSCI.1389-08.2008. (Available from: http://dx.doi.org/10.1523/JNEUROSCI.1389-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagon Y, Hur E, Zheng B, et al. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab. 2012;16(1):104–112. doi: 10.1016/j.cmet.2012.05.010. (Available from: http://dx.doi.org/10.1016/j.cmet.2012.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jonghe BC, Hayes MR, Bence KK. Melanocortin control of energy balance: evidence from rodent models. Cell Mol Life Sci. 2011;68(15):2569–2588. doi: 10.1007/s00018-011-0707-5. (Available from: http://dx.doi.org/10.1007/s00018-011-0707-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253(1):100–109. doi: 10.1006/excr.1999.4683. (Available from: http://dx.doi.org/10.1006/excr.1999.4683) [DOI] [PubMed] [Google Scholar]
- 13.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. doi: 10.1016/S0140-6736(05)66378-7. (Available from: http://dx.doi.org/10.1016/S0140-6736(05)66378-7) [DOI] [PubMed] [Google Scholar]
- 14.Eisen EJ, Leatherwood JM. Predicting percent fat in mice. Growth. 1981;45(2):100–107. [PubMed] [Google Scholar]
- 15.Fingar DC, Salama S, Tsou C, et al. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16(12):1472–1487. doi: 10.1101/gad.995802. (Available from: http://dx.doi.org/10.1101/gad.995802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116(2):337–350. doi: 10.1016/s0092-8674(03)01081-x. (Available from: http://dx.doi.org/10.1016/S0092-8674(03)01081-X) [DOI] [PubMed] [Google Scholar]
- 17.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, et al. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46(3):212–223. doi: 10.3164/jcbn.09-83. (Available from: http://dx.doi.org/10.3164/jcbn.09-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garfield AS, Lam DD, Marston OJ, et al. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab. 2009;20(5):203–215. doi: 10.1016/j.tem.2009.02.002. (Available from: http://dx.doi.org/10.1016/j.tem.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 19.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371–1374. doi: 10.1126/science.280.5368.1371. (Available from: http://dx.doi.org/10.1126/science.280.5368.1371) [DOI] [PubMed] [Google Scholar]
- 20.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. doi: 10.1172/JCI10842. (Available from: http://dx.doi.org/10.1172/JCI10842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappeler L, de Magalhaes Filho C, Leneuve P, et al. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009;150(1):314–323. doi: 10.1210/en.2008-0981. (Available from: http://dx.doi.org/10.1210/en.2008-0981) [DOI] [PubMed] [Google Scholar]
- 22.Kawasome H, Papst P, Webb S, et al. Targeted disruption of p70 (S6K) defines its role in protein synthesis and rapamycin sensitivity. PNAS. 1998;95(9):5033–5038. doi: 10.1073/pnas.95.9.5033. (Available from: http://dx.doi.org/10.1073/pnas.95.9.5033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56(8):1960–1968. doi: 10.2337/db07-0111. (Available from: http://dx.doi.org/10.2337/db07-0111) [DOI] [PubMed] [Google Scholar]
- 24.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49(2):79–90. [PubMed] [Google Scholar]
- 26.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–318. doi: 10.1038/nrm2672. (Available from: http://dx.doi.org/10.1038/nrm2672) [DOI] [PubMed] [Google Scholar]
- 27.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441(1):1–21. doi: 10.1042/BJ20110892. (Available from: http://dx.doi.org/10.1042/BJ20110892) [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Kanou Y, Murata Y, et al. Molecular cloning of a novel thyroid hormone-responsive gene, ZAKI-4, in human skin fibroblasts. J Biol Chem. 1996;271(24):14567–14571. doi: 10.1074/jbc.271.24.14567. (Available from: http://dx.doi.org/10.1074/jbc.271.24.14567) [DOI] [PubMed] [Google Scholar]
- 29.Mizuno Y, Kanou Y, Rogatcheva M, et al. Genomic organization of mouse ZAKI-4 gene that encodes ZAKI-4 α and β isoforms, endogenous calcineurin inhibitors, and changes in the expression of these isoforms by thyroid hormone in adult mouse brain and heart. Eur J Endocrinol. 2004;150(3):371–380. doi: 10.1530/eje.0.1500371. (Available from: http://dx.doi.org/10.1530/eje.0.1500371) [DOI] [PubMed] [Google Scholar]
- 30.Monteiro R, de Castro PM, Calhau C, et al. Adipocyte size and liability to cell death. Obes Surg. 2006;16(6):804–806. doi: 10.1381/096089206777346600. (Available from: http://dx.doi.org/10.1381/096089206777346600) [DOI] [PubMed] [Google Scholar]
- 31.Mori H, Inoki K, Munzberg H, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9(4):362–374. doi: 10.1016/j.cmet.2009.03.005. (Available from: http://dx.doi.org/10.1016/j.cmet.2009.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morton GJ, Cummings DE, Baskin DG, et al. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. (Available from: http://dx.doi.org/10.1038/nature05026) [DOI] [PubMed] [Google Scholar]
- 33.Ohanna M, Sobering AK, Lapointe T, et al. Atrophy of S6K1−/− skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7(3):286–294. doi: 10.1038/ncb1231. (Available from: http://dx.doi.org/10.1038/ncb1231) [DOI] [PubMed] [Google Scholar]
- 34.Reed DR, Lawler MP, Tordoff MG. Reduced body weight is a common effect of gene knockout in mice. BMC Genet. 2008;9:4. doi: 10.1186/1471-2156-9-4. (Available from: http://dx.doi.org/10.1186/1471-2156-9-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roa J, Tena-Sempere M. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metab. 2010;21(9):519–528. doi: 10.1016/j.tem.2010.05.003. (Available from: http://dx.doi.org/10.1016/j.tem.2010.05.003) [DOI] [PubMed] [Google Scholar]
- 36.Rogers P, Webb GP. Estimation of body fat in normal and obese mice. Br J Nutr. 1980;43(1):83–86. doi: 10.1079/bjn19800066. (Available from: http://dx.doi.org/10.1079/BJN19800066) [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MW, Porte DJr. Diabetes, obesity, and the brain. Science. 2005;307(5708):375–379. doi: 10.1126/science.1104344. (Available from: http://dx.doi.org/10.1126/science.1104344) [DOI] [PubMed] [Google Scholar]
- 38.Shima H, Pende M, Chen Y, et al. Disruption of the p70s6k/p85s6k gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17(22):6649–6659. doi: 10.1093/emboj/17.22.6649. (Available from: http://dx.doi.org/10.1093/emboj/17.22.6649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith J, Al-Amri M, Dorairaj P, et al. The adipocyte life cycle hypothesis. Clin Sci (Lond) 2006;110(1):1–9. doi: 10.1042/CS20050110. (Available from: http://dx.doi.org/10.1042/CS20050110) [DOI] [PubMed] [Google Scholar]
- 40.Smith MA, Katsouri L, Irvine EE, et al. Ribosomal S6K1 in POMC and AgRP neurons regulates glucose homeostasis but not feeding behavior in mice. Cell Rep. 2015;11(3):335–343. doi: 10.1016/j.celrep.2015.03.029. (Available from: http://dx.doi.org/10.1016/j.celrep.2015.03.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. (Available from: http://dx.doi.org/10.2337/db07-0767) [DOI] [PubMed] [Google Scholar]
- 42.Sun XY, Hayashi Y, Xu S, et al. Inactivation of the Rcan2 gene in mice ameliorates the age- and diet-induced obesity by causing a reduction in food intake. PLOS ONE. 2011;6(1):e14605. doi: 10.1371/journal.pone.0014605. (Available from: http://dx.doi.org/10.1371/journal.pone.0014605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surwit RS, Kuhn CM, Cochrane C, et al. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 44.Surwit RS, Feinglos MN, Rodin J, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44(5):645–651. doi: 10.1016/0026-0495(95)90123-x. (Available from: http://dx.doi.org/10.1016/0026-0495(95)90123-X) [DOI] [PubMed] [Google Scholar]
- 45.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. (Available from: http://dx.doi.org/10.1038/nature02866) [DOI] [PubMed] [Google Scholar]
- 46.Um SH, Sticker-Jantscheff M, Chau GC, et al. S6K1 controls pancreatic β cell size independently of intrauterine growth restriction. J Clin Invest. 2015;125(7):2736–2747. doi: 10.1172/JCI77030. (Available from: http://dx.doi.org/10.1172/JCI77030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. 2015;161(1):119–132. doi: 10.1016/j.cell.2015.03.008. (Available from: http://dx.doi.org/10.1016/j.cell.2015.03.008) [DOI] [PubMed] [Google Scholar]
- 48.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8(3):523–534. doi: 10.1016/0031-9384(72)90340-x. (Available from: http://dx.doi.org/10.1016/0031-9384(72)90340-X) [DOI] [PubMed] [Google Scholar]
- 49.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. (Available from: http://dx.doi.org/10.1172/JCI200319246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. (Available from: http://dx.doi.org/10.1038/414782a) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Individual mouse data on NCD
Table S2 Individual mouse data on HFD
Table S3 Individual mouse data for pair-feeding
Table S4 Individual mouse data for double-mutants