Abstract
Rhodopsins are the major photopigments in the fruit fly Drosophila melanogaster. Drosophila express six well-characterized Rhodopsins (Rh1–Rh6) with distinct absorption maxima and expression pattern. In 2000, when the Drosophila genome was published, a novel Rhodopsin gene was discovered: Rhodopsin 7 (Rh7). Rh7 is highly conserved among the Drosophila genus and is also found in other arthropods. Phylogenetic trees based on protein sequences suggest that the seven Drosophila Rhodopsins cluster in three different groups. While Rh1, Rh2 and Rh6 form a “vertebrate-melanopsin-type”–cluster, and Rh3, Rh4 and Rh5 form an “insect-type”-Rhodopsin cluster, Rh7 seem to form its own cluster. Although Rh7 has nearly all important features of a functional Rhodopsin, it differs from other Rhodopsins in its genomic and structural properties, suggesting it might have an overall different role than other known Rhodopsins.
Keywords: Rhodopsins, Opsins, GPCR, Drosophila, Vision, Phototransduction
Introduction
Higher animals are able to perceive their environment through various senses, and vision is possibly the most crucial one. Not only is it well-developed in many animals but it is also the most sensitive sense. A single photon is sufficient to activate a signaling cascade in which many different phototransduction proteins are involved (Nikolic et al., 2010). Rhodopsins, photon-receiving G-protein coupled proteins, play a decisive role in this context. Rhodopsins are seven helix membrane proteins that are covalently coupled to a light sensitive chromophore, called retinal. Photon absorption leads to the isomerization of retinal, which results in the conformational change of Rhodopsin to Metarhodopsin and subsequent activation of the G-protein coupled cascade (Devary et al., 1987; Britt et al., 1993; Kiselev & Subramaniam, 1994).
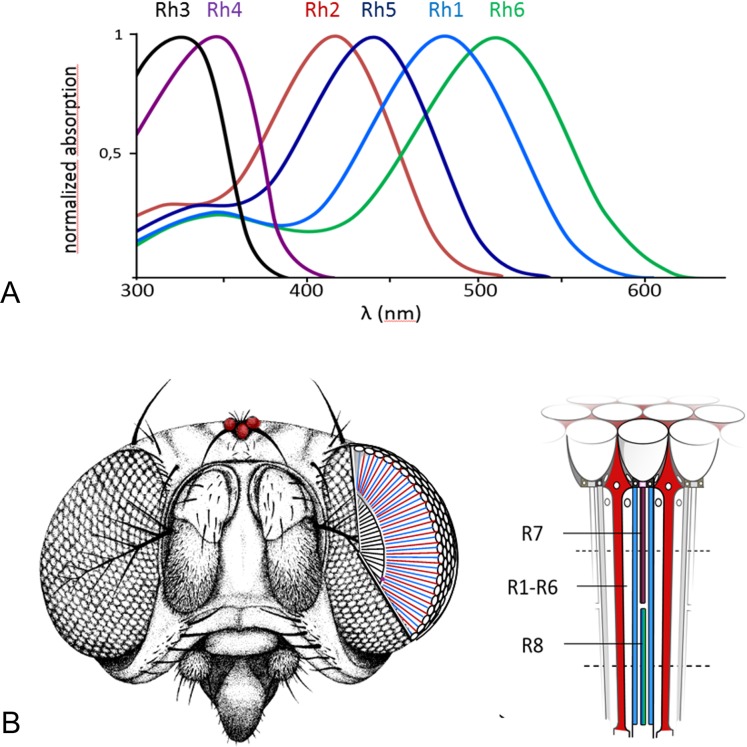
To have a broad and sensitive visual system, many animals express more than one Rhodopsin molecule. The fruit fly Drosophila melanogaster possesses six well-characterized Rhodopsins with distinct spectral sensitivities, named Rh1–Rh6 (Fig. 1A). While Rh1, Rh2 and Rh5 are sensitive to blue light (absorption maxima of 486, 418 and 442 nm), Rh3 and Rh4 are sensitive to UV light (331 and 355 nm), and Rh6 is sensitive to green light (515 nm) (Salcedo et al., 1999).
Figure 1. Six known Drosophila Rhodopsins.
(A) All known Rhodopsins have a distinct absorption maxima ranging from UV light (331 nm) to visible green light (515 nm) (adapted from Stavenga & Arikawa (2008)). (B) While Rh2 is expressed in fly’s ocelli, all other known Rhodopsins are primarily expressed in different receptor cells of the compound eye (modified from Helfrich-Förster (2014) and Grebler (2015)).
Besides differences in spectral sensitivity, each Rhodopsin also exhibits differences in expression site (Chou et al., 1999) (Fig. 1B). While Rh2 is expressed in the dorsal ocelli, the simple primitive eye found in many invertebrates, all other Rhodopsins (Rh1, Rh3, Rh4, Rh5, and Rh6) are expressed in different photoreceptor cells of the compound eye. The compound eye consists of approximately 800 identical optical units, termed ommatidia, and each ommatidium contains eight photoreceptor cells (R1–R8). Each photoreceptor cell expresses only one type of Rhodopsin: the outer photoreceptor cells (R1–R6) express the major Rhodopsin Rh1, which is encoded by the gene ninaE (O’Tousa et al., 1985; Zuker, Cowman & Rubin, 1985), the distal inner receptor cell R7 expresses either Rh3 or Rh4 whereas the proximal inner receptor cell R8 express either Rh5 or Rh6 (Rister, Desplan & Vasiliauskas, 2013; Behnia & Desplan, 2015). Two types of ommatidia, pale and yellow, are randomly interspersed in the compound eye, i.e. pale ca. 30% and yellow ca. 70%. The pale-type ommatidia contain Rh3 in the distal inner cell R7 cell and Rh5 in the proximal inner cell R8, while the yellow-type ommatidia contain Rh4 in the distal inner cell R7 and Rh6 in the proximal inner cell R8 (Chou et al., 1996; Huber et al., 1997; Papatsenko, Sheng & Desplan, 1997).
However, Rhodopsin expression seems not to be restricted to the eyes and to the ocelli. Two Rhodopsins, Rh5 and Rh6, are also found in the Bolwig’s organ in the larvae (Yasuyama & Meinertzhagen, 1999; Sprecher & Desplan, 2008) and in the H.B-eyelet (Hofbauer & Buchner, 1989; Helfrich-Förster et al., 2001) of the adult fly. Though not as much as in in visual organs, Rhodopsins are also found in non-visual organs, e.g. in the testis (Alvarez, Robison & Gilbert, 1996), in the body wall (Shen et al., 2011), and in the second antennal segment (Senthilan et al., 2012). This might indicate that Rhodopsins are more than only visual proteins as they appear to be involved in audition and thermosensation. In addition, Rhodopsins are important for synchronizing the fly circadian clock to the environmental light-dark-cycles (Hanai & Ishida, 2009; Rieger, Stanewsky & Helfrich-Förster, 2003; Schlichting et al., 2014). In mammals, a special Rhodopsin, Melanopsin, that has all the characteristics of an invertebrate Rhodopsin, has a prominent role in synchronizing the circadian clock (reviewed in Lucas, 2013). In the fly, Melanopsin is not present but the existence of a further Rhodopsin was predicted from a study showing that flies that lack all Rhodopsins plus the blue-light photopigment Cryptochrome still respond to light by decreasing activity in the morning, even though the presence of this Rhodopsin alone was not sufficient to entrain the endogenous clock to the light-dark cycles (Helfrich-Förster et al., 2001).
Indeed, when the genome of the fruit fly Drosophila melanogaster was published in the year 2000, a novel Rhodopsin gene appeared. This gene CG5638 was then called Rhodopsin 7 (Rh7) due to the sequence similarity to other Rhodopsin genes. In this study, we tested the structural and evolutionary characteristics of Rh7 and compared it with other known Rhodopsins to get a first idea about its putative function. Not to be biased by putative pseudogenes, we compared the sequences of the Rhodopsin proteins and not those of the genes.
Materials and Methods
Protein sequences
All Drosophila Rhodopsin sequences were obtained from Flybase (http://www.flybase.org; FB2016_02, released March 30, 2016). To calculate the E-values, the Flybase BLAST (http://flybase.org/blast/; blastp 2.2.18 Mar-02-2008) was used (Altschul et al., 1997).
Based on annotated protein databases and the blastp program in NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi), we identified putative homologues for the Drosophila Rhodopsins and for other opsins. Putative homologues, especially from Rh7, were re-blasted in http://flybase.org/blast/ to confirm that they are indeed homologues of the Drosophila Rhodopsins. All Protein sequences were then exported and characterized in GENtle V.1.9.4. (http://gentle.magnusmanske.de).
Sequence identities and similarities
Sequence identities and similarities were calculated with Sequence Identities and Similarities (SIAS) (SIAS, April, 2008; http://imed.med.ucm.es/Tools/sias.html). For the similarity calculation, the default parameters offered by SIAS were chosen, in which all positively charged amino acids (Arg, Lys, and His), all negatively charged amino acids (Asp and Glu), and all aliphatic amino acids (Val, Iso, and Leu) were considered as similar. Additionally the aromatic amino acids Phe, Tyr, and Trp, the polar amino acids Asn and Gln, and the small amino acids Ala, Thr, and Ser were treated as similar. To calculate the normalized similarity score, the BLOSUM62 matrix was chosen. Protein sequences were aligned with the Clustal-W algorithm in the GENtle software.
Phylogeny analyses
Multiple alignments were then exported to Phylogeny.fr (http://www.phylogeny.fr/). Phylogenic tree analyses were then performed using the One Click method. In this method the MUSCLE 3.8.31 multiple alignment, the Gblocks 0.91 b Alignment refinement, the PhyML 3.1/3.0 aLRT Phylogeny (using the maximum likelihood), and the TreeDyn 198.3 tree rendering are used by default (Dereeper et al., 2008; Dereeper et al., 2010).
Further in silico analysis
The hydrophobicity analyses were performed with PolyPhobius (http://phobius.sbc.su.se/poly.html), a combined transmembrane topology and signal peptide predictor (Käll, Krogh & Sonnhammer, 2005). To find out whether Rhodopsin Core Sequence I (RCSI) is located in the genome of different Drosophila species, the sequence of the genomic region was obtained from http://flybase.org/. All further characterizations were performed using the GENtle software.
qPCR
To test whether further Rh7 isoforms exist in D. melanogaster, qPCR analysis was performed. Total RNA was extracted from the retinas of 3–4 wildtype flies (strain CantonS) using the Quick-RNA™ MicroPrep Kit from Zymo Research and reversely transcribed using the Qiagen QuantiTect Reverse Transcription Kit. qPCRs were then carried out with the Bioline SensiFAST SYBR No-ROX Kit in combination with the Qiagen Rotor-Gene Q machine and 0.1 μM PCR primers. For each primer pair, three biological replicates were examined and for each replicate two qPCRs were run. The relative mRNA levels were calculated by using the ΔCT–equation and alpha-tubulin was used as the reference gene. The following genes and primers (sequences 5’–3’) were used:
Rh7 ex2-3: ACCACGACAAGCACGTGAATG; TGGCTCGAACTGTAGCCAAAAG Rh7 hyp(a): GGCTTATGAAGTTGCAAAAAGAATTC; TGGCTCGAACTGTAGCCAAAAG
Rh7 hyp(b): CAATACAATGTCAAATAGTTCTGAAACCA; TGGCTCGAACTGTAGCCAAAAG
Alpha-tub: TCTGCGATTCGATGGTGCCCTTAAC; GGATCGCACTTGACCATCTGGTTGGC
Results
Sequence homology
To inquire into the protein sequence conservation between Rh7 and all other Rhodopsins BLAST analyses with corresponding protein sequences were performed. This analysis revealed that the presumed Rh7 protein is closely related to other known Drosophila Rhodopsins. Expectation (e)-values are the most reliable and sensitive indicator of likely sequence homology. A similarity score of e < 0.01 or e < 0.001 simply says that this score should occur by chance once in 100 or once in 1,000 database searches, respectively (Pearson, 2016). Rh7 had the following e-values, when compared to the different Rhodospins: 1.7e−46 for Rh1, 7.2e−49 for Rh2, 4.9e−58 for Rh3, 2.9e−55 for Rh4, 7.8e−52 for Rh5, and 1.6e−41 for Rh6 were obtained and revealed that Rh3, Rh4, and Rh5 are the closest relatives of Rh7 (Table 1; Fig. 2). All three Rhodopsins displayed one common feature: they were all expressed in the inner receptor cells of the compound eye.
Table 1. Sequence identities between Rhodopsins in percentage.
Pairwise comparison of amino acid sequences of Rhodopsins (http://imed.med.ucm.es/Tools/sias.html). Rhodopsins from the same group exhibit very strong identities about 70% and Rhodopsins from different groups exhibit about 30% identities. All Drosophila Rhodopsins are related to vertebrate Melanopsins and have amino acid identities of about 30%. However, Rh1, Rh2, and Rh6 are the most similar Rhodopsins to Melanopsin. Rh7 is the farthermost, when compared with Bos taurus melanopsin.
| Dmel Rh1 | 100% | ||||||||
| Dmel Rh2 | 69.16% | 100% | |||||||
| Dmel Rh3 | 35.38% | 34.38% | 100% | ||||||
| Dmel Rh4 | 35.12% | 35.71% | 73.01% | 100% | |||||
| Dmel Rh5 | 32.17% | 32.28% | 40.83% | 44.44% | 100% | ||||
| Dmel Rh6 | 51.76% | 51.21% | 31.16% | 32.79% | 30.35% | 100% | |||
| Dmel Rh7 | 29.75% | 28.87% | 30.28% | 30.42% | 30.36% | 27.1% | 100% | ||
| Btau Opsin1 | 24.71% | 24.42% | 22.7% | 23.56% | 22.41% | 25% | 23.27% | 100% | |
| Btau Melanopsin | 31.09% | 30.44% | 28.45% | 29.89% | 27.22% | 31.7% | 24.47% | 25.57% | 100% |
| Dmel Rh1 | Dmel Rh2 | Dmel Rh3 | Dmel Rh4 | Dmel Rh5 | Dmel Rh6 | Dmel Rh7 | Btau Opsin1 | Btau Melanopsin |
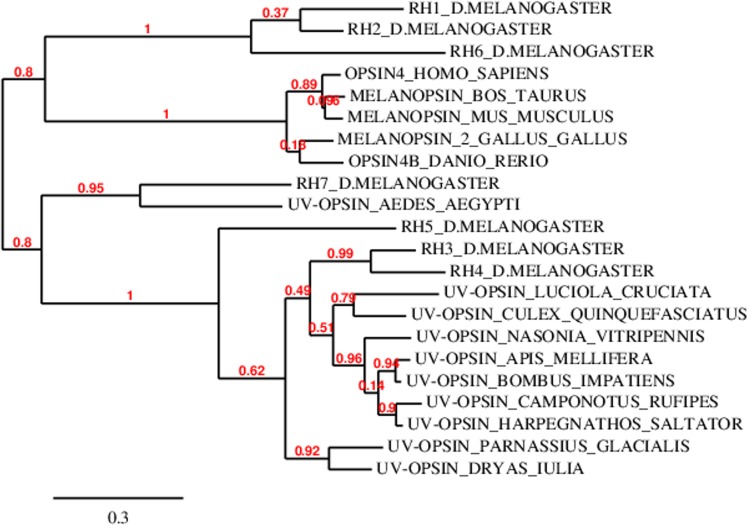
Figure 2. Phylogenic analysis of Drosophila Rhodopsins and other randomly chosen opsins from other animals.
While Rh1, Rh2, and Rh6 form a cluster with vertebrate melanopsins, Rh3, Rh4, Rh5, and Rh7 form an insect specific cluster. The branch length is proportional to the number of substitutions per site. Branch support values are given as red numbers (with 1 as the highest value).
In the next step, the amino acid sequence of Rh7 was compared with all other Drosophila Rhodopsins as well as with the vertebrate Rhodopsin and Melanopsin of Bos Taurus (Table 1). The protein sequence of Rh7 is very similar to that of other Drosophila Rhodopsins (Fig. S1; Table 1): about 30% of Rh7 the amino acids were identical to amino acids of other Drosophila Rhodopsins (Rh1: 29.75%, Rh2: 28.87%, Rh3: 30.28%, Rh4: 30.42%, Rh5: 30.36%, and Rh6: 26.51%) and about 24% to vertebrate opsins (Rhodopsin: 23.27%, Melanopsin: 24.47%) (Table 1). Sequence identity between the other Drosophila Rhodopsins was variable and ranged from ∼30 to 69% (Table 1). Similarly to Rh7, the sequence identity between the other Drosophila Rhodopsins and vertebrate opsins was lower (between 22.4 and 31.1%) than within the Drosophila Rhodopsins.
Phylogenetic analyses
Phylogenetic analysis of protein sequences of all Drosophila Rhodopsin yielded a tree with two large clades with Rh1, Rh2, and Rh6 forming the first cluster, and Rh3, Rh4, Rh5, and Rh7 forming the second cluster. Although all Drosophila Rhodopsins are related to vertebrate melanopsins, Rh1, Rh2, and Rh6 are the closest relatives, while Rh3, Rh4, and Rh5 are closer related to other insect-type opsins. Rh7, although in the same cluster with Rh3, Rh4, and Rh5, is outlying when compared with the other three Rhodopsins (Fig. 2).
To investigate the evolutionary role of Rh7, we searched for putative Rh7 homologues in all so far sequenced organisms. Putative Rh7 homologues were found in the phylum of arthropods. A second phylogenetic analysis with all presumptive Rh7 homologues (see Table S1) revealed that they are in the same lineage with Rh7. This suggests the presence of a third Rhodopsin cluster: the Rh7-group (Fig. 3). Rh7 homologues are namely present in water fleas (Daphnia), spider mites (Tetranynchus), bugs (Cimex and Halyomorpha), butterflies (Papilio), aphids (Diuraphis and Acyrthosiphon), dragonflies (Anax, Orthertrum, etc.), sawflies (Athalia, Neodiprion), mosquitos (Anopheles, Aedes, etc.), and ‘real’ fruit flies (Ceratitis, Bactrocera). Additionally, ancient animals like the atlantic horseshoe crab Limulus polyphemus own Rh7 homologues. Some animals (e.g. aphids and water fleas) even possess more than one Rh7 relative in their genome. Other insects, such as bees and ants belonging to the derived apocrita group of hymenoptereans may have lost Rh7 during evolution, since the more basic hymenopterean sawflies (symphyta) possess Rh7. However, none of the animals living in a pelagic, aphotic environment were found to possess Rh7.
Figure 3. Phylogenetic analysis of all probable Rh7 homologues.
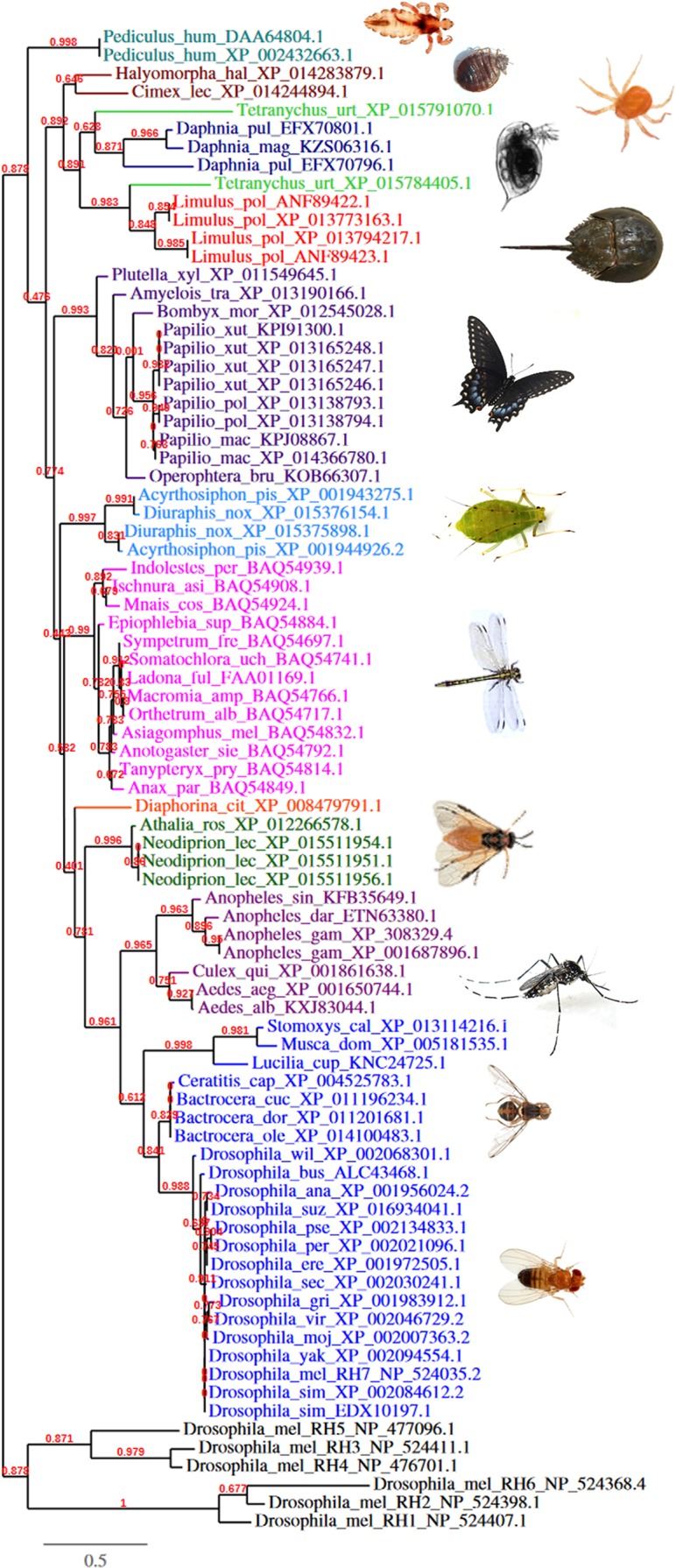
Probable Rh7 homologues were obtained by BLAST analysis with http://blast.ncbi.nlm.nih.gov/Blast.cgi (Table S1). The branch length is proportional to the number of substitutions per site. Branch support values are given as red numbers. Only proteins that cluster indeed with Rh7 and not with other Rhodopsins are shown. The phylogenetic tree reveal that Rh7 and its homologues form a third Rhodopsin cluster, when compared with other Drosophila Rhodopsins.
Structure of Rhodopsin 7
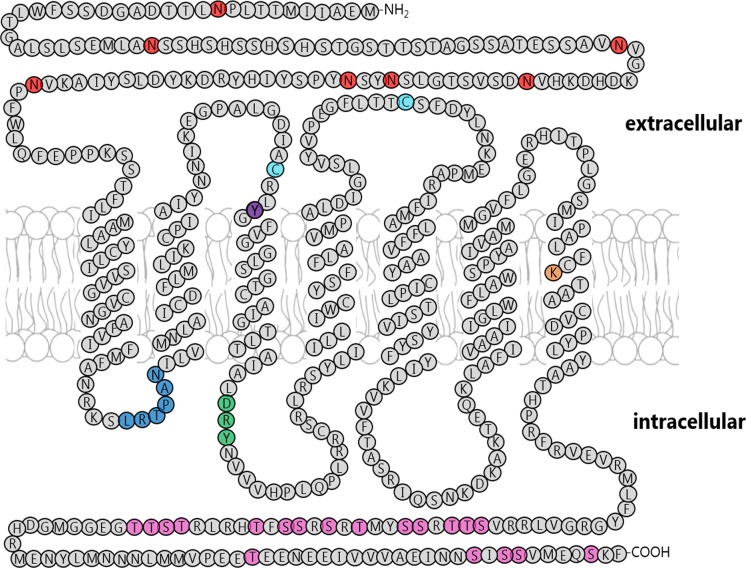
The tertiary structure of the assumed Rh7 protein is very similar to that of other Rhodopsins and contains several highly conserved features that are important for a functional protein (Figs. 4 and S1). Hydrophobicity analysis by PolyPhobius revealed that Rh7 possess seven transmembrane domains like all other Rhodopsins. Rhodopsins are characterized by an extracellular N-Terminus with many asparagines (N) that are glycosylated and an intracellular C-Terminus with several serines (S) and threonines (T) that serve as possible light-dependent phosphorylation sites for the Rhodopsin kinase (Ohguro et al., 1996; Papatsenko, Sheng & Desplan, 1997). Both tails are important for signal-transduction whereby the N-terminus tails of the different Rhodopsins differ in general very much (Fig. S3). Only the N-termini of Rh1 and Rh2, as well as the N-termini of Rh3 and Rh4 share about 50% similar amino acids. The lowest sequence similarity of 8% is found between the N-termini of Rh1 and Rh5. Rh7 has a sequence similarity of its N-terminus with the N-termini of the other Rhodopsins ranging between 12 and 22% (Fig. S3). Compared to other Rhodopsins, the N-terminus of Rh7 is very long: Rh1 has an N-terminus tail of about 50 amino acids. In Rh2 the tail contains 57 amino acids, in Rh3 58 amino acids, in Rh4 54 amino acids, in Rh5 50 amino acids, and in Rh6 45 amino acids. The N-terminus of Rh7 contains instead 115 amino acids and is therefore twice as long as the others. This is also true for the C-terminus: In Rh1 the C-terminus contains 42 amino acids, while in Rh2 it contains 43 amino acids, in Rh3 42 amino acids, in Rh4 41 amino acids, in Rh5 48 amino acids, and in Rh6 41 amino acids. In Rh7, the C-terminus is 96 amino acids long hence again twice as long as the others. The prolonged N- and C-termini lead Rh7 to possessing many more asparagines (N) in the N-terminus as well as serines (S) and threonines (T) in the C-terminus compared to other Rhodopsins.
Figure 4. Tertiary structure of the presumptive Rh7 protein.
The assumed Rh7 protein share several highly conserved features with other Rhodopsins. This includes: seven transmembrane domains, an extracellular N-Terminus, an intracellular C-Terminus, the chromophore binding lysine in the seventh transmembrane domain, the LRTPXN motif in the first cytoplasmic loop, the DRY motif in the second cytoplasmic loop, two cysteines (C) in the second and third extracellular loops, and a conserved tyrosine residue in the third transmembrane domain. However, Rh7 differs from other Rhodopsins with its extended N- and C-Termini and by the lack of the QAKK motif in the third cytoplasmic loop.
The chromophore binding lysine (K)-residue is another important feature of all Rhodopsins. This highly conserved residue is bound via a Schiff’s base linkage by the retinal chromophore. Rh7 also possess this lysine, which is found at position 374 in the seventh transmembrane domain.
The LRTPXN motif in the first cytoplasmic loop, of which the leucine (L) and asparagine (N) are necessary for the formation of a functional Rhodopsin is also found in Rh7. Rh7 also owns the DRY motif in the second cytoplasmic loop. This motif is necessary for the interaction with the G-protein (Franke et al., 1992) as well as the two cysteines (C) in the second and third extracellular loop that form a disulfide bond (Maden, 1995). Rh1 possess a conserved tyrosine (Y) residue three amino acids apart from the first cysteine in the third transmembrane domain. This tyrosine can also be found in all other Rhodopsins that detect visible light i.e. Rh2, Rh5, and in Rh6, while this residue is changed by a phenylalanine (F) in UV-light sensitive Rh3 and Rh4 (Chou et al., 1996). In this position, Rh7 possesses a tyrosine like visible light detecting Rhodopsins, thus suggesting that Rh7 also respond to visible light.
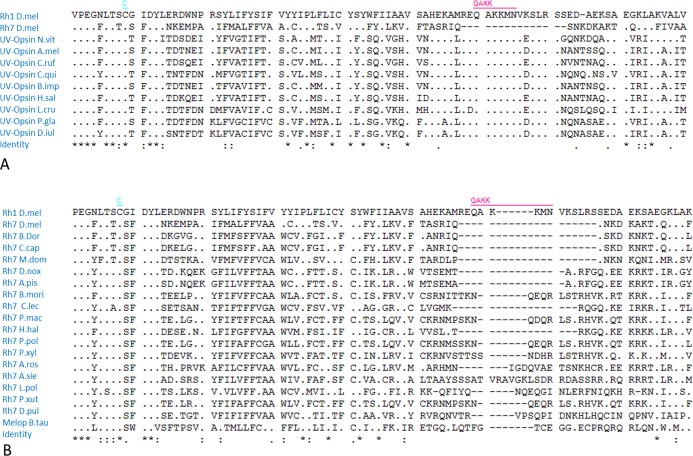
The Rh7 sequence strongly differs from all other known insect rhodopsins in the third cytoplasmic loop. This part of the protein has the conserved QAKK motif, which mediates the interaction with the G-protein along with the DRY motif in the second cytoplasmic loop. Rh7 lacks the QAKK motif in its third cytoplasmic loop. Although the QAKK motif is highly conserved in many so far characterized insect opsins (Fig. 5A), it is absent in vertebrate opsins (e.g. the Bos taurus opsin), as well as in all insect opsins that cluster with Drosophila Rh7 (Fig. 5B).
Figure 5. Multiple alignment of the amino acid sequence of diverse Opsin proteins.
(A) The primary structure of known Opsins reveal that the QAKK domain is highly conserved among insect Opsins, but missing in Drosophila Rh7. (B) However all homologues of the Drosophila Rh7 as well as vertebrate melanopsins lack this motif.
Rh7 locus
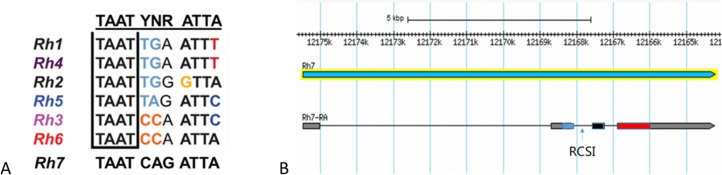
To search for further conserved Rhodopsin regions, we examined its genomic region. All Drosophila genes coding for proteins that are involved in the phototransduction cascade, such as Rhodopsins, G-proteins, arrestins, TRP and TRPL ion channels, scaffolding proteins, phospholipase C, etc. possess a conserved cis-regulatory element with the nucleotide sequence TAATYNRATTA, called RCSI, about 100 bps upstream of the transcription start site (Rister et al., 2015). While this sequence is palindromic in phototransduction genes that are broadly expressed in each photoreceptor cell, the last nucleotide differs in Rhodopsin genes, indicating their receptor cell-specific expression. An exception is Rh6, in which the ending is still “ATTA,” but the middle sequences differ from the broadly expressed phototransduction genes. Rh7 also possesses a RCSI with the nucleotide sequence TAATCAGATTA (Fig. 6A) which is palindromic like that of broadly expressed phototransduction genes and thereby differs from other Rhodopsin genes. In addition, instead of being located 100 bps upstream of the transcription start site, the RCSI is found within the second intron of the Rh7 gene (Fig. 6B).
Figure 6. The cis-regulatory Rhodopsin Core Seqeunce I (RCSI) is highly conserved in the Rhodopsin gene locus.
(A) Like all other Drosophila Rhodopsin genes, Rh7 also possess the RCSI motif. (B) The RCSI motif of Rh7 is located in the second intron of the Rh7 gene and not 100 bps upstream from the transcription start site.
To investigate whether Rh7 may have lost its original function in the course of evolution and is no longer expressed in the photoreceptor cells of Drosophila melanogaster, which is suggested by the RCSI no longer appearing 100 bp before the transcription start site, we screened the genomic region of twelve other sequenced Drosophila species for the RCSI. Five out them (D. melanogaster, D. simulans, D. sechellia, D. yakuba, and D. erecta) possess the RCSI in the Rh7 gene locus. In D. ananassae the sequence seem to be bit modified and all other species (D. pseudoobscura pseudoobscura, D. persimilis, D. willistoni, D. mojavensis, D. virilis, and D. grimshawi) did not exhibit the RCSI. Thus, the expression pattern of Rh7 might have indeed changed during evolution.
In all species that possess the RCSI, this motif is located in an intron of the Rh7 gene and not upstream of the putative transcription start site. Most interestingly, the RCSI motif is always followed by the next exon starting with the coding sequence NYSNY and the Rh7 amino acid sequences of the different Drosophila species are highly conserved downstream of the NYSNY sequence, whereas they are highly variable upstream of it (Figs. S2 and S3). This suggests that the real transcription start site might not locate upstream but downstream the RCSI motif as in the other Rhodopsins. Indeed, there are two hypothetical translation start sites in the region downstream the RCSI. If translation starts at one of these sites, the N-terminus of Rh7 will be only ∼50 amino acids long and will show a higher homology to the N-termini of the other Rhodopsins. To test this possibility, we performed qPCR analysis on D. melanogaster retinas with primers in the region of the intron containing the RCSI motif. However, we obtained only PCR products for the previously described Rh7 isoform with the extended and non-conserved N-terminus and not for the hypothetical ones (Fig. S4). This does not only show that the shorter isoforms of Rh7 are not existent, but also that Rh7 with the long N-terminus tail is indeed expressed in the retina, suggesting that the RCSI is functional in D. melanogaster.
Discussion
The six previously identified Drosophila Rhodopsins fall into two groups: the vertebrate-melanopsin-type-opsins, and the insect-type-opsins. Whereas Rh3, Rh4, and Rh5 are very similar to other insect-opsins (e.g. UV-opsin Apis mellifera), Rh1, Rh2, and Rh6 are more closely related to vertebrate-melanopsins. The sequence similarity between Rhodopsins belonging to the same group is very high. Rh1 shares 69.16% of its amino acids with Rh2, and 51.65% with Rh6, while Rh3 is 73.01% identical to Rh4 and 40.83% to Rh5.
However, Rh7 only shares about 30% of its amino acids with other Drosophila Rhodopsins. This seems to be a small overlap compared to other Rhodopsin identities from the same group. However, even members from the two known groups only share 30–35% of their amino acids (Rh1, Rh2, and Rh6 compared with Rh3, Rh4, and Rh5). This already indicates that Rh7 might belong to a third, so far uncharacterized, Rhodopsin group.
Phylogenic tree analyses support the idea that Rh7 might belong to a novel Rhodopsin group. As expected Rh1, Rh2, and Rh6 cluster together with vertebrate melanopsins, while Rh3, Rh4, and Rh5 cluster with other insect opsins. Rh7 though, despite related to the insect-type-opsin group, seems to form its own unique group: the “Rh7-like” group. The fact that Rh7 homologues are already present in ancient animals like Limulus polyphemus and Daphnia pulex indicates that the Rh7 gene is indeed a conserved protein coding gene, otherwise random mutations would have appeared in the coding region of the gene. However, like all Rhodopsin genes, the Rh7 gene also seems to be duplicated in some animals. Both aphids as well as Daphnia possess more than one Rh7 relative in their genome, yet whether these multiple replicates are real protein coding genes or merely pseudogenes remains to be studied. Although many different arthropods possess Rh7 homologues, hymenopterans belonging to the group of apocrita for example don’t possess Rh7 homologues. Since Athalia rosae and Neodiprion lecontei having its place within the symphyta (sawflies), the more basic group of hymenoptera, still possess Rh7, it is likely that the apocrita may have lost Rh7 during evolution due to different habituation.
Although the protein structure of Rh7 is very similar to all other known Rhodopsins, it slightly differs from the other Drosophila Rhodopsins. With its prolonged N- and C-termini, Rh7 possesses many more asparagines (N), serines (S) and threonines (T) than other Rhodopsins do. All these amino acids are important for protein-protein-interaction, thus Rh7 may interact with more and other interaction partners as compared to the known Drosophila Rhodopsins. In addition, it is notable that the N-termini of all Drosophila Rhodopsins differ a lot, when compared to each other (Fig. S3). This suggests that the N-terminus of each Rhodopsin has unique properties. Rh7 has the most conspicuous N-terminus. It is not only long, but has also the highest potency to undergo interactions with other proteins, perhaps even with other Rhodopsins. G-protein-coupled receptors (GPCRs) including Rhodopsin-like GPCRs can form dimers (Gurevich & Gurevich, 2008a; Gurevich & Gurevich, 2008b; Hiller, Kühhorn & Gmeiner, 2013). This dimerization seems not necessary for G-protein binding and activation, but it seems to influence signaling in the phototransduction cascade (White et al., 2007).
The assumption that Rh7 may interact with another Rhodopsin to exert its function is supported by the fact that Rh7 and its homologues miss the highly conserved QAKK motif, which is, together with the DRY motif, important for G-protein binding and consequently for the activation of the G-protein-coupled cascade. Thus, Rh7 might signal via a second Rhodopsin and not by itself. The presence of more than one Rhodopsin in one receptor cell has been shown in vertebrates and invertebrates (Applebury et al., 2000; Hu et al., 2014; Mazzoni, Desplan & Çelik, 2004; Stavenga & Arikawa, 2008) and makes this possible. Nevertheless, it is also imaginable that the conserved DRY motif alone, that is present in Rh7, suffices for G-protein binding. The latter is suggested by a study in the mosquito Aedes aegypti (Hu et al., 2014) showing that the Rh7 homologue of the mosquito, Op10, is a functional Rhodopsin although it lacks the QAKK motif. Op10 can even activate the phototransduction cascade in R1–6 of D. melanogaster. This suggests that G-protein binding is still possible even in the absence of the QAKK motif, but it does not exclude the possibility that Rh7 dimerizes with a second Rhodopsin and by this way affects signaling.
The distinctiveness of Rh7 is also confirmed by its genomic structure: first it possesses a palindromic RCSI, which is atypical for Rhodopsins, and, second, it possesses the RCSI in the middle of the gene while all other Rhodopsin genes possess the RCSI about 100 bp upstream of the transcription starting site. The hypothesis that the crucial role of Rh7 may have been lost in D. melanogaster during evolution, but may still present in other Drosophila species living in different habitats, is unsupported by the fact that some of the tested Drosophila species don’t even own the RCSI in their genomic locus. These results might indicate that Rh7 is differently activated by other transcription factors than other Rhodopsin genes and that it could therefore be expressed in other tissues and developmental stages than other known Rhodopsins. Nevertheless, our qPCR results show that Rh7 with the long N-terminus tail is expressed in the retina of D. melanogaster.
In summary, we show here that Rh7 is a peculiar Rhodopsin with unusual properties. Its presence in most groups of arthropods, excluding those living in aphotic environments, suggests that it has a conserved function in light-signaling either as visual or non-visual Rhodopsin. Rh7 might play a role in basic insect circadian photoreception and thus play a similar role in insects as melanopsin plays in mammals. It will be most promising to unravel this putative function of Rh7 by using the arsenal of genetic techniques available in D. melanogaster. We are currently pursuing this endeavor.
Conclusion
Rh7 seems to be an ancient type of Rhodopsin, which is conserved among different arthropods but lost in other. Rh7 possess nearly all important features for a functional visual protein, but it differs from other known Rhodopsins in its genomic characteristics, suggesting that Rh7 may have a different role than other known Rhodopsins. The palindromic RCSI in the first intron, which is even lost in some species suggests that Rh7 is transcribed differently from other Rhodopsins. In addition, the structural properties of Rh7 suggest that it might undergo different pathways compared to all other known Rhodopsins.
Supplemental Information
The transmembrane domains are shown in grey, the LRTPXN motif is shown in blue, the disulfide bridge binding cysteines are shown in cyan, the visual light sensitive tyrosine is shown purple, the DRY motif is shown in green, the chromophore binding lysine is shown in orange, and the proper location of the QAKK motif is shown magenta.
The N-termini of all Drosophila Rhodopsins were aligned. The amino acid sequences before the first transmembrane domain starts were extracted for the alignment. The N-termini of each Rhodopsin seem to be kept very variable.
BLAST analysis was performed in http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins using the Rh7 protein sequence.
Pairwise comparison of amino acid sequences of Rhodopsins (http://imed.med.ucm.es/Tools/sias.html). The N-terminus of all Rhodopsins, with the exception of Rh1/Rh2 and Rh3/Rh4, differ a lot, while the whole protein identities (Table 1) are at least about 30% similar.
Acknowledgments
We are grateful to Rudi Grebler for fruitful discussions and for providing pictures. We like to thank Marta Beauchamp and Pamela Menegazzi for their constructive comments and suggestions.
Funding Statement
This study was funded by the German Research Foundation (DFG), Collaborative Research Center SFB 1047 “Insect Timing” Project A2. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Pingkalai R. Senthilan conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Charlotte Helfrich-Förster contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Data Deposition
The following information was supplied regarding data availability:
All Drosophila Rhodopsin sequences were obtained from Flybase (www.flybase.org; FB2016_02, released March 30, 2016).
References
- Altschul et al. (1997).Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, Robison & Gilbert (1996).Alvarez CE, Robison K, Gilbert W. Novel Gq alpha isoform is a candidate transducer of rhodopsin signaling in a Drosophila testes-autonomous pacemaker. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(22):12278–12282. doi: 10.1073/pnas.93.22.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebury et al. (2000).Applebury ML, Antoch MP, Baxter LC, Chun LLY, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27(3):513–523. doi: 10.1016/S0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Behnia & Desplan (2015).Behnia R, Desplan C. Visual circuits in flies: beginning to see the whole picture. Current Opinion in Neurobiology. 2015;34:125–132. doi: 10.1016/j.conb.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt et al. (1993).Britt SG, Feiler R, Kirschfeld K, Zuker CS. Spectral tuning of rhodopsin and metarhodopsin in vivo. Neuron. 1993;11(1):29–39. doi: 10.1016/0896-6273(93)90268-V. [DOI] [PubMed] [Google Scholar]
- Chou et al. (1996).Chou W-H, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17(6):1101–1115. doi: 10.1016/S0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Chou et al. (1999).Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126(4):607–616. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- Dereeper et al. (2010).Dereeper A, Audic S, Claverie J-M, Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evolutionary Biology. 2010;10(1):8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper et al. (2008).Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J-F, Guindon S, Lefort V, Lescot M, Claverie J-M, Gascuel O. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research. 2008;36(suppl 1):W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary et al. (1987).Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(19):6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke et al. (1992).Franke RR, Sakmar TP, Graham RM, Khorana HG. Structure and function in rhodopsin. Studies of the interaction between the rhodopsin cytoplasmic domain and transducin. Journal of Biological Chemistry. 1992;267(21):14767–14774. [PubMed] [Google Scholar]
- Grebler (2015).Grebler R. Dissertation. Universität Würzburg; 2015. Investigation of Rhodopsin 7 and Cryptochrome in Drosophila melanogaster vision. [Google Scholar]
- Gurevich & Gurevich (2008a).Gurevich VV, Gurevich EV. GPCR monomers and oligomers: it takes all kinds. Trends in Neurosciences. 2008a;31(2):74–81. doi: 10.1016/j.tins.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich & Gurevich (2008b).Gurevich VV, Gurevich EV. Rich tapestry of G protein-coupled receptor signaling and regulatory mechanisms. Molecular Pharmacology. 2008b;74(2):312–316. doi: 10.1124/mol.108.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai & Ishida (2009).Hanai S, Ishida N. Entrainment of Drosophila circadian clock to green and yellow light by Rh1, Rh5, Rh6 and CRY. Neuroreport. 2009;20(8):755–758. doi: 10.1097/WNR.0b013e32832a7c4e. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster (2014).Helfrich-Förster C. From Neurogenetic studies in the fly brain to a concept in circadian biology. Journal of Neurogenetics. 2014;28(3–4):329–347. doi: 10.3109/01677063.2014.905556. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster et al. (2001).Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30(1):249–261. doi: 10.1016/S0896-6273(01)00277-X. [DOI] [PubMed] [Google Scholar]
- Hiller, Kühhorn & Gmeiner (2013).Hiller C, Kühhorn J, Gmeiner P. Class A G-protein-coupled receptor (GPCR) dimers and bivalent ligands. Journal of Medicinal Chemistry. 2013;56(17):6542–6559. doi: 10.1021/jm4004335. [DOI] [PubMed] [Google Scholar]
- Hofbauer & Buchner (1989).Hofbauer A, Buchner E. Does Drosophila have seven eyes? Naturwissenschaften. 1989;76(7):335–336. doi: 10.1007/BF00368438. [DOI] [Google Scholar]
- Hu et al. (2014).Hu X, Leming MT, Whaley MA, O’Tousa JE. Rhodopsin coexpression in UV photoreceptors of Aedes aegypti and Anopheles gambiae mosquitoes. Journal of Experimental Biology. 2014;217(6):1003–1008. doi: 10.1242/jeb.096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber et al. (1997).Huber A, Schulz S, Bentrop J, Groell C, Wolfrum U, Paulsen R. Molecular cloning of Drosophila Rh6 rhodopsin: the visual pigment of a subset of R8 photoreceptor cells. FEBS Letters. 1997;406(1–2):6–10. doi: 10.1016/S0014-5793(97)00210-X. [DOI] [PubMed] [Google Scholar]
- Käll, Krogh & Sonnhammer (2005).Käll L, Krogh A, Sonnhammer ELL. An HMM posterior decoder for sequence feature prediction that includes homology information. Bioinformatics. 2005;21(Suppl 1):i251–i257. doi: 10.1093/bioinformatics/bti1014. [DOI] [PubMed] [Google Scholar]
- Kiselev & Subramaniam (1994).Kiselev A, Subramaniam S. Activation and regeneration of rhodopsin in the insect visual cycle. Science. 1994;266(5189):1369–1373. doi: 10.1126/science.7973725. [DOI] [PubMed] [Google Scholar]
- Lucas (2013).Lucas RJ. Mammalian inner retinal photoreception. Current Biology. 2013;23(3):R125–R133. doi: 10.1016/j.cub.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Maden (1995).Maden BE. Opsin genes. Essays in Biochemistry. 1995;29:87–111. [PubMed] [Google Scholar]
- Mazzoni, Desplan & Çelik (2004).Mazzoni EO, Desplan C, Çelik A. ‘One receptor’ rules in sensory neurons. Developmental Neuroscience. 2004;26(5–6):388–395. doi: 10.1159/000082281. [DOI] [PubMed] [Google Scholar]
- Nikolic et al. (2010).Nikolic K, Loizu J, Degenaar P, Toumazou C. A stochastic model of the single photon response in Drosophila photoreceptors. Integrative Biology. 2010;2(7–8):354–370. doi: 10.1039/c0ib00031k. [DOI] [PubMed] [Google Scholar]
- O’Tousa et al. (1985).O’Tousa JE, Baehr W, Martin RL, Hirsh J, Pak WL, Applebury ML. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40(4):839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- Ohguro et al. (1996).Ohguro H, Rudnicka-Nawrot M, Buczyłko J, Zhao X, Taylor JA, Walsh KA, Palczewski K. Structural and enzymatic aspects of rhodopsin phosphorylation. Journal of Biological Chemistry. 1996;271(9):5215–5224. doi: 10.1074/jbc.271.9.5215. [DOI] [PubMed] [Google Scholar]
- Papatsenko, Sheng & Desplan (1997).Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124(9):1665–1673. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- Pearson (2016).Pearson WR. Finding protein and nucleotide similarities with FASTA. Current Protocols in Bioinformatics. 2016;24(53):3.9.1–3.9.25. doi: 10.1002/0471250953.bi0309s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger, Stanewsky & Helfrich-Förster (2003).Rieger D, Stanewsky R, Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. Journal of Biological Rhythms. 2003;18(5):377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- Rister, Desplan & Vasiliauskas (2013).Rister J, Desplan C, Vasiliauskas D. Establishing and maintaining gene expression patterns: insights from sensory receptor patterning. Development. 2013;140(3):493–503. doi: 10.1242/dev.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister et al. (2015).Rister J, Razzaq A, Boodram P, Desai N, Tsanis C, Chen H, Jukam D, Desplan C. Single-base pair differences in a shared motif determine differential Rhodopsin expression. Science. 2015;350(6265):1258–1261. doi: 10.1126/science.aab3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo et al. (1999).Salcedo E, Huber A, Henrich S, Chadwell LV, Chou W-H, Paulsen R, Britt SG. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. Journal of Neuroscience. 1999;19(24):10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting et al. (2014).Schlichting M, Grebler R, Peschel N, Yoshii T, Helfrich-Förster C. Moonlight detection by Drosophila’s endogenous clock depends on multiple photopigments in the compound eyes. Journal of Biological Rhythms. 2014;29(2):75–86. doi: 10.1177/0748730413520428. [DOI] [PubMed] [Google Scholar]
- Senthilan et al. (2012).Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, Pauls S, Winkler M, Möbius W, Howard J, Göpfert MC. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150(5):1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2011).Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331(6022):1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- Sprecher & Desplan (2008).Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454(7203):533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga & Arikawa (2008).Stavenga DG, Arikawa K. One rhodopsin per photoreceptor: Iro-C genes break the rule. PLoS Biology. 2008;6(4):e2427. doi: 10.1371/journal.pbio.0060115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White et al. (2007).White JF, Grodnitzky J, Louis JM, Trinh LB, Shiloach J, Gutierrez J, Northup JK, Grisshammer R. Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12199–12204. doi: 10.1073/pnas.0705312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama & Meinertzhagen (1999).Yasuyama K, Meinertzhagen IA. Extraretinal photoreceptors at the compound eye’s posterior margin in Drosophila melanogaster. Journal of Comparative Neurology. 1999;412(2):193–202. doi: 10.1002/(SICI)1096-9861(19990920)412:2<193::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zuker, Cowman & Rubin (1985).Zuker CS, Cowman AF, Rubin GM. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The transmembrane domains are shown in grey, the LRTPXN motif is shown in blue, the disulfide bridge binding cysteines are shown in cyan, the visual light sensitive tyrosine is shown purple, the DRY motif is shown in green, the chromophore binding lysine is shown in orange, and the proper location of the QAKK motif is shown magenta.
The N-termini of all Drosophila Rhodopsins were aligned. The amino acid sequences before the first transmembrane domain starts were extracted for the alignment. The N-termini of each Rhodopsin seem to be kept very variable.
BLAST analysis was performed in http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins using the Rh7 protein sequence.
Pairwise comparison of amino acid sequences of Rhodopsins (http://imed.med.ucm.es/Tools/sias.html). The N-terminus of all Rhodopsins, with the exception of Rh1/Rh2 and Rh3/Rh4, differ a lot, while the whole protein identities (Table 1) are at least about 30% similar.