Abstract
Objective: To explore the efficacy of ursolic acid in sensitizing colon cancer cells to chemotherapy under hypoxia and its underlying mechanisms. Methods: Three colon cancer cell lines (RKO, LoVo, and SW480) were used as in vitro models. 5-Fluorouracil (5-FU) and oxaliplatin were used as chemotherapeutic drugs. Cell viability and apoptosis were tested to evaluate the sensitivity of colon cancer cells to chemotherapy. The transcription and expression levels of hypoxia-inducible factor-1α (HIF-1α), multidrug resistance gene 1 (MDR1), and vascular endothelial growth factors (VEGF) were assessed by quantitative real-time polymerase chain reaction (qRT-PCR) and immunoblotting. Cycloheximide and MG132 were used to inhibit protein synthesis and degradation, respectively. In vitro tube formation assay was used to evaluate angiogenesis. Results: We demonstrated the chemosensitizing effects of ursolic acid with 5-FU and oxaliplatin in three colon cancer cell lines under hypoxia. This effect was correlated to its inhibition of MDR1 through HIF-1α. Moreover, ursolic acid was capable of inhibiting HIF-1α accumulation with little effects on its constitutional expression in normoxia. In addition, ursolic acid also down-regulated VEGF and inhibited tumor angiogenesis. Conclusions: Ursolic acid exerted chemosensitizing effects in colon cancer cells under hypoxia by inhibiting HIF-1α accumulation and the subsequent expression of the MDR1 and VEGF.
Keywords: Ursolic acid, Colon cancer, Hypoxia-inducible factor-1α (HIF-1α), Multidrug resistance gene 1 (MDR1), Drug resistance
1. Introduction
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related death worldwide (Siegel et al., 2016). In China, the morbidity of CRC has been greatly increasing due to the change of people’s life style (Chen et al., 2016). Although the survival rates of stages I, II, and III CRC are relatively high if detected early, the prognosis of stage IV CRC is still poor, and most patients need chemotherapy because of synchronic or postoperative metastasis. Although advances in chemotherapy have occurred in the past decades, the overall response rates remain unsatisfactory (Gustavsson et al., 2015). Chemoresistance of tumors is believed to be responsible for the failure of this strategy. Therefore, it is urgently vital to overcome chemotherapy resistance in CRC patients.
CRC undergoes hypoxia like many other solid tumors. There is a general understanding that the hypoxic microenvironment can promote tumor progression as well as drug resistance (Selvakumaran et al., 2013; Zhang et al., 2013; Liu et al., 2015). One of the first reported molecular mechanisms explaining the contribution of hypoxia to drug resistance was the finding that the hypoxia-inducible factor 1α (HIF-1α) was able to activate the multidrug resistance gene 1 (MDR1), which encodes for the P-glycoprotein (P-gp) that decreases intracellular drug concentration by acting as a drug efflux pump (Bellamy, 1996; Chen et al., 2014). It is therefore highly valuable to find novel ways to block the expressions of HIF-1α and MDR1 in tumor cells under hypoxic stress (Nabekura, 2010).
Ursolic acid (3β-hydroxyurs-12-en-28-oic acid, UA) is a pentacyclic triterpenic acid found in a variety of natural plants, including Chinese medicinal herbs. Hon-Yeung CHEUNG is the first scientist reporting on this compound, which is found in Hedyotic diffusa Willd. and Prunell avulgaris L. (Cheung et al., 2006; Cheung and Zhang, 2008). It exhibits a broad range of pharmacological effects such as anti-inflammatory, antiviral, antioxidant, and hepatoprotective activities (Feuillolay et al., 2016; Kashyap et al., 2016; Siegel et al., 2016), and has proven to be effective in treating various types of cancers including CRC (Pathak et al., 2007; Prasad et al., 2012; Lin et al., 2013; Kadioglu and Efferth, 2015). Several mechanisms by which UA acts as a chemosensitizer have been proposed, such as pro-apoptosis (Meng et al., 2015) and anti-angiogenesis (Lin et al., 2013). In addition, UA was found to be capable of affecting multiple signaling pathways including nuclear factor-κB (NF-κB) (Prasad et al., 2012), signal transducer and activator of transcription-3 (STAT3) (Pathak et al., 2007), and phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) (Meng et al., 2015), among others. Here we showed that UA could sensitize colon cancer cells to both 5-fluorouracil (5-FU) and oxaliplatin under hypoxia by inhibiting the expressions of HIF-1α and its downstream effector MDR1.
2. Materials and methods
2.1. Reagents
UA (purity 98%, analytical standard; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (Sigma-Aldrich), aliquoted, and stored at −20 °C. Cycloheximide and MG132 were purchased from Sigma. 5-FU (fluorouracil injection, 10 ml:0.25 g) was purchased from the Shanghai Xudonghaipu Pharmaceutical Co. Ltd., China. Oxaliplatin for injection (50 mg/bottle) was purchased from Sanofi (Hangzhou, China). HIF-1α small interfering RNA (siRNA) and MDR1 siRNA were purchased from GenePharma (Shanghai, China). The primary antibodies including HIF-1α and MDR1 were purchased from Cell Signaling Technology (Danvers, MA, USA). The secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
2.2. Cell culture
Human colon cancer cell lines (RKO, LoVo, and SW480) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). SW480 cells were cultured in Leibovitz’s L-15 medium, LoVo cells were cultured in F-12K medium, and RKO cells were cultured in Eagle’s minimum essential medium. These mediums were supplemented with 10% (v/v) fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin. Cell lines were maintained in a humidified atmosphere of 5% CO2 at 37 °C. Hypoxic conditions (1% O2) were established in a sealed chamber using the BBL GasPak Plus anaerobic system envelopes with a palladium catalyst (Becton Dickinson, Cockeysville, MD, USA).
2.3. Cell proliferation assay
Cell proliferation was evaluated by measuring the mitochondrial dehydrogenase activity, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as the substrate. The absorbance was measured at 570 nm using an MRX Revelation 96-well multiscanner (Dynex Technologies, Chantilly, VA, USA).
2.4. Apoptosis assay
We used a fluorescein-conjugated Annexin V (Annexin V-FITC)/propidium iodide (PI) staining kit (Sigma-Aldrich) to assess the apoptosis of the cells. Briefly, 1×106 cells were treated with indicated doses of chemicals for 48 h. Cells were harvested and stained with assay reagents, and cell apoptosis was determined by flow cytometry using FACSCaliber (Becton Dickinson, Franklin Lakes, NJ, USA).
2.5. RNA extraction and quantitative real-time PCR analysis
Total RNA was isolated from cells treated with chemicals for 48 h using QiaShredder columns and the RNeasy kit from Qiagen (Hilden, Germany) according to the manufacturer’s instructions. The primers used were: 5'-GTTTGATTTTACTCATCCAT-3' and 5'-TTCATAGTTCTTCCTCGG-3' for HIF-1α; 5'-CT TGGCAGCAATTAGAAC-3' and 5'-TCAGCAGGA AAGCAGCAC-3' for MDR1; RNA was reverse-transcribed using the PrimeScript™ RT reagent kit (TaKaRa-Bio, Tokyo, Japan) to generate the first strand complementary DNA (cDNA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on a StepOnePlus™ quantitative real-time PCR system (Thermo Fisher Scientific). β-Actin was used as the internal control. SYBR® Premix Ex Taq™ was used according to the instructions of the manufacturer (TaKaRa-Bio). All primers were synthesized by Sangon Biotech (Shanghai, China).
2.6. Immunoblotting
Western blot analysis was performed as described before (Shan et al., 2016) with minor adjustments. Briefly, 5×105 cells were incubated in the presence or absence of UA or in combination with oxaliplatin for 48 h and were lysed in a sample buffer (Pierce, Rockford, IL, USA). Protein (60 μg) was loaded into a 5% (0.05 g/ml) to 10% (0.10 g/ml) gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membrane was then incubated with the respective primary and secondary antibodies. Signals were detected using a chemiluminescence kit (Pierce).
2.7. HIF-1α and MDR1 knockdown in CRC cell lines
Using siRNA, the expressions of HIF-1α and MDR1 were knocked down in three cell lines. Pre-miRNA RNA interference (RNAi) sequences for the target genes HIF-1α and MDR1 were designed and synthesized by Sangon Biotech (Shanghai, China). Transfections were performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Six hours later, the transfection medium was replaced using a complete medium. HIF-1α and MDR1 siRNAs were transfected into cells at a concentration of 100 nmol/L. The efficiency of siRNA interference was evaluated after 48 h using a Western blot as previously described (Shan et al., 2016).
2.8. In vitro tube formation assay
In vitro tube formation assay was performed according to the manufacturer’s instructions (Chemicon, Shanghai, China). Briefly, 1×105 human umbilical vein endothelial cells (HUVEC) were serum-starved in 0.2% (v/v) FBS-containing media for 18 h, and then incubated in the presence or absence of UA (20 μmol/L) or in combination with 5-FU for 24 h under hypoxia. Tube formation was monitored for 2–6 h in a conditioned medium. Cell images were taken using a microscope.
2.9. Statistical analysis
All experiments were repeated at least three times and the data are presented as a mean and standard deviation (SD). Prism 6 (GraphPad, San Diego, CA, USA) was used to perform statistical analysis. The Student’s t-test was used for comparison between each treatment group and its corresponding control group, and a value of P<0.05 was considered statistically significant.
3. Results
3.1. Hypoxia-induced drug resistance of colon cancer cells
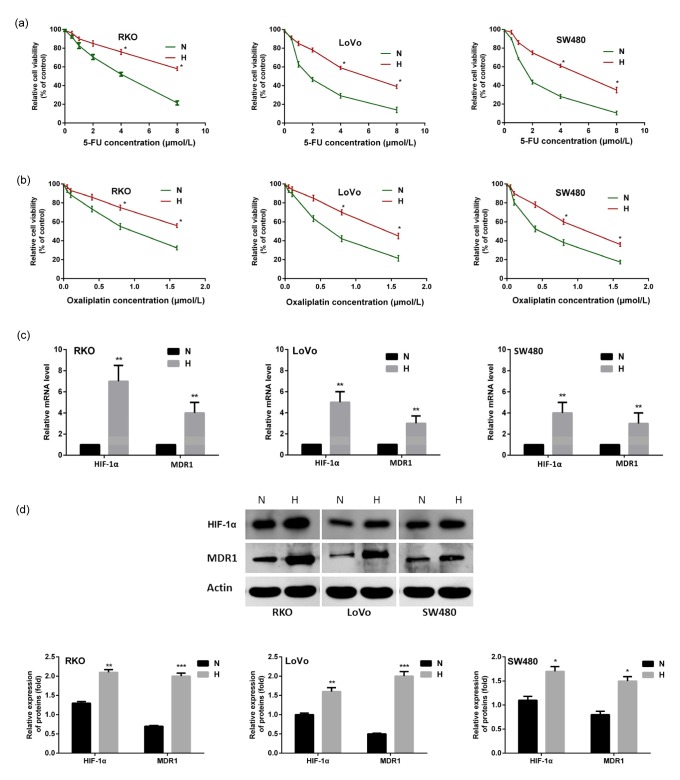
To exam the effect of hypoxia on colon cancer cells that were exposed to chemotherapeutic drugs, we compared the cell viabilities of three colon cancer cell lines (RKO, LoVo, and SW480) treated with 5-FU and oxaliplatin under normoxic and hypoxic conditions. As shown in Figs. 1a and 1b, cells were more resistant to the chemotherapeutic drugs when experiencing hypoxia. Overexpressions of HIF-1α and MDR1 were detected in both the mRNA and protein levels (Figs. 1c and 1d).
Fig. 1.
More resistance of colon cancer cells to chemotherapy under hypoxia, accompanied by HIF-1α and MDR1 overexpression
Three colon cancer cells were cultured under normoxic (20% O2) or hypoxic (1% O2) conditions with 5-FU (a) or oxaliplatin (b) for 24 h, and cell viability was examined using MTT assays. Overexpressions of HIF-1α and MDR1 were detected in both mRNA (c) and protein (d) levels after the cancer cells were exposed to hypoxia. Data are expressed as mean±SD of triplicate experiments. * P<0.05, ** P<0.01, *** P<0.005, compared to normoxia. N: normoxia; H: hypoxia
3.2. Role of UA in chemoresistance of colon cancer cells under hypoxia
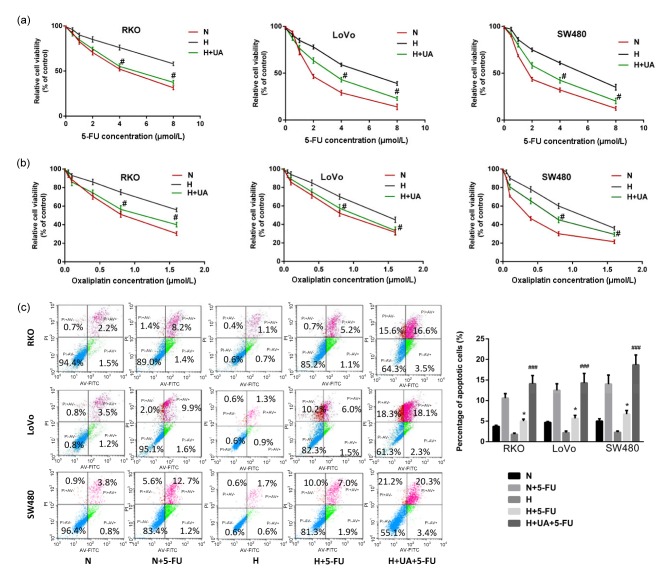
We then tested the role of UA in hypoxia-induced chemoresistance in colon cancer cells. Encouragingly, the addition of UA recovered chemo-sensitivity in all cell lines tested (Figs. 2a and 2b). Flow cytometry analysis also showed that the addition of UA restored the sensitivity of cells to 5-FU under hypoxia compared to normoxia (Fig. 2c). These results suggest that UA could restore the sensitivity of colon cancer cells to chemotherapeutic drugs under hypoxia.
Fig. 2.
Effect of UA on sensitivity of colon cancer cells to chemotherapeutic drugs under hypoxia
Cells were pretreated with UA (20 μmol/L) for 24 h in normoxia. Cells were then cultured under normoxic (20% O2) or hypoxic (1% O2) conditions with 5-FU (a) or oxaliplatin (b) for 24 h. Cell viability was evaluated using MTT assays. (c) Flow cytometry analysis of apoptotic colon cancer cells treated with 5-FU alone or combined with UA under normoxia and hypoxia. Data are expressed as mean±SD of triplicate experiments. * P<0.05, compared to N+5-FU; # P<0.05, ### P<0.005 compared to hypoxia. N: normoxia; H: hypoxia
3.3. Inhibitive effect of UA on MDR1 expression under hypoxia
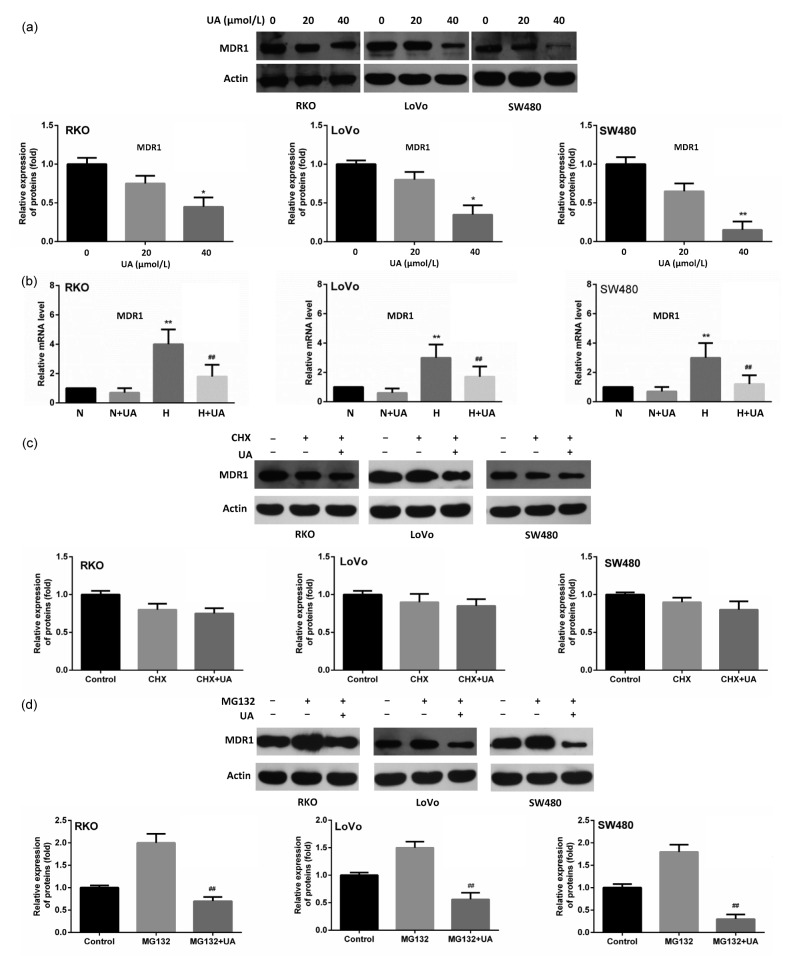
To figure out the mechanism by which UA acts as a chemosensitizer, we focused on MDR1 which has previously proven to be induced by hypoxia. Different from the previous findings that UA could inhibit the function of MDR1 (Nabekura et al., 2010), we found that UA decreased MDR1 expression under hypoxia in a dose-dependent manner (Fig. 3a). However, it did not influence its constitutional expression under normoxia (Fig. 3b). We further used cycloheximide to stop protein synthesis, and found that the addition of UA did not have an obvious effect on protein stability (Fig. 3c). In contrast, UA significantly reversed the up-regulation of MDR1 induced by MG123, a specific proteasome inhibitor that can block protein degradation in all the three cell lines (Fig. 3d).
Fig. 3.
Inhibitive effect of UA on MDR1 expression under hypoxia
(a) Cells were cultured under hypoxia and were treated with UA (0, 20 and 40 μmol/L, respectively) for 24 h. MDR1 expression was detected using immunobloting. * P<0.05, ** P<0.01, compared to 0 μmol/L UA group. (b) Cells were treated with 20 μmol/L UA under normoxic and hypoxic conditions and MDR1 mRNA was examined by quantitative RT-PCR. ** P<0.01, compared to normoxia; ## P<0.01, compared to hypoxia. (c) Cells were treated with 50 μg/ml of cycloheximide (CHX) for 24 h, followed with subsequent treatment of UA (20 μmol/L) for another 24 h. MDR1 abundance was examined in all three cell lines using Western blot. (d) Cells were treated with 100 μmol/L of MG132 for 24 h, followed with subsequent treatment of UA (20 μmol/L) for another 24 h. MDR1 expression was detected using Western blot. Data are expressed as mean±SD of triplicate experiments. ## P<0.01, compared to MG132 group
3.4. Inhibitive effect of UA on MDR1 through HIF-1α inhibition
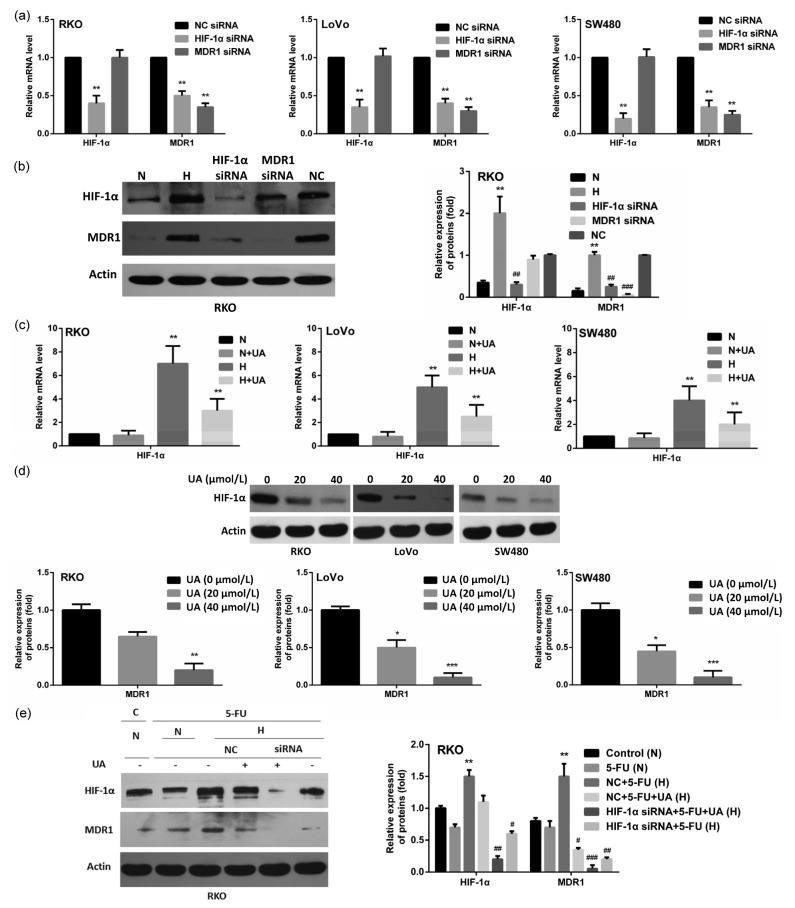
Since MDR1 is an HIF-1α target gene and it was reported that UA was capable of inhibiting HIF-1α (Wang et al., 2016), we wondered whether HIF-1α mediates UA-induced MDR1 down-regulation. We observed that under hypoxia, inhibition of HIF-1α resulted in lower MDR1 levels, while MDR1 inhibition did not influence the expression of HIF-1α, confirming the transcriptional regulation of MDR1 by HIF-1α (Figs. 4a and 4b). UA significantly reduced hypoxia-induced accumulation of HIF-1α mRNA with little effect on its constitutional expression under normoxia (Fig. 4c). In hypoxia, UA also down-regulated the expression of HIF-1α in a dose-dependent manner (Fig. 4d). Moreover, the knocking down of HIF-1α using its specific siRNA resulted in a similar reduction of both HIF-1α and MDR1 to that of UA treatment (Fig. 4e). The addition of UA in HIF-1α knockdown cells further abolished the residual HIF-1α and completely abrogated MDR1 (Fig. 4e). These data strongly suggested that UA inhibited MDR1 through inhibiting HIF-1α.
Fig. 4.
UA-induced MDR1 down-regulation by inhibiting HIF-1α
(a) Cells were transfected with either 100 nmol/L non-silencing (control) siRNA or HIF-1α- or MDR1-specific siRNA. The expressions of HIF-1α and MDR1 mRNA were quantified using qRT-PCR 48 h after transfection. ** P<0.01, compared to non-silencing (control) siRNA. (b) Cells were either untreated or treated with 100 nmol/L non-silencing siRNA or HIF-1α- or MDR1-specific siRNA for 48 h. The expressions of HIF-1α and MDR1 were detected using immunobloting. Cells under normoxic conditions were used as the control. ** P<0.01, compared to normoxia; ## P<0.01, ### P<0.005, compared to hypoxia. (c) Cells were treated with 20 μmol/L UA under normoxic and hypoxic conditions and HIF-1α mRNA was examined by using quantitative RT-PCR. ** P<0.01, compared to normoxia. (d) Cells were cultured under hypoxia and were treated with UA (0, 20, and 40 μmol/L, respectively) for 24 h. MDR1 expression was detected using immunobloting. * P<0.05, ** P<0.01, *** P<0.005, compared to UA (0 μmol/L). (e) RKO cells were treated with or without HIF-1α-specific siRNA or UA under normoxia and hypoxia in the presence of 5-FU and the expressions of HIF-1α and MDR1 were detected using Western blot. ** P<0.01, compared to control; # P<0.05, ## P<0.01, ### P<0.005, compared to NC+5-FU. Data are expressed as mean±SD of triplicate experiments. N: normoxia; H: hypoxia; C: control; NC: non-specific control
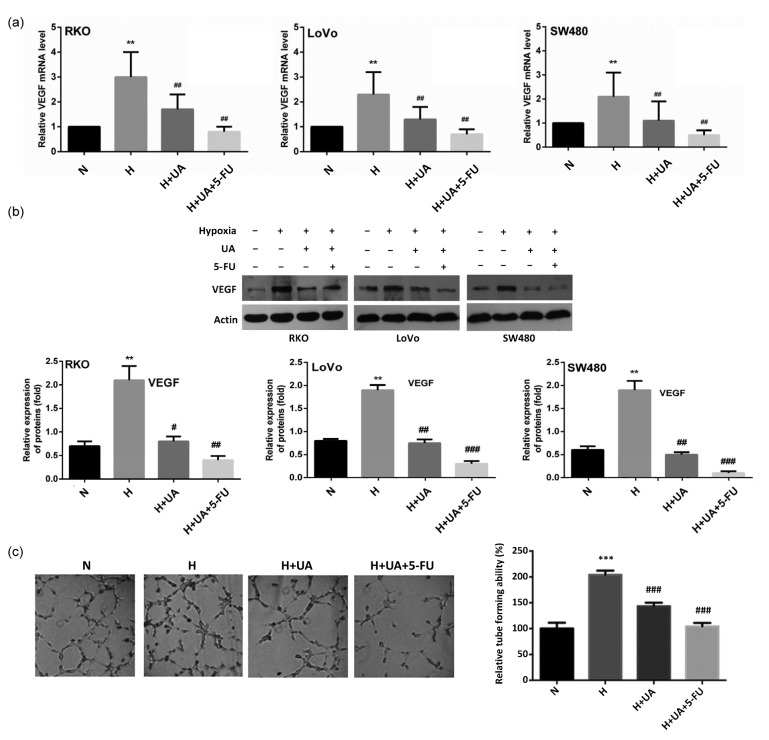
3.5. Inhibitive effect of UA on hypoxia-induced VEGF expression and angiogenesis
Given that many downstream genes, including VEGF, are regulated by HIF-1α, we also investigated the effects of UA on VEGF expression and angiogenesis. Hypoxia significantly induced VEGF overexpression, and UA was capable of blocking this induction. This effect was further improved by combining with 5-FU (Figs. 5a and 5b). Using HUVEC cells as an in vitro model, UA was found to inhibit hypoxia-induced angiogenesis with or without 5-FU combination (Fig. 5c).
Fig. 5.
Inhibitive effect of UA on VEGF expression and subsequent angiogenesis
(a, b) In all the three colon cancer cell lines tested, UA (20 μmol/L) with or without 5-FU inhibited hypoxia-induced VEGF expression. (c) Tube formation of HUVEC cells was disturbed by UA with or without 5-FU in hypoxia. Data are expressed as mean±SD of triplicate experiments. ** P<0.01, *** P<0.005, compared to normoxia; # P<0.05, ## P<0.01, ### P<0.005, compared to hypoxia. N: normoxia; H: hypoxia
4. Discussion
Currently, many strategies have been developed to enhance the effects of chemotherapy, including the use of biomaterial vectors (Kang et al., 2015), combination of synergistic small-molecule chemicals (Bai et al., 2014), nanoparticle formulation (Ni et al., 2015), etc. Apart from its direct tumor-suppressing role reported in many types of cancers (Li et al., 2010; Prasad et al., 2016), UA has also been identified as a promising natural material showing chemosensitizing effects (Nabekura, 2010; Weng et al., 2014). However, the underlying mechanisms are not fully understood. In this study, we showed that UA was able to inhibit hypoxia-induced expression of HIF-1α and its downstream effector MDR1, through which it sensitized colon cancer cells to chemotherapeutic drugs under hypoxic conditions.
CRC cells are exposed to the hypoxic environment pathologically, and HIF-1α accumulates in those cells partly due to impaired degradation (Lee et al., 2010). In addition, treatment of chemotherapeutic drugs can also stimulate HIF-1α transcription even in cells under normoxic conditions (Cao et al., 2013). As a result, cancer cells either under hypoxic stress or exposed to chemotherapeutic drugs are supposed to have high levels of HIF-1α. On the contrary, normal cells rarely suffer from hypoxic stress and therefore only have physiological levels of HIF-1α. As UA could only inhibit pathologically accumulated HIF-1α but not its constitutional expression, it seems to be a good choice in this context.
Our study reported that UA reversed chemoresistance of three colon cancer cells to 5-FU and oxaliplatin under hypoxia through three aspects as follows: hypoxia conferred chemoresistance to colon cancer cells by inducing the overexpressions of HIF-1α and MDR1; UA sensitized colon cancer cells to chemotherapeutic drugs under hypoxia; UA inhibited MDR1 expression through regulating HIF-1α but not protein stability. To our best knowledge, this is the first report showing that UA acts as a chemosensitizer through the HIF-1α-MDR1 axis in colon cancer cells to enhance the cytotoxicity of chemotherapeutic drugs under hypoxia.
As an HIF-1α inhibitor, UA could in theory modify all the downstream events linked with increased HIF-1α. For instance, HIF-1α is associated with enhanced apoptosis resistance (Kilic et al., 2007), while UA could promote apoptosis in many conditions and various types of cancer (Kashyap et al., 2016). Moreover, we also detected decreased apoptotic tumor cells by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay under hypoxia, which could be reversed by UA (data not shown). Additionally, inhibition of angiogenesis by UA was also confirmed by our current study and previous works (Shan et al., 2009; 2011), which was also involved with HIF-1α accumulation. Therefore, UA could be a promising candidate to combine with current chemotherapy to reverse drug resistance and enhance tumor cell apoptosis under hypoxia.
5. Conclusions
In conclusion, we demonstrated that UA was able to inhibit MDR1 expression by down-regulating hypoxia-induced HIF-1α accumulation, which may, at least partially, explain the role of UA in reversing chemoresistance of colon cancer cells under hypoxia.
Footnotes
Project supported by the Zhejiang Science and Technology Research Program of China (No. 2013C33229) and the Traditional Chinese Medicine Program of Zhejiang Province of China (Nos. 2013ZA081 and 2016ZA129)
Compliance with ethics guidelines: Jian-zhen SHAN, Yan-yan XUAN, Qi ZHANG, and Jian-jin HUANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Bai XL, Zhang Q, Ye LY, et al. Inhibition of protein phosphatase 2A enhances cytotoxicity and accessibility of chemotherapeutic drugs to hepatocellular carcinomas. Mol Cancer Ther. 2014;13(8):2062–2072. doi: 10.1158/1535-7163.MCT-13-0800. (Available from: http://dx.doi.org/10.1158/1535-7163.MCT-13-0800) [DOI] [PubMed] [Google Scholar]
- 2.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol. 1996;36:161–183. doi: 10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Eble JM, Moon E, et al. Tumor cells upregulate normoxic HIF-1α in response to doxorubicin. Cancer Res. 2013;73(20):6230–6242. doi: 10.1158/0008-5472.CAN-12-1345. (Available from: http://dx.doi.org/10.1158/0008-5472.CAN-12-1345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Ding Z, Peng Y. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLOS ONE. 2014;9(6):e98882. doi: 10.1371/journal.pone.0098882. (Available from: http://dx.doi.org/10.1371/journal.pone.0098882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Baade PD. Cancer statistics in China, 2015. CA: Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. (Available from: http://dx.doi.org/10.3322/caac.21338) [DOI] [PubMed] [Google Scholar]
- 6.Cheung HY, Zhang QF. Enhanced analysis of triterpenes, flavonoids and phenolic compounds in Prunella vulgaris L. by capillary zone electrophoresis with the addition of running buffer modifiers. J Chromatogr A. 2008;1213(2):231–238. doi: 10.1016/j.chroma.2008.10.033. (Available from: http://dx.doi.org/10.1016/j.chroma.2008.10.033) [DOI] [PubMed] [Google Scholar]
- 7.Cheung HY, Cheung SH, Law ML, et al. Simultaneous determination of key bioactive components in Hedyotis diffusa by capillary electrophoresis. J Chromatogr. 2006;834(1-2):195–198. doi: 10.1016/j.jchromb.2006.02.007. (Available from: http://dx.doi.org/10.1016/j.jchromb.2006.02.007) [DOI] [PubMed] [Google Scholar]
- 8.Feuillolay C, Pecastaings S, le Gac C, et al. A Myrtus communis extract enriched in myrtucummulones and ursolic acid reduces resistance of Propionibacterium acnes biofilms to antibiotics used in acne vulgaris. Phytomedicine. 2016;23(3):307–315. doi: 10.1016/j.phymed.2015.11.016. (Available from: http://dx.doi.org/10.1016/j.phymed.2015.11.016) [DOI] [PubMed] [Google Scholar]
- 9.Gustavsson B, Carlsson G, Machover D, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14(1):1–10. doi: 10.1016/j.clcc.2014.11.002. (Available from: http://dx.doi.org/10.1016/j.clcc.2014.11.002) [DOI] [PubMed] [Google Scholar]
- 10.Kadioglu O, Efferth T. Pharmacogenomic characterization of cytotoxic compounds from Salvia officinalis in cancer cells. J Nat Prod. 2015;78(4):762–775. doi: 10.1021/np501007n. (Available from: http://dx.doi.org/10.1021/np501007n) [DOI] [PubMed] [Google Scholar]
- 11.Kang L, Gao Z, Huang W, et al. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm Sin B. 2015;5(3):169–175. doi: 10.1016/j.apsb.2015.03.001. (Available from: http://dx.doi.org/10.1016/j.apsb.2015.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashyap D, Tuli HS, Sharma AK. Ursolic acid (UA): a metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi: 10.1016/j.lfs.2016.01.017. (Available from: http://dx.doi.org/10.1016/j.lfs.2016.01.017) [DOI] [PubMed] [Google Scholar]
- 13.Kilic M, Kasperczyk H, Fulda S, et al. Role of hypoxia inducible factor-1α in modulation of apoptosis resistance. Oncogene. 2007;26(14):2027–2038. doi: 10.1038/sj.onc.1210008. (Available from: http://dx.doi.org/10.1038/sj.onc.1210008) [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Kang JE, Park SK, et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1α via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010;80(7):982–989. doi: 10.1016/j.bcp.2010.06.018. (Available from: http://dx.doi.org/10.1016/j.bcp.2010.06.018) [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Xing D, Chen Q, et al. Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-κB using ursolic acid. Int J Cancer. 2010;127(2):462–473. doi: 10.1002/ijc.25044. (Available from: http://dx.doi.org/10.1002/ijc.25044) [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Chen Y, Wei L, et al. Ursolic acid inhibits colorectal cancer angiogenesis through suppression of multiple signaling pathways. Int J Oncol. 2013;43(5):1666–1674. doi: 10.3892/ijo.2013.2101. (Available from: http://dx.doi.org/10.3892/ijo.2013.2101) [DOI] [PubMed] [Google Scholar]
- 17.Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(1):32–43. doi: 10.1631/jzus.B1400221. (Available from: http://dx.doi.org/10.1631/jzus.B1400221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Y, Lin ZM, Ge N, et al. Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/Akt/mTOR pathway. Am J Chin Med. 2015;43(7):1471–1486. doi: 10.1142/S0192415X15500834. (Available from: http://dx.doi.org/10.1142/S0192415X15500834) [DOI] [PubMed] [Google Scholar]
- 19.Nabekura T. Overcoming multidrug resistance in human cancer cells by natural compounds. Toxins. 2010;2(6):1207–1224. doi: 10.3390/toxins2061207. (Available from: http://dx.doi.org/10.3390/toxins2061207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabekura T, Yamaki T, Hiroi T, et al. Inhibition of anticancer drug efflux transporter P-glycoprotein by rosemary phytochemicals. Pharmacol Res. 2010;61(3):259–263. doi: 10.1016/j.phrs.2009.11.010. (Available from: http://dx.doi.org/10.1016/j.phrs.2009.11.010) [DOI] [PubMed] [Google Scholar]
- 21.Ni D, Ding H, Liu S, et al. Superior intratumoral penetration of paclitaxel nanodots strengthens tumor restriction and metastasis prevention. Small. 2015;11(21):2518–2526. doi: 10.1002/smll.201403632. (Available from: http://dx.doi.org/10.1002/smll.201403632) [DOI] [PubMed] [Google Scholar]
- 22.Pathak AK, Bhutani M, Nair AS, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5(9):943–955. doi: 10.1158/1541-7786.MCR-06-0348. (Available from: http://dx.doi.org/10.1158/1541-7786.MCR-06-0348) [DOI] [PubMed] [Google Scholar]
- 23.Prasad S, Yadav VR, Sung B, et al. Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: chemosensitization with capecitabine. Clin Cancer Res. 2012;18(18):4942–4953. doi: 10.1158/1078-0432.CCR-11-2805. (Available from: http://dx.doi.org/10.1158/1078-0432.CCR-11-2805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad S, Yadav VR, Sung B, et al. Ursolic acid inhibits the growth of human pancreatic cancer and enhances the antitumor potential of gemcitabine in an orthotopic mouse model through suppression of the inflammatory microenvironment. Oncotarget. 2016;7(11):13182–13196. doi: 10.18632/oncotarget.7537. (Available from: http://dx.doi.org/10.18632/oncotarget.7537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvakumaran M, Amaravadi RK, Vasilevskaya IA, et al. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 2013;19(11):2995–3007. doi: 10.1158/1078-0432.CCR-12-1542. (Available from: http://dx.doi.org/10.1158/1078-0432.CCR-12-1542) [DOI] [PubMed] [Google Scholar]
- 26.Shan JZ, Xuan YY, Zheng S, et al. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ-Sci B. 2009;10(9):668–674. doi: 10.1631/jzus.B0920149. (Available from: http://dx.doi.org/10.1631/jzus.B0920149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan JZ, Xuan YY, Ruan SQ, et al. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin J Integr Med. 2011;17(8):607–611. doi: 10.1007/s11655-011-0815-y. (Available from: http://dx.doi.org/10.1007/s11655-011-0815-y) [DOI] [PubMed] [Google Scholar]
- 28.Shan JZ, Xuan YY, Zhang Q, et al. Ursolic acid synergistically enhances the therapeutic effects of oxaliplatin in colorectal cancer. Protein Cell. 2016;7(8):571–585. doi: 10.1007/s13238-016-0295-0. (Available from: http://dx.doi.org/10.1007/s13238-016-0295-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. (Available from: http://dx.doi.org/10.3322/caac.21332) [DOI] [PubMed] [Google Scholar]
- 30.Wang WJ, Sui H, Qi C, et al. Ursolic acid inhibits proliferation and reverses drug resistance of ovarian cancer stem cells by downregulating ABCG2 through suppressing the expression of hypoxia-inducible factor-1α in vitro. Oncol Rep. 2016;36(1):428–440. doi: 10.3892/or.2016.4813. (Available from: http://dx.doi.org/10.3892/or.2016.4813) [DOI] [PubMed] [Google Scholar]
- 31.Weng H, Tan ZJ, Hu YP, et al. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int. 2014;14(1):96. doi: 10.1186/s12935-014-0096-6. (Available from: http://dx.doi.org/10.1186/s12935-014-0096-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Bai X, Chen W, et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013;34(5):962–973. doi: 10.1093/carcin/bgt027. (Available from: http://dx.doi.org/10.1093/carcin/bgt027) [DOI] [PubMed] [Google Scholar]