Significance
Terminal differentiation of endocytosed rhizobia into nitrogen-fixing bacteroids inside of legume root nodules is orchestrated by a large family of host-encoded cysteine-rich signaling peptides. These peptides have diverse effects on bacteria, but their molecular mechanisms of action are still unknown. The presence of highly conserved cysteine motifs in this peptide family results in an additional layer of complexity because each individual peptide can potentially form several disulfide–cross-linked regioisomers under oxidative conditions. By demonstrating several distinct patterns of relative activities between the three disulfide–cross-linked regioisomers of NCR247, its reduced form, and a variant lacking cysteines, our work suggests that disulfide cross-linking can augment the rich complexity of Rhizobium-legume chemical communication required for symbiosis.
Keywords: host--microbe interactions, host peptides, signaling peptides, oxidative folding, regioisomers
Abstract
Interactions of rhizobia with legumes establish the chronic intracellular infection that underlies symbiosis. Within nodules of inverted repeat-lacking clade (IRLC) legumes, rhizobia differentiate into nitrogen-fixing bacteroids. This terminal differentiation is driven by host nodule-specific cysteine-rich (NCR) peptides that orchestrate the adaptation of free-living bacteria into intracellular residents. Medicago truncatula encodes a family of >700 NCR peptides that have conserved cysteine motifs. NCR247 is a cationic peptide with four cysteines that can form two intramolecular disulfide bonds in the oxidized forms. This peptide affects Sinorhizobium meliloti transcription, translation, and cell division at low concentrations and is antimicrobial at higher concentrations. By preparing the three possible disulfide–cross-linked NCR247 regioisomers, the reduced peptide, and a variant lacking cysteines, we performed a systematic study of the effects of intramolecular disulfide cross-linking and cysteines on the activities of an NCR peptide. The relative activities of the five NCR247 variants differed strikingly among the various bioassays, suggesting that the NCR peptide-based language used by plants to control the development of their bacterial partners during symbiosis is even greater than previously recognized. These patterns indicate that certain NCR bioactivities require cysteines whereas others do not. The results also suggest that NCR247 may exert some of its effects within the cell envelope whereas other activities occur in the cytoplasm. BacA, a membrane protein that is critical for symbiosis, provides protection against all bactericidal forms of NCR247. Oxidative folding protects NCR247 from degradation by the symbiotically relevant metalloprotease HrrP (host range restriction peptidase), suggesting that disulfide bond formation may additionally stabilize NCR peptides during symbiosis.
Establishment of the ecologically, agriculturally, and economically important nitrogen-fixing symbiosis between legumes and rhizobia requires a mutual exchange of signals between the plant and its endosymbiotic bacterial partner (1). Development of the symbiosis is initiated when particular flavonoids secreted by the legume host induce the secretion of bacterial Nod factors. Nod factors activate signal transduction networks in the host plant, establish the nodule meristem necessary for nodule growth, stimulate the root hair curling that entraps rhizobia, and stimulate expression of an exopolysaccharide receptor. Synthesis of one or more rhizobial exopolysaccharides is then required for growth of infection threads, plant-derived tubes that permit the entrapped rhizobia to passage down the root hairs and penetrate into the interior of the developing nodule. In the infection zone of the nodule (the nondividing cells just after the nodule meristem), the bacteria are endocytosed into host membrane compartments, termed symbiosomes, where they undergo the major physiological changes necessary to enable nitrogen fixation (1).
Medicago truncatula and Medicago sativa (alfalfa) are inverted repeat-lacking clade (IRLC) legumes that form indeterminate nodules. After being endocytosed into the plant cytoplasm, their symbiotic partner Sinorhizobium meliloti undergoes terminal differentiation into polyploid nitrogen-fixing bacteroids (2). It has been shown that one factor in promoting differentiation is the low levels of free oxygen in the plant cytoplasm due to leghemoglobin and the triggering of a hemoprotein sensor kinase, FixL, which starts a cascade to regulate nif gene transcription. A more recent advance in understanding the signaling events required for symbiosis has been the discovery that a family of >700 defensin-like nodule-specific cysteine-rich (NCR) peptides drive many of the complex physiological changes that characterize S. meliloti differentiation. These changes include alterations of the bacterial cell cycle to permit rounds of endoreduplication, major changes in patterns of transcription and translation, alterations of membrane permeability, and major morphological changes, including elongation and frequent branching (3–8). The NCR peptides are expressed only in nodules and are induced in successive waves as coordinated nodule development and rhizobial infection occur (5). The peptides are delivered to the symbiosome compartments through an endoplasmic reticulum-dependent secretion system that requires the action of the DNF1 nodule-specific signal peptidase (3, 4). The mature peptides are usually 20–55 aa long and vary greatly in their amino acid sequences; many can be clustered into two subgroups, NCR motif-A (four cysteines) and NCR motif-B (six cysteines) (9). Recent studies of two symbiotically defective M. truncatula mutants have shown that NCR169 (10) and NCR211 (11) are each required for a functional nitrogen-fixing symbiosis. Additionally, if S. meliloti carries a plasmid encoding the HrrP (host range restriction peptidase) metalloprotease capable of degrading NCR peptides, symbiotic outcomes are affected at a late developmental stage (12). Although NCR peptides clearly play signaling roles in symbiosis, some members are bactericidal at higher concentrations. The membrane protein BacA plays a critical role in symbiosis by protecting S. meliloti cells in the symbiosome from the potential bactericidal action of NCR peptides (13, 14).
Prokaryotes, as well as lower and higher eukaryotes, use peptides as signaling molecules, and the cysteine-rich peptides (CRPs) constitute one of the most important families of these molecules. Particularly well-studied CRPs include conotoxins, neurotoxins produced by cone snails, and defensins, important effectors of innate immunity in vertebrates, invertebrates, insects, and plants (15). Although, M. truncatula’s >700 NCR peptides are distinct from its ∼80 defensins (5), the shared structural feature of multiple disulfide bonds is thought to afford a compact structure and resistance to proteolytic and chemical degradation in both peptide families (16). Furthermore, reports indicate that reduced and oxidized forms of some CRP peptides have different biological activities (17–20). In principle, motif-A NCR peptides, which have four cysteine residues, can form three possible disulfide–cross-linked regioisomers whereas motif-B NCR peptides, which have six cysteine residues, can form 15 possible disulfide–cross-linked regioisomers. Nevertheless, there has been no systematic study of the relative effects of the various oxidized regioisomers and the reduced isoform on the symbiotic and bactericidal activities of any NCR peptide.
To address the issue of how NCR peptide structure and oxidation state relate to function, we chose the intensively studied NCR247 peptide (Fig. 1). NCR247 is composed of 24 aa, contains four cysteine residues, has a cationic charge (+5.6) at neutral pH, and also has the highest Boman index (4.6) of any of the NCR peptides (5). NCR247 is expressed in the older cells in the infection zone (zone II), where S. meliloti cells stop dividing and start differentiating, and also in interzone II and III, where a sudden growth of bacteroids is visible (21). Gene-expression profiling revealed that treatment of a synchronized S. meliloti population with a sublethal level of NCR247 affects ∼15% of the genome, including three symbiotically important regulons (7). Two of these regulons are controlled by the ExoS/ChvI and FeuQ/FeuP two-component systems, respectively, and the other by the master cell cycle regulator ctrA. Sublethal levels of NCR247 permit the cell to complete one round of DNA replication but then lead to inhibition of cell division, Z-ring formation, and septation (7). NCR247 also inhibits protein synthesis (21). Potential interacting partners of NCR247 by using affinity chromatography include FtsZ, numerous ribosomal proteins, the GroEL chaperone, and RNA polymerase units (21). Both the reduced isoform of NCR247 (NCR247red) and one disulfide-linked NCR247 regioisomer have been shown to be bactericidal at higher concentrations (3, 22).
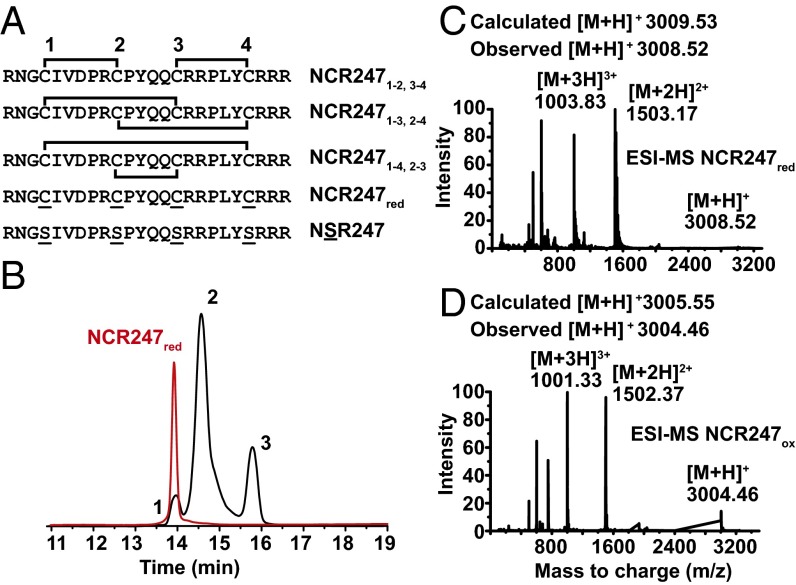
Fig. 1.
Generation and analyses of NCR247 regioisomers. (A) Primary sequences and illustration of disulfide bond linkages in NCR247ox. The brackets indicate the disulfide bond linkages in the oxidized peptides. (B) HPLC trace (10–60% acetonitrile over 30 min, 1 mL/min, 280 nm) showing that oxidation of NCR247red in 20% DMSO generated three new peaks, which are assigned as the NCR247ox regioisomers. (C and D) Deconvoluted ESI-MS spectra of NCR247red and NCR247ox variants indicate the oxidation state of the peptides.
Our results demonstrate that the particular disulfide–cross-linked regioisomers of NCR peptides and the reduced NCR peptides can differ strikingly in their abilities to cause various biological effects. Taken together, these observations suggest that the NCR peptide-based language that plants use to control the development of their bacterial partner during symbiosis may be more complex than currently appreciated.
Results
Preparation of the Three Disulfide-Linked Regioisomers of NCR247.
Previous studies of the biological activities of NCR247 have used the reduced isoform NCR247red (3, 22) or the disulfide-linked NCR2471-2,3-4 regioisomer (7, 14) (Fig. 1A). The latter form has been proposed (22) to be the isoform present in root nodules on the basis of Chou–Fasman predictions (14). To gain insights into the potential importance of disulfide bonds and cysteines to the biological activities of NCR247, we prepared the three disulfide-linked regioisomers of NCR247 (Fig. 1A). We then compared the biological activities of these oxidized peptides with those of NCR247red and also of NSR247 (22). NSR247 is an NCR247 variant that cannot form disulfide bridges because all four cysteine residues are replaced by serines.
We obtained NCR247red by Fmoc solid-phase peptide synthesis and used DMSO in oxidative folding assays. This mild oxidant has been used to fold basic and hydrophobic peptides, including human β-defensin 3 (23). We found that incubating NCR247red in a 20% (vol/vol) DMSO solution (room temperature for 20 h) resulted in the formation of three species, which could be separated by HPLC (high-performance liquid chromatography) (Fig. 1B). Mass spectrometry revealed that each peak was a new species that had an m/z value 4 units less than that of NCR247red. Thus, all three NCR247ox regioisomers formed under these conditions, each of which contains two intramolecular disulfide bonds (Fig. 1C and D).
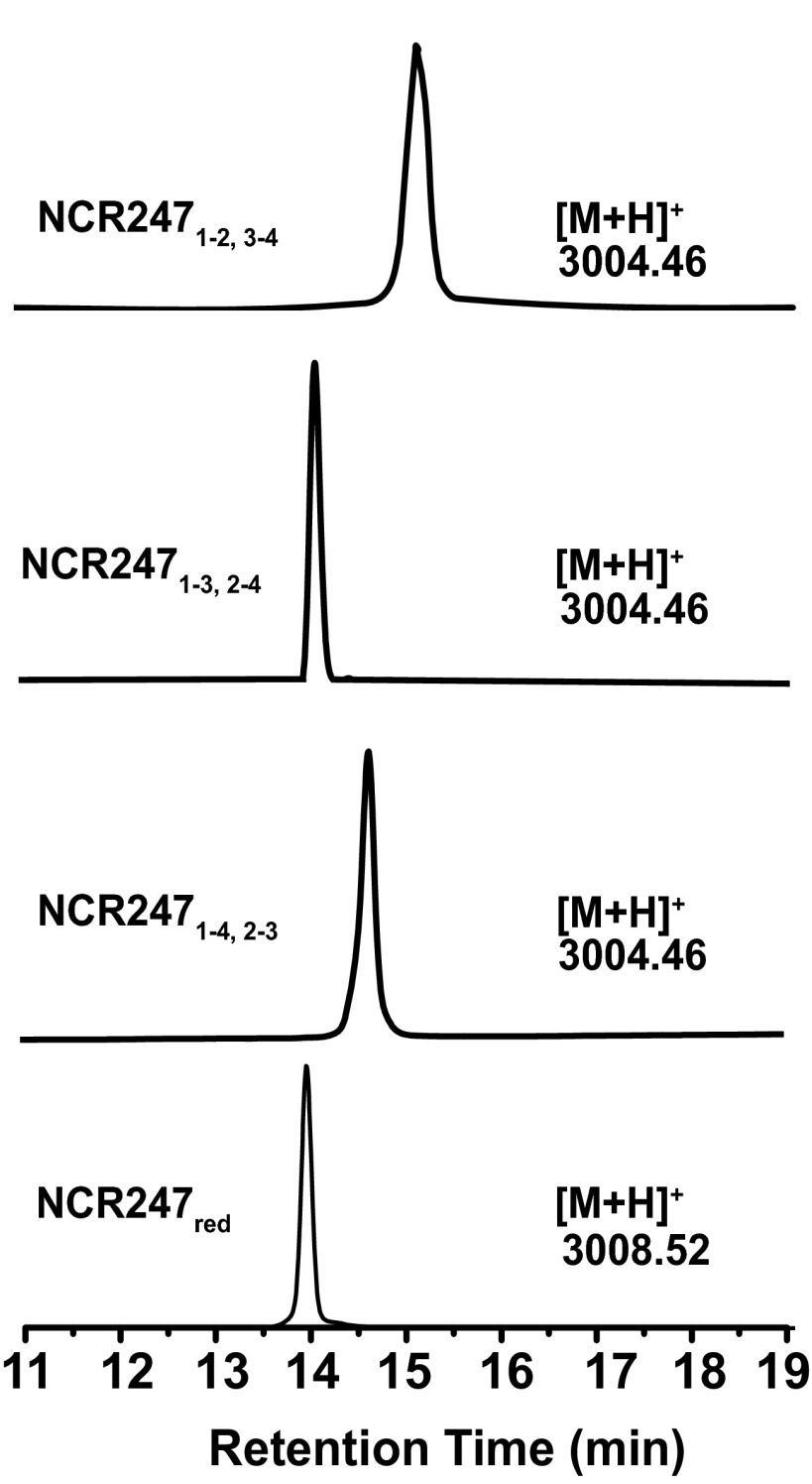
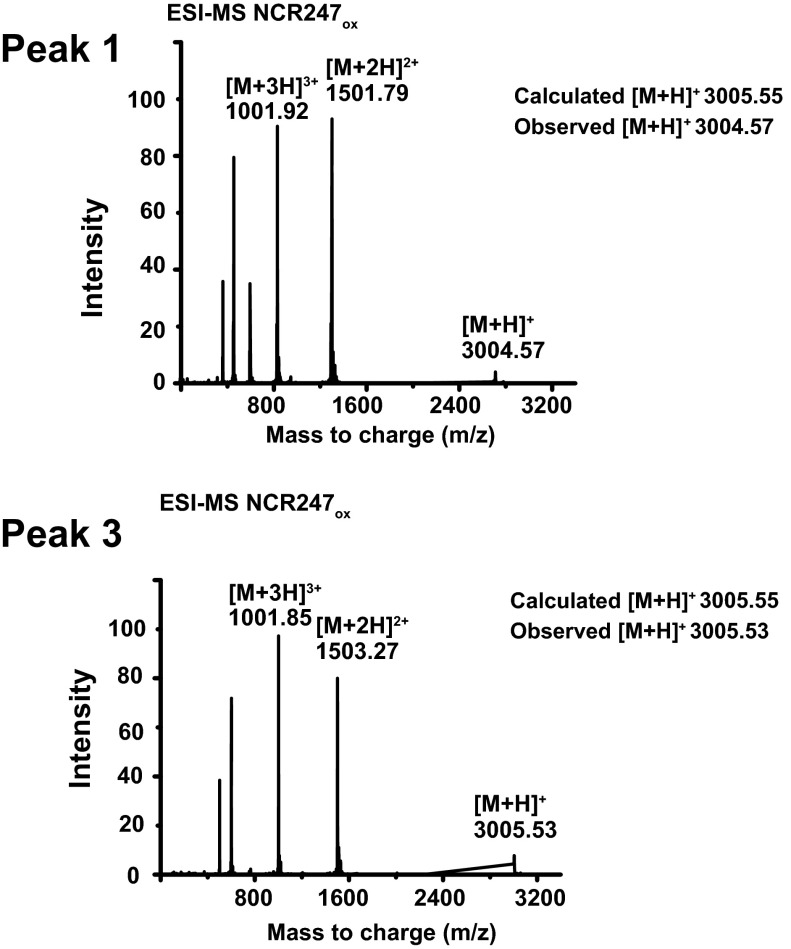
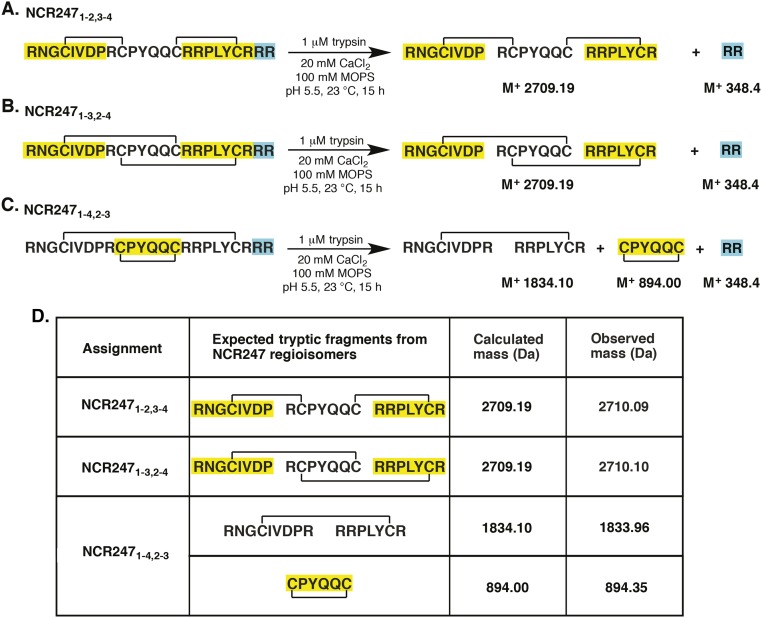
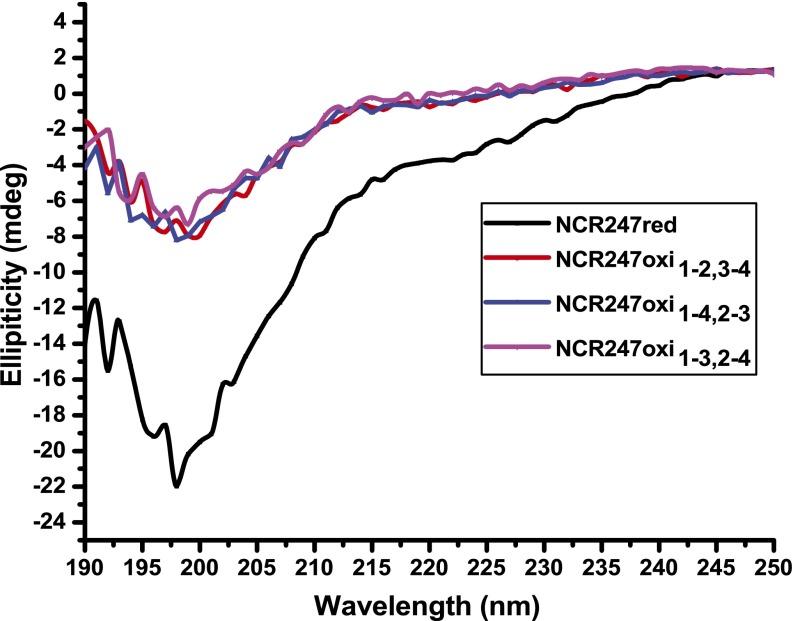
After HPLC separation and purification of each regioisomer (Figs. S1 and S2), we mapped the disulfide linkages of each peptide. We assigned the major peak (peak 2) to the NCR2471-4,2-3 regioisomer by identifying the peptide fragments generated by extended trypsin digestion (Fig. S3). To distinguish between NCR2471-2,3-4 and NCR2471-3,2-4, which yield peptide fragments of identical mass after trypsin digestion, we synthesized the NCR2471-2,3-4 regioisomer by orthogonal protection of cysteines and subsequent pairwise deprotection and oxidation to use as an HPLC standard. Authentic NCR2471-2,3-4 displayed the same HPLC retention time as peak 3, thereby allowing us to infer that peak 1 is the NCR2471-3,2-4 regioisomer (Fig. S1). Our results suggest that NCR2471-4,2-3 is the thermodynamically most stable regioisomer of oxidized NCR247, rather than NCR2471-2,3-4 as had been proposed (14). It is possible that NCR2471-4,2-3 may be the most abundant isoform present in M. truncatula nodules, but other factors could influence the in vivo distribution of regioisomers. Circular dichroism (CD) spectroscopy did not reveal classical secondary structure features in any of the disulfide–cross-linked regioisomers (Fig. S4).
Fig. S1.
RP-HPLC analysis of NCR247red and NCR247ox variants. Purified peptides were analyzed by C18 RP-HPLC with a linear gradient of 10–60% acetonitrile over 30 min. Observed mass values are shown in the figure.
Fig. S2.
Deconvoluted ESI-MS spectra of peak 1 and peak 3 indicate the oxidation state of peptides.
Fig. S3.
(A–C) Determination of the disulfide connectivity in NCR247ox regioisomers by trypsin digestion and mass spectrometry. (D) Scheme of theoretical trypsin digestion of NCR247ox variants.
Fig. S4.
CD spectra of NCR247 variants (20 μM) in 10 mM, pH 7.0 sodium phosphate buffer.
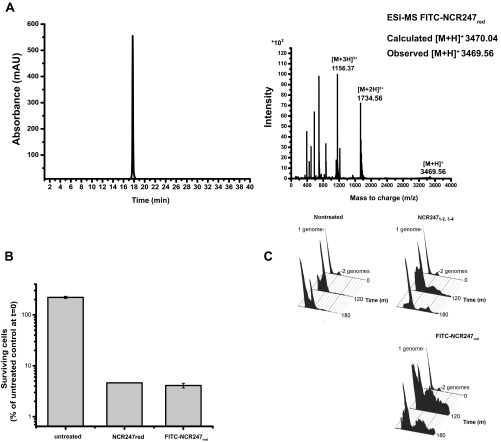
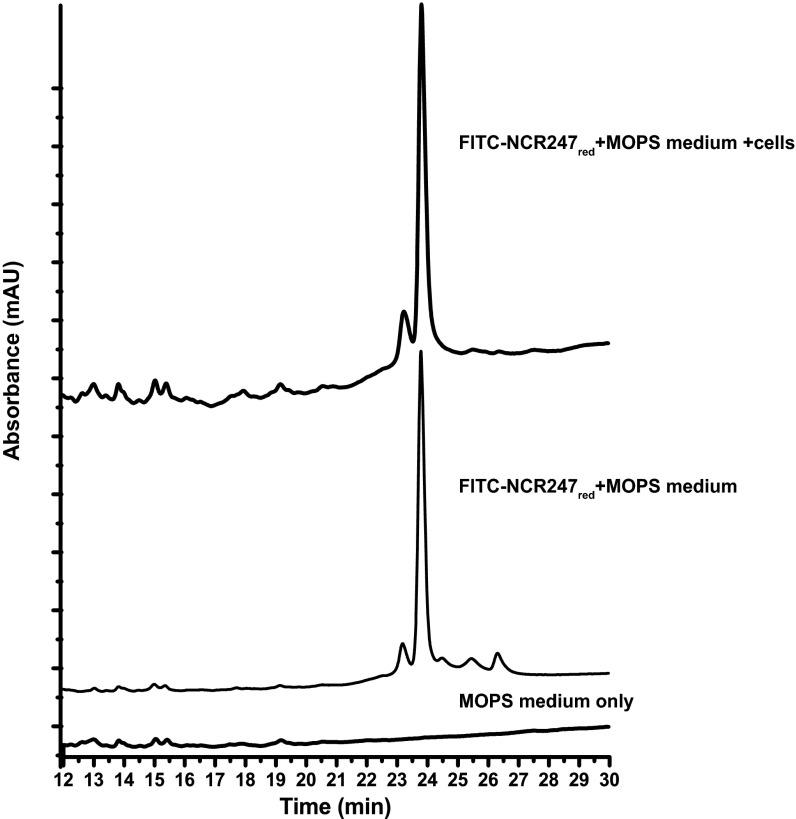
We also synthesized a derivative of NCR247red in which fluorescein isothiocyanate was conjugated to the N terminus (FITC-NCR247red). This derivative has cell division inhibition and bactericidal activity comparable with unmodified NCR247red (Fig. S5). Because the robust optical signal provided by the fluorophore allows detection of relatively low concentrations of the peptide by HPLC, we used this peptide to evaluate whether NCR247red stays reduced under our in vivo experimental conditions. After incubating a sublethal concentration (4 µM) of FITC-NCR247red aerobically with S. meliloti cells for 3 h, the supernatant was analyzed by HPLC and mass spectrometry. The retention time and mass of the peptide recovered from the supernatant were the same as FITC-NCR247red (Fig. S6). It is possible that some oxidation occurs within the bacterial cells; however, based on data presented below that indicate differences between oxidized and reduced forms in some bioassays, we reason that oxidation does not happen to a great extent.
Fig. S5.
(A) RP-HPLC and ESI-MS analysis of the reduced FITC-NCR247red peptide dissolved in Milli-Q water at a concentration of 0.5 mg⋅mL−1. ESI-MS analyses are in agreement with peptides of 3,465.05 Da and specify that FITC-NCR247red is in the reduced form. (B) Antibacterial activity of FITC-NCR247red at 20 µM in Mops-GS buffered medium. (C) Cell division inhibition activity of FITC-NCR247red at 6 µM.
Fig. S6.
FITC-NCR247red peptide is retained in the reduced state, when incubated with S. meliloti.
We then systematically examined the relative abilities of sublethal doses of the three disulfide-linked regioisomers of NCR247, NCR247red, and NSR247 to affect several physiological responses thought to be relevant to symbiosis: (i) the expression of representative ExoS/ChvI- and FeuQ/FeuP-controlled genes and the key regulatory gene ctrA (7), (ii) protein synthesis (21), and (iii) cell division, Z ring formation, and septum formation (3, 7). Additionally, we examined their relative bactericidal activities and resistance to proteolysis.
Effect of NCR247 Disulfide–Cross-Linked Regioisomers on ExoS/ChvI and FeuQ/FeuP Controlled Genes and ctrA.
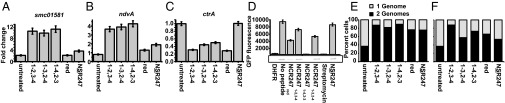
Our previous gene-expression profiling study of a synchronized population of S. meliloti treated with a sublethal concentration (4 μM) of NCR2471-2,3-4 (7) revealed the up-regulation of two symbiotically important regulons controlled by the ExoS/ChvI and FeuQ/FeuP two-component regulatory systems, respectively. The sensors, ExoS and FeuQ, are histidine kinases that phosphorylate their respective response regulators, ChvI and FeuP. The ExoS/ChvI regulon includes numerous genes required for the synthesis of the symbiotically important exopolysaccharide succinoglycan, as well as the known direct ChvI target smc01581 (24). The FeuQ/FeuP regulon includes ndvA, which encodes an ABC cyclic glucan exporter required for symbiosis (25). In contrast to up-regulation of these two regulons, our transcriptional analysis showed that a sublethal concentration of the NCR peptide decreased the expression of ctrA (7), which encodes a critical cell cycle regulator and master regulator of symbiosis (26).
Using quantitative PCR (qPCR) to compare the expression levels of smc01581 and ndvA genes after treatment of S. meliloti cultures with the NCR247 variants, we found that all disulfide–cross-linked regioisomers significantly up-regulated expression of both genes to similar extents. In contrast, NCR247red had no effect, and NSR247 had only low activity in up-regulating smc01581 and ndvA expression (Fig. 2 A and B). We observed a different pattern of the relative bioactivities of the five NCR247 peptides when we examined their effects on ctrA expression (Fig. 2C). All three oxidized NCR247 regioisomers were again equivalently bioactive, reducing ctrA expression to similar degrees, and NSR247 was inactive. However, in contrast to having no effect on the expression of the ExoS/ChvI- and FeuQ/FeuP-regulated genes, NCR247red was as active as the oxidized peptides in reducing ctrA expression. Taken together, these results reveal that the activity of the reduced isoform NCR247red relative to that of the oxidized NCR247 regioisomers depends on the biological attribute being assayed.
Fig. 2.
Treatment with NCR247 variants affects expression levels of genes regulated by the ExoS-ChvI, FeuQ-FeuP regulons and of the master cell-cycle regulator CtrA. (A and B) Fold change in smc01581 (A) and ndvA (B) expression relative to smc00128 after 15 min with no peptide or with a sublethal concentration (4 μM) of peptide-treated synchronized cultures. (C) The ctrA expression in nontreated and peptide-treated synchronized cultures relative to smc00128. (D) NCR247red and NCR2471-4,2-3 specifically inhibit protein synthesis in vitro. (D, Upper) NCR247 variants (40 μM) were added to the “in vitro translation reaction” mixture, and GFP fluorescence was monitored by using a fluorescence plate reader after 3 h (λex = 485 nm, λem = 530 nm). (D, Lower) In-gel fluorescence of GFP reporter protein after 3 h of treatment with peptide. (E and F) Level of cell division inhibition activity of NCR247 is affected by redox state and oxidative folding. (E and F) DNA content per cell during synchronized growth of S. meliloti cultures treated with or without 4 μM (E) and 2 μM (F) of NCR247 variants. DNA content per cell during synchronized growth of S. meliloti cultures treated with or without NCR247 variants. Flow cytometry was used to measure the percentage of cells with one genome or two genomes. All error bars represent SD from the mean, based on triplicate experiments.
NCR247 Regioisomers Selectively Inhibit Bacterial in Vitro Translation.
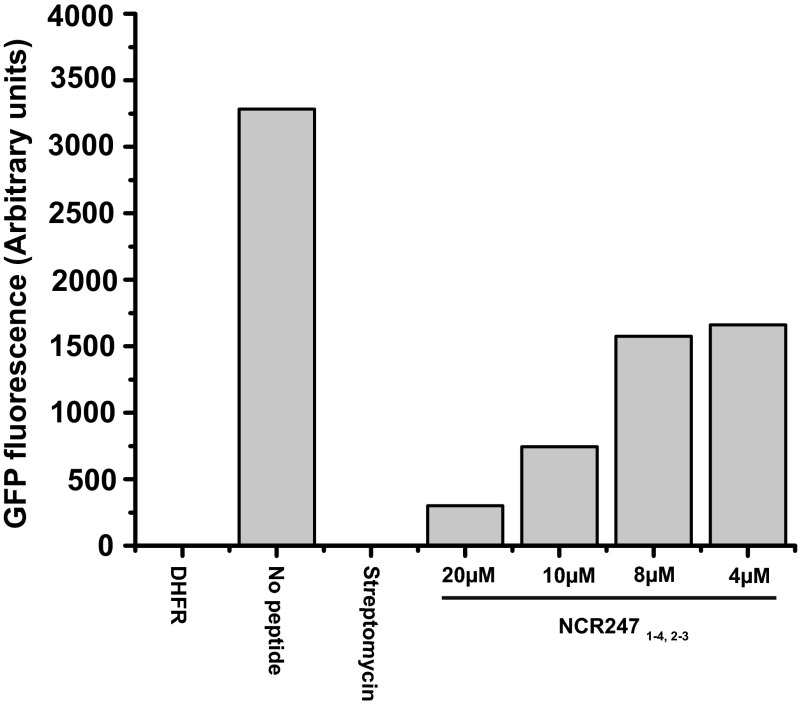
The protein translation machinery is one of the major targets of several signaling peptides in plant–microbe and insect–microbe symbioses (27). Farkas et al. (21) observed that the set of NCR247-interacting proteins identified by affinity chromatography includes ribosomal proteins and subsequently discovered that NCR247red can inhibit protein synthesis in vitro (21). Consequently, we sought to determine whether the other NCR247 variants inhibit protein synthesis. Guided by the reported assay (21), we used an in vitro translation system in which translation of green fluorescent protein (GFP) mRNA can be monitored by measuring GFP fluorescence. We confirmed that NCR247red (40 μM) substantially inhibits protein synthesis and observed that the relative abilities of the NCR247 variants to inhibit translation were strikingly different from those we had observed in our transcription assays (Fig. 2D). NCR2471-2,3-4 and NCR2471-3,2-4 inhibited translation, albeit to a lesser degree than NCR247red. In striking contrast, NCR2471-4,2-3 was highly effective at inhibiting the translation of GFP (Fig. 2D) and could even interfere with translation at the sublethal level of 4 μM (Fig. S7). Moreover, NSR247 was less effective at inhibiting translation compared to NCR247red, which indicates that the cysteine residues are generally important but not essential for this activity (Fig. 2D).
Fig. S7.
NCR2471-4,2-3 (20 μM, 10 μM, 8 μM, and 4 μM) was added to the “in vitro translation reaction” mixture, and GFP fluorescence was monitored by using a fluorescence plate reader after 3 h (λex = 485 nm, λem = 530 nm).
NCR247 Cell Division Inhibition Activity Is Independent of Disulfide Connectivity, and Cysteines Are Not Required.
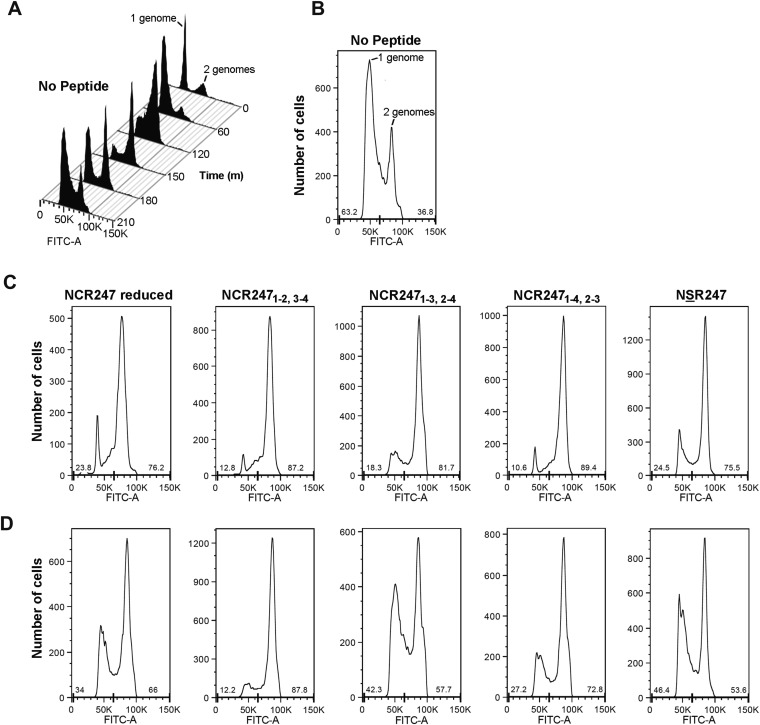
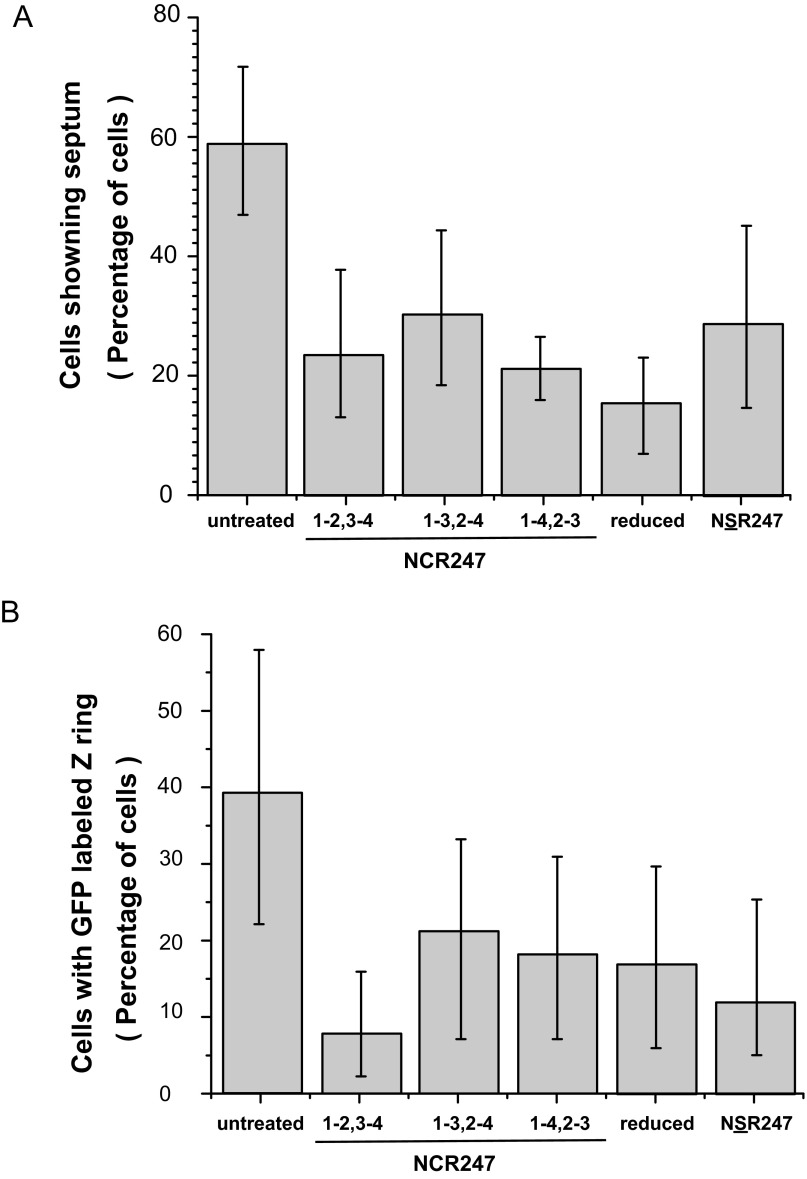
We have previously shown that a sublethal concentration (4 μM) of NCR2471-2,3-4 does not alter the initiation or progression of DNA replication, such that the cellular DNA content increases from 1C to 2C, for synchronized S. meliloti cultures at the G1 phase. However, NCR2471-2,3-4 specifically and robustly blocks cell division in S. meliloti in G2 (7). At the sublethal dose of 4 µM, the three NCR247ox regioisomers, NCR247red, and NSR247 all exhibited similar abilities to prevent the 2C cells from dividing to yield daughter cells with 1C DNA content (Fig. 2E). Lowering the peptide concentration to 2 µM revealed that one of the minor regioisomers, NCR2471-2,3-4, was somewhat more active than the other peptides (Fig. 2F and Fig. S8). We have previously shown that NCR2471-2,3-4 causes an early block in cell division because very little septation is observed and Z-ring formation is inhibited (7). The other two NCR247ox regioisomers, NCR247red, and NSR247, also inhibited the appearance of septa to a similar degree (Fig. S9A). Moreover, when we used an inducible FtsZ-GFP translational fusion protein to visualize the Z-ring in cells in synchronous cultures at the G2 phase (7), we found that each of the five NCR247 variants (4 μM) exhibited similar abilities to inhibit Z-ring formation (Fig. S9B).
Fig. S8.
The level of cell division inhibition activity of NCR247 is affected by redox state and folding. (A) Flow cytometry data of synchronized, nontreated cells progressing through the cell cycle. The DNA stain SYTOX green was used to monitor DNA content per cell during the cell cycle. (B) Distribution of nontreated cells with 1 genome or 2 genomes after 210 min of incubation. The percent of cells with 1 genome or 2 genomes is shown at the bottom of the plot. (C and D). Distribution of cells with 1 genome or 2 genomes after 210 min of incubation with 2 μM (D) or 4 μM (C) NCR247red, NCR2471-2,3-4, NCR2471-3,2-4, NCR2471-4,2-3, or NSR247 peptide. The percent of cells with 1 genome or 2 genomes is shown at the bottom of the plots.
Fig. S9.
Treatment with NCR247 variants blocks cell division by antagonizing Z-ring formation or stability. (A) Frequency of septating cells in nontreated and NCR247-treated synchronized cultures at the G2 phase (120 min) of the cell cycle. Treated bacteria analyzed with a light microscope at (G2 phase) 120 min after treatment. Data represent >1,000 cells from one experiment. (B) Relative abundance of cells with FtsZ-GFP–labeled Z-ring in synchronized cultures treated with or without 4 μM NCR247 variants. Treated bacteria were analyzed with a fluorescence microscope at (G2 phase) 120 min after treatment. Isopropyl β-d-1-thiogalactopyranoside (0.125 mM) was added at time 0 to induce FtsZ-GFP expression. Error bars indicate SD.
Taken together, these results show that the pattern of activities of the various NCR peptides in inhibiting cell division differs from the activity patterns obtained from the transcription and translation bioassays. Even though NCR2471-2,3-4 was the most active at 2 µM, the fact that the three disulfide–cross-linked regioisomers had approximately the same activity as NCR247red at 4 µM indicates that disulfide bond formation is not crucial. Our observation that the NSR247 peptide is active further indicates that none of the cysteines are required for inhibition of cell division, thereby suggesting a different underlying molecular mechanism from those involved in regulation of transcription and translation.
Disulfide Cross-Linking Influences the Relative Bactericidal Activities of NCR247 Peptides in Wild-Type S. meliloti.
Although both NCR247red and NCR2471-2,3-4 induce bacteroid-like features in free-living S. meliloti at sublethal concentrations (3, 7, 14), both peptides reduce the colony-forming ability of S. meliloti at higher concentrations (14, 22). We compared the bactericidal activity of the three NCR247ox regioisomers (Fig. 3A) and observed that NCR2471-4,2-3 and NCR2471-2,3-4 are both ≥10-fold less active than NCR247red and NCR2471-3,2-4. In this respect, our results for NCR2471-3,2-4 differ from those of Haag et al. (22), who reported that NCR2471-3,2-4 is less bactericidal than NCR2471-2,3-4 (22). However, they used a preparation of NCR2471-3,2-4 that was known to be impure (22), and we now have shown that the impurities in a sample of their peptide are not other NCR247ox regioisomers as they had speculated (22) (Fig. S10). Thus, our data indicate that disulfide bonds are not required for NCR247 to be bactericidal against S. meliloti although they can modulate its activity. Of the oxidized species, the most abundant regioisomer, NCR2471-4,2-3, has the weakest bactericidal activity against S. meliloti, an observation of potential physiological significance. NSR247 has negligible bactericidal activity (Fig. 3A), which indicates that one or more cysteines residues are important for the bactericidal activity of NCR247 against S. meliloti even though disulfide bonds do not seem to be required.
Fig. 3.
Antimicrobial activity of NCR247 depends on the regioisomer. (A) S. meliloti Sm1021 WT and ΔbacA mutant strains were treated with 20 μM NCR247 variants for 180 min in MOPS-GS buffer supplemented with 1% casamino acids. (B) E. coli MG1655 WT and ΔsbmA mutant strains were treated with 6 μM NCR247 variants for 180 min in 10 mM sodium phosphate buffer (pH 7.0). (C) Bars show the relative survival of the defined S. meliloti strains with either empty control plasmid pRK404, plasmid pAI351 harboring the E. coli sbmA gene, or plasmid pJG51A harboring the S. meliloti bacA gene. Cells were treated with 20 µM NCR2471-2,3-4 for 180 min in MOPS-GS buffer supplemented with 1% casamino acids. Bars show bacterial survival assessed relative to the cfu⋅mL−1 at t = 0 min. All error bars represent mean ± SD (n = 3) based on three technical replicates representing trends observed in at least two independent experiments. Significant values are **P ≤ 0.01 and ***P ≤ 0.001.
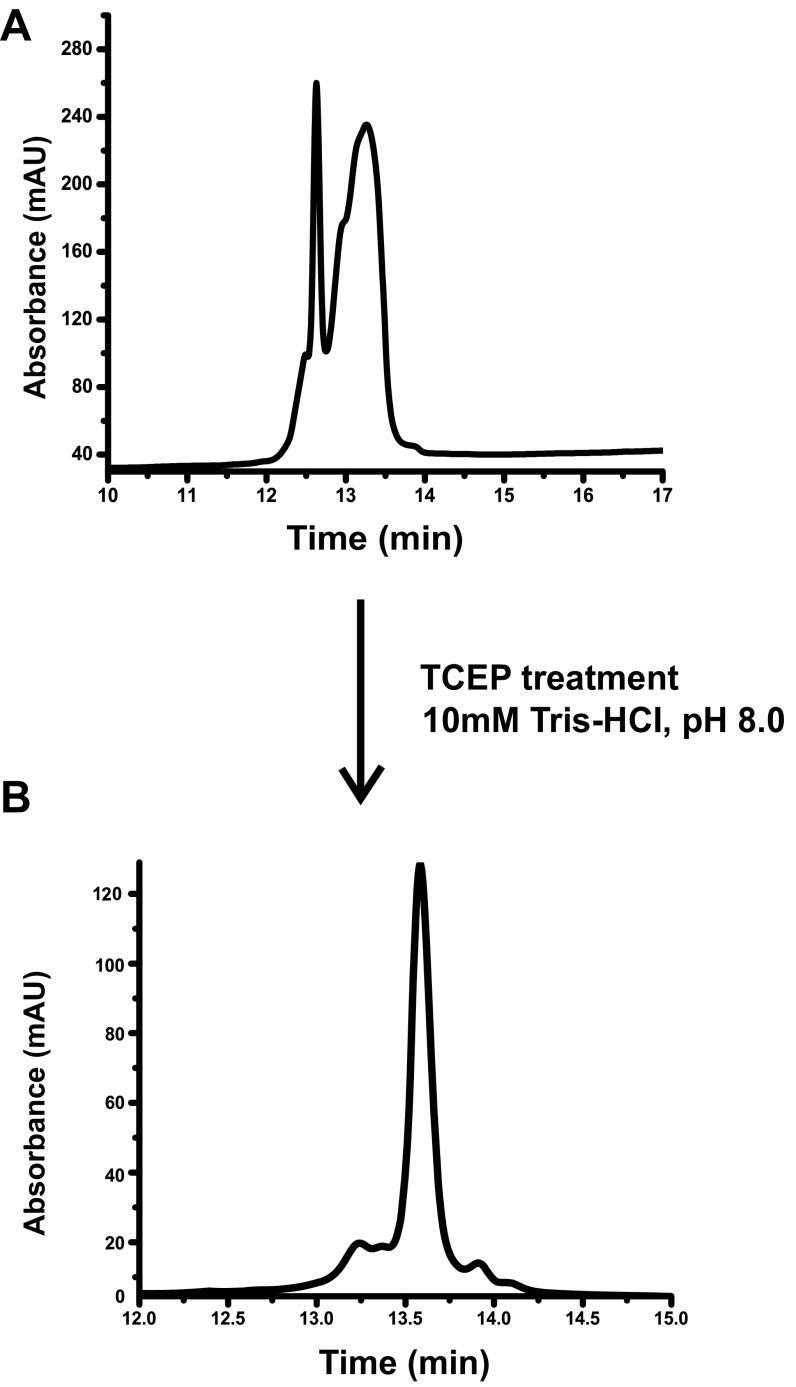
Fig. S10.
(A) RP-HPLC of the NCR2471-3,2-4 peptide used in a previous study (22) dissolved in Milli-Q water at a concentration of 166 µM. (B) RP-HPLC of NCR2471-3,2-4 peptide dissolved in 10 mM Tris⋅HCl, pH 8.0, at a concentration of 0.5 mg⋅mL−1 and treated with 1 mM TCEP for 15 min. After 15 min, the reaction was quenched by addition of 6% vol/vol TFA in Milli-Q water to afford 2% vol/vol TFA.
BacA Function Attenuates the Bactericidal Activity of All NCR247 Regioisomers and NCR247red Against S. meliloti.
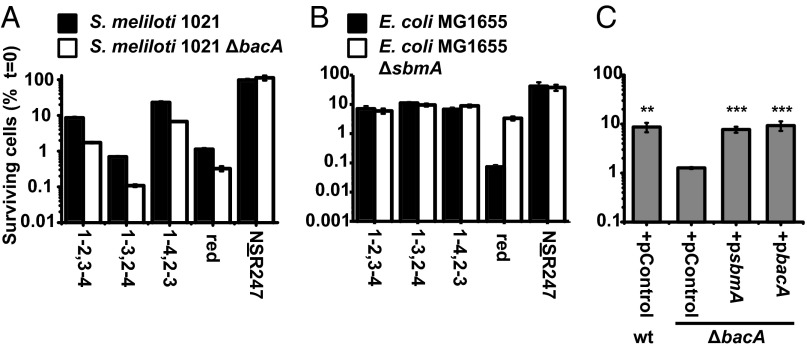
The membrane protein BacA is required for S. meliloti to establish the chronic intracellular infection that underlies its symbiosis (13) and also for the related mammalian pathogen Brucella abortus to establish the chronic intracellular infection that underlies its pathogenesis (28). We found that an S. meliloti ΔbacA mutant also displayed increased sensitivity to NCR2471-3,2-4 and NCR2471-4,2-3, as well as NCR247red. Thus, our results indicate that the molecular mechanism for BacA-mediated resistance of S. meliloti to bactericidal NCR247 peptide works equally well for all conformations of the NCR247 peptide.
As shown for NCR2471-2,3-4, the sensitivity of the ΔbacA mutant to killing by all of the NCR247 peptides can be restored to that of a WT strain by expressing a plasmid-borne copy of the S. meliloti bacA+ gene or its isofunctional Escherichia coli ortholog, sbmA+ gene (Fig. 3C). Interestingly, despite E. coli SbmA being isofunctional with BacA when expressed in S. meliloti (29), we observed that an E. coli ΔsbmA mutant did not display increased sensitivity to killing by any of the NCR247ox regioisomers and was more resistant, rather than more sensitive, to NCR247red (Fig. 3B). E. coli ΔsbmA mutants have previously been shown to exhibit resistance to killing by several proline-rich peptides. The mechanism of S. meliloti’s BacA-mediated resistance to NCR247 will require further investigation and may be complex.
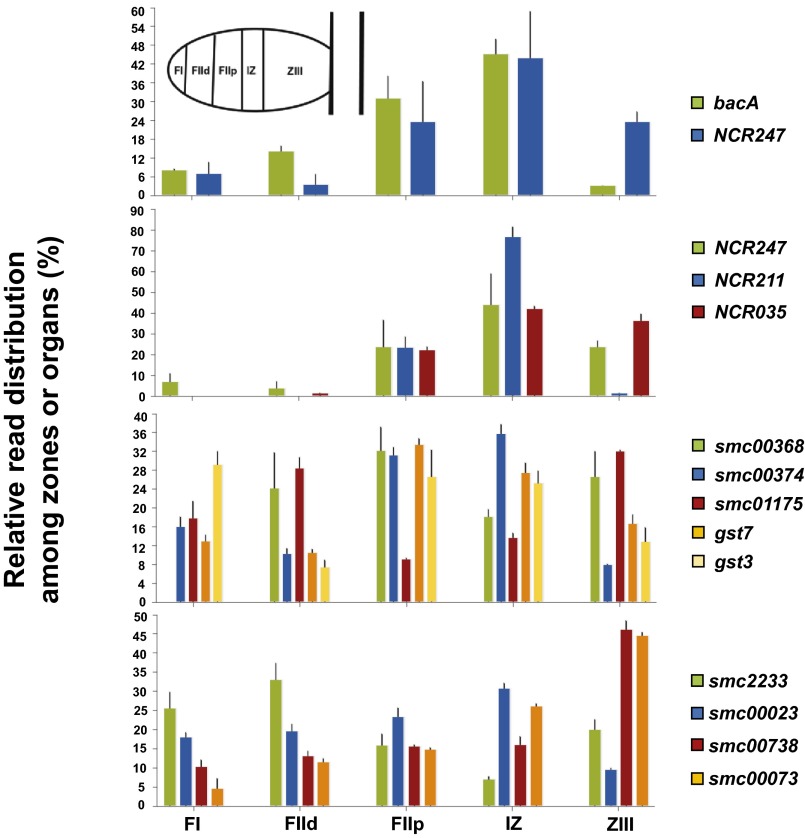
Indeed, BacA/SbmA has been shown to affect both the modification of S. meliloti and B. abortus lipid A by very long chain fatty acids (VLCFAs) and import of proline-rich peptides. Furthermore, the differing responses of the S. meliloti bacA and E. coli sbmA mutants to NCR247 peptides indicate that the physiological role of the conserved BacA/SbmA protein in a particular bacterium can also be influenced by other cellular functions. As symbiosis proceeds, the expression of NCR247 and two other intensively studied NCR peptides (NCR211 and NCR169) roughly coincides with bacA, consistent with BacA protecting the nascent bacteroids from the bactericidal property of the NCR247 peptide. The expression of genes encoding functions that could influence the properties and functions of NCR peptides, such as bacterial periplasmic disulfide, oxidoreductases, and glutathione-S-transferases, also varies during the development of the symbiosis (30) (Fig. S11).
Fig. S11.
Gene expression patterns for several bactericidal NCR peptides, bacA, disulfide isomerases, oxidoreductases, and glutathione S-transferases (GST) enzymes. Data were generated using the laser capture microdissection/RNA seq approach published by Roux et al. (30).
Disulfide Bonding in NCR247 Protects Against Degradation by HrrP.
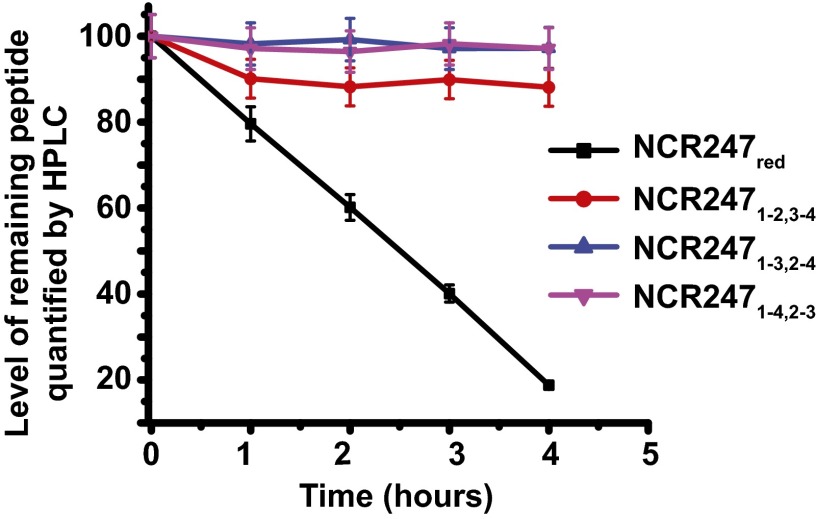
Although NCR peptides have been proposed to be oxidized in planta (9), our results confirm that NCR247red is active in several symbiotically related bioassays. A possible role for disulfide cross-linking of NCR peptides could be to stabilize them against proteolytic degradation (31). Expression of the plasmid-encoded metalloprotease HrrP, which is capable of degrading a range of NCR peptides in vitro, has recently been shown to restrict nodule development and nitrogen fixation of certain plant hosts (12). To examine whether disulfide bond formation modulates the proteolytic stability of NCR247, we performed degradation assays using HrrP and catalytically inactive variant HrrP (E62A). The assays were done using the oxidized and reduced NCR variants as substrates. All three NCR247ox regioisomers were markedly less susceptible to degradation by HrrP than NCR247red (Fig. 4). At a time point when NCR247red was 80% degraded, NCR2471-3,2-4 and NCR2471-4,2-3 exhibited virtually no degradation, and NCR2471-2,3-4 was only 10% degraded. Thus, in addition to the effects that disulfide cross-linking may have on various specific bioactivities of the NCR peptides, our results are consistent with the disulfide bonds also providing resistance to proteolytic degradation during the development of the symbiosis.
Fig. 4.
Disulfide bonding in NCR247 is important for peptide stability against HrrP. NCR247ox (40 μM) and NCR247red (40 μM) incubated with 2.5 µg of HrrP for various time points at 30 °C. Enzymatic activity was determined by the decrease in substrate concentration compared with matched assays using HrrP (E62A). Time course represents the percentage of the remaining peptide as a function of time.
Discussion
In this work, we report a systematic investigation of how disulfide bond formation affects the biological activities of an NCR peptide. Using chemical synthesis, we prepared the three possible oxidized regioisomers of M. truncatula NCR247 and compared the activities of these peptides with NCR247red and NSR247 in a variety of bioassays related to symbiosis. Our studies revealed that the relative activities of these NCR247 variants are strikingly different depending on the bioassay. More than 700 NCR peptides have been identified to date, and this work indicates that disulfide cross-linking provides an additional source of structural and functional diversity. Thus, the subtleties and complexities of how plant NCR peptides manipulate and fine-tune activities of their endosymbiotic partner may be even greater than previously recognized. Our observations are consistent with a growing body of evidence that the disulfide–cross-linked and reduced forms of defensins can have different biological activities (20). Our results also suggest that disulfide cross-linking may be important because it protects the NCR peptides from degradation by host or bacterial proteases during symbiosis.
Several distinct patterns of relative activities of the NCR247 variants for symbiotically relevant functions are evident in our data. One pattern is seen for genes controlled by the ExoS/ChvI and FeuQ/FeuP two-component regulatory systems. The three NCR247ox regioisomers exhibit similar inducing activity whereas NCR247red and NSR247 are inactive or have only weak activities. Both the ExoS and FeuQ sensors span the membrane and have a periplasmic sensing domain and a cytoplasmic domain that activates their cognate response regulator. One possible explanation for our results is that disulfide cross-linking protects the peptide from proteolytic degradation from bacterial outer membrane, periplasmic, or membrane proteases, so that it can act in the periplasm or membrane to activate the sensors, whereas the non–cross-linked forms are either rapidly degraded in the periplasm or membrane or imported into the cytoplasm. Another possibility is that the oxidized peptides affect the cellular redox balance in a manner that can be detected directly or indirectly by the ExoS/ChvI and FeuQ/FeuP two-component regulatory systems. The similar activities of the three regioisomers suggest that they do not act by binding to one special site on a regulatory protein. However, we cannot rule out the possibility of a rapid interconversion between the regioisomers catalyzed by a periplasmic disulfide isomerase, with one particular regioisomer being capable of such binding.
A second pattern was seen with respect to the reduction of ctrA expression, with all three NCR247ox regioisomers and NCR247red being approximately equally effective, but NSR247 being inactive. Because many of the key upstream regulators of ctrA expression are cytoplasmic proteins (26, 32), these observations are consistent with models in which the effects of NCR247 are exerted after it has entered the cytoplasm (21) and the disulfide–cross-linked regioisomers become reduced. Nevertheless, our data suggest that at least one cysteine of NCR247 is required for ctrA regulation.
A third pattern was observed in the assays for inhibition of cell division, Z-ring formation, and septation, with all five NCR247 variants exhibiting similar activity. Because the oxidized forms of NCR peptides are likely to be reduced upon entry into the cytoplasm, this pattern is consistent with the reduced form acting inside the cell. Notably, unlike ctrA regulation, there is no apparent requirement for the NCR247 peptide to contain a cysteine for these activities. These observations are consistent with the suggestion by Farkas et al. (21) that NCR247 may exert some of its effects by interacting with intracellular proteins such as FtsZ. The high Boman index of NCR247 is consistent with the evidence that it is capable of interactions with multiple partner proteins (21).
A fourth pattern is evident in the case of inhibition of in vitro translation: NCR2471-4,2-3 is a potent inhibitor, NCR247red and NSR247 are less active inhibitors, and the other two regioisomers are very weak inhibitors. These data suggest that NCR2471-4,2-3 binds to a specific target in the translational apparatus that is poorly bound by the other two regioisomers because of their differing 3D shapes and that NCR247red and NSR247 may be able to adopt conformations similar to that of NCR2471-4,2-3 when they bind to this target. As noted, one would expect oxidized forms of NCR247 to be converted to NCR247red upon entry into the cytoplasm. However, rhizobia are subjected to oxidative stress while establishing the symbiosis (33), and prolonged exposure to NCR247 might possibly lead to oxidative stress, as has been reported for certain antimicrobial peptides. Bessette et al. (34) have shown that the bacterial cytoplasm can be made sufficiently oxidizing to allow the formation of native disulfide bonds without compromising cell viability.
The pattern of the relative activities of the NCR247 variants for bactericidal action has some resemblance to that for ctrA regulation, in that all three regioisomers and NCR247red are active whereas NSR247 is inactive. However, in this case, NCR2471-4,2-3 and NCR2471-2,3-4 are both at least ten times less active than NCR247red and NCR2471-3,2-4. Thus, not only does the specific nature of the disulfide cross-linking influence the bactericidal potency of the NCR247 regioisomer, but also the NCR247 peptide must contain one or more cysteines to kill cells even when it is reduced.
The exquisite potency and selectivity that conotoxins display for a range of ion channels and receptors illustrates the high degree of biological specificity that can be achieved by cysteine-rich peptides. NCR peptides have been shown to be undergoing rapid and recent evolution, which suggests that the family of NCR genes is rapidly changing to adapt to different environments and to acquire new functions (35). Nevertheless, the conservation of the 4- and 6-cysteine motifs indicates their importance for NCR function. Horváth et al. (10) have shown that replacement of any of the four cysteines in NCR169 prevents it from carrying out its role in symbiosis. However, it is not yet clear whether this observation is due to an effect on peptide stability, peptide signaling functions, or both.
Antimicrobial peptides are an ancient component of the innate immune system whereas the legume–Rhizobium symbiosis arose only about 60 million years ago. Furthermore, the involvement of NCR peptides in the host–microbe interaction is thought to have been a relatively recent innovation within a subclass of legumes (35) that may lead to more efficient nitrogen fixation or to increased release of rhizobia into the environment after senescence (5). Thus, the bactericidal activity that some of the NCR peptides exhibit at relatively high concentrations may simply reflect their evolutionary relationship to defensins (35, 36). Nevertheless, it is also possible that this activity may help the plant to maintain an internal monoculture by killing any susceptible bacterium that manages to coinvade along with the rhizobial symbiont. Along these lines, the BacA protein plays a critical role in symbiosis by preventing the rhizobia from being killed by the concentrations of NCR peptides present in the symbiosome, so that the signaling roles of the NCR peptides can predominate.
NCR peptides affect multiple components of bacterial physiology to achieve a successful symbiosis, some inducing the final terminal differentiated state and others affecting bacteroid persistence. Some NCR peptides may work cooperatively with other NCR peptides to exert positive or negative effects on affected pathways whereas multiple NCR peptides may bind to the same target to achieve their ultimate biological effects. Although some NCR peptides are likely to be at least partially functionally redundant with other members of the large NCR peptide family, at least two have unique functions essential for symbiosis (NCR169 and NCR211) (10, 11). Which physiological functions require peptide interactions with membranes and which may require interactions with specific cellular proteins remain to be clarified. Cationic peptides can disrupt cellular membranes, consequently some cationic NCR peptides may affect membrane permeability, leading to physiological outcomes and also permitting other classes of NCR peptides to enter the bacteria. Interactions of an NCR peptide with a specific protein target would probably be strongly affected by the pattern of disulfide cross-linking.
Materials and Methods
Detailed materials and methods can be found in SI Materials and Methods.
Bacterial Growth Conditions.
For all experiments, S. meliloti 1021 WT and ΔbacA-null mutant strains were grown in Lysogeny broth (LB) prepared with 5 g⋅L−1 NaCl and supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4 (LB-MC) for 48 h at 30 °C. E. coli WT and ΔsbmA-null mutant strains were grown in LB (5 g⋅L−1 NaCl) at 37 °C. Unless indicated otherwise, S. meliloti cultures were supplemented with 200 μg⋅mL−1 streptomycin (Sm).
Folding and Disulfide Formation.
The folding reaction typically proceeded at room temperature overnight in a sealed vial with gentle stirring. Oxidation of NCR247red was done in 20% DMSO in water (23, 37).
SI Materials and Methods
Bacterial Growth Conditions.
For all experiments, Sinorhizobium meliloti 1021 WT and ΔbacA-null mutant strains were grown in Lysogeny broth (LB) prepared with 5 g⋅L−1 NaCl and supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4 (LB-MC) for 48 h at 30 °C. Escherichia coli WT and ΔsbmA-null mutant strains were grown in LB (5 g⋅L−1 NaCl) at 37 °C. Unless indicated otherwise, S. meliloti cultures were supplemented with 200 μg⋅mL−1 streptomycin (Sm).
Solid-Phase Peptide Synthesis of NCR247 and Variants.
The synthesis of NCR247 and its variants was performed using a semiautomated approach with a custom-made stainless steel fritted reaction vessel on a 0.038-mmol scale (37). Standard solid-phase methodology for Fmoc chemistry was used, and preloaded Fmoc-Arg (Pbf)-NovaSyn TGA resin was used (0.19 mmol/g loading). Fmoc-protected amino acids (1 mmol) were coupled at 60 °C after dissolution in 2.5 mL of dimethylformamide (DMF) containing 0.38 mM HBTU (2-(1H-benzotriazol-1-yl)-1, 1, 3, 3-tetramethyluronium hexafluorophosphate). After addition of DIPEA (N, N-diisopropylethylamine) (200 µL for Cys, 450 µL for all other amino acids), the activated amino acid was administered to the warmed reactor at a flow rate of 6 mL/min, and then the resin was washed with DMF (60 s at 20 mL/min). The Fmoc was removed with 20% piperidine in DMF (25 s at 20 mL/min), and the resin was washed with DMF (70 s at 20 mL/min).
After coupling the N-terminal Fmoc-Arg (Pbf)-OH, Fmoc deprotection was performed using 20% piperidine in DMF, and the resin was washed with DMF and transferred to a 50-mL centrifuge tube. The resin was dried, and global deprotection was performed using 20 mL of solution containing a TFA:1, 2-ethanedithiol (EDT):H2O:triisopropylsilane (TIS) (94:2.5:2.5:1) mixture for 10 min at 60 °C. The mixture was concentrated to a volume of ∼5 mL by using a gentle stream of nitrogen. Ice-cold diethyl ether was added to the resulting concentrate, which resulted in precipitation of the crude peptide, and the mixture was centrifuged in a Beckman Coulter GS-6R rotor (3,500 rpm × 15 min, 4 °C). The organic supernatant was decanted, and the pelleted precipitate was redissolved in 5 mL of 5% TFA in 3:1 H2O/MeCN. Preparative RP-HPLC purification (10–60% B acetonitrile over 30 min, 10 mL/min) afforded reduced NCR247 (25 mg, 18% overall yield).
The combination of the cysteine thiol protecting groups trityl (Trt) and acetamidomethyl (Acm) was used for the regioselective formation of NCR2471-2,3-4 regioisomer (38). Trt was used for cysteine at positions 3-4, and Acm was used for cysteine at positions 1-2. To create the first disulfide bridge in NCR247 between the third and fourth cysteine residues, the Trt groups were selectively removed using TFA:EDT:H2O:TIS (94:2.5:2.5:1) as described above. The purified peptide solution (30 mL) was then diluted with a 1:2 mixture of water-DMSO, and the solution was stirred for 24 h. The folding was monitored by analytical RP-HPLC. Complete conversion to the Acm-protected NCR2473-4 peptide was confirmed by LC-MS analysis of the reaction mixture. Once folding was complete, the peptide was purified by semipreparative RP-HPLC. Semipreparative HPLC was performed on a Zorbax C-18 column (5-μm pore, 9.4 × 250 mm, Agilent Technologies, Inc.) at a flow rate of 4 mL/min. For semipreparative HPLC, solvent A was Milli-Q water containing 0.1% TFA that was passed through a 0.2-μm filter before use and solvent B was HPLC-grade MeCN containing 0.1% TFA. To create the second disulfide bridge between the first and second cysteine residues, the purified peptide (10 mg) was dissolved in acetic acid (30 mL) and iodine (17 mg) was then added, and the reaction was stirred at room temperature for an additional 60 min. During this time, the reaction was monitored by RP-HPLC. Removal of the Acm groups and complete formation of the second disulfide bond to afford NCR2471-2,3-4 was confirmed by liquid chromatography–mass spectrometry (LC-MS). The excess iodine was quenched by 2 mL of ascorbic acid (1 M in water), and the solvent was evaporated under vacuum. NCR2471-2,3-4 was purified to homogeneity by semipreparative RP-HPLC. The semipreparative column was equilibrated with 10% eluent A [0.1% trifluoroacetic acid (TFA) in water]. For semipreparative HPLC, a Zorbax C-18 column (5-μm pore, 9.4 × 250 mm, Agilent Technologies, Inc.) was employed. Peptides were eluted with a linear gradient from 10 to 60% eluent B (0.1% TFA in acetonitrile) in 15 min at 3.0 mL/min flow rate at 25 °C. The eluate was monitored at 220 and 280 nm. The specific pairing was confirmed by tryptic digestion and LC-MS.
HPLC of Peptides.
Peptides were analyzed by RP-HPLC using an Agilent 1200 HPLC system equipped with a diode array detector (DAD). Samples were loaded onto a Clipeus C18, 5-µm, 300-Å (150 × 4.6 mm) HPLC column (Higgins Analytical, Inc.) equilibrated with 10% eluent A [0.1% trifluoroacetic acid (TFA) in water]. Peptides were eluted with a linear gradient from 10 to 60% eluent B (0.1% TFA in acetonitrile) in 15 min at 1.0 mL/min flow rate at 25 °C. The eluate was monitored at 220 and 280 nm. Peptide stock solution concentrations were routinely quantified by using calculated extinction coefficients. Extinction coefficients were calculated using a web-based tool (web.expasy.org/protparam/). The calculated extinction coefficients (280 nm) are as follows: NCR247ox, 3,230 M−1⋅cm−1; NCR247red, 2,980 M−1⋅cm−1. Purified peptide samples were stored as lyophilized powders or as aqueous stock solutions at −20 °C in very dilute hydrochloric acid.
ESI-MS.
Electrospray ionization–mass spectrometry (ESI-MS) was performed on an LC-MS system comprised of an Agilent 1260 LC outfitted with a Poroshell 120 EC, C-18 column (2.7-μm pore, 3.0 × 50 mm; Agilent Technologies, Inc.) and connected to an Agilent 6230 TOF system housing an Agilent Jetstream ESI source. LC-MS–grade water and MeCN containing 0.1% formic acid (Fluka Chemicals) were used as solvent A and solvent B, respectively. The samples were run at a flow rate of 0.4 mL/min using a gradient of 0–95% of acetonitrile over 5 min.
Protease Digests and Regioisomer Identification.
To identify the regioisomers of NCR247ox, a 100-μL solution of each peptide (25 μM) was prepared in an aqueous solution containing 20 mM CaCl2 in 100 mM 2-(N-morpholino) ethanesulfonic acid (MES) buffer, pH 5.5. A 50-μL aliquot was removed to use as the no-enzyme control. A 15-μL aliquot of tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin from Sigma (0.025 mg/mL in 20 mM CaCl2, 0.001% Triton X-100) was added to the remaining 150-μL solution of peptide. The mixture was incubated at room temperature for 14−16 h and quenched by addition of 6% aqueous TFA vol/vol (15 μL). The quenched samples were vortexed, centrifuged (15,588 × g × 10 min, 4 °C) and stored on ice. The peptide fragments were separated and isolated by analytical HPLC (10−60% B in 30 min).The isolated fractions were lyophilized to dryness, and the resulting products were dissolved in 0.1% TFA and analyzed by LC-MS-ESI mass spectrometry. The m/z values of the peptide fragments were assigned and used to identify the disulfide linkages as detailed in Fig. S3.
Synthesis of FITC-NCR247red.
The synthesis of FITC-NCR247red was performed using standard Fmoc solid-phase peptide synthesis techniques in a custom-made 25-mL glass reaction vessel outfitted with a medium porosity frit and a “T”-bore for N2 gas bubbling (37). Fluorescein-5-isothiocyanate (FITC “Isomer I”; Molecular Probes) was obtained as a 9:1 mixture of isomers and used without further purification. The synthesis of NCR247 was performed on a 0.01-mmol scale of Fmoc-Arg (Pbf)-Novasyn©TGA resin as described above except that, at amino acid position 3, Gly was replaced with Lys (NCR247 [G3K]). Fmoc-Lys (Alloc)-OH was coupled thrice using Fmoc-Lys(Alloc)-OH (10 equiv) dissolved in DMF (15 mL) containing HATU (1-[Bis (dimethylamino) methylene]-1H-1, 2, 3-triazolo [4, 5-b] pyridinium 3-oxid hexafluorophosphate) (10 equiv), HOAt (1-hydroxy-7-azabenzotriazole) (10 equiv), and DIPEA (20 equiv). After coupling of the N-terminal Arg residue, the resin-bound peptide was subjected to Alloc deprotection under mild conditions by treatment with PhSiH3 (25 equiv) and catalytic [Pd(Ph3P)4] (0.10 equiv) in 10 mL of CH2Cl2 at room temperature for 30 min. The deprotection reaction was repeated three times. After Alloc deprotection of Lys3, Fmoc-NCR247 [G3K]-resin was thoroughly washed with CH2Cl2 before coupling of the FITC to the free ε-amino group of Lys3. The coupling reaction was repeated three times with the 10 mg FITC dissolved in 10 mL of DMF containing 2 mL of DIPEA for 2 h in the dark. Then, the resin was thoroughly washed with CH2Cl2, the Fmoc was removed as described above, and the cleavage reaction was performed to obtain crude FlTC-NCR247 [G3K] as described above. The crude product was reduced by addition of 1 mM tris(2-carboxy)ethyl-1-phosphine (TCEP) in Tris⋅HCl, pH 8.0, and purified to obtain FITC-NCR247red [G3K].
Circular Dichroism Spectroscopy.
Peptide solutions (20 μM, 280 μL) were prepared in 5 mM sodium phosphate buffer, pH 7.0. A 1-mm path-length quartz CD cell (Hellma) was used for all measurements. The CD spectra were collected from 260 to 190 nm at 1-nm intervals (10 s averaging time, three independent scans per wavelength). The data obtained from three scans were averaged by using Microcal Origin and plotted. The samples were incubated at room temperature for ∼10 min, and the CD spectra were subsequently collected as described above.
Antimicrobial Activity Assays.
NCR247 sensitivity of S. meliloti and E. coli cells was determined as described previously with modifications using early exponential phase cultures in 3-(N-morpholino) propanesulfonic acid (MOPS) buffered minimal medium (50 mM MOPS, 1 mM MgSO4, 0.25 mM CaCl2, 19 mM glutamic acid, 0.004 mM biotin, pH 7.0) Mops-GS supplemented with 1% casamino acids for S. meliloti and 10 mM sodium phosphate buffer (pH 7.0) for E. coli (7, 14).
In Vitro Translation Reactions.
In vitro translation reactions were performed with the “Pure Express in Vitro Protein Synthesis” (Invitrogen) in the presence of 40 μM NCR247 variants and various controls [dihydrofolate reductase (DHFR and streptomycin]. GFP fluorescence was measured in a Spectramax M5 plate reader (Molecular Devices) at various time intervals. In the vector used (a gift from the Baker laboratory, Massachusetts Institute of Technology), production of the GFP was controlled by the T7 promoter.
Protease Susceptibility Assays.
To determine the relative susceptibility of NCR247 regioisomers and NCR247red to HrrP, we calculated the decrease in the amount of substrate peptide compared with matched assays using WT and nonfunctional HrrP (E62A). In brief, 200-μL solutions of 80-μM peptide were prepared in 40 mM sodium phosphate buffer, pH 7.0, containing 100 nM ZnSO4. A 5-μL aliquot of HrrP (460 μg/mL) or HrrP (E62A) was added, and the reactions were mixed with a pipet and incubated at room temperature. A 40-μL aliquot of each reaction was removed at t = 0, 1, 2, 3, and 4 h and quenched via addition of 1% vol/vol aqueous TFA (10 μL). The samples were vortexed immediately, stored on ice, centrifuged (15,588 × g × 10 min, 4 °C) to remove any precipitate, and analyzed by analytical HPLC using a solvent gradient of 10−60% B over 30 min. The peak areas were calculated and the plotted as the percentage of peptide left after the enzymatic activity compared with matched assays using HrrP (E62A).
Acknowledgments
We thank all members of the G.C.W. and E.M.N. laboratories and Caroline Koehrer for helpful discussions. We thank Ms. Deborah Pheasant (Biophysical Instrumentation Facility) for help in the CD spectroscopy experiments and the BioMicro Center for RT-qPCR facilities. This work was supported by National Institutes of Health (NIH) Grant GM31010 (to G.C.W.), NIH Grant 1DP2OD007045 (to E.M.N.), NIH Grant P30 ES002109 to the MIT Center for Environmental Health Sciences, and a Human Frontier Science Fellowship (to M.S.). J.S.G. and P.A.P. were supported by National Science Foundation Grant IOS-1054980 and J.S.G. was also supported by USDA/National Institute of Food and Agriculture Grant 2015-67013-22915. H.T.B. was supported by the Elisabeth Meurer Foundation. G.C.W. is an American Cancer Society Professor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610724113/-/DCSupplemental.
References
- 1.Gibson KE, Kobayashi H, Walker GC. Molecular determinants of a symbiotic chronic infection. Annu Rev Genet. 2008;42:413–441. doi: 10.1146/annurev.genet.42.110807.091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mergaert P, et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci USA. 2006;103(13):5230–5235. doi: 10.1073/pnas.0600912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Velde W, et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science. 2010;327(5969):1122–1126. doi: 10.1126/science.1184057. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, et al. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science. 2010;327(5969):1126–1129. doi: 10.1126/science.1184096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maróti G, Downie JA, Kondorosi É. Plant cysteine-rich peptides that inhibit pathogen growth and control rhizobial differentiation in legume nodules. Curr Opin Plant Biol. 2015;26:57–63. doi: 10.1016/j.pbi.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Kondorosi E, Mergaert P, Kereszt A. A paradigm for endosymbiotic life: Cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu Rev Microbiol. 2013;67:611–628. doi: 10.1146/annurev-micro-092412-155630. [DOI] [PubMed] [Google Scholar]
- 7.Penterman J, et al. Host plant peptides elicit a transcriptional response to control the Sinorhizobium meliloti cell cycle during symbiosis. Proc Natl Acad Sci USA. 2014;111(9):3561–3566. doi: 10.1073/pnas.1400450111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiricz H, et al. Antimicrobial nodule-specific cysteine-rich peptides induce membrane depolarization-associated changes in the transcriptome of Sinorhizobium meliloti. Appl Environ Microbiol. 2013;79(21):6737–6746. doi: 10.1128/AEM.01791-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mergaert P, et al. A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol. 2003;132(1):161–173. doi: 10.1104/pp.102.018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horváth B, et al. Loss of the nodule-specific cysteine rich peptide, NCR169, abolishes symbiotic nitrogen fixation in the Medicago truncatula dnf7 mutant. Proc Natl Acad Sci USA. 2015;112(49):15232–15237. doi: 10.1073/pnas.1500777112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M, et al. An antimicrobial peptide essential for bacterial survival in the nitrogen-fixing symbiosis. Proc Natl Acad Sci USA. 2015;112(49):15238–15243. doi: 10.1073/pnas.1500123112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price PA, et al. Rhizobial peptidase HrrP cleaves host-encoded signaling peptides and mediates symbiotic compatibility. Proc Natl Acad Sci USA. 2015;112(49):15244–15249. doi: 10.1073/pnas.1417797112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazebrook J, Ichige A, Walker GC. A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev. 1993;7(8):1485–1497. doi: 10.1101/gad.7.8.1485. [DOI] [PubMed] [Google Scholar]
- 14.Haag AF, et al. Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol. 2011;9(10):e1001169. doi: 10.1371/journal.pbio.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulaj G, Olivera BM. Folding of conotoxins: Formation of the native disulfide bridges during chemical synthesis and biosynthesis of Conus peptides. Antioxid Redox Signal. 2008;10(1):141–155. doi: 10.1089/ars.2007.1856. [DOI] [PubMed] [Google Scholar]
- 16.Tam JP, Wang S, Wong KH, Tan WL. Antimicrobial peptides from plants. Pharmaceuticals (Basel) 2015;8(4):711–757. doi: 10.3390/ph8040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Cougnon FBL, Wanniarachchi YA, Hayden JA, Nolan EM. Reduction of human defensin 5 affords a high-affinity zinc-chelating peptide. ACS Chem Biol. 2013;8(9):1907–1911. doi: 10.1021/cb400340k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroeder BO, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469(7330):419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 19.Masuda K, Sakai N, Nakamura K, Yoshioka S, Ayabe T. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J Innate Immun. 2011;3(3):315–326. doi: 10.1159/000322037. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, et al. Reduction impairs the antibacterial activity but benefits the LPS neutralization ability of human enteric defensin 5. Sci Rep. 2016;6:22875. doi: 10.1038/srep22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farkas A, et al. Medicago truncatula symbiotic peptide NCR247 contributes to bacteroid differentiation through multiple mechanisms. Proc Natl Acad Sci USA. 2014;111(14):5183–5188. doi: 10.1073/pnas.1404169111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haag AF, et al. Role of cysteine residues and disulfide bonds in the activity of a legume root nodule-specific, cysteine-rich peptide. J Biol Chem. 2012;287(14):10791–10798. doi: 10.1074/jbc.M111.311316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human β-defensin 3. Proc Natl Acad Sci USA. 2003;100(15):8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen EJ, Fisher RF, Perovich VM, Sabio EA, Long SR. Identification of direct transcriptional target genes of ExoS/ChvI two-component signaling in Sinorhizobium meliloti. J Bacteriol. 2009;191(22):6833–6842. doi: 10.1128/JB.00734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffitts JS, et al. A Sinorhizobium meliloti osmosensory two-component system required for cyclic glucan export and symbiosis. Mol Microbiol. 2008;69(2):479–490. doi: 10.1111/j.1365-2958.2008.06304.x. [DOI] [PubMed] [Google Scholar]
- 26.Pini F, et al. The DivJ, CbrA and PleC system controls DivK phosphorylation and symbiosis in Sinorhizobium meliloti. Mol Microbiol. 2013;90(1):54–71. doi: 10.1111/mmi.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Login FH, et al. Antimicrobial peptides keep insect endosymbionts under control. Science. 2011;334(6054):362–365. doi: 10.1126/science.1209728. [DOI] [PubMed] [Google Scholar]
- 28.LeVier K, Phillips RW, Grippe VK, Roop RM, 2nd, Walker GC. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science. 2000;287(5462):2492–2493. doi: 10.1126/science.287.5462.2492. [DOI] [PubMed] [Google Scholar]
- 29.Ichige A, Walker GC. Genetic analysis of the Rhizobium meliloti bacA gene: Functional interchangeability with the Escherichia coli sbmA gene and phenotypes of mutants. J Bacteriol. 1997;179(1):209–216. doi: 10.1128/jb.179.1.209-216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux B, et al. An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to RNA sequencing. Plant J. 2014;77(6):817–837. doi: 10.1111/tpj.12442. [DOI] [PubMed] [Google Scholar]
- 31.Sevier CS, Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol. 2002;3(11):836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 32.Pini F, et al. Cell cycle control by the master regulator CtrA in Sinorhizobium meliloti. PLoS Genet. 2015;11(5):e1005232. doi: 10.1371/journal.pgen.1005232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro CW, Alloing G, Mandon K, Frendo P. Redox regulation of differentiation in symbiotic nitrogen fixation. Biochim Biophys Acta. 2015;1850(8):1469–1478. doi: 10.1016/j.bbagen.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Bessette PH, Åslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci USA. 1999;96(24):13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nallu S, Silverstein KAT, Zhou P, Young ND, Vandenbosch KA. Patterns of divergence of a large family of nodule cysteine-rich peptides in accessions of Medicago truncatula. Plant J. 2014;78(4):697–705. doi: 10.1111/tpj.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham MA, Silverstein KAT, Cannon SB, VandenBosch KA. Computational identification and characterization of novel genes from legumes. Plant Physiol. 2004;135(3):1179–1197. doi: 10.1104/pp.104.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tam JP, Wu CR, Liu W, Zhang JW. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J Am Chem Soc. 1991;113(17):6657–6662. [Google Scholar]
- 38.Simon MD, et al. Rapid flow-based peptide synthesis. Chembiochem. 2014;15(5):713–720. doi: 10.1002/cbic.201300796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuthbertson A, Indrevoll B. Regioselective formation, using orthogonal cysteine protection, of an alpha-conotoxin dimer peptide containing four disulfide bonds. Org Lett. 2003;5(16):2955–2957. doi: 10.1021/ol035105w. [DOI] [PubMed] [Google Scholar]