Abstract
Prolonged healing and scar formation are two major challenges in the treatment of soft tissue trauma. Adipose mesenchymal stem cells (ASCs) play an important role in tissue regeneration, and recent studies have suggested that exosomes secreted by stem cells may contribute to paracrine signaling. In this study, we investigated the roles of ASCs-derived exosomes (ASCs-Exos) in cutaneous wound healing. We found that ASCs-Exos could be taken up and internalized by fibroblasts to stimulate cell migration, proliferation and collagen synthesis in a dose-dependent manner, with increased genes expression of N-cadherin, cyclin-1, PCNA and collagen I, III. In vivo tracing experiments demonstrated that ASCs-Exos can be recruited to soft tissue wound area in a mouse skin incision model and significantly accelerated cutaneous wound healing. Histological analysis showed increased collagen I and III production by systemic administration of exosomes in the early stage of wound healing, while in the late stage, exosomes might inhibit collagen expression to reduce scar formation. Collectively, our findings indicate that ASCs-Exos can facilitate cutaneous wound healing via optimizing the characteristics of fibroblasts. Our results provide a new perspective and therapeutic strategy for the use of ASCs-Exos in soft tissue repair.
While soft tissue trauma is a common occurrence, many chronic wounds, such as diabetic ulcers1, are difficult to heal2. Prolonged healing soft tissue wound are more likely to form scars, which means less favorable healing quality3,4. This not only causes functional disability, but also can affect the mental health of patients. Therefore, shortening healing time and reducing the scar formation in soft tissue trauma recovery represent urgent clinical needs. Current conventional methods to accelerate the healing and reduce scar formation include skin grafting5,6, laser therapy7 and the local application of some growth factors8 or gene therapy9. However these methods may lead to atrophic scar, pigment abnormalities, skin necrosis and other undesirable consequences10. Additionally, local injected factors can easily be degraded by body fluid, and their dosage and concentration are often highly variable at wound site11. Hence, it is necessary to find a new stable, efficient and safe method to promote soft tissue wound healing.
Fat tissue is widely distributed in human body, and plays a vital role in supporting and protecting adjacent soft tissue in physiological state12. At the same time, as an active endocrine organ, fat tissue is also crucial element of metabolism, growth and development, inflammation resistance, among other biological processes13,14. In recent years, fat tissue has been extensively studied for its role in wound repair. Autologous adipose graft was applied for complicated wound repair15, and also often used to regenerate soft tissues in plastic surgery. Its efficacy andsafety are widely accepted16,17, but there is lack of direct evidence for its mechanism.
Exosomesare a kind of membrane lipid vesicles with 30–100 nm in diameter, and they were previously thought to be metabolic products of cells18,19. According to previous studies, exosomes represent an essential medium for intercellular communication as a variety of miRNAs and proteins are sorted in exosomes20,21,22. Studies have demonstrated that exosomes derived stem cell may aid in tissue repair due to their advantages of high stability, non-immune rejection, homing effect, easy control of dosage and concentration23,24,25. Exosomes secreted by umbilical cord mesenchymal stem cells can effectively repair the damage of myocardial ischemia reperfusion injury26,27, liver fibrosis28 and acute kidney injury29. In the pathogenesis and progression of breast cancer, exosomes secreted by adipose mesenchymal stem cells (ASCs-Exos) can affect cancer cell migration through Wnt signaling pathway. ASCs-Exos also contain a high level of enkephalinase, which is helpful for Alzheimer treatment. In our previous study, we found that conditioned medium of cultured adipose stem cells (ASC-CM) could promote migration, proliferation and collagen synthesis of fibroblasts30, and we extracted a number of exosomes from the conditioned medium. Herein, we hypothesized that exosomes secreted by adipose derived stem cells have a positive role in promotion of skin or mucosal soft tissue wound repair. To address this hypothesis, we compared the healing rate of wound with fat layer and without fat layer, and examined the expression of exosomes in these two sites of wound. In vitro and in vivo tracking of exosome were also explored. We found that exosomes derived from ASCs can result in changes to cell proliferation, migration and collagen synthesis, which can benefit wound healing. This study confirmed that ASCs-Exoscan shorten the healing time and reduce scar formation of mouse skin incision wound. These data present strong in vitro and in vitro evidence that ASCs-Exos have promising potential for clinical application in soft tissue wound healing.
Results
Subcutaneous fat tissue was benefit for skin wound healing
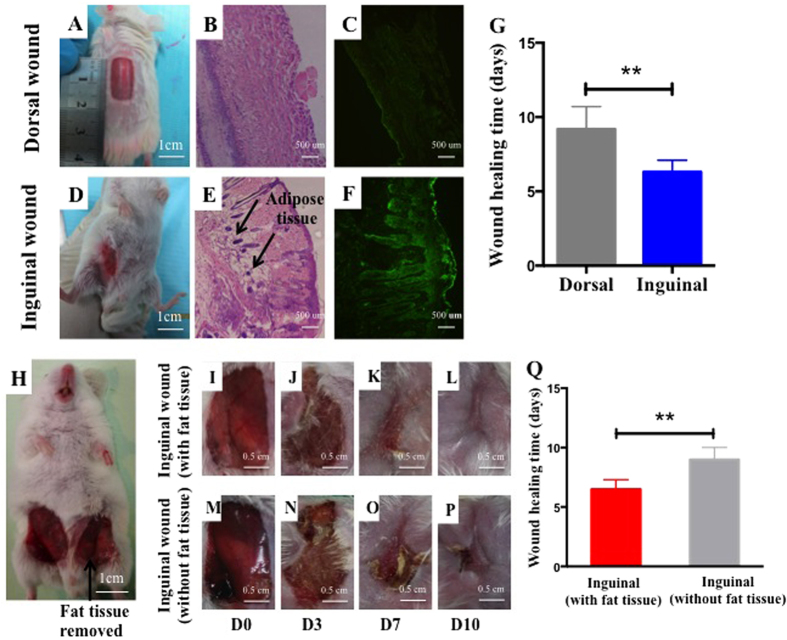
To investigate the influence of subcutaneous fat tissue on skin wound healing, we performed a preliminary study to comparethe healing rate of skin wounds with or without a fat layer. Briefly, we created the same size of inguinal wound (with fat layer) and dorsal wound (without fat layer) on mice (Fig. 1A,B,D,E). Our results demonstrated that CD63, a specific marker of exosomes, was expressed at significantly higher levels in the inguinal wound area (Fig. 1C,F). Moreover, the dorsal wound took a longer time to completely heal than inguinal wound (p < 0.01), (Fig. 1G). We also compared the wound healing of a inguinal wound with fat layer and a inguinal wound without fat layer (fat tissue was removed form the wound) on same mouse (Fig. 1H), and found that inguinal wound with fat layer healed better than inguinal wound without fat layer, as the former wound performing less scar and shorter healing time (Fig. 1I–P and Fig. 1Q). To exclude the possible effects of the intrinsic differences between the inguinal and dorsal fibroblasts on wound healing, we cultured mice inguinal and dorsal fibroblasts separately in vitro, and compared their proliferation and migration abilities. The results presented no difference between these two fibroblasts (Supplementary Fig.1). These results suggest that subcutaneous fat tissue was beneficial for skin wound healing, and this phenomenon might be closely relevant with the production of exosomes in fat layer.
Figure 1. Subcutaneous fat tissue was beneficial for skin wound healing.
(A) The same size of wounds (1.5 × 1 cm) were created respectively on the back and groin area (D) of mice. H&E staining showed that abundant fat tissue existed under inguinal wound (B), while no obvious fat tissue existed under dorsal wound (E). Immunofluorescence staining indicated wide distribution of CD63 in inguinal wound area (C,F). (G) Dorsal wound took longer time to completely heal than inguinal wound (**P < 0.01). The samesize of wounds (1.5 × 1 cm) were also created respectively on the two sides of groin areaof each mouse, and the fat layer of one side wound was removed (H). Pictures for wound area of two sides wound showed that wound with fat layer healed faster than inguinal wound without fat layer (I–P,Q).
Characterization of human ASCs-Exos
We isolated exosomes from the supernatants of ASCs. The cup-shaped morphology of exosomes was observed by electron microscopic analysis,and their diameters almost ranged from 30 to 100 nm (Fig. 2A–C). The particle size distribution, concentration and dynamic tracking were measured by using NanoSight analysis (Fig. 2D–G). Western blot showed some exosomal markers, such as CD9 and CD63 (Fig. 2H), were expressed in exosomes. To detect the purity of the exosome isolated from ASCs, we measured the expression of Tubulin (cytosolic marker) and Lamin A/C(nuclear marker) in the ASCs and exosomes by western blot. A barely detectable expression of Tubulin and Lamin A/C were presented in exosomes, which means no contamination of cellular components in isolated exosomes (Fig. 2I). The results indicated that exosomes from human ASC were successfully isolated and consistent with the defined exosomes. Fibroblasts also can secrete exosomes, and the concentrations of exosomes secreted by equal amount of mice inguinal ASCs, inguinal fibroblasts and dorsal fibroblasts are nearly the same (Supplementary Fig.2), but the components of exosomes secreted by these cells are theoretically different, which needs further study.
Figure 2. Characterization of human ASCs-Exos.
Microscope images of human ASCs-Exos morphology(A,B). These particles size distribution displayed about 85% range from 30 to 100 nm (C). Characterizations of exosomes were measured by NanoSight analysis (D–G). Size distribution (D), concentration analysis (E), vesicles’ trajectory of Brownian motion (F), and Dynamic tracking video capture (G). Detection of CD63 and CD9 expression in exosomes by western blotting (H). The purity of ASCs was detected by western blotting analysis of Tubulin (cytosolic marker) andLamin A/C (nuclear marker)(I).
Internalization of exosomes by fibroblasts
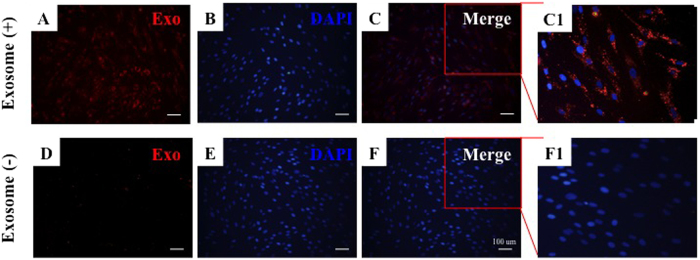
Studies have reported that exosomes can enter into target cells, thereby regulating their biological behavior. As such, we investigated whether exosomes from human ASCs can enter into fibroblasts. In our in vitro tracking experiment, the labeled exosomes were incubated with fibroblasts for 24 h, and the cellular uptake of ASCs-Exos was evaluated via fluorescence microscopy (Fig. 3A–C1). This analysis demonstrated that exosomes can enter into the cytoplasm of fibroblasts, mainly localizing to the perinuclear region, implying that ASCs-Exos can be internalized by fibroblasts.
Figure 3. Cellular internalization of ASCs-Exos by fibroblasts.
Fibroblasts were incubated with CM-Dil dye labeled exosomes for 24 h (A–C), and cells were also incubated with dye without exosomes as a negative control to examine carryover of CM-Dildye (D–F). Numerous dye labeled exosomes were observed inside the fibroblasts (C1), while there was no dye present in the control group (F1).
ASCs-Exos promoted fibroblasts migration, proliferation, collagen synthesis in vitro
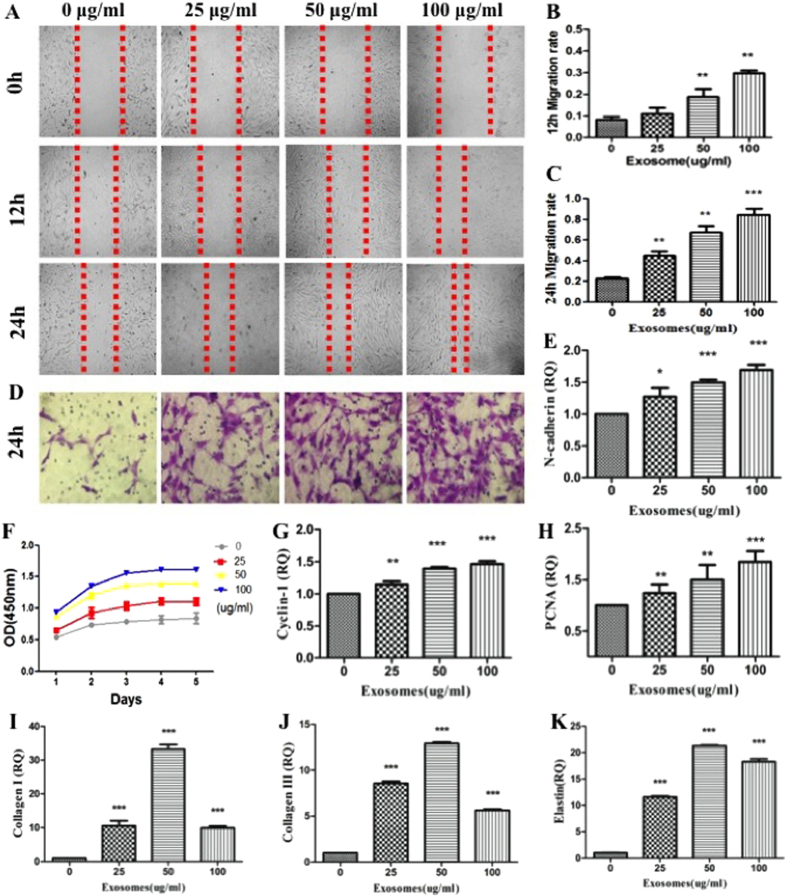
Our previous studies have found that human adipose stem cells conditioned medium (ASCs-CM) can significantly promote human skin dermis fibroblasts (FBs) migration, proliferation and collagen synthesis. We hypothesized that this effect may be partly due to the participation of exosomes. Scratch closure test results demonstrated that the migration of fibroblasts increased at 12 h and 24 h in the presence of exosomes as compared to control group p < 0.001) (Fig. 4A–C). Similarly, a Transwell migration assay detected that exosomes could promote fibroblasts migration in a dose-dependent manner (Fig. 4D). CCK-8 analysis of cell proliferation showed that exosomes also promoted fibroblasts proliferation with the increase of concentration of exosomes at Day 1, 2, 3, 4, 5 (Fig. 4F). In addition, mRNA expression of N-cadherin (Fig. 4E), Cyclin-1 (Fig. 4G) and PCNA (p < 0.001, Fig. 4H) suggested that increased exosome concentration significantly induced expression of genes related to cell migration and proliferation, respectively.
Figure 4. The changes of characteristics of human dermal fibroblasts with stimulation of exosomes.
The images (A) and migration rates of human fibroblasts following co-culture with 0, 25, 50, or 100 μg/mL ASCs-Exos for 12 h (B) or 24 h (C). Transwelltest of fibroblasts with stimulation of different concentrations of exsomes (D). Growth curves of fibroblasts co-cultured with 0, 25, 50, or 100 μg/mL ASC-Exos (F). The N-calcium (E) Cyclin-1 (G) and PCNA (H) mRNA expression of human fibroblasts. The Col I (I), III (J) and elastin (K) mRNA expression of human fibroblasts treated with ASCs-Exos.*P < 0.05, **P < 0.01, ***P < 0.001.
Further in vitro studies were performed to determine that whether exosomes can affect collagen synthesis ability of dermal fibroblasts. After stimulating fibroblasts with ASCs-Exos, Collagen I (Fig. 4I), III (Fig. 4J) gene expression and elastin protein production (Fig. 4K) were significantly increased dose-dependently (p < 0.05), and the optimal concentration of exosomesis 50 ug/ml.
In vivo tracking of intravenous injected exosomes
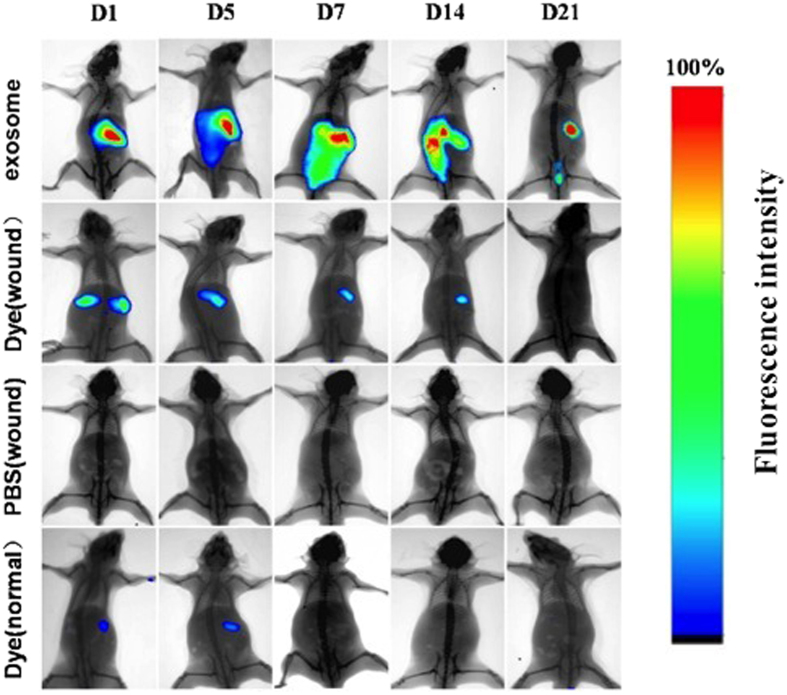
To evaluate the contributions of exosomes in wound healing, we monitored the migration and effect of exosomes after they were injected into mice sufferring a back wound. As a control, dye without exosomes was also injected into normal and wounded mice to observe their metabolic processes. Bioluminescence imaging showed (Fig. 5) that fluorescence gathered in the area of the wound in 7 days (D7) and could still be detected until day 21 (D21) when the fluorescence became weak and gradually disappeared. No fluorescence signal wasdetected in control mice where only Dye was injected.
Figure 5. Tracking experiments in vivo.
Assessment of bioluminescence imaging signals evaluate whether ASCs-Exos can migrate to the wound site. Representative bioluminescence imaging of animals injected with fluorescent labeled exosomes. Dye or PBS after sufferring a back wound (2 × 1.5 cm) were collected at indicated time points of Day 1, 5, 7, 14, 21. To observe the metabolism of dyes in normal mice, dye was injected into the tail vein of the control group.
ASCs-Exos promoted cutanenous wound healing in vivo
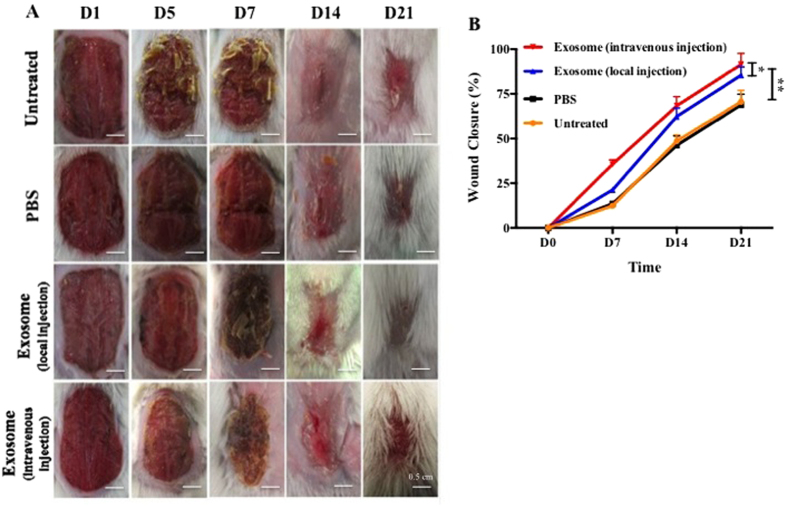
As shown in Fig. 6A,B, wound closure of exosomes treatment mice was accelerated, illustrated by smaller wound areas measured at day1, 5, 7, 14, and 21 post-wounding when compared with control groups(untreated and PBS-treated). Most notably,intravenous exosomes injection resulted in a 50% closure by day 7 post-wound,was ~75% closed by day 14 and ~90%closed by day 21. Wound healing occurred significantly faster when injected with exosomes than local injection group(p < 0.05).
Figure 6. ASCs-Exos promoted wound healing in vivo.
Representative wound closure imaging of animals injected locally or intravenously with exosomesor PBS after sufferring a back wound (2 × 1.5 cm) were collected at indicated time points of Day 1, 5, 7, 14, 21 (A). Untreated and PBS injection severed as control. Quantitative analysis of wound closure at different time points (B). *P < 0.05, **P < 0.01.
ASCs-Exos exosomes promoted collagen expression during wound healing
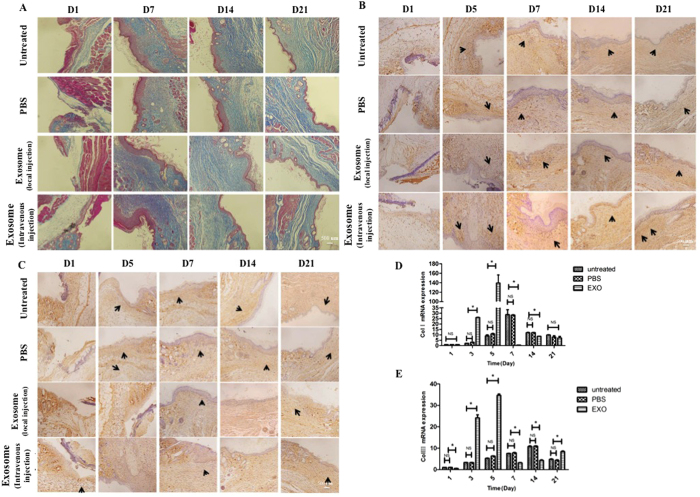
To evaluate scarless wound healing, we detected collagen deposition by Masson’s staining, showing that the collagen production by Day 7 and the collagen maturity in the late stage of day 14 and 21 in the intravenous injection groups and local injection groups were higher than PBS groupsand untreated groups, respectively (Fig. 7A).
Figure 7. ASCs-Exos promoted collagen expression and secretion during wound healing in vivo.
Evaluation of collagen synthesis secretion of wounds following treatment with PBS, injected locally or intravenously with exosomesat Day 1, 7, 14, 21 post-wounding, untreated animals served as control (A). Immunohistochemical and RT-PCR analysis of collagen synthesis of fibroblasts. The results of immunohistochemical analysis of collagen I (B) and collagen III (C) were same as above (arrows indicate Col I or Col III positive), withcollagen I (D) and collagen III (E)were obviously upregulated in the early stage. *P ≤ 0.05; NS: no significant difference.
We also performed Immunohistochemical observation and qRT-PCR analysis to assess collagen I (Fig. 7B,D) and III (Fig. 7C,E) expressions in the wound area. Our results showed that these two types of collagen were expressed higher in the groups that received intravenous injection, and reached a peak value at day 5. These results suggested that exosomes can promote the expression of collagen I and III in the early stage of wound healing. While in the late stage, exosomes might inhibit collagen expression.
Discussion
Fat tissue, which contains a lot of mesenchymal stem cells, is an active endocrine organ, and is thought to play an important role in the repair of soft tissue trauma31,32. However, there is no direct evidence for this effect, and the underlying mechanism remains enigmatic. Recent studies have demonstrated that exosomes are very important for paracrine activity of stem cells33. In this study, the effects of ASCs-Exos on fibroblasts activity and skin wound repair were explored.
The results of our study demonstrate that inguinal wound (with fat layer) healed faster, and had more exosomes distribution in the surrounding of the wound regionthan those with dorsal wound (without fat layer), indicating that both that the presence of a fat layer was beneficial for soft tissue wound healing and that exosomes of adipose tissue may promote this effect.
Exosomes are small membrane vesicles that form by the inward budding of cellular compartments that fuses with the plasma membrane34,35. Fibroblasts are the main effector cells in the wound healing of soft tissue36. Their migration, proliferation and collagen synthesis are important for the quality of wound healing37. We found that human ASCs-secreted exosomes can be internalized by fibroblasts anddose-dependently optimized their characteristics, such as the migration and proliferation abilities, collagen synthesis and elastic secretion capacity. The role of exosomes, derived from stem cell, in promoting tissue repair hasbeen reported by numerous sources. Exosomes secreted by umbilical cord mesenchymal stem cells have positive effects on migration,proliferation and tube formation of endothelial cells38, while iPS-Exos(human induced pluripotent stem cells) can promote collagen synthesis39. It is believed that exosomes are able to exert this affect due to their rich composition of of RNAs and proteins, which are related to the functions of fibroblasts.
Our in vitro tracking results showed that exosomes could enter into the cytoplasm of fibroblasts. Similar observations have previously been reported in other cell types. Ramesh et al., co-cultured dye labeled exosomes of tumor cells with different cell lines, and confirmed the physical entry of exosomes into the cell cytoplasm40. Lipid raft-mediated endocytosis is responsible for exosomes uptake through ERK1/2-Heat Shock Protein 27 Signaling41. Reports suggest that miRNAs and proteins derived from exosomes can mediate signal transduction in target cells following endocytosis, or membrane fusion42. Our in vitro tracking results confirmed that ASCs-Exos released their active substances after they entered into cells to promote migration, proliferation and collagen secretion of fibroblasts.
Local injection of exosomes has also been reported to promote regeneration of damaged tissue, but there is no report regarding the effects of intravenous injection of exosomes. Our in vivo tracing experiment found that exosomes can be recruited to wound area via tail vein blood circulation, assembling around the wound on day 7 post-injection, aiding in the healing process. This phenomenon might be similiar to the homing function of stem cells. The cytomembrane of stem cells invaginates into the cytoplasm, and coats miRNAs and proteins to form multivesicular endosome, which combines with cytomembrane to release exosomes to surround environment43. Due to surface receptors and adhesion molecules of stem cells44,45, exosomes may present similar homing abilities as their parent cells, and can be recruited to the wound region. It has been postulated that exosomes can avoid recognition/detection by the immune system, and maintain the integrity of cell membrane to avoid degradation. However, the specific mechanisms through which exosomes contributes to wound healing requires further investigation.
We applied exosomes to mouse skin wound through local injection and intravenous injection, and found that the exosome-treated mice healed faster than the control mice, indicating that exosomes derived from human adipose mensenchymal stem cells can accelerate cutaneous wound healing. Interestingly, intravenous injection was superior at wound healing as compared to local injectionand we speculated loss of exosomes during local injection may contribute to this difference. Moreover, when injecting exosomes directly into the wound, inevitably the wound can be further disturbed, thus disrupting the wound healing process. After entering into blood, exosomes could be recruited to damage area through receptors or adhesion molecules of membrane surface, targeted fibroblasts to promote wound healing by. In vivo studies showed that collagen I and III distributions were promoted by exosomes in the early stage of wound healing, a result that was confirmed by with increased expressions of collagen I and III. These results suggest that exosomes promote the early stages of wound healing by shortening healing time, while in the late stages, they might inhibit collagen synthesis to reduce scar formation. This tendency follows the histological changes observed during natural healing of soft tissue wounds, that is, in the early phase of healing, collagen deposition is more important, while in the late phase of healing, matrix reconstruction is more critical.
In summary, exosomes secreted by human adipose stem cells are easily obtained and can be effectively used in research and clinical treatment. Exosomes can optimize the characteristics of fibroblasts, such as promoting the migration, proliferation and collagen synthesis of fibroblasts, thereby accelerating wound healing of soft tissue. Our findings suggest that ASCs-Exos may represent a novel therapeutic tool in soft tissue wound healing.
Methods and Materials
Isolation and identification of exosomes derived from human adipose stem cells(HASCs)
HASCs were isolated as previously described46. Human subcutaneous fat tissue was obtained from healthy mother aged from 18 to 30 years old with informed consent approved by the Committee of Wuhan Union Hospital and the following protocols including all relevant details were performed in accordance with the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (IORG No: IORG0003571, and the ethics statement is in the supplementary information). HASCs were cultured in serum-free medium for 24 hours to collect conditioned medium, and cell debris were removed by centrifuging at 3,000 g for 15 min, and then passed through a 0.22 um filter (Millipore). Supernatants were concentrated using 100 KDa molecular weighAmicon® Ultra-15 Centrifugal Filter Devicest (Millipore) and then incubated with ExoQuick-TC exosome precipitation solution (System Biosciences) overnight. Exosome pellets were resuspended in PBS, and the purified exosomes were passed through a 0.22 um filter. After diluted with PBS, 1 ul exosomes were used to quantitate their concentration by BCA protein assay kit, as suggested by the manufacturer(Beyotime Instituteof Biotechnology). The collected exosomes morphologies were observed by 100 kv transmission electron microscopy (HITACHIH-7000FA, Japan). The size, concentration and particle size distribution of exosomes were identified by NanoSight LM10 (Nanosight) and Nanoparticle Tracking Analysis software version 2.2 (NanoSight). Antibodies against CD9 (Abcam) and CD63 (Abcam) proteins were used to analyse their expression by western blotting.
Scratch closure test
Primary human dermal fibroblasts were isolated using previously described protocols47. 3 × 105 Skin fibroblasts were seeded into 6-well platesand scratched by a sterile 100 ul pipette tip. Fresh serum-free culture medium containing ASCs-Exos (0, 25, 50, 100 ug/ml) were added. We took images of the scratched area at 0, 12, and 24 h, andmeasured widths of the scratched by the Image-Pro Plus 6.0 software.
In vitro Migration Assay
The migration of fibroblasts exposed to exosomes was also determined by Transwell assays using transwell chambers with 8 um pore filters (USA), according to the manufacturer’s recommendation. Approximately 1 × 105 fibroblasts suspended were seeded into the upper compartment and ASCs-Exos (0, 25, 50, 100 ug/ml) were added into the lower compartment. Cells were allowed to migrate for 24 hours, after which the upper chamber was washed three times and wiped toremove nonmigrated cells. Migrated cells were stained with 0.4% crystal violet, and cell counts were performed via microscopy.
Cell proliferation assay
We used Counting Kit-8 (CCK-8) (Dojindo) to determine proliferation of skin fibroblasts when they were co-cultured with ASCs-Exos, according tothe previously described protocols47.
Collagen synthesis of fibroblasts with exosomes stimulation
Fibroblasts were serum starved for 24 h, and different concentrations of ASCs-Exos (0, 25, 50, 100 ug/ml) with fresh serum-free culture medium were changed for additional 48 hours culture, and cells were collected and collagen expression level was examined by qRT-PCR. The primer sequences were listed in Table 1.
Table 1. Primer Sequences and Products of Reverse Transcription–Polymerase Chain Reaction.
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| Col1a1 (mus) | AAGAAGCACGTCTGGTTTGGAG | GGTCCATGTAGGCTACGCTGTT |
| Col3a1 (mus) | GTGGCAATGTAAAGAAGTCTCTGAAG | GGGTGCGATATCTATGATGGGTAG |
| GAPDH (mus) | AGGAGCGAGACCCCACTAACA | AGGGGGGCTAAGCAGTTGGT |
| Col1a1 (homo) | CAAGACGAAGACATCCCACCAATC | ACAGATCACGTCATCGCACAACA |
| Col3a1 (homo) | TCGCTCTGCTTCATCCCACTAT | CTTCCAGACATCTCTATCCGCAT |
| ELASTIN (homo) | GGGTTGTGTCACCAGAAGCA | CAACCCCGTAAGTAGGAATGC |
| PCNA (homo) | AGCCATATTGGAGATGCTGTTG | CTGAGTGTCACCGTTGAAGAAGAGAG |
| N-Cadherin(homo) | AAGAGGCAGAGACTTGCGAAAC | TGGAGTCACACTGGCAAACCTT |
| Cyclin-D1(homo) | GCATCTACACCGACAACTCCATC | CGCGTGTTTGCGGATGATCT |
| GAPDH (homo) | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
In vitro Tracking
Human ASCs-Exos were fluorescently labeled with CM-Dil dye(CM-Dil;Molecular Probes). The labeled exosomes were passed through 100-kDa filter (Microcon YM-100, Millipore) and resuspended in PBS for 3 times to remove excess dye. Mixtures were co-cultured with fibroblasts in FBS-free medium overnight at 37 °C. Following fixed with 4% paraformaldehyde and 4′, 6-diamidino-2-phenylindole (DAPI, sigma), the cells were observed under a fluorescence microscope.
Mice skin wound model and treatment
Adult male Balb/c mice (6–8 weeks) were purchased from the Animal Centre of disease control and prevention. All animal procedures including all relevant details were performed in accordance with the Animal Care Committee of Tongji Medical College, Huazhong University of Science andTechnology and approved by Ethics Committee of Tongji Medical College, Huazhong University of Science andTechnology (IORG No: IORG0003571)
Mice model of skin wound was established as previously described. After anesthetizing and shaving the mice, a dorsal wound and an inguinal wound with same size(1 × 1.5 cm) were created. Half the mice were sacrificed at day 7 post-surgery, and the tissues of wound area were cut for H&E staining. CD63 staining of the wound area was analyzed by immunofluorescence. The remaining micewere observed to compare the healing rate of dorsal wound and inguinal wound.
We created a 2 × 1.5 cm full-thickness wound on the back of mice to confirm the effects of ASCs-Exos on cutaneous wound healing. Mice were randomly divided into four groups: untreated group, 200 μl PBS subcutaneous injection group, 200 μg exosome in 200 μl PBS subcutaneous injection group, 200 μg exosome in 200 μl PBS intravenous injection group. The animals were housed individually, and were imaged prior to surgery and regularly everyday post-surgery. Mice were killed at day 1, 3, 5, 7, 14, and 21 following treatment, and wound areas were measured and calculated using image analysis software. Half of the wound skin tissues were collected for qRT-PCR analysis. The rest of the half sampleswere used for immunohistochemistry staining.
In vivo tracking
ASCs-Exos were labeled with DIR (DIR;Molecular Probes) according to the manufacturer’s protocol. Labeled exosomes, dye mixture (not contain exosomes) and PBS were tail intravenously injected into Balb/c mice with a 1.5 × 2 cm dorsal wound, and intravenous injection of pure dye mixture into normal mice were used to as negative control. Mice were anesthetized for observation under bioluminescence system at Day 1, 3, 7, 14 and 21, and fluorescence images for exosomes distribution were acquired with 740 nm excitation and 790 nm emission filters.
Real-Time PCR
Total RNA was extracted from fibroblasts with the RNeasy Mini Kit (Qiagen), and then reversed-transcribed into complementary DNA (cDNA), and the appropriate primers for PCR were designed as shown in Table1.
Immunohistochemistry
For immunohistochemical staining of collagen synthesis of fibroblasts, formalin-fixed and paraffin embedded skin tissues were deparaffinized to prevent non-specific protein binding. Samples were then incubated with diluted primary antibodies overnight at 4 °C. After the wash steps, the sections were incubated with secondary antibodies for 2 h. Antibody binding of tissue sections were visualized by incubating with DAB substrate, and counterstained with hematoxylin before the slides were mounted. The collagen expressions were evaluated by high-power light microscopy examination.
Statistical analyses
The data were assessed using Student’s t-tests (t-tests), one-way or two-wayanalysis of variance (ANOVA) comparing the differences between groups by GraphPad Prism 5 software. P values < 0.05 were considered statistically significant. *P < 0.05, **P < 0.01, ***P < 0.0001. The values are shown in the figures.
Additional Information
How to cite this article: Hu, L. et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 6, 32993; doi: 10.1038/srep32993 (2016).
Supplementary Material
Acknowledgments
This work was supported by Major Project from the National Natural Science Foundation of China No. 31110103905 (to L.L Chen), National Outstanding Youth Science Fund of China No. 31422022 (to L.L Chen), Science and Technology Supporting Fund of Hubei Province No. 2015BAA031 (to H.D Zhang) and National Natural Science Foundation of China No. 81500831 (to Li Hu).
Footnotes
Author Contributions L.H., J.W. and X.Z. designed and performed the experiments, collected and analyzed data and drafted the manuscript; Z.X. performed several in vitro experiments and collected data; J.Z. and R.Y. performed several in vivo experiments and collected data; F.H. and H.Z. contributed to analysis of data; L.C. conceived and designed the experiments, oversaw the results collection and data interpretation, and finalized the manuscript.
References
- Blakytny R. & Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabetic medicine : a journal of the British Diabetic Association 23, 594–608, doi: 10.1111/j.1464-5491.2006.01773.x (2006). [DOI] [PubMed] [Google Scholar]
- Harding K. G, M H. L. & Patel G. K. Science, medicine, and the future Healing chronic wounds. Bmj 160–163 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- P M. Wound healing–aiming for perfect skin regeneration. Science 276, 75 (1997). [DOI] [PubMed] [Google Scholar]
- Longaker M T. et al. Studies in Fetal Wound Healing, VI. Second and Early Third Trimester Fetal Wounds Demonstrate Rapid Collagen Deposition Without Scar Formation. PediatricSurger 25, 1 (1990). [DOI] [PubMed] [Google Scholar]
- Hu Z. C. et al. Randomized clinical trial of autologous skin cell suspension combined with skin grafting for chronic wounds. The British journal of surgery 102, e117–e123, doi: 10.1002/bjs.9688 (2015). [DOI] [PubMed] [Google Scholar]
- Johnson T. M., Ratner D. & Nelson B. R. Soft tissue reconstruction with skin grafting. Journal of the American Academy of Dermatology 27, 151–165 (1992). [DOI] [PubMed] [Google Scholar]
- Loreti E. H. et al. Use of laser therapy in the healing process: a literature review. Photomedicine and laser surgery 33, 104–116, doi: 10.1089/pho.2014.3772 (2015). [DOI] [PubMed] [Google Scholar]
- Martino M M. et al. Growth Factors Engineered for Super-Affinity to the Extracellular Matrix Enhance Tissue Healing. science 343, 21 (2014). [DOI] [PubMed] [Google Scholar]
- Andreadis S. T. & Geer D. J. Biomimetic approaches to protein and gene delivery for tissue regeneration. Trends in biotechnology 24, 331–337, doi: 10.1016/j.tibtech.2006.05.001 (2006). [DOI] [PubMed] [Google Scholar]
- Orgill D. P. & Ogawa R. Discussion: The embrace Device Significantly Decreases Scarring following Scar Revision Surgery in a Randomized Controlled Trial. Plastic and reconstructive surgery 133, 406–407, doi: 10.1097/01.prs.0000436812.73412.a4 (2014). [DOI] [PubMed] [Google Scholar]
- Bernuzzi G. et al. Use of platelet-rich plasma in the care of sports injuries: our experience with ultrasound-guided injection. Blood transfusion = Trasfusione del sangue 12 Suppl 1, s229–s234, doi: 10.2450/2013.0293-12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D. W. Fat Prospects for Healing Cutaneous Wounds: New Activities Under the Sheets. Diabetes 64, 2717–2719, doi: 10.2337/db15-0533 (2015). [DOI] [PubMed] [Google Scholar]
- Eto H. S. H., Matsumoto D. et al. Characterization of structure and cellular components of aspiratedand excised adipose tissue. Plast Reconstr Surg 124, 1087–1097 (2009). [DOI] [PubMed] [Google Scholar]
- Marcelino H. et al. A role for adipose tissue de novo lipogenesis in glucose homeostasis during catch-up growth: a Randle cycle favoring fat storage. Diabetes 62, 362–372, doi: 10.2337/db12-0255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviglia H. et al. Is it possible to use autologous adipose graft for wound repair in patients with coagulation disorders? Haemophilia: the official journal of the World Federation of Hemophilia , doi: 10.1111/hae.12804 (2015). [DOI] [PubMed] [Google Scholar]
- Eun S. C. Stem cell and research in plastic surgery. Journal of Korean medical science 29 Suppl 3, S167–S169, doi: 10.3346/jkms.2014.29.S3.S167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducic Y. Fat grafting in trauma and reconstructive surgery. Facial plastic surgery clinics of North America 16, 409–416, v–vi, doi: 10.1016/j.fsc.2008.05.003 (2008). [DOI] [PubMed] [Google Scholar]
- RM P. B. J. Fate of the transferfin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. cell 33, 967–978 (1983). [DOI] [PubMed] [Google Scholar]
- Record M., Carayon K., Poirot M. & Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochimica et biophysica acta 1841, 108–120, doi: 10.1016/j.bbalip.2013.10.004 (2014). [DOI] [PubMed] [Google Scholar]
- T B. C. T. Exosomes: new players in cell—cell communication. Int J Biochem Cell Biol 44, 2060–2064 (2012). [DOI] [PubMed] [Google Scholar]
- Waldenstrom A. & Ronquist G. Role of exosomes in myocardial remodeling. Circulation research 114, 315–324, doi: 10.1161/CIRCRESAHA.114.300584 (2014). [DOI] [PubMed] [Google Scholar]
- Gross J. C., Chaudhary V., Bartscherer K. & Boutros M. Active Wnt proteins are secreted on exosomes. Nature cell biology 14, 1036–1045, doi: 10.1038/ncb2574 (2012). [DOI] [PubMed] [Google Scholar]
- Januszyk K. & Lima C. D. The eukaryotic RNA exosome. Current opinion in structural biology 24, 132–140, doi: 10.1016/j.sbi.2014.01.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio S. R., Baldini P. D. & Mesenchymal N. stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 3, 359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W. K. M., Geuze H. J. & Raposo G. The biogenesis andfunctions of exosomes. Traffic 3, 321 (2002). [DOI] [PubMed] [Google Scholar]
- Lai R. C. et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4, 214–222 (2010). [DOI] [PubMed] [Google Scholar]
- Sahoo S. & Losordo D. W. Exosomes and cardiac repair after myocardial infarction. Circulation research 114, 333–344, doi: 10.1161/CIRCRESAHA.114.300639 (2014). [DOI] [PubMed] [Google Scholar]
- Li T. et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22, 845–854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Zhao J., Liu J., Gong N. & Chen L. Effects ofadipose stem cell-conditioned medium on the migration of vascular endothelial cells, fibroblasts and keratinocytes. Exp Ther Med 5, 701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hiroshi Mizuno M. T. & Cagri Uysal A. Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem cells 30, 804–810 (2012). [DOI] [PubMed] [Google Scholar]
- Liu S. et al. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair.. Tissue Eng Part A17, 725 (2011). [DOI] [PubMed] [Google Scholar]
- Liang X. et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell transplantation 23, 1045–1059, doi: 10.3727/096368913X667709 (2014). [DOI] [PubMed] [Google Scholar]
- Ludwig A. K. & Giebel B. Exosomes: small vesicles participating in intercellular communication. The international journal of biochemistry & cell biology 44, 11–15, doi: 10.1016/j.biocel.2011.10.005 (2012). [DOI] [PubMed] [Google Scholar]
- Mathivanan S., Ji H. & Simpson R. J. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics 73, 1907–1920, doi: 10.1016/j.jprot.2010.06.006 (2010). [DOI] [PubMed] [Google Scholar]
- Singer A. J. & Cutaneous C. R. wound healing. N Engl J Med 341, 738–746 (1999). [DOI] [PubMed] [Google Scholar]
- Ghahary A. S. Y., Scott P. G. et al. Enhanced expression of mRNA for transforming growth factor-beta, type I and type III procollagen in human post-burn hypertrophic scar tissues. J Lab ClinMed 122, 465–473 (1993). [PubMed] [Google Scholar]
- Zhao Y. et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Acute Myocardial Ischemic Injury. Stem cells international 2015, 761643, doi: 10.1155/2015/761643 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of translational medicine 13, 49, doi: 10.1186/s12967-015-0417-0 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. et al. Exosomes: a role for naturally occurring nanovesicles in cancer growth, diagnosis and treatment. Current gene therapy 15, 182–192 (2015). [DOI] [PubMed] [Google Scholar]
- Svensson K. J. et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J Biol Chem. 288, 17713 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A. & Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends in cell biology 18, 199–209, doi: 10.1016/j.tcb.2008.03.002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M. & Raposo G. Exosomes--vesicular carriers for intercellular communication. Current opinion in cell biology 21, 575–581, doi: 10.1016/j.ceb.2009.03.007 (2009). [DOI] [PubMed] [Google Scholar]
- Corrado C. et al. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. International journal of molecular sciences 14, 5338–5366, doi: 10.3390/ijms14035338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. & Zhao R. C. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem cell reviews 8, 243–250, doi: 10.1007/s12015-011-9293-z (2012). [DOI] [PubMed] [Google Scholar]
- Hu L. et al. Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissuederived mesenchymal stem cells. Biomed Res Int (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao J. et al. The Effects of Macrophage-Stimulating Protein on the Migration, Proliferation, and Collagen Synthesis of Skin FibroblastsIn Vitroand In Vivo. Tissue Engineering Part A21, 982–991, doi: 10.1089/ten.tea.2013.0726 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.