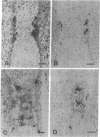
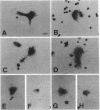
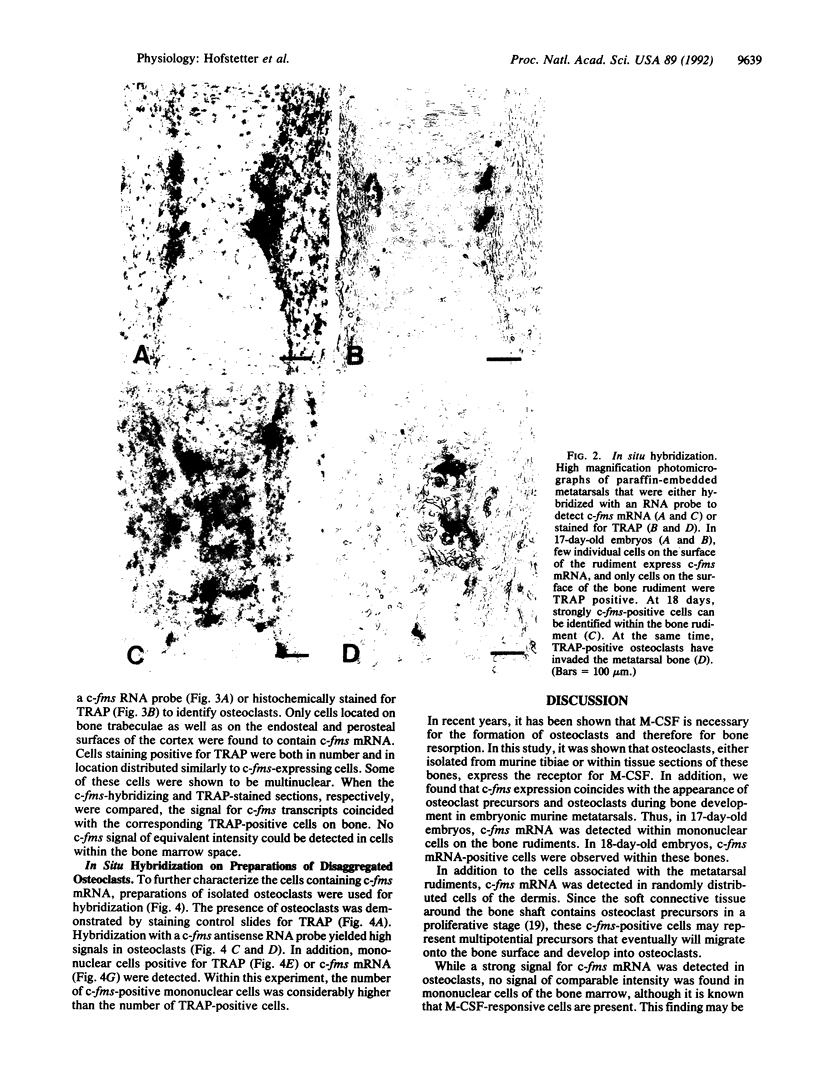
Abstract
Macrophage colony-stimulating factor (M-CSF), whose action is restricted to the cell populations of the mononuclear phagocyte system, has recently been found to be required for osteoclastogenesis and bone resorption. To investigate the cells involved in the action of M-CSF in these processes, expression of c-fms mRNA, encoding the M-CSF receptor, was studied by in situ hybridization. Paws from murine embryos and newborn mice, tibiae from 2-day-old animals, as well as isolated osteoclasts, were hybridized with a c-fms-specific RNA probe. In bone, c-fms mRNA was detected only in cells at the late stages of osteoclastogenesis and in mature osteoclasts. The findings strengthen the relation between osteoclasts and the mononuclear phagocyte system. Furthermore, they suggest that M-CSF acts directly on osteoclast precursors and on mature osteoclasts during osteoclastogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athanasou N. A., Quinn J. Immunophenotypic differences between osteoclasts and macrophage polykaryons: immunohistological distinction and implications for osteoclast ontogeny and function. J Clin Pathol. 1990 Dec;43(12):997–1003. doi: 10.1136/jcp.43.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelmez S. H., Sacca R., Stanley E. R. Lineage specific receptors used to identify a growth factor for developmentally early hemopoietic cells: assay of hemopoietin-2. J Cell Physiol. 1985 Mar;122(3):362–369. doi: 10.1002/jcp.1041220305. [DOI] [PubMed] [Google Scholar]
- Bartocci A., Mastrogiannis D. S., Migliorati G., Stockert R. J., Wolkoff A. W., Stanley E. R. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6179–6183. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Dunn C. J. The effect of parathyroid hormone, 1,25-dihydroxycholecalciferol and prostaglandins on the cytoplasmic activity of isolated osteoclasts. J Pathol. 1982 Jul;137(3):193–203. doi: 10.1002/path.1711370304. [DOI] [PubMed] [Google Scholar]
- Corboz V. A., Cecchini M. G., Felix R., Fleisch H., van der Pluijm G., Löwik C. W. Effect of macrophage colony-stimulating factor on in vitro osteoclast generation and bone resorption. Endocrinology. 1992 Jan;130(1):437–442. doi: 10.1210/endo.130.1.1727717. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Hofstetter W., Elford P. R., Stutzer A., Fleisch H. Impairment of macrophage colony-stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res. 1990 Jul;5(7):781–789. doi: 10.1002/jbmr.5650050716. [DOI] [PubMed] [Google Scholar]
- Gordon S., Crocker P. R., Morris L., Lee S. H., Perry V. H., Hume D. A. Localization and function of tissue macrophages. Ciba Found Symp. 1986;118:54–67. doi: 10.1002/9780470720998.ch5. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Dorey E., Horton M. A., Chambers T. J. Human macrophage colony-stimulating factor inhibits bone resorption by osteoclasts disaggregated from rat bone. J Cell Physiol. 1988 Oct;137(1):199–203. doi: 10.1002/jcp.1041370125. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Kerby J. A., Chambers T. J. Identification of osteoclast precursors in multilineage hemopoietic colonies. Endocrinology. 1991 Jan;128(1):259–262. doi: 10.1210/endo-128-1-259. [DOI] [PubMed] [Google Scholar]
- Hogg N., Shapiro I. M., Jones S. J., Slusarenko M., Boyde A. Lack of Fc receptors on osteoclasts. Cell Tissue Res. 1980;212(3):509–516. doi: 10.1007/BF00236514. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Loutit J. F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984 Mar;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. J., Simmons D. J. Investigation of cell lineage in bone using a chimaera of chick and quial embryonic tissue. Nature. 1975 Nov 27;258(5533):325–327. doi: 10.1038/258325a0. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N., Civin C., Roodman G. D. Osteotropic factor responsiveness of highly purified populations of early and late precursors for human multinucleated cells expressing the osteoclast phenotype. J Bone Miner Res. 1991 Mar;6(3):257–261. doi: 10.1002/jbmr.5650060307. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Lane P. W. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976 Jan-Feb;67(1):11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Mueller C., Gershenfeld H. K., Lobe C. G., Okada C. Y., Bleackley R. C., Weissman I. L. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J Exp Med. 1988 Mar 1;167(3):1124–1136. doi: 10.1084/jem.167.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Specificity of action of colony-stimulating factors in the differentiation of granulocytes and macrophages. Ciba Found Symp. 1986;118:7–28. doi: 10.1002/9780470720998.ch2. [DOI] [PubMed] [Google Scholar]
- Regenstreif L. J., Rossant J. Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev Biol. 1989 May;133(1):284–294. doi: 10.1016/0012-1606(89)90319-9. [DOI] [PubMed] [Google Scholar]
- Rothwell V. M., Rohrschneider L. R. Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res. 1987 Sep-Oct;1(4):311–324. [PubMed] [Google Scholar]
- Scheven B. A., Burger E. H., Kawilarang-de Haas E. W., Wassenaar A. M., Nijweide P. J. Effects of ionizing irradiation on formation and resorbing activity of osteoclasts in vitro. Lab Invest. 1985 Jul;53(1):72–79. [PubMed] [Google Scholar]
- Scheven B. A., Kawilarang-De Haas E. W., Wassenaar A. M., Nijweide P. J. Differentiation kinetics of osteoclasts in the periosteum of embryonic bones in vivo and in vitro. Anat Rec. 1986 Apr;214(4):418–423. doi: 10.1002/ar.1092140413. [DOI] [PubMed] [Google Scholar]
- Scheven B. A., Visser J. W., Nijweide P. J. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986 May 1;321(6065):79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Udagawa N., Akatsu T., Tanaka H., Isogai Y., Suda T. Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology. 1991 Apr;128(4):1792–1796. doi: 10.1210/endo-128-4-1792. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Udagawa N., Akatsu T., Tanaka H., Shionome M., Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res. 1991 Sep;6(9):977–985. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Tas M. C., van der Meer J. W., Burger E. H. Growth of osteoclast precursor-like cells from whole mouse bone marrow: inhibitory effect of CSF-1. Bone Miner. 1987 Nov;3(2):97–110. [PubMed] [Google Scholar]