Significance
We describe a human disease linked to mutations in the linear deubiquitinase (DUB) OTULIN, which functions as a Met1-specific DUB to remove linear polyubiquitin chains that are assembled by the linear ubiquitin assembly complex (LUBAC). OTULIN has a role in regulating Wnt and innate immune signaling complexes. Hydrolysis of Met1-linked ubiquitin chains attenuates inflammatory signals in the NF-κB and ASC-mediated pathways. OTULIN-deficient patients have excessive linear ubiquitination of target proteins, such as NEMO, RIPK1, TNFR1, and ASC, leading to severe inflammation. Cytokine inhibitors have been efficient in suppressing constitutive inflammation in these patients. This study, together with the identification of haploinsufficiency of A20 (HA20), suggests a category of human inflammatory diseases, diseases of dysregulated ubiquitination.
Keywords: OTULIN, linear deubiquitinase, NF-κB pathway, autoinflammatory disease, cytokines
Abstract
Systemic autoinflammatory diseases are caused by mutations in genes that function in innate immunity. Here, we report an autoinflammatory disease caused by loss-of-function mutations in OTULIN (FAM105B), encoding a deubiquitinase with linear linkage specificity. We identified two missense and one frameshift mutations in one Pakistani and two Turkish families with four affected patients. Patients presented with neonatal-onset fever, neutrophilic dermatitis/panniculitis, and failure to thrive, but without obvious primary immunodeficiency. HEK293 cells transfected with mutated OTULIN had decreased enzyme activity relative to cells transfected with WT OTULIN, and showed a substantial defect in the linear deubiquitination of target molecules. Stimulated patients’ fibroblasts and peripheral blood mononuclear cells showed evidence for increased signaling in the canonical NF-κB pathway and accumulated linear ubiquitin aggregates. Levels of proinflammatory cytokines were significantly increased in the supernatants of stimulated primary cells and serum samples. This discovery adds to the emerging spectrum of human diseases caused by defects in the ubiquitin pathway and suggests a role for targeted cytokine therapies.
Posttranslational modifications by ubiquitination are important for the regulation of many signaling complexes (1). Linear ubiquitin chains, also known as Met1-linked chains, are generated by the linear ubiquitin assembly complex (LUBAC) (2). LUBAC-mediated Met1 ubiquitination is critical for regulation of immune signaling and cell death (3). Absence of LUBAC attenuates NF-κB signaling and patients with loss-of-function mutations in LUBAC present with paradoxical features of susceptibility to infection and systemic inflammation, the latter due to increased responsiveness to IL-1β in monocytes (3–5). OTULIN and CYLD are deubiquitinases (DUBs) that cleave Met1-linked chains (6). Although OTULIN functions exclusively as a Met1 deubiquitinase (7, 8), CYLD may also hydrolyze Lys63-linked ubiquitin (9). OTULIN is an evolutionarily highly conserved protein, and in mice complete deficiency is embryonically lethal (8). Recently, we reported patients with heterozygous germline mutations in TNFAIP3/A20 (10), which has DUB activity for K63-linked polyubiquitin chains. Both OTULIN and A20 are important gatekeepers of innate immunity (7, 11).
Results
Identification of Loss-of-Function Mutations in OTULIN in Three Patients.
Using a combination of exome sequencing and candidate gene screening, we identified three homozygous mutations in the OTULIN/FAM105B gene in unrelated families of Pakistani and Turkish descent (Fig. 1, Fig. S1, Table 1, and Tables S1 and S2). Unaffected parents and siblings were carriers for the respective mutations. None of the mutations was reported in public databases or detected in 1,630 Turkish healthy controls. Two missense mutations, p.Leu272Pro and p.Tyr244Cys, are predicted to be deleterious by multiple algorithms (Table S3) and affect highly conserved amino acid residues (Fig. S2A). Similar to A20 disease-causing mutations (10), all three OTULIN mutations are located in the ovarian tumor (OTU) domain (Fig. S2B). The p.Gly174Aspfs*2 mutation introduces a premature stop codon, and mutant truncated protein was detectable by overexpression in HEK293 cells (Fig. 2A) but not in the patient’s fibroblasts (Fig. 2B, patient 3). The missense mutations likely affect the S1 site of the linear ubiquitin-binding domain (Fig. S2C). They reduce OTULIN protein stability (Fig. 2B) and may result in the instability of the LUBAC complex subunits, SHARPIN and HOIP (Fig. 2B).
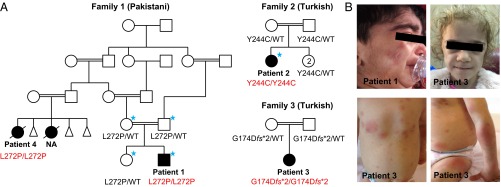
Fig. 1.
Mutations in OTULIN cause severe early-onset systemic autoinflammatory disease. (A) Pedigrees and the identified genotypes in three families with mutations in OTULIN. WT indicates wild-type OTULIN alleles. The individuals selected for exome sequencing are marked with blue asterisks. NA: an affected cousin of patient 1 had similar disease, but her DNA sample was not available for this study. (B) Clinical manifestations of three patients with otulipenia. Top two panels show facial features of the patients, including cushingoid appearance (Left) and prominent fat loss (lipodystrophy) (Right). Bottom two panels show erythematous skin lesions and subcutaneous nodules.
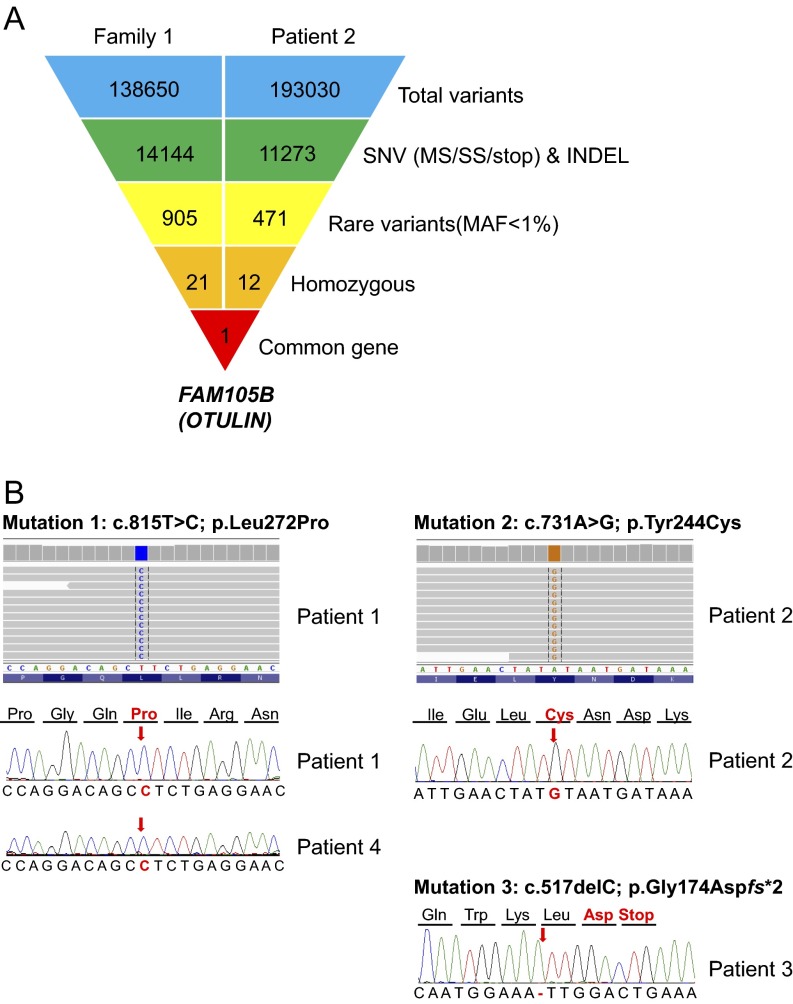
Fig. S1.
Identification of FAM105B/OTULIN mutations using exome sequencing and Sanger sequencing. (A) Whole-exome sequencing leads to identification of a common gene FAM105B. Schematic representation of the exome data-filtering approach used to select for novel and homozygous inherited variants segregating with disease in family 1 and patient 2. OTULIN is the only gene in common between the family 1 and patient 2. INDEL, frameshift and nonframeshift insertions and deletions; SNV, single-nucleotide variants including missense variants, splice site variants, and stop codon variants. (B) Electropherograms for the three OTULIN mutations identified in four patients from three families.
Table 1.
OTULIN mutations identified in three consanguineous families
| Family | Ancestry | Nucleotide alteration† | cDNA alteration‡ | Amino acid alteration | Domain | ExAC | Turkish population | Software prediction§ | Conservation¶ |
| 1 | Pakistani | chr5: 14690368T>C | c.815T>C | p.Leu272Pro | OTU | 0/122,972 | 0/3,260 | Damaging | Conserved |
| 2 | Turkish | chr5: 14690284A>G | c.731A>G | p.Tyr244Cys | OTU | 0/122,972 | 0/3,260 | Damaging | Conserved |
| 3 | Turkish | chr5: 14687678delC | c.517delC | p.Gly174Aspfs*2 | OTU | 0/122,972 | 0/3,260 | / | / |
Genome reference: GRCh37 (hg19).
cDNA reference: NM_138348.4.
SIFT, PolyPhen2, LRT, Mutation Taster, and CADD.
GERP, SiPhy 29 way, and CLUSTALW.
Table S1.
List of candidate homozygous variants in family 1 based on exome sequencing
| Chr | Position | Reference | Variant | Gene | Consequence | Our 616 exomes | Clinseq | 1000 Genomes | ExAC | Polyphen | Patient 1 | Father | Mother | Healthy sibling |
| chr1 | 38397501 | C | T | INPP5B | c.439G>A:p.A147T | 0.0045 | 0.0016 | 0 | 3.22E-03 | Probably damaging | TT | CT | CT | CT |
| chr1 | 54371889 | G | A | DIO1 | c.459G>A:p.M153I | 0.0041 | 0.0011 | 0 | 2.21E-03 | Probably damaging | AA | AG | AG | AG |
| chr1 | 55166996 | G | A | MROH7 | c.3285+1G>A | 0.0043 | 0 | 0 | 4.33E-04 | / | AA | AG | AG | AG |
| chr1 | 63329777 | G | T | ATG4C | c.1324G>T:p.D442Y | 0.0065 | 0.0032 | 0.0005 | 1.55E-03 | Possibly damaging | TT | GT | GT | GT |
| chr3 | 39226171 | G | A | XIRP1 | c.4766C>T:p.T1589I | 0.0033 | 0.0011 | 0 | 5.55E-03 | Benign | AA | AG | AG | GG |
| chr3 | 52537419 | A | G | STAB1 | c.754A>G:p.K252E | 0.0041 | 0.0005 | 0 | 2.26E-03 | Benign | GG | AG | AG | AG |
| chr3 | 111842564 | C | T | GCSAM | c.281G>A:p.R94Q | 0.0033 | 0 | 0 | 1.63E-05 | Benign | TT | CT | CT | CC |
| chr3 | 112648018 | A | G | CD200R1 | c.539T>C:p.L180S | 0.0033 | 0.0011 | 0 | 4.77E-03 | Benign | GG | AG | AG | AA |
| chr3 | 119911827 | G | A | GPR156 | c.433C>T:p.L145F | 0.0033 | 0 | 0.0014 | 7.40E-04 | Probably damaging | AA | AG | AG | GG |
| chr3 | 126255146 | C | T | CHST13 | c.130C>T:p.L44F | 0.0033 | 0 | 0 | 3.43E-03 | Benign | TT | CT | CT | CC |
| chr3 | 127983506 | C | T | EEFSEC | c.668C>T:p.P223L | 0.0049 | 0.0021 | 0.0005 | 1.97E-03 | Probably damaging | TT | CT | CT | CC |
| chr5 | 5463514 | C | T | KIAA0947 | c.4067C>T:p.T1356I | 0.0042 | 0 | 0 | 0 | Benign | TT | CT | CT | CT |
| chr5 | 14690368 | T | C | FAM105B | c.815T>C:p.L272P | 0.0025 | 0 | 0 | 0 | Probably damaging | CC | CT | CT | CT |
| chr6 | 131179287 | C | T | EPB41L2 | c.2548G>A:p.G850R | 0.0041 | 0.0016 | 0 | 2.02E-03 | Probably damaging | TT | CT | CT | CT |
| chr12 | 10464155 | C | T | KLRD1 | c.256C>T:p.R86W | 0.0049 | 0 | 0 | 5.69E-05 | Probably damaging | TT | CT | CT | CT |
| chr13 | 53603108 | T | A | OLFM4 | c.137T>A:p.F46Y | 0.0033 | 0 | 0 | 3.60E-03 | Benign | AA | AT | AT | TT |
| chr15 | 34077949 | G | A | RYR3 | c.9355G>A:p.E3119K | 0.0058 | 0.001 | 0 | 2.47E-03 | Probably damaging | AA | AG | AG | GG |
| chr15 | 43512935 | C | T | EPB42 | c.89G>A:p.S30N | 0.0041 | 0.0011 | 0.0027 | 1.35E-03 | Benign | TT | CT | CT | CT |
| chr16 | 56709804 | C | A | MT1IP | c.134C>A:p.A45E | 0.0129 | 0 | 0.0046 | 2.25E-03 | NA | AA | AC | AC | AC |
| chr16 | 57602000 | C | T | GPR114 | c.1054C>T:p.R352C | 0.0041 | 0 | 0.0005 | 9.03E-04 | Probably damaging | TT | CT | CT | CT |
| chr19 | 1117467 | G | A | SBNO2 | c.1559C>T:p.A520V | 0.015 | 0 | 0 | 3.31E-05 | Probably damaging | AA | AG | AG | AG |
Boldface type indicates the only gene in common between family 1 and patient 2.
Table S2.
List of candidate homozygous variants in patient 2 based on exome sequencing
| Chr | Position | Reference | Variant | Gene | Consequence | Our 616 exomes | Clinseq | 1000 Genomes | ExAC | Polyphen | Patient 2 |
| chr1 | 55068396 | A | G | ACOT11 | c.1084A>G:p.R362G | 0.0018 | 0 | 0 | 8.42E-06 | Probably damaging | GG |
| chr3 | 169587465 | G | A | LRRC31 | c.131C>T:p.S44F | 0.0016 | 0.0027 | 0.0005 | 1.62E-03 | Benign | AA |
| chr5 | 14690284 | A | G | FAM105B | c.731A>G:p.Y244C | 0.0016 | 0 | 0 | 0 | Probably damaging | GG |
| chr5 | 24593481 | C | A | CDH10 | c.119G>T:p.R40L | 0.0016 | 0 | 0 | 8.13E-06 | Benign | AA |
| chr5 | 39377259 | C | T | DAB2 | c.1630G>A:p.V544I | 0.0065 | 0.0053 | 0.0018 | 3.64E-03 | Benign | TT |
| chr9 | 135202325 | A | C | SETX | c.4660T>G:p.C1554G | 0.0098 | 0.0011 | 0.0027 | 5.81E-03 | Probably damaging | CC |
| chr9 | 136280025 | G | A | REXO4 | c.332C>T:p.S111L | 0.0082 | 0.0064 | 0.0023 | 5.68E-03 | Benign | AA |
| chr19 | 40392655 | C | G | FCGBP | c.7849G>C:p.G2617R | 0.0023 | 0 | 0 | 7.59E-05 | Probably damaging | GG |
| chr19 | 42583629 | C | T | ZNF574 | c.1141C>T:p.R381C | 0.0025 | 0.0016 | 0.0005 | 5.61E-04 | Probably damaging | TT |
| chr19 | 43023175 | C | T | CEACAM1 | c.1171G>A:p.A391T | 0.0016 | 0 | 0 | 0 | Benign | TT |
| chr19 | 45791007 | C | T | MARK4 | c.1177C>T:p.R393W | 0.0257 | 0 | 0.0027 | 6.54E-03 | Benign | TT |
| chr19 | 49207114 | G | A | FUT2 | c.901G>A:p.G301R | 0.0024 | 0 | 0.0005 | 1.89E-03 | Probably damaging | AA |
Boldface type indicates the only gene in common between family 1 and patient 2.
Table S3.
Bioinformatic predictions for pathogenicity of the OTULIN candidate missense mutations
| Amino acid alteration | SIFT | PolyPhen2 | LRT | Mutation taster | CADD | GERP | SiPhy 29way | CUPSAT | I-Mutant (protein stability) | DiANNA | |
| Overall stability | Torsion | ||||||||||
| p.Leu272Pro | Deleterious | Probably damaging | Deleterious | Disease causing | 27.5 | 6.07 | 15.81 | Stabilizing | Unfavorable | Decrease | / |
| p.Tyr244Cys | Deleterious | Probably damaging | Deleterious | Disease causing | 19.96 | 4.76 | 11.635 | Destabilizing | Unfavorable | Decrease | Create disulfide bond |
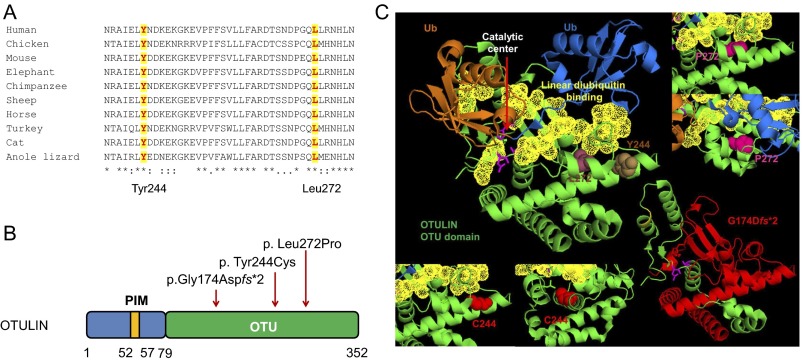
Fig. S2.
Schematic of OTULIN protein domains, and positions and conservation of the three disease-associated mutations. (A) Amino acid sequence alignment of the OTU domain of OTULIN orthologs. The two missense mutations, p.Tyr244Cys and p.Leu272Pro, affect amino acid residues that are highly conserved. The protein sequences of different species were aligned by using CLUSTALW. The two conserved amino acids Tyr244 and Leu272 are highlighted. (B) OTULIN is a 352-residue protein that consists of N-terminal LUBAC-binding PUB-interacting motif (PIM) and C-terminal ovarian tumor (OTU) domain that mediates deubiquitinase activity of OTULIN (79–352 aa). All three mutations are located in the OTU domain, and red arrows indicate their positions. (C) In silico modeling of OTULIN mutations based on the crystal structure of human OTULIN (3ZNZ). The distal Ub is marked in blue, the proximal Ub is in brown, and the OTU domain is in green. The linear ubiquitin binding regions (95–96 aa, 124–126 aa, 255–259 aa, 283–289 aa, and 336–338 aa) are shown in yellow, and catalytic sites (residues Asp126, Cys129, and His339) are shown as pink sticks. The left top panel shows the native residue of Leu272 (in raspberry) and Tyr244 (in brown). The left bottom two panels show the mutant Cys244 from front and side views. The right top two panels show the mutant Pro272 from two different angles. The mutations Tyr244Cys and Leu272Pro are located near or on the S1 distal Ub binding site. These two missense mutations likely affect enzymatic activity or linear ubiquitin binding. The right bottom panel shows location of the p.G174Dfs*2 mutation, with the truncated part of the OTU domain shown in red.
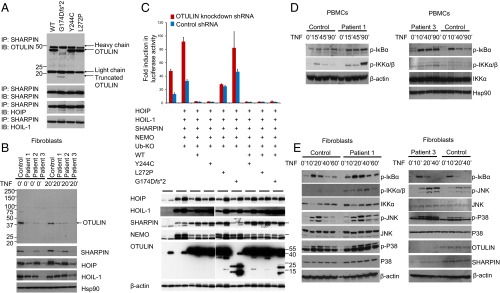
Fig. 2.
Induced NF-κB activity in cells with mutant OTULIN. (A) OTULIN mutants do not disrupt interaction with LUBAC. HEK293 cells were transiently transfected with plasmids encoding the LUBAC components (SHARPIN, HOIP, and HOIL-1), and WT or mutant OTULIN plasmids. Whole-cell lysates were collected 36 h later, and subjected to immunoprecipitation with antibodies against SHARPIN. The precipitates were then immunoblotted with OTULIN, SHARPIN, HOIP, and HOIL-1. (B) OTULIN and LUBAC complex expression in patients’ fibroblasts. Whole-cell lysates from the OTULIN-deficient patients and one healthy donor were immunoblotted with antibodies for OTULIN, SHARPIN, HOIP, HOIL-1, and Hsp90. (C) NF-κB luciferase assay in transiently transfected HEK293 cells with endogenous OTULIN down-regulated by shRNA. OTULIN mutants p.L272P and p.G174Dfs*2 do not inhibit LUBAC-induced NF-κB activation in HEK293 cells transfected with firefly NF-κB reporter plasmid, a Renilla luciferase control vector, and expression plasmids for WT or mutant OTULIN, together with LUBAC (SHARPIN, HOIL-1, HOIP), Ub-KO (ubiquitin mutant with all lysines mutated to arginines, which only forms linear polyubiquitin chains), and LUBAC linear ubiquitination substrate NEMO. Results are plotted as firefly normalized to Renilla luciferase activity to account for variance in transfection efficiency and cell number. One representative result of three independent experiments is shown. Values are reported as the means of technical triplicates ± SEM. (Lower) Whole-cell lysates from transfected cells were immunoblotted with antibodies for HOIP, HOIL-1, SHARPIN, NEMO, OTULIN, and β-actin. (D) PBMCs from OTULIN-deficient patients showed increased levels of phosphorylated IκBα and phosphorylated IKKα/IKKβ compared with a healthy control. Whole-cell lysates from TNF-stimulated PBMCs were immunoblotted for respective target proteins. (E) Stimulated fibroblasts from OTULIN-deficient patients sustained increased levels of phosphorylated IκBα, increased phosphorylated IKKα/IKKβ, and increased phosphorylated JNK and P38. Fibroblasts from patients 1 and 3 were stimulated with TNF for the time periods indicated. Whole-cell lysates were immunoblotted for respective target proteins. Two healthy individuals’ fibroblasts served as controls.
Clinical Manifestations of OTULIN Deficiency.
Patient 1, from a large consanguineous family, was born prematurely and soon after birth presented with fever and rash (Fig. 1 and Table S4). Two of his first cousins died from a similar disease in early childhood. Only one DNA sample was available for genotyping and was found to have the same homozygous mutation as patient 1. Other findings included failure to thrive, joint swelling, lipodystrophy, and diarrhea. Treatment with an IL-1β inhibitor (anakinra) was not steroid sparing; however, within 1 mo of starting a TNF inhibitor (infliximab) at the age of 3 y, his fevers and rash subsided. Eight years after initiation of the treatment, he is normal size for his age and fully functional (Fig. S3). Patient 2 presented at the age of 4.5 mo with prolonged fevers and pustular, scarring rashes. Skin biopsy revealed panniculitis and neutrophilic dermatosis. Initially, she responded to treatment with steroids, and subsequently symptoms improved on treatment with anakinra (Table S4). Patient 3 presented with neonatal-onset fever and prominent cutaneous lesions including an erythematous rash with painful skin nodules (Fig. 1B). Her skin biopsy showed a predominantly septal panniculitis with vasculitis of small and medium-sized blood vessels. Other manifestations included arthralgia, progressive lipodystrophy, and developmental delay. Her disease is partially controlled with a TNF inhibitor (etanercept), but she is still steroid dependent (Table S4). Patients did not have clear evidence for primary immunodeficiency and suffered from infections related to use of immunosuppressive therapies. Patients 1 and 3 had normal to high levels of T, B, and natural killer cells (Table S5), Ig levels were normal to high, and IgA was elevated in the two patients. T- and B-cell proliferative responses were normal (Fig. S4A). Patients had adequate specific antibody responses to vaccines or natural infections when tested.
Table S4.
Clinical manifestations of patients with OTULIN mutations
| Clinical features | Patient 1 | Patient 2 | Patient 3 |
| Ancestry | Pakistani | Turkish | Turkish |
| Consanguinity | Yes | Yes | Yes |
| Gender | Male | Female | Female |
| Current age | 11 y | 4 y | 11 y |
| Age of onset | 1 mo | 4.5 mo | 1 mo |
| Mutation | Homozygous p.Leu272Pro | Homozygous p.Tyr244Cys | Homozygous p.Gly174Aspfs*2 |
| Fevers | Yes | Yes (fever lasting for 20 d) | Yes (fever lasting 2–3 wk) |
| Rash/nodular panniculitis | Erythematous with skin nodules first noted in the neonatal period | Pustular rash, multiple scars | Erythematous with painful skin nodules first noted in the neonatal period. Occasional pustular rash. |
| Lipodystrophy | Yes | Yes | Yes |
| Skin biopsy | Neutrophil-rich panniculitis with fat necrosis but no frank vasculitis | Panniculitis and neutrophilic dermatosis | Septal panniculitis, vasculitis of small and medium-sized arteries and capillaries |
| Arthralgias/myalgias | Yes/yes | Yes/no | Yes/yes |
| Lymphadenopathy | Yes | No, parotitis sialadenitis (accompanying 3-d attack) | Yes |
| Abdominal pain/diarrhea | Yes/yes | No/no | No/no |
| Therapies | Prednisone, partial response to a dose of 2 mg/kg of anakinra. Infliximab started at the age of 3 y. Methotrexate, 2.5 mg weekly. | Prednisone, anakinra treatment started at the age of 1 y, responsive to a dose of 6 mg/kg. Never been treated with anti-TNF therapy | Prednisone, she developed macrophage activation syndrome (MAS) on 1 dose of canakinumab. Anakinra was not effective at a dose of 1–3 mg/kg. Etanercept started at age of 3 y. Partial response, still requires prednisone for flares. |
| History of infections | RSV pneumonia, two episodes of varicella and shingles, both well tolerated† | No | Frequent lymphadenitis typically during flares, varicella† |
| Additional medical history | Born at 28 wk of gestation, failure to thrive | Born at 38 wk of gestation, failure to thrive | Born at 38 wk of gestation, failure to thrive, convulsions, hepatosplenomegaly, sterile encephalitis‡ |
| Family history | Two affected cousins with similar disease | No family history | No family history |
| Lab results at the time of NIH visit | ESR (8 mm/h), CRP (1 mg/L) | NA | ESR (60 mm/h), CRP (63 mg/L) |
| WBC, 10K | WBC, 14K | ||
| Lab results before coming to NIH | Before infliximab: CRP (147 mg/L), WBC, 31.6K | Before anakinra: ESR (59 mm/h); CRP, 91 mg/L; WBC, 27,4K | On etanercept: ESR (94 mm/h); CRP (>200 mg/L); WBC, 26,6K |
| 2 mo after infliximab: CRP, <1 mg/L | After anakinra: ESR (52 mm/h); CRP, 1.5 mg/L; WBC, 18,4K | ||
| Autoantibodies | No | No | No |
CRP, C-reactive protein; ESR, erythrocytes sedimentation rate; NA, not available; WBC, white blood cell.
Intercurrent infections during immunosuppressive therapy.
Possibly related to immunosuppressive therapy.
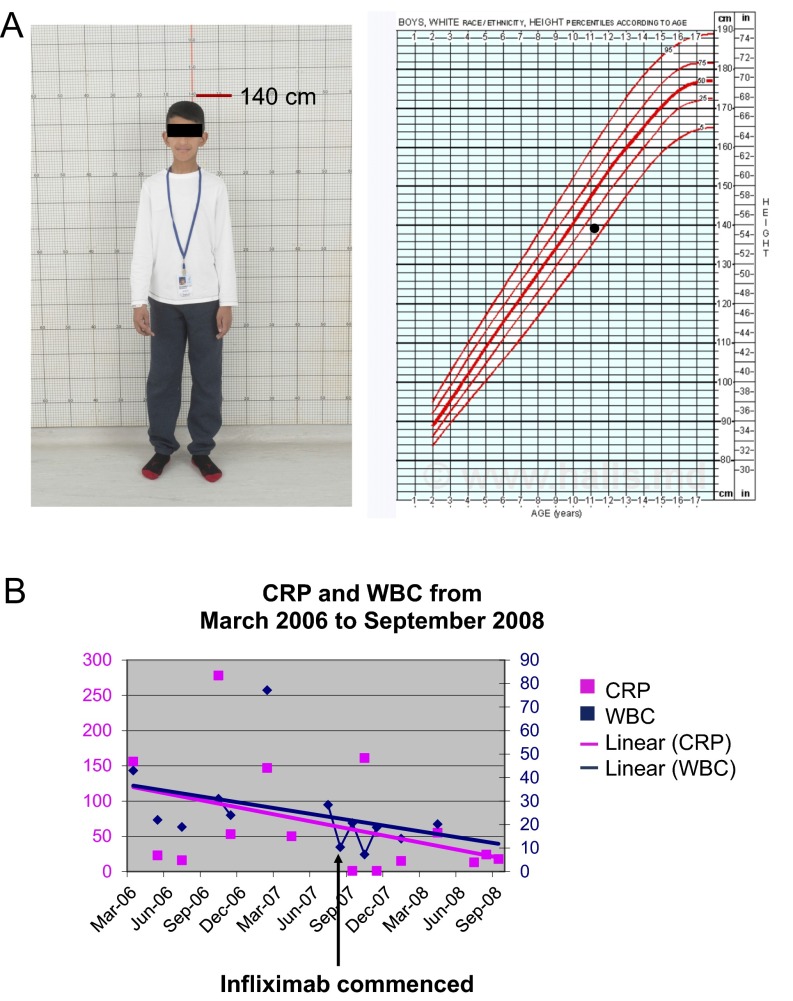
Fig. S3.
Patient 1 responded to infliximab treatment. (A) Image of patient 1 at the age of 11 y, 4 mo (Left); his height is at the 10th to 25th percentile for his age (Right). (B) Patient 1’s CRP and white blood cell (WBC) level before and after infliximab treatment from March 2006 to September 2008. Patient 1 was treated with prednisone but continued to have fevers and skin lesions before the start of infliximab treatment.
Table S5.
Clinical manifestations of patients with OTULIN mutations
| Cell types | Patient 1 | Patient 3 | Normal range | ||
| CD3, no. | 3,789 | H | 6,072 | H | 1,900–3,700 |
| CD4/CD3, % | 52.6 | 39.5 | 60–76% | ||
| CD4/CD3, no. | 2,404 | H | 2,876 | H | 650–1,500/μL |
| CD8/CD3, % | 22.3 | 31.7 | 11.2–34/8% | ||
| CD8/CD3, no. | 1,019 | H | 2,308 | H | 370–1,100/μL |
| CD19, % | 10 | L | 12.8 | L | 13–27% |
| CD19, no. | 457 | 932 | H | 270–860/μL | |
| NK cells, % | 7 | 3.7 | L | 4–17% | |
| NK cells, no. | 320 | 269 | 100–480/μL |
Lymphocyte phenotyping in patients 1 and 3. Patient 2 was not available for immunophenotyping. NK, natural killer.
Fig. S4.
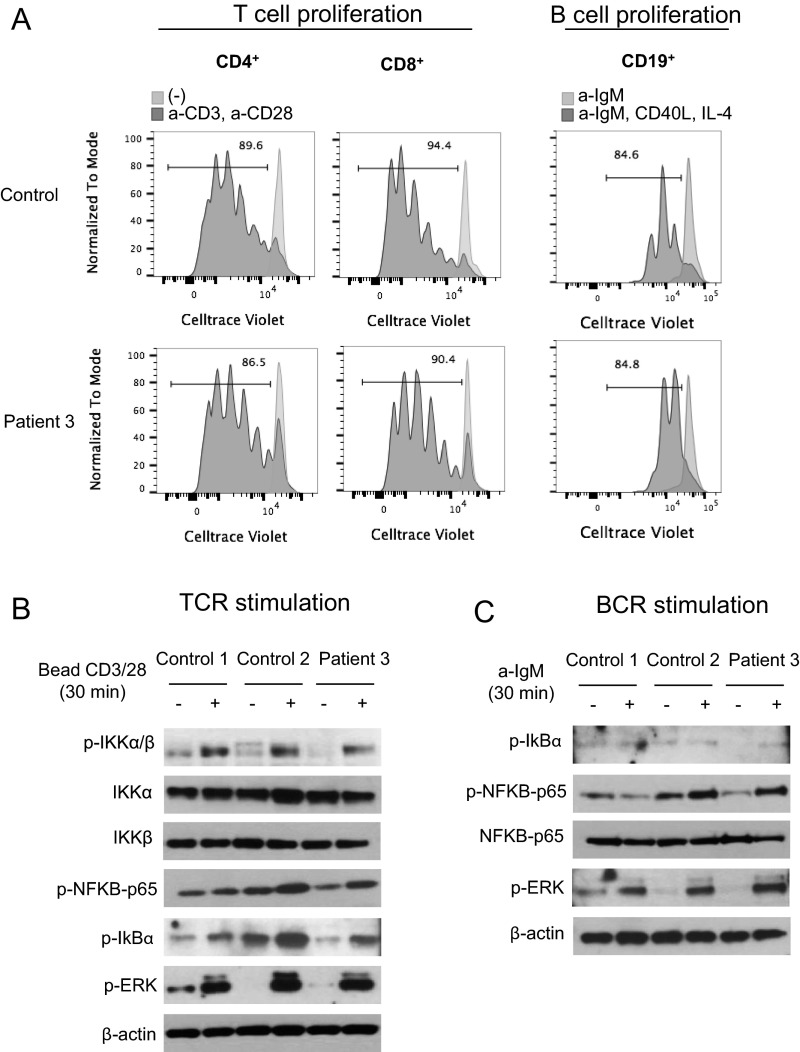
Normal T- and B-cell proliferation and normal NF-κB activation in response to TCR and B-cell receptor (BCR) stimulation in patient 3 compared with controls. (A) Normal T- and B-cell proliferation in patient 3 compared with a healthy control. Total peripheral blood mononuclear cells (PBMCs) were stained with CellTrace Violet and cultured with (dark gray) or without (light gray) anti-CD3 and anti-CD28 antibodies (1 μg/mL) for 4 d. For B-cell proliferation, PBMCs were stimulated with a-IgM (10 μg/mL), CD40L (1 μg/mL), and IL-4 (50 ng/mL) for 4 d. Numbers indicate percentage of cells having undergone at least one cellular division, assessed by dye dilution. (B) Normal NF-κB activation in response to TCR stimulation in patient 3 compared with controls. (C) Normal NF-κB activation in response to BCR stimulation in patient 3 compared with controls.
Mutations Do Not Disrupt OTULIN Interaction with LUBAC.
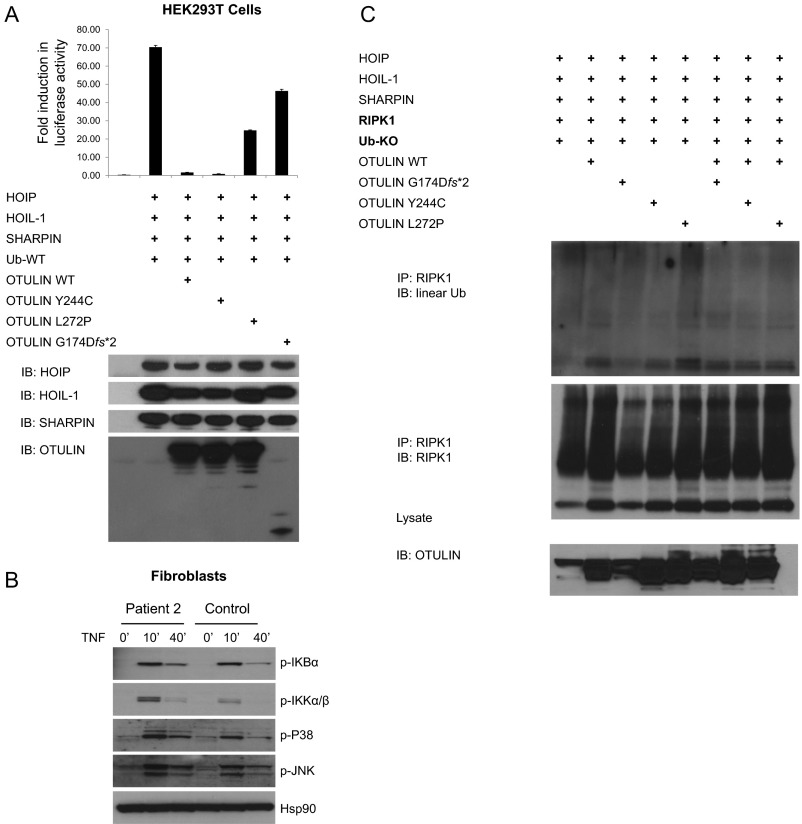
OTULIN is a 352-residue protein that consists of an N-terminal LUBAC-binding domain and a C-terminal ovarian tumor (OTU) domain (Fig. S2B). OTULIN interacts with the PUB domain of HOIP, and their interaction is required for the recruitment of OTULIN to the TNF receptor complex (12). The three mutations do not disrupt the OTULIN interaction with LUBAC, and mutant proteins maintain the intact N-terminal domain necessary for LUBAC interaction (Fig. 2A).
Increased NF-κB Signaling in OTULIN-Deficient Cells.
OTULIN restricts NF-κB signaling activity (7). We performed NF-κB luciferase assays to study the function of mutant OTULIN proteins in human embryonic kidney (HEK) 293 cells. Overexpressed mutant OTULIN plasmid p.Leu272Pro and p.Gly174Aspfs*2 failed to restrain NF-κB activity compared with WT OTULIN (Fig. 2C and Fig. S5A). Mutant p.Tyr244Cys plasmid suppressed the LUBAC-induced NF-κB activity similar to WT OTULIN. These results indicate that p. Leu272Pro and p.Gly174Aspfs*2 mutations affect the OTULIN enzyme activity, whereas the p.Tyr244Cys mutation retains sufficient residual OTULIN activity in the overexpression experiment, or may affect the protein function in a different manner. We then studied the activity of the NF-κB pathway in stimulated patients’ fibroblasts and peripheral blood mononuclear cells (PBMCs) (Fig. 2 D and E, and Fig. S5B). Sequential phosphorylation of IKKs and IκBα are essential steps in activation of the canonical NF-κB pathway (13). Patient-derived mononuclear leukocytes and fibroblasts sustained higher levels of phosphorylated IKKα/IKKβ and IκBα, and showed increased phosphorylation of P38 and JNK MAP kinases compared with healthy controls. These results demonstrate enhanced signaling of the NF-κB and MAPK pathways in OTULIN-deficient patients. Our data also suggest that NF-κB activation was not affected in patient’s lymphocytes in the context of T-cell receptor and B-cell receptor stimulation (Fig. S4 B and C).
Fig. S5.
Increased NF-κB in OTULIN-deficient cells. (A) NF-κB luciferase assay in transiently transfected HEK293T cells. OTULIN mutants p.L272P and p.G174Dfs*2 do not inhibit LUBAC-induced NF-κB activation in HEK293T cells transfected with firefly NF-κB reporter plasmid, a Renilla luciferase control vector, and expression plasmids for WT or mutant OTULIN, together with LUBAC (SHARPIN, HOIL-1, HOIP), Ub-WT. Results are plotted as firefly luciferase activity normalized to Renilla luciferase activity to account for variance in transfection efficiency differences and cell number differences. One representative result of three independent experiments is shown. Values are shown reported as the means of technical triplicates ± SEM. (B) Increased NF-κB signaling in fibroblasts from patient 2 compared with a healthy control. Stimulated fibroblasts from patient 2 showed increased levels of phosphorylated IκBα, increased phosphorylated IKKα/IKKβ, and increased phosphorylation of JNK and P38. Fibroblasts derived from patient 2 and a healthy control were stimulated with TNF for the time periods indicated. Whole-cell lysates were immunoblotted for respective target proteins. (C) Ubiquitination defect of RIPK1 was less noticeable in cells transfected with the mutant monoubiquitin plasmid (Ub-KO), which can only form linear ubiquitin chains.
Defect in the Deubiquitinase Function of Mutant OTULIN Proteins.
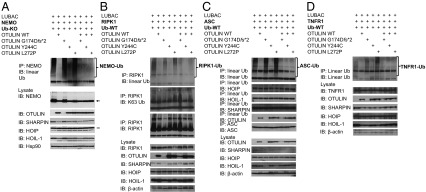
OTULIN cleaves Met1-linked linear polyubiquitin chains from target substrates, such as NEMO (IKKγ), RIPK1, ASC, and TNFR1 to restrict signaling activation and propagation (7, 14, 15). To investigate the effect of OTULIN mutations on its deubiquitinase function, we cotransfected WT and mutant OTULIN plasmids into HEK293 cells along with plasmids encoding the LUBAC subunits, mono specific-ubiquitin plasmid, and each of the OTULIN substrates NEMO (Fig. 3A), RIPK1 (Fig. 3B), ASC (Fig. 3C), and TNFR1 (Fig. 3D). Cells transfected with mutant p.Leu272Pro and p.Gly174Aspfs*2 proteins showed substantial defects in deubiquitination of the target substrate as indicated by accumulated high–molecular-weight linear-ubiquitin aggregates (Fig. 3 A–D). The defect in RIPK1 deubiquitination was more noticeable in cells transfected with WT monoubiquitin plasmid (Fig. 3B) than in cells transfected with the mutant monoubiquitin plasmid (Ub-KO), which can only form linear ubiquitin chains (Fig. S5C). RIPK1 and TNFR1 require the assembly of K63 and linear polyubiquitin chains for proper signaling activity, and they are subject to deubiquitination by A20 and OTULIN (14). The K63 ubiquitination of RIPK1 was not affected by the presence of mutant OTULIN proteins (Fig. 3B, second panel). Cells transfected with p.Tyr244Cys plasmid showed only mild, if any, defect compared with the other two mutant proteins (Fig. 3 A–D). The in vitro-observed defect in DUB activity of mutant proteins was rescued by cotransfection with WT OTULIN (Fig. 3 A–D).
Fig. 3.
Mutant OTULIN plasmids show impaired deubiquitinase function in transfected HEK293 cells. (A–D) OTULIN mutants failed to deubiquitinate Met1-linked linear ubiquitin chains from molecules targeted by LUBAC. HEK293 cells were transiently transfected with expression plasmids for one of the OTULIN target proteins, including NEMO (A), RIPK1 (B), ASC (C), or TNFR1 (D) together with plasmids for the LUBAC (SHARPIN, HOIL-1, and HOIP), Ub plasmids (Ub-KO: ubiquitin mutant with all lysines mutated to arginines, which only forms linear polyubiquitin chains; Ub-WT: WT ubiquitin, which forms linear polyubiquitin chains and other polyubiquitin chains, such as K48-Ub, K63-Ub chains), and WT or mutant OTULIN plasmids. Whole-cell lysates were subjected to immunoprecipitation with antibodies against NEMO (A), RIPK1 (B), ASC (C), and linear ubiquitination chain (C and D). High–molecular-weight (HMW) ubiquitin aggregates (Top) were detected by immunoblotting the precipitates with linear Ub antibody (A–D). Cell lysates were also blotted with antibody against NEMO, RIPK1, TNFR1, ASC, HOIP, SHARPIN, HOIL-1, OTULIN, and Hsp90 or β-actin.
Increased Linear Ubiquitination in Patients’ PBMCs and Fibroblasts.
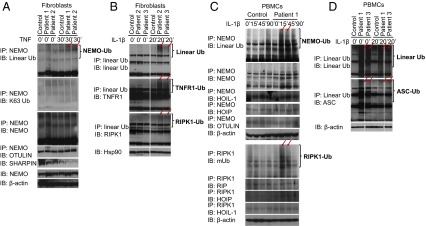
Consistent with the data from overexpressed mutant proteins, TNF- or IL-1β–stimulated OTULIN-deficient primary patients’ cells showed accumulation of linear-ubiquitinated NEMO (Fig. 4 A and C), TNFR1 (Fig. 4B), RIPK1 (Fig. 4 B and C), and ASC (Fig. 4D), and accumulation of high-molecular linear Ub aggregates (Fig. 4 B and D) compared with healthy controls. In ex vivo experiments with cells from patient 2, who carries the p.Tyr244Cys mutation, we noted an increase in the linear ubiquitinated NEMO, TNFR1, RIPK1 (Fig. 4 A and B), and accumulation of high-molecular linear Ub aggregates (Fig. 4B). This ex vivo experiment supports the strong genetic data for pathogenicity of the p.Tyr244Cys mutation identified in patient 2. The combination of in vitro and ex vivo experiments provides compelling evidence that loss-of-function mutations in OTULIN result in increased linear ubiquitination of signaling molecules and lead to enhanced TNFR1, NF-κB, and ASC-dependent inflammation.
Fig. 4.
Patient-derived PBMCs and fibroblasts lose their ability to deubiquitinate Met1-linked linear ubiquitin chains. Whole-cell lysates were immunoprecipitated with antibodies for NEMO (A and C), linear Ub (B and D), and RIPK1 (C), and the precipitates were blotted with antibodies against linear ubiquitin. The precipitates were also blotted with antibodies against NEMO, TNFR1, RIPK1, K63-linked ubiquitin, HOIP, HOIL-1, or OTULIN, and the cell lysates were blotted with antibodies against Hsp90 or β-actin. Red arrows point to the differences for comparison. (A) Patients’ TNF-stimulated fibroblasts showed increased abundance and molecular weight of linear-ubiquitinated NEMO as a result of the impaired OTULIN deubiquitinase activity. K63-ubiquitinated NEMO is mainly unaffected (second panel). Fibroblasts from patient 1, patient 2, and a healthy control were stimulated with TNF for 30 min. (B) Patients’ IL-1β–stimulated fibroblasts showed increased abundance of linear ubiquitinated TNFR1 and RIPK1 and accumulation of high-molecular linear-ubiquitin chains. Fibroblasts from patient 2, patient 3, and a healthy control were stimulated with IL-1β for 20 min. (C and D) Patients’ IL-1β–stimulated PBMCs showed increased linear ubiquitination of NEMO (C, Upper), RIPK1 (C, Lower), and ASC (D, Lower), and accumulation of high-molecular linear-ubiquitin chains (D, Upper). PBMCs from patients 1 and 3, and a healthy control were stimulated with IL-1β for the indicated time. mUB is a mouse monoclonal antibody that is not linear Ub specific (C).
A Strong Inflammatory Signature in OTULIN-Deficient Patients’ Cells.
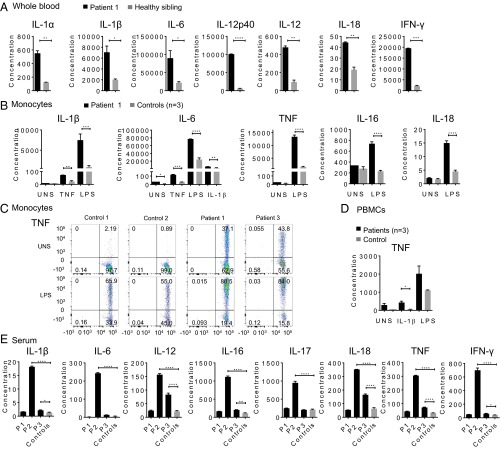
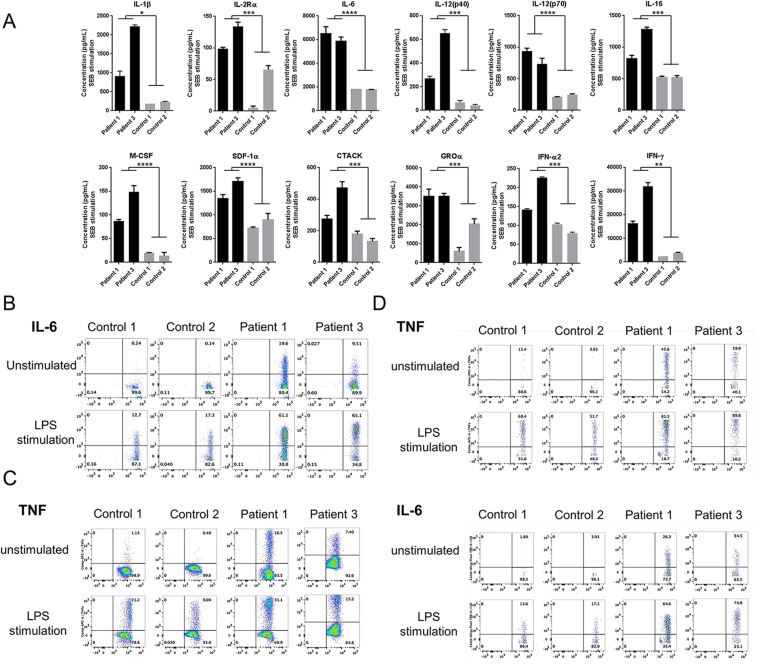
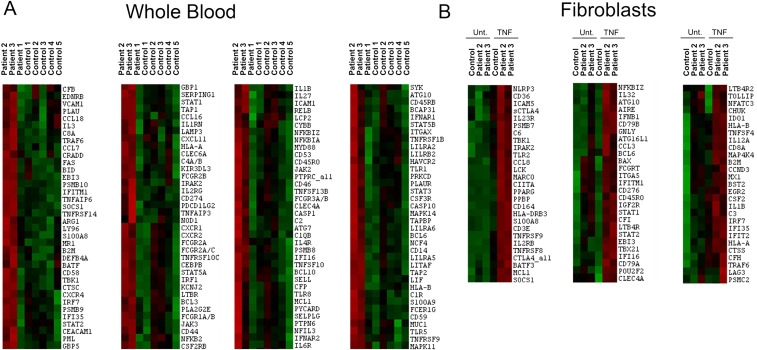
Stimulated patient whole-blood samples showed an increased production of IL-1β, IL-6, IL-12, IL-18, and IFN-γ in response to LPS, and increased levels of multiple cytokines and chemokines in response to staphylococcal enterotoxin B stimulation (Fig. 5A and Fig. S6A). Purified patients’ monocytes had significantly higher secretion of IL-1β, IL-6, IL-16, IL-18, and TNF in response to LPS, TNF, or IL-1β stimulation relative to cells from healthy controls (Fig. 5B). Intracellular staining for TNF and IL-6 from patient 1 and 3 was higher at basal levels in monocytes (Fig. 5C and Fig. S6B), T cells (Fig. S6C), and dendritic cells (Fig. S6D) than in controls. Fig. 5D represents the average of TNF responses assayed on the three patients individually. Cytokine profiling in serum samples was consistent with disease activity. Patient 2, who had the most active disease at the time of sampling, had the highest levels of proinflammatory cytokines. Patients 1 and 3 had less active disease phenotypes at the time of sampling and substantially lower cytokine levels (Table S4 and Fig. 5E). Transcriptome profiling of patient whole-blood samples and stimulated fibroblasts showed similar results. Patients 2 and 3 displayed strong inflammatory signatures enriched for NF-κB, Jak-STAT, and TNF signaling (Fig. S7 A and B). In contrast, patient 1, whose disease was both clinically and biochemically inactive at the time of visit, had a transcriptome profile similar to controls. These data provide evidence that a malfunction in linear deubiquitination leads to an up-regulation in cytokine production and that the disease is amenable to targeted anticytokine treatment.
Fig. 5.
Patient-derived immune cells display a strong inflammatory signature. Cytokine profiles are compared for OTULIN-deficient patients and healthy controls. Cytokine concentration shown in y axis is in picograms per milliliter. Values are represented as means ± SEM. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. (A) Whole-blood samples from patient 1 and his unaffected sibling were stimulated with bacterial LPS (1 μg/mL) for 24 h. A total of 48 cytokines or growth factors listed in SI Materials and Methods were assayed in duplicates. (B) Cytokine levels from the supernatant of stimulated purified monocytes from patient 1 compared with three healthy controls. Cells were unstimulated, TNF stimulated (20 ng/mL), LPS stimulated (1 μg/mL), or IL-1β stimulated (10 ng/mL) for 48 h. A total of 48 cytokines or growth factors were assayed; however, only the most significant results are shown. Samples were assayed in duplicates. (C) Intracellular staining of TNF in monocytes from patient 1 and patient 3 compared with two healthy controls before stimulation and following LPS stimulation (1 μg/mL). PBMCs from patient 2 were not available due to sample limitation. (D) TNF levels in the supernatants of PBMCs derived from three patients and one healthy control, at the basal level and after stimulation with IL-1β (10 ng/mL), and LPS (1 μg/mL). Samples were assayed in triplicates. (E) Serum cytokine levels from 3 patients and 12 healthy controls. Patient 1 (P1) has been treated with the TNF inhibitor, infliximab, and had no evidence of active disease at the time of sampling. Patient 2 (P2) has been treated with the IL-1 inhibitor, anakinra, and had active disease at the time of sampling. Patient 3 (P3) has been treated with the TNF inhibitor, etanercept, and still had some symptoms at the time of sampling. Patients’ samples were assayed in triplicates.
Fig. S6.
Increased cytokine production in patients. (A) SEB stimulation in whole blood. Cytokine profiles are compared for OTULIN-deficient patients and healthy controls. Cytokine concentration shown in y axis is in picograms per milliliter. Values are represented as means ± SD. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Whole-blood samples from patient 1 and patient 3 compared with age-matched healthy controls were stimulated with staphylococcal enterotoxin B (SEB) at 1 μg/mL for 24 h. A total of 48 cytokines or growth factors listed in SI Materials and Methods were assayed in triplicates. (B) Intracellular cytokine staining for IL-6 in PBMCs gated on CD14+/CD11+ for monocytes. (C) Intracellular cytokine staining TNF in PBMCs gated on CD3+/CD45RO+ for T cells. Flow cytometry showed higher TNF levels at baseline in T cells of patients compared with two healthy controls. (D) Intracellular cytokine staining for TNF and IL-6 in PBMCs gated on CD14+/CD11+/CD123− for dendritic cells. Flow cytometry showed increased TNF and IL-6 staining in dendritic cells, at basal level and after LPS stimulation, from two patients compared with two healthy controls.
Fig. S7.
TNF-induced inflammatory signature in whole blood and fibroblasts. (A) Gene expression of immunologically related genes in unstimulated whole-blood samples from three patients and five healthy controls by Nanostring assay. Transcriptional profiles reflect the disease activity of each patient at the time of sampling. Patient 1 had inactive disease, and his profile is similar to control samples. Patient 2 and patient 3 had more active disease as shown by red color in the heat map. (B) TNF-induced inflammatory signature in fibroblasts. Gene expression profiles in stimulated fibroblasts from two patients and a healthy control. Fibroblasts from patients 2 and 3 showed increased inflammatory signatures enriched for NF-κB, Jak-STAT, and TNF signaling.
Discussion
We describe a recessively inherited autoinflammatory disease caused by excessive linear ubiquitination in innate immune signaling pathways, which we denote as “otulipenia.” We show that OTULIN deficiency leads to increased linear ubiquitination of target proteins, which is associated with enhanced NF-κB activity, increased TNFR1 signaling, and NLRP3 inflammasome activity. The phenotype is very severe and potentially lethal if left untreated.
This is a report of a human disease caused by excessive linear ubiquitination. Conversely, patients with LUBAC deficiency have impaired linear ubiquitination of the same target molecules, which leads to immunodeficiency due to decreased NF-κB activity in fibroblasts, and a concomitant inflammatory phenotype due to hyperresponsiveness to IL-1β in monocytes (4). These latter studies demonstrate cell type-specific functions of the LUBAC subunits HOIP and HOIL-1. No human disease has yet been linked to SHARPIN deficiency. In contrast, OTULIN-deficient patients have a broader constitutive inflammatory phenotype in fibroblasts and monocytes and no overt primary immunodeficiency. Heterozygote carriers of these mutations are asymptomatic, which suggests that OTULIN expression levels do not appear to be critical for immune homeostasis. The importance of the linear ubiquitin pathway in the regulation of innate immune responses has been demonstrated in murine models. Mice deficient in LUBAC subunits have variable degrees of inflammation, from a mild phenotype in HOIL-1–deficient mice (16) to more severe inflammation and dermatitis in SHARPIN KO (2, 17) to defective vascularization and embryonic lethality in HOIP KO (18). Consistent with the essential function of OTULIN in regulation of multiple signaling pathways, OTULIN-deficient mice (gumby/gumby) are embryonic lethal due to vascular and neuronal defects caused by dysregulation in canonical Wnt signaling (8).
This is the second report of human germline mutations in a deubiquitinase protein leading to an inflammatory phenotype, the first being mutations in DUB A20 (10). In contrast, deficiency of another deubiquitinase CYLD, which hydrolyzes both Met1 and K63 ubiquitin chains, leads to cylindromatosis (19). Although A20 and OTULIN have roles in attenuating common signaling pathways, patients with otulipenia have a more severe inflammatory phenotype than patients with A20 haploinsufficiency (HA20) likely for two reasons: (i) OTULIN has a unique and nonredundant function in regulation of the linear ubiquitin pathway, and (ii) patients with otulipenia have a more profound protein deficiency than patients with HA20, who still retain 50% of nonmutant A20 protein. The discoveries of otulipenia, HA20, and LUBAC deficiencies demonstrate a complex interplay between LUBAC and deubiquitinases in controlling immune signaling complexes.
Materials and Methods
Patients.
We studied three patients in this report. All subjects and their family members were enrolled in an Institutional Review Board-approved protocol and provided written informed consent. Samples from patient 1 were available for all experiments, whereas samples from patients 2 and 3 were limited. More detailed information is reported in SI Materials and Methods.
Genetic and Functional Analysis.
We performed whole-exome sequencing in patients 1 and 2 and their family members, candidate-gene sequencing in patient 3 and her parents, and mutation-specific genotyping in 1,630 DNA samples from the Turkish population. To study protein function, we used short hairpin RNA (shRNA) knockdowns in 293 cells and NF-κB luciferase assay, and Met1-linked linear polyubiquitin deubiquitination assay in 293 cells. Immunoprecipitation and immunoblotting, flow cytometry, Nanostring, intracellular cytokine staining, and cytokine profiling were performed on samples from the patients and healthy controls. SI Materials and Methods describes the methods used for all these procedures.
SI Materials and Methods
Human Subjects.
Patients 1 and 3 were evaluated at the NIH Clinical Center, patients 2 and 3 were evaluated at the Hacettepe University Faculty of Medicine Department of Pediatric Nephrology and Rheumatology in Turkey and Familial Mediterranean Fever Arthritis Vasculitis and Orphan Disease Research Center, Gulhane Military Medical Academy, in Turkey. All of the three patients enrolled in this study were evaluated under protocols approved by the respective institutional review boards and all patients and family members provided written informed consent including consent to publish (the National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Diabetes and Digestive and Kidney Diseases combined institutional review board at the NIH, and the Hacettepe University Institutional Review Board in Turkey), and all patients and family members provided written informed consent including consent to publish.
Whole-Exome Sequencing.
Genomic DNA samples from patients and family members were isolated from peripheral blood. We performed whole-exome sequencing on patient 1 and his three family members as well as patient 2 from the second family (Fig. S1A). Whole-exome sequencing and data analysis were performed as previously described (10). Exonic variants including missense, splice site, and stop codon variants as well as small INDELs (frameshift and nonframeshift insertions and deletions) were filtered by ExAC, 1000 Genomes, dbSNP, NHLBI GO Exome Sequencing Project, ClinSeq database, and our internal database with over 600 exomes, based on a minor allele frequency below 1%. The variants were further filtered by homozygous inheritance due to the consanguinity of the patients.
Sanger Sequencing and Single-Base Extension Genotyping.
We performed Sanger sequencing to confirm the OTULIN mutations identified by exome sequencing and the candidate gene screening in additional patients with similar clinical presentations including patient 3. We used the BigDye Terminator, Version 1.1, Cycle Sequencing kit (Applied Biosystems) for sequencing the OTULIN exons. Sequencing was performed on a 3130xl Genetic Analyzer (Applied Biosystems), and Sequencher (Gene Codes) was used to analyze the sequencing data.
We performed single-base extension genotyping for the three OTULIN mutations (p.Leu272Pro, p.Tyr244Cys, p.Gly174Aspfs*2) in a total of 1,630 Turkish healthy controls using the Sequenom iPLEX gold method (Sequenom). Genotypes were determined with Typer 4.0 software (Sequenom).
Antibodies and Expression Plasmids.
We used the following antibodies for immunoprecipitation and immunoblotting. Antibodies specific for NEMO (sc-8330), ASC (N-15, sc-22514-R), and HOIL1 (sc-365523) were from Santa Cruz; phospho-IKKα/β (#2697), IKKα (#11930), IκBα (#4814, #9242), phospho-IκBα (#2859), TNFR1 (#3736), RIP (#3493), phospho-p38 MAPK (#4511), phospho-SAPK/JNK (#4668), SAPK/JNK (#9252), p38 MAPK (#8690), SHARPIN (#12541), OTULIN (#14127), β-actin (#4970), and HSP90 (#4874) were from Cell Signaling; HOIP (TA329873) was from Origene; polyubiquitin (sc-271289) was from Santa Cruz; linear polyubiquitin (AB130) was from Lifesensors; and linear polyubiquitin (1F11/3F5/Y102L) and K63 polyubiquitin (Apu3.A8) were from Genentech.
The antibodies used for intracellular cytokine staining were anti-TNF (554514; BD Biosciences), anti-IL6 (56-7069-42; eBioscience), and anti-CD14 (555398; BD Biosciences).
The WT OTULIN plasmid (RC224840) was from Origene, and the three mutant OTULIN plasmids (Y244C, L272P, and G174Dfs*2) were constructed by site-directed mutagenesis. Plasmids for RIPK1 (HW1506051), NEMO (SC117828), TNFRSF1A (RC204008), and ASC (RC215592) were from Origene; plasmids for SHARPIN (#50014), HOIP (#50015), and HOIL-1 (#50016) were from Addgene; GFP-Ub (#11928) and GFP-Ub KO (#11934) were also from Addgene, and Ub-WT and Ub-KO were constructed by site-directed mutagenesis of removing the GFP tag from GFP-Ub and GFP-Ub KO, respectively.
Cell Cultures, Peripheral Blood Mononuclear Cell Preparation, and Monocyte Isolation and Stimulation.
Human embryonic kidney 293 cells (HEK293 cells) (negative for mycoplasma, originally obtained from the American Type Culture Collection) and skin fibroblast cells derived from OTULIN-deficient patients or normal donors were grown in DMEM (Life Technologies) plus 10% (vol/vol) fetal bovine serum (FBS) and 1× antibiotics (Life Technologies). Heparinated peripheral blood mononuclear cells (PBMCs) were separated by Ficoll (Ficoll-Paque PLUS; GE Healthcare) gradient centrifugation. Monocytes were purified from PBMCs by negative selection (Monocyte Isolation Kit II; Miltenyi Biotec). Monocytes were then suspended in monocyte attachment medium (PromoCell) and seeded at a density of 150,000 per cm2 for 2 h. After washing out suspension cells, cells were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS, 2 mM l-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL) for 1 h.
Recombinant human TNF (PeproTech; 20 ng/mL) and human recombinant IL-1β (PeproTech; 10 ng/mL) were used to stimulate PBMCs and fibroblasts for certain time periods indicated in the figures.
OTULIN Gene Silencing by Short Hairpin RNA Transduction.
Human short hairpin RNA (shRNA) constructs for OTULIN (TL304698) and scrambled controls were purchased from Origene and prepared according to manufacturer’s protocol. HEK293 cells were transduced by incubating with shRNA virus. Puromycin (2 μg/mL) was added to the medium [DMEM (Life Technologies) plus 10% FBS and 1× antibiotics (Life Technologies)] to generate stable knockdown cell lines.
NF-κB Reporter Assay.
NF-κB pathway reporter plasmids (Promega) (pGL4.32[luc2P/NF-κB-RE/Hygro] luciferase reporter and the pRLCMV-Renilla vector) were used to cotransfect in HEK293 cells with shRNA knocked-down OTULIN, together with expression plasmids for OTULIN WT or mutants, LUBAC (SHARPIN, HOIL-1, and HOIP), and Ub-KO with or without NEMO using Lipofectamine 2000 reagents (Invitrogen). Luciferase activity was measured 36 h later by the DualGlo Luciferase Assay System (Promega). Results for NF-κB activity are expressed as fold induction by normalizing firefly luciferase activity to Renilla luciferase activity.
Immunoprecipitation and Immunoblotting.
Whole-cell lysates were prepared using ice-cold cell lysis buffer containing 20 mM Tris⋅HCl, pH 7.5, 140 mM NaCl, and 1% Triton X-100, and supplemented with protease inhibitor mixture (Roche) and 1% phosphatase inhibitor (Thermo Fisher). For immunoprecipitations, antibodies (5 μg) were added to 1 mg of total protein extract and incubated overnight at 4 °C. For linear-polyubiquitin immunoprecipitation, cells were lysed in a cell lysis buffer containing 8 M urea (Qiagen) and incubated with an antibody in cell lysis buffer containing 7 M urea at room temperature. Protein G-agarose beads (Thermo Fisher) were added to the samples and incubated for 2 h at 4 °C. Then beads were washed three times with lysis buffer and resuspended in 1× NuPAGE LDS sample buffer (Thermo Fisher) with 1% 2-mercaptoethanol (Sigma-Aldrich). Proteins were separated by SDS/PAGE and transferred to PVDF membranes. Immunoreactive proteins were visualized using ECL Plus Western blotting substrate (Thermo Fisher).
T- and B-Cell Stimulation.
For B-cell activation, human B cells were purified by negative selection using the StemSep Human B-Cell Enrichment kit according to the manufacturer’s instructions. B cells were stimulated with F(ab′)2 anti-IgM (10 μg/mL; Jackson ImmunoResearch Laboratory) for 30 min. For T-cell receptor (TCR) stimulation, total PBMCs (1 × 106) were stimulated with CD3/CD28 (Dynabeads T-cell activator from Thermo Fisher Scientific) for 30 min. Cell lysates [50 mM Tris-Cl, pH 7.4, 0.5% Nonidet P-40, 0.5% Triton X-100, 0.15 M NaCl, 2 mM EDTA, protease inhibitor (Thermo Fisher Scientific)] were prepared and resolved on NuPAGE Novex 4-12% Bis–Tris gels (polyacrylamide concentration: 4–12% gradient) (Thermo Fisher Scientific). Proteins were transferred on a nitrocellulose membrane and probed using indicated antibodies.
Met1-Linked Linear Polyubiquitin Deubiquitination Assay.
For deubiquitination assay, HEK293 cells were transfected with plasmids expressing target protein (NEMO, ASC, RIP1, or TNFR1), together with Ub-KO or Ub-WT, SHARPIN, HOIP, HOIL-1, OTULIN WT or mutants, or WT together with one mutant. Cells were harvested 36 h after transfection. Cell lysates were prepared and immunoprecipitated as previously described.
Immune Cells Cytokine Production and Serum Cytokine Detection.
Whole blood was stimulated with bacterial lipopolysaccharide (LPS) (Sigma) and staphylococcal enterotoxin B (SEB) (Sigma) for 24 h. Purified monocytes (1 × 106 cells per mL) were stimulated with LPS (Sigma; 10 ng/mL), human recombinant TNF (PeproTech; 20 ng/mL), or human recombinant IL-1β (PeproTech; 10 ng/mL) for 48 h. The concentrations of cytokines in the supernatants of stimulated and nonstimulated whole blood, monocytes, and serum were determined using Bio-Plex Pro Human Cytokine 27-plex and 21-plex immunoassay kits (Bio-Rad Hercules). The Bio-Plex pro human cytokine standard group I and group II were used as standards for the assays. The 48 cytokines and growth factors assayed are as follows: IL-1α, IL-2Rα, IL-3, IL-12 (p40), IL-16, IL-18, CTACK, GRO-α, HGF, IFN-α2, LIF, MCP-3, M-CSF, MIF, MIG, β-NGF, SCF, SCGF-β, SDF-1α, TNF-β, TRAIL, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF.
PBMCs (2 × 106 cells per mL) were stimulated with human recombinant IL-1β (PeproTech; 10 ng/mL), LPS (Sigma) for 24 h. The concentrations of cytokines in the supernatants of stimulated and nonstimulated PBMCs were determined using ProcartaPlex 13plex (Affymetrix). The 13 cytokines are IL-1α, IL-1β, IL-1ra, IL17A, IL-17F, IL-2, IL-6, IL-8, IP-10, MCP-1, MIP-1β, RANTES, and TNF. The differences in the cytokine concentrations were statistically analyzed using unpaired t test, and plotted with GraphPad Prism software package.
Intracellular Cytokine Staining.
Intracellular cytokine staining for TNF and IL-6 following LPS stimulation in PBMCs gated on CD14+ CD11+ for monocytes, gated on CD14+CD11+CD123− for dendritic cells, and gated on CD3+CD45RO+ for T cells were performed as previously described. Briefly, cells were washed twice with PBS, stained with the Live/Dead Fixable Aqua Dead Cell Stain Kit (Invitrogen) for 15 min at 4 °C, immediately fixed with 4% paraformaldehyde for 10 min at room temperature and permeabilized with cold methanol at −20 °C overnight. Cells were stained as indicated with the following antibodies: CD3-Qdot605 (Life Technologies), CD11c-PB (BD Biosciences), CD14-PE (BD Biosciences), CD123-PEcy5 (BD Biosciences), CD45RO-Texas-Red (Beckman Coulter), and TNF-FITC and IL6-AF700 (BD Biosciences). All events were collected on an LSRFortessa (BD Biosciences) and analyzed with FlowJo (Tree Star).
NanoString Assay.
RNA was extracted by PAXgene Blood RNA Kit (Qiagen), and gene expression analysis was conducted using the nCounter Analysis System (NanoString Technologies) with a codeset designed to target 594 immunologically related genes. A total of 100 ng of total RNA was mixed with the codeset and reporter probes and hybridized for 24 h. The complexes were then bound to the imaging surface using the nCounter Prep Station and then were quantified on the nCounter digital analyzer. nSolver software was used for data analysis. The expression data were normalized by using a geometric mean of 15 housekeeping genes (EEF1G, TUBB, TBP, POLR2A, GUSB, HPRT1, GAPDH, SDHA, OAZ1, PPIA, G6PD, RPL19, POLR1B, ABCF1, and ALAS1).
Accession Codes: Referenced Accessions.
NCBI reference sequence: NM_138348.4.
URLs.
URLs are as follows: Exome Aggregation Consortium (ExAC) database, exac.broadinstitute.org/; 1000 Genomes Project, www.1000genomes.org/; dbSNP, www.ncbi.nlm.nih.gov/projects/SNP/; Picard, broadinstitute.github.io/picard/; Genome Analysis Toolkit (GATK), https://software.broadinstitute.org/gatk/; ANNOVAR, www.openbioinformatics.org/annovar; CLUSTALW, www.genome.jp/tools/clustalw/.
Acknowledgments
We thank all the patients and their families, and the healthy controls, for their enthusiastic support during this research study. We thank Drs. Alejandra Negro and Xiaodong Fu from National Heart, Lung, and Blood Institute (NHLBI) for technical support, and Dr. Eric P. Hanson from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) for helpful suggestions. This research was supported by the Intramural Research Programs of the National Human Genome Research Institute, NIAMS, NHLBI, National Institute of Allergy and Infectious Diseases, and the NIH Clinical Center. E.D. received grant support from Tübitak 1003, Primary Subjects R&D Funding Program (Project 315S122), which is supported by the Scientific and Technological Research Council of Turkey.
Footnotes
Conflict of interest statement: S.Ö. received royalties for consulting and speaking from Novartis and SOBI. All other authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612594113/-/DCSupplemental.
References
- 1.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 2.Tokunaga F, et al. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature. 2011;471(7340):633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 3.Boisson B, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol. 2012;13(12):1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boisson B, et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J Exp Med. 2015;212(6):939–951. doi: 10.1084/jem.20141130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ombrello MJ, Kastner DL, Milner JD. HOIL and water: The two faces of HOIL-1 deficiency. Nat Immunol. 2012;13(12):1133–1135. doi: 10.1038/ni.2471. [DOI] [PubMed] [Google Scholar]
- 6.Draber P, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13(10):2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keusekotten K, et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell. 2013;153(6):1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivkin E, et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature. 2013;498(7454):318–324. doi: 10.1038/nature12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrdinka M, et al. CYLD limits Lys63- and Met1-linked ubiquitin at receptor complexes to regulate innate immune signaling. Cell Rep. 2016;14(12):2846–2858. doi: 10.1016/j.celrep.2016.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2016;48(1):67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma A, Malynn BA. A20: Linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12(11):774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffer V, et al. Binding of OTULIN to the PUB domain of HOIP controls NF-κB signaling. Mol Cell. 2014;54(3):349–361. doi: 10.1016/j.molcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Israël A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2(3):a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wertz IE, et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528(7582):370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers MA, et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med. 2014;211(7):1333–1347. doi: 10.1084/jem.20132486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDuff DA, et al. Phenotypic complementation of genetic immunodeficiency by chronic herpesvirus infection. eLife. 2015;4:4. doi: 10.7554/eLife.04494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda F, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471(7340):637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltzer N, et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Reports. 2014;9(1):153–165. doi: 10.1016/j.celrep.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 19.Bignell GR, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25(2):160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]