Abstract
Objectives:
To determine the prevalence and dose-response relationship of chronic periodontitis among smokers in Pakistan.
Methods:
This is a cross-sectional study among participants seeking dental care in Karachi Medical and Dental College, Karachi, Pakistan. A total of 443 participants with a mean age of 44.3 (±6.5) participated in the study from April 2011 to December 2011. Males comprised 64.7%, and females comprised 35.2%. Participants were interviewed on social demographics and oral habits. Participants with shallow pockets (3.5-5.5 mm) and deep pockets (>5.5 mm) were considered suffering from chronic periodontitis. The characteristics of participants were assessed using frequency distribution for categorical variables and mean (standard deviation) for continuous variables.
Results:
Among 443 participants, smokers were distributed as 55.1% and non-smokers as 44.9%. Smoking was found to be significantly related to young adults (p<0.007), male gender (p<0.001), and lower education level (p<0.01). Overall prevalence of chronic periodontitis among smokers was estimated at 81.6%. Heavy smoking was found to have significantly high prevalence (p<0.001) and severity (p<0.001) of periodontitis as compared with moderate and light smokers. The multivariate unadjusted model depicted 3.5 times higher risk of chronic periodontitis among smokers (p<0.001).
Conclusion:
Chronic periodontitis had a high prevalence among smokers. Heavy smoking was found to have a higher risk for having periodontitis.
Tobacco smoking is a global health issue. In 2010, tobacco smoking was considered as a leading risk factor for the global burden of diseases.1 It is expected that by 2030, tobacco smoking will increase annual mortality rates to 8.3 million.2 In Pakistan, the prevalence of tobacco smokers is estimated as 31.5%.3 It has been associated as a risk factor for various systemic diseases including chronic periodontitis.4 Chronic periodontitis is a recognized global public health problem. The worldwide prevalence of periodontal disease in the general adult population is 30-35%.5 According to WHO estimates, the prevalence of chronic periodontitis estimates in the Pakistani population is 30%.5 Chronic periodontitis is a result of the interplay between bacterial challenge and host immune response.6 Dental plaque is the main etiological factor for periodontal disease, which together with inflammatory and immunological response causes damage to the periodontal tissues.7 Furthermore, chronic periodontitis is found associated with several predisposing and modifying factors like local tooth factors, stress, low social, economic status, systemic diseases, alcohol and smoking.8 Smoking is a potential modifying factor for periodontal disease.9 Smoking affects by causing upheld host immune response, through alteration in neutrophil function, antibody production, fibroblast activities and vascular factors and inflammatory mediators production.10 Several studies have demonstrated epidemiological and biological plausibility between smoking and chronic periodontitis.11-13 Epidemiological studies provide evidence that smoking has a negative impact on periodontal health.14 The United States (US), National health and Nutrition Examination Survey (NHANES III)14 reported 41.9% of periodontitis was attributed to smoking, and 10.9% to former smoking. Considering these findings, we can concur that smoking is a potential risk factor for periodontitis, and its increase dosage and intensity could be a risk factor for worsened periodontal health. Hence, the current study aims to determine the prevalence of chronic periodontitis in the Pakistani population, and also to determine the dose response relationship between smoking and chronic periodontitis.
Methods
This cross-sectional study was carried out in adult population visiting the Dental Hospital, Faculty of Dentistry, Karachi Medical and Dental College, Karachi, Pakistan. The target population was general adult population, aged >18 years. The participants of the study were recruited using a randomized sampling technique, using the frame based on registration number. Informed consent was obtained from the participant after explaining the nature of the study, including the associated potential benefits and the drawbacks. The Medical Ethics Committee of the Faculty of Dentistry, Karachi Medical and Dental College approved the study. The study protocol was designed in compliance with the Declaration of Helsinki.
The inclusion criteria included participants ≥18 years of age. Exclusion criteria were as follows: participants who had received periodontal treatment in the last 4 months, participants on antibiotics within the past 4 months. Also, participants were excluded if they were pregnant women and lactating mothers, mentally handicapped, or on prophylactic antibiotics, or systemic/topical steroidal anti-inflammatory drugs for the last 4 months. Based on fulfilment of inclusion and exclusion criteria, participants were invited to participate in the study. All participants underwent an interviewed questionnaire and clinical periodontal assessment.
There were 3 calibrated interviewers, which interviewed the participants on social demographic characteristics, oral health behavior, and habits of smoking. The smokers were subcategorized into 2 categories based on the frequency of cigarettes consumed per day, namely, heavy smokers (≥5 cigarettes per day) and light/moderate smokers (<5 cigarettes per day). The medical history was obtained, which helped in precluding of participants based on exclusion criteria. After the interview, participants underwent clinical periodontal examination.
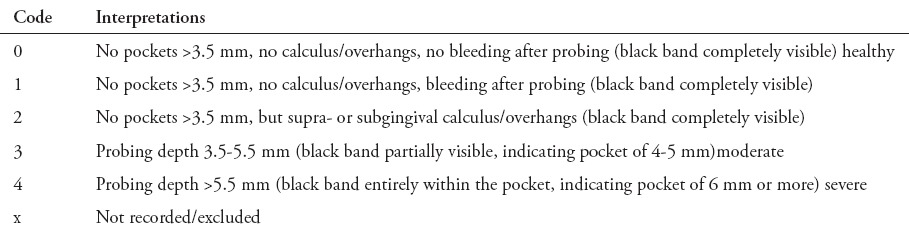
The clinical examination was conducted by 3 calibrated qualified trained dentists, with inter (kappa scores of 0.82) and intra examiner reliability (kappa scores of 0.85). The oral examination was carried out on an equipped dental unit with an adequate light source. No radiographs were obtained. A basic periodontal examination (BPE) was carried out for the periodontal screening of all participants. Examination of all permanent fully erupted teeth was carried out excluding the third molars. For periodontal assessment using basic periodontal examination, the mouth was divided into 6 sextants. The WHO probe with calibrated markings (Hu-Friedy Mfg. Co. Inc., Chicago, IL, USA) was used for measurement. Basic periodontal examination is an important tool in the periodontal screening of participants in epidemiological surveys, with a scoring system as shown in Table 1. The current study classified the BPE scores in 2 categories: i) no periodontitis (score 0, 1, or 2) and ii) chronic periodontitis (score 3 or 4).
Table 1.
Classification of basic periodontal examination in 443 participants.
Statistical analysis was carried out using the Statistical Package for Social Sciences Software Version 22, (Armonk, NY: IBM Corp). The characteristics of participants were assessed using frequency distribution for categorical variables and mean (standard deviation) for continuous variables. The Chi-square test was used to estimate the relationship between smoking status and intensity of smoking (heavy smokers versus light/moderate smokers) in relation to chronic periodontitis. Multivariate regression models were used to estimate risk association between smoking status of participants and number of cigarettes smoked per day in relation to chronic periodontitis. Following is the equation used to define multivariate logistic regression.
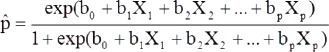
Results
The current study constituted of a total of 443 participants, with a mean age of 44.3 (±6.5), distributed as smokers (55.1%) and non-smokers (44.9%). Almost half of the studied population were young adults (18-34 years). Males were predominant and more than 55% of the participants belonged to the Mohajir ethnicity, approximately 70% of the participants had secondary education. Diabetes mellitus (DM) has a prevalence of 55.7%. Seventy percent of the participants suffered from chronic periodontitis (Table 2). Table 2 shows the distribution of participants among smoking and non-smoking group. Based on smoking habit, approximately 50% were young adult’s smokers. The male gender, Mohajir ethnic group, and DM had a higher prevalence of smoking habit. Prevalence of chronic periodontitis was estimated to be almost 82% among smokers, and 56% in non-smokers (Table 2).
Table 2.
Distribution of Pakistani participants among the smoker and non-smoker groups.
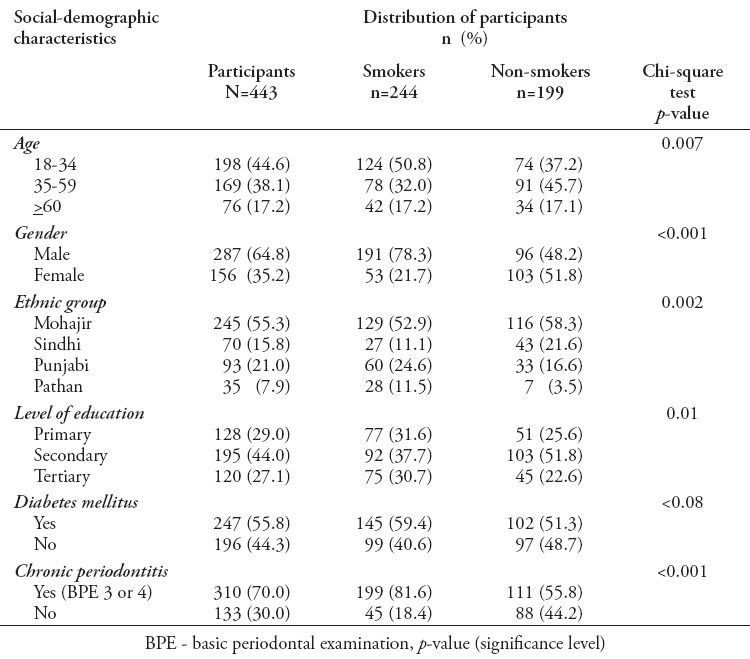
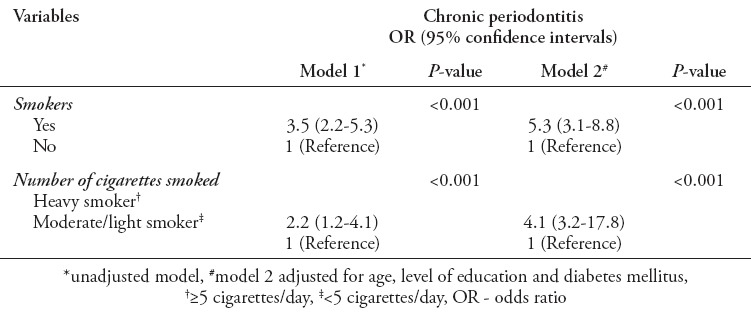
Table 3 shows the prevalence and severity of chronic periodontitis to numbers of cigarettes consumed per day. Heavy smokers were associated with increased burden of chronic periodontitis (96.7%) as compared with moderate/light smokers (66.1%). The intensity of cigarettes smoked was found significantly related to increased prevalence of chronic periodontitis (p<0.001). Furthermore, the severity of chronic periodontitis was significant among heavy smokers (p<0.002). Multivariate logistic regression models were used to evaluate the association of chronic periodontitis with smoking and frequency of cigarette consumption (Table 4). Model 1 was an unadjusted model, it showed odds ratio (OR) of 3.5 (2.2-5.3), which was associated with significantly greater risk of having chronic periodontitis as compared with non-smokers (p<0.001). Furthermore, increased cigarettes consumption, namely, heavy smoking was found to have higher OR 2.2 (1.2-4.1) of having chronic periodontitis (p<0.001). Model 2 represented adjusted models for covariates of age, level of education, and DM. Model 2 showed smoking habit (p<0.001) and increased cigarette consumption (p<0.001) with significantly higher OR (5.3 [3.1-8.8] and 4.1 [3.2-17.8]) of having chronic periodontitis.
Table 3.
Number of cigarette consumed and its relationship to prevalence and severity of chronic periodontitis.
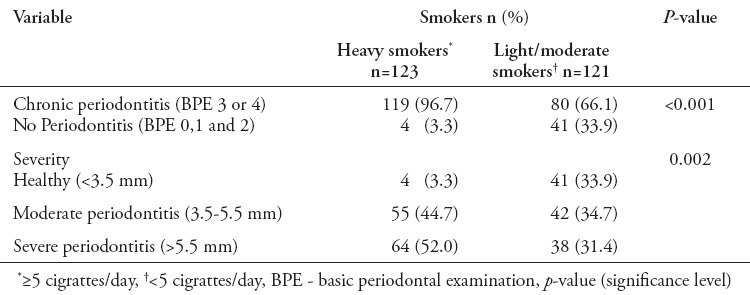
Table 4.
Multivariate regression models of association of chronic periodontitis and smoking.
Discussion
Smoking is a recognized public health issue. The WHO has distinct goals to control the tobacco smoking consumption and mortality rates around the world. The impact of tobacco smoking is high on general and systemic health. In the current study, the overall prevalence of smokers in the studied population was 55%. Also, approximately 50% of these smokers were young adults. The rampage of smoking habits could be improved by implementation of laws, which bans media advertisement for promoting smoking and a ban on smoking in public places. Smoking is a potential risk factor for various systemic diseases including periodontal disease.15,16 Previous studies16,17 demonstrated a significant biological plausibility on the relationship of smoking and periodontal disease. However, there is a gap in relation to dose response of smoking and periodontal disease. The present study provided convincing evidence on smoking and chronic periodontitis association in the Pakistani population, with the prevalence of chronic periodontitis at 81.6% among smokers. This prevalence of chronic periodontitis is twice that of the general adult population worldwide, and that reported previously in Pakistan.5 Similar findings were reported in the Brazilian population,18 where smoking was found to have a significantly higher prevalence of chronic periodontitis as compared with non-smokers. A study in the Mexican populations also demonstrated significantly high prevalence of chronic periodontitis among smokers.19 The high prevalence of chronic periodontitis in previous studies could be due to upheld host response associated with smoking, resulting in an alteration in neutrophil function, antibody production, fibroblast activities, vascular factors and inflammatory mediator production.11
The current scenario depicted the significant relationship between chronic periodontitis and frequency of cigarettes smoked per day (p<0.001). This finding is in line with Susin et al’s18 study that showed a higher prevalence of chronic periodontitis in heavy smokers as compared with light/moderate smoker. Previous study20 shows increased duration, and intensity of smoking is known to be related to increased severity of periodontal disease. Hence, speculation can be made that the increased frequency of smoking is associated with a disturbance in cellular and humoral immune response and deterioration of periodontal health.
In the present study, smokers were found to have a significantly higher risk association with chronic periodontitis (p<0.001). This association confirms the association of smoking and chronic periodontitis in the Pakistani population, comparable to that reported in previous studies.18,21 Also, the unadjusted and adjusted models showed heavy smoking as a potential risk factor for chronic periodontitis. This finding is in accordance with Susin et al’s18 study that reported heavy smokers with a greater risk of chronic periodontitis as compared with moderate/light smokers and non-smokers. Similar demonstrations were made in previous studies,22-24 showing a positive association between smoking and periodontitis. This may be due to the long-term exposure of periodontal tissues to tobacco smoking, which induces a host inflammatory response, resulting in deterioration of periodontal tissues and bone loss.25-27
Study limitations
Firstly, this study was a cross-sectional study, which decreases the ability to develop a causal relationship between smoking and chronic periodontitis. The design limits our ability to assess the association of chronic periodontitis and smoking, which can be better carried out with longitudinal cohort studies. Secondly, the current study employed BPE as a screening measure to define periodontal disease, which is relative to estimate rather than a true prevalence of chronic periodontitis. Hence, future studies are advised to use full-mouth periodontal examination with measures of probing pocket depth and clinical attachment loss to measure the true burden of periodontal disease. Also, it is advised to carry out longitudinal cohort studies among smokers to better understand its role in progression of periodontal disease.
In conclusion, smoking was found to be a potential risk factor for chronic periodontitis. Smoking is found to be a deteriorating factor for periodontal health affecting overall quality of life and the well-being of an individual. Hence, it is advised to clinicians and dental professionals for educate their patients in terms of potential hazards of smoking to their general and oral health. Also, implementation of smoking cessation and preventive programs could help improve the oral and overall health of an individual.
Acknowledgment
The authors gratefully acknowledge the Faculty of Dentistry, Karachi Medical and Dental College, Karachi, Pakistan for allowing us to conduct the study.
Footnotes
Clinical Practice Guidelines.
Clinical Practice Guidelines must include a short abstract. There should be an Introduction section addressing the objective in producing the guideline, what the guideline is about and who will benefit from the guideline. It should describe the population, conditions, health care setting and clinical management/diagnostic test. Authors should adequately describe the methods used to collect and analyze evidence, recommendations and validation. If it is adapted, authors should include the source, how, and why it is adapted? The guidelines should include not more than 50 references, 2-4 illustrations/tables, and an algorithm.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam TH, Hedley AJ. Respiratory diseases. In: Detels R, McEwen J, Beaglehole R, Tanaka H, editors. Oxford Textbook of Public Health. 4th ed. Oxford (UK): Oxford University Press; 2002. pp. 859–861. [Google Scholar]
- 3.Sreeramareddy CT, Pradhan P, Mir IA, Sin S. Smoking and smokeless tobacco use in nine South and Southeast Asian countries: prevalence estimates and social determinants from Demographic and Health Surveys. Popul Health Metr. 2014;12:22. doi: 10.1186/s12963-014-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellappally S, Fiala Z, Smejkalová J, Jacob V, Somanathan R. Smoking related systemic and oral diseases. Acta Medica (Hradec Kralove) 2007;50:161–166. [PubMed] [Google Scholar]
- 5.World Health Organization. The WHO global oral health data bank. Geneva (CH): World Health Organization; 2007. Available from URL: http://www.who.int/oral_health . [Google Scholar]
- 6.Page RC, Simpson DM, Ammons WF. Host tissue response in chronic inflammatory periodontal disease IV. The periodontal and dental status of a group of aged great apes. J Periodontol. 1975;46:144–155. doi: 10.1902/jop.1975.46.3.144. [DOI] [PubMed] [Google Scholar]
- 7.Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 8.Khan S, Saub R, Vaithilingam RD, Safii SH, Vethakkan SR, Baharuddin NA. Prevalence of chronic periodontitis in an obese population: a preliminary study. BMC Oral Health. 2015;15:1–7. doi: 10.1186/s12903-015-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:138–153. doi: 10.1111/j.1600-0757.2010.00340.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson GK, Guthmiller JM. The impact of cigarette smoking on periodontal disease and treatment. Periodontol 2000. 2007;44:178–194. doi: 10.1111/j.1600-0757.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 11.Javed F, Ahmed HB, Romanos GE. Association between environmental tobacco smoke and periodontal disease: A systematic review. Environ Res. 2014;133:117–122. doi: 10.1016/j.envres.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Han DH, Lim S, Kim JB. The association of smoking and diabetes with periodontitis in a Korean population. J Periodontol. 2012;83:1397–1406. doi: 10.1902/jop.2012.110686. [DOI] [PubMed] [Google Scholar]
- 13.Javed F, Al-Askar M, Samaranayake LP, Al-Hezaimi K. Periodontal disease in habitual cigarette smokers and nonsmokers with and without prediabetes. Am J Med Sci. 2013;345:94–98. doi: 10.1097/MAJ.0b013e31824d5337. [DOI] [PubMed] [Google Scholar]
- 14.Tomar SL, Asma S. Smoking-Attributable Periodontitis in the United States: Findings From NHANES III. J Periodontol. 2000;71:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 15.Nociti FH, Casati MZ, Duarte PM. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontol 2000. 2015;67:187–210. doi: 10.1111/prd.12063. [DOI] [PubMed] [Google Scholar]
- 16.Visvanathan R, Mahendra J, Ambalavanan N, Pandisuba C. Effect of Smoking on Periodontal Health. J Clin Diagn Res. 2014;8:ZC46. doi: 10.7860/JCDR/2014/8359.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 18.Susin C, Haas AN, Valle PM, Oppermann RV, Albandar JM. Prevalence and risk indicators for chronic periodontitis in adolescents and young adults in south Brazil. J Clin Periodontol. 2011;38:326–333. doi: 10.1111/j.1600-051X.2011.01699.x. [DOI] [PubMed] [Google Scholar]
- 19.Minaya-Sánchez M, Medina-Solís CE, Maupomé G, Vallejos-Sánchez AA, Casanova-Rosado JF, Marquez-Corona ML. Prevalence of and risk indicators for chronic periodontitis in males from Campeche, Mexico. Revista de Salud Pública. 2007;9:388–398. doi: 10.1590/s0124-00642007000300007. [DOI] [PubMed] [Google Scholar]
- 20.Salem A, Hilow H, Khraisat A, Smadi L, Ryalat S. Association between intensity of smoking and periodontal pockets among young university students. Odontostomatol Trop. 2008;31:5–10. [PubMed] [Google Scholar]
- 21.Natto S, Baljoon M, Bergström J. Tobacco smoking and periodontal health in a Saudi Arabian population. J Periodontol. 2005;76:1919–1926. doi: 10.1902/jop.2005.76.11.1919. [DOI] [PubMed] [Google Scholar]
- 22.Haber J, Kent RL. Cigarette smoking in a periodontal practice. J Periodontol. 1992;63:100–106. doi: 10.1902/jop.1992.63.2.100. [DOI] [PubMed] [Google Scholar]
- 23.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27:61–68. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 24.Calsina G, Ramón JM, Echeverría JJ. Effects of smoking on periodontal tissues. J Clin Periodontol. 2002;29:771–776. doi: 10.1034/j.1600-051x.2002.290815.x. [DOI] [PubMed] [Google Scholar]
- 25.Baljoon M, Natto S, Bergström J. Long-term effect of smoking on vertical periodontal bone loss. J Clin Periodontol. 2005;32:789–797. doi: 10.1111/j.1600-051X.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 26.Holm G. Smoking as an Additional Risk for Tooth Loss. J Periodontol. 1994;65:996–1001. doi: 10.1902/jop.1994.65.11.996. [DOI] [PubMed] [Google Scholar]
- 27.Campos ML, Corrêa MG, Júnior FH, Casati MZ, Sallum EA, Sallum AW. Cigarette smoke inhalation increases the alveolar bone loss caused by primary occlusal trauma in a rat model. J Periodontal Res. 2014;49:179–185. doi: 10.1111/jre.12091. [DOI] [PubMed] [Google Scholar]


