Significance
Proteins destined for secretion from cells enter the secretory pathway in coat protein complex II (COPII)-coated vesicles budding from the endoplasmic reticulum (ER). Most cargo proteins are small and exit the ER in 60-nm vesicles. However, some secretory cargos are too large to enter such carriers; in particular, the procollagen precursor of the extracellular matrix exits the ER as a 300- to 400-nm fibril. Recent research suggests that procollagen may be packaged into large COPII-coated tubules, guided by the receptor molecule TANGO1/cTAGE5. We show that each TANGO1/cTAGE5 receptor protein has a multiplicity of binding sites to recruit and concentrate COPII proteins. We propose the model that TANGO1/cTAGE5 instructs the COPII coat to form large tubular carriers.
Keywords: vesicle traffic, coat protein, procollagen
Abstract
The supramolecular cargo procollagen is loaded into coat protein complex II (COPII)-coated carriers at endoplasmic reticulum (ER) exit sites by the receptor molecule TANGO1/cTAGE5. Electron microscopy studies have identified a tubular carrier of suitable dimensions that is molded by a distinctive helical array of the COPII inner coat protein Sec23/24•Sar1; the helical arrangement is absent from canonical COPII-coated small vesicles. In this study, we combined X-ray crystallographic and biochemical analysis to characterize the association of TANGO1/cTAGE5 with COPII proteins. The affinity for Sec23 is concentrated in the proline-rich domains (PRDs) of TANGO1 and cTAGE5, but Sec23 recognizes merely a PPP motif. The PRDs contain repeated PPP motifs separated by proline-rich linkers, so a single TANGO1/cTAGE5 receptor can bind multiple copies of coat protein in a close-packed array. We propose that TANGO1/cTAGE5 promotes the accretion of inner coat proteins to the helical lattice. Furthermore, we show that PPP motifs in the outer coat protein Sec31 also bind to Sec23, suggesting that stepwise COPII coat assembly will ultimately displace TANGO1/cTAGE5 and compartmentalize its operation to the base of the growing COPII tubule.
The coat protein complex II (COPII)-coated vesicles transport secretory and plasma membrane proteins from the endoplasmic reticulum (ER). COPII vesicle budding involves a stepwise assembly reaction: Membrane-bound Sar1-GTP recruits Sec23/24 to form the inner coat complex that, in turn, recruits the Sec13/31 outer coat protein (1). Sec13/31 self-assembles into a polyhedron, and in the process, it sculpts the ER membrane into a bud, producing a COPII-coated vesicle with a diameter of ∼60 nm (2–4).
Small cargo molecules are captured during the budding reaction through mechanisms that are well established (5). However, the formation of canonical 60-nm COPII vesicles cannot explain the packaging of large cargos, such as the procollagen fibril with a length of 300–400 nm. Procollagen follows the conventional route of secretion taken by other extracellular proteins, and its ER export evidently depends on COPII carriers because mutations in genes encoding COPII proteins result in collagen secretion blockade, impaired extracellular matrix deposition, and abnormal craniofacial development (6–8). A key discovery was the identification of the receptor TANGO1 and its coreceptor cTAGE5 as ER-localized machinery for loading procollagen into COPII carriers (9, 10). TANGO1 has a luminal SH3 domain shown to interact with collagen VII and a cytosolic domain that interacts with Sec23/24. Both TANGO1 and cTAGE5 are required for collagen export from the ER (9, 10). The TANGO1 knockout mouse has generalized defects in extracellular matrix formation, owing to a block in ER export of multiple collagen types (11).
Recent research has implicated post-ER membranes in TANGO1-mediated carrier formation (12, 13); however, the mechanism of action of TANGO1/cTAGE5 in procollagen export remains unclear. The possibility that it acts as a receptor linking luminal procollagen cargo to cytosolic COPII is challenged by the observation that TANGO1 does not depart in the COPII carrier (9). Alternatively, TANGO1/cTAGE5 might constitute machinery that switches COPII coat assembly from small spherical vesicles to large carriers, but such a mechanism has not been explored to date. Important in this respect is the observation of tubular COPII carriers with dimensions commensurate with procollagen cargo; such tubules form, along with small vesicles, in budding reactions in vivo and in vitro (14–16). COPII-coated tubules are observed as straight-sided tubes (17), or with a beads-on-a-string appearance in which regular constrictions may arise from aborted attempts at membrane fission (14, 16). Whereas vesicles contain a Sec23/24 inner coat and a self-assembled Sec13/31 cage with isometric (cubic) symmetry, tubules are coated with a distinctive, close-packed Sec23/24 helical lattice and a rhomboidal Sec13/31 cage (17). The realization that COPII coat proteins can form two distinct and mutually incompatible lattices suggests the hypothesis that COPII carrier formation involves two self-assembly reactions that compete to determine the shape of the COPII carrier—either the isometric small vesicle or the helical tubule—with the outcome of budding influenced by regulatory proteins that promote one or other lattice.
In this study, we have characterized the interaction of TANGO1/cTAGE5 with COPII proteins. We report that TANGO1/cTAGE5 binds to the inner coat protein Sec23 via a simple PPP motif. The flexible cytosolic regions of TANGO1 and cTAGE5 molecules contain repeated PPP motifs separated by proline-rich linkers, so a single TANGO1/cTAGE5 receptor can bind multiple copies of coat protein in a close-packed array. From these results, we propose a mechanism for TANGO1/cTAGE5-assisted assembly of large COPII carriers.
Results
Interaction of TANGO1 and cTAGE5 with COPII Proteins.
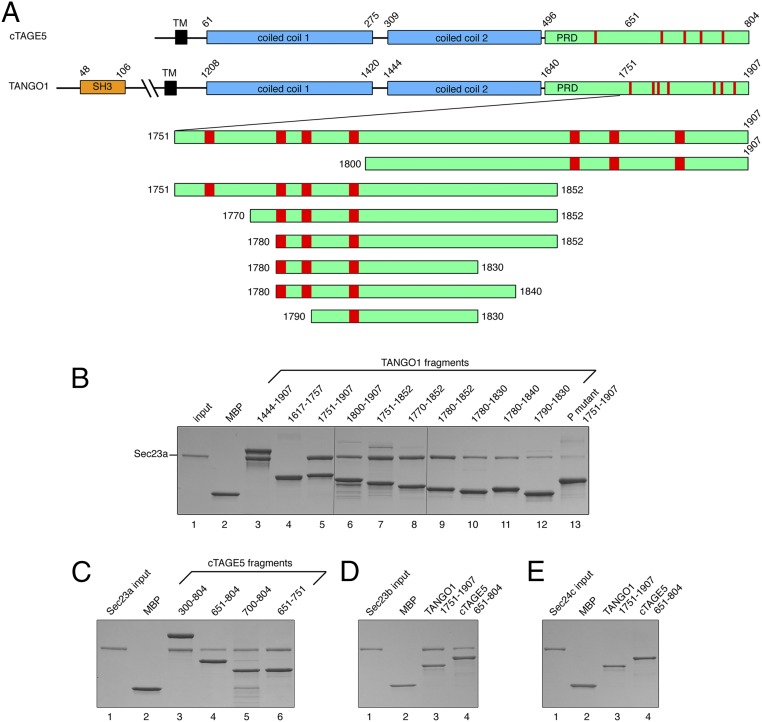
Interactions between TANGO1/cTAGE5 and the COPII inner coat protein Sec23/24 were identified by yeast two-hybrid analysis in two published studies; the homologous proline-rich domains (PRDs) of TANGO1 and cTAGE5 were both shown to interact with human Sec23a (9, 10). The PRD of TANGO1 is also required for ER exit site localization, presumably by virtue of its interaction with Sec23/24 (9). The schematic in Fig. 1A indicates the position of the PRD at the C terminus of the TANGO1 and cTAGE5 polypeptides.
Fig. 1.
Dissection of TANGO1 and identification of Sec23-binding regions. (A) Upper diagram shows the domain organization of the homologous human cTAGE5 and TANGO1 proteins. Cytosolic regions are drawn to the right of the transmembrane domain. The PRDs of TANGO1 and cTAGE5 are colored green, and the repeated PPP sequences that our experiments identify as the Sec23-binding motif are indicated in red. TANGO1 and cTAGE5 interact via their second (C-terminal) coiled coil domains (10). SH3 denotes the luminal Src homology 3 domain of TANGO1. In addition to the single transmembrane domain, TANGO1 has an adjacent membrane-binding sequence that penetrates but does not cross the bilayer (9). The lower diagram shows the series of constructs of the TANGO1 PRD that were prepared to explore the interaction with Sec23/24. (B) TANGO1 polypeptides fused to MBP were immobilized on amylose resin beads, which were then incubated with a mixture containing 0.8 mg/mL human Sec23a. Proteins were analyzed by 4–20% (wt/vol) SDS/PAGE and Coomassie blue staining. (C) Sec23a also binds to the PRD of cTAGE5. (D) TANGO1 and cTAGE5 both bind to the alternative isoform Sec23b. (E) No binding is detected between human Sec24c and the PRDs of TANGO1 or cTAGE5.
To map the Sec23/24 binding site to a circumscribed region of TANGO1, we prepared a series of polypeptides (Fig. 1A, Lower) and tested these for binding to purified Sec23 and Sec24 proteins (Materials and Methods). TANGO1 polypeptides, expressed as C-terminal fusions to maltose binding protein (MBP), were immobilized on amylose resin beads and probed for binding to human Sec23a. Sec23a bound to a construct encompassing the entire PRD plus coiled coil 2 of TANGO1, residues 1444–1907 (Fig. 1B, lane 3). No appreciable binding was observed between Sec23a and MBP alone (lane 2). The affinity for Sec23 was contained in the C-terminal half of the TANGO1 PRD (lane 5), and no significant binding was observed to the N-terminal half (lane 4). We repeated this analysis with cTAGE5 (Fig. 1C), and found that cTAGE5 residues 651–804 bound to Sec23a (Fig. 1C, lane 4), as did shorter cTAGE5 fragments (lanes 5 and 6). These data are consistent with two-hybrid interactions reported (9, 10). In addition to Sec23a, the paralog Sec23b bound to TANGO1 and cTAGE5 (Fig. 1D). Finally, we tested for interactions with the inner coat protein Sec24c; neither TANGO1 nor cTAGE5 PRD bound to Sec24c, because binding was at background levels (Fig. 1E). This finding contrasts with experiments that reported a two-hybrid interaction with Sec24c, albeit significantly weaker than Sec23a interactions with TANGO1 and cTAGE5 (10). Taken together, we suggest that TANGO1/cTAGE5 interacts specifically with the Sec23 component of the COPII inner coat complex.
Next, we tested the series of short TANGO1 polypeptides (Fig. 1A) to pinpoint the interaction with Sec23a and to define a complex for crystallographic analysis. The tight-binding TANGO1 construct 1751–1907 was used as a starting point (Fig. 1B, lane 5). N-terminal truncation of this construct reduced binding to Sec23a (Fig. 1B, compare, for example, lanes 7 and 8), in particular beyond residue 1780 (compare lanes 10 and 12). Likewise, C-terminal truncations also reduced binding (Fig. 1B, compare, for example, lanes 6 and 12). Because the TANGO1 PRD is an intrinsically disordered polypeptide, a clear cutoff effect might not be expected from these experiments; nevertheless, it was puzzling to find that affinity for Sec23a depends on TANGO1 residues that are dispersed along 157 residues of polypeptide.
The biochemical analysis indicated that TANGO1 residues 1780–1840 retained appreciable binding to Sec23a (Fig. 1B, lane 11), albeit weaker than longer forms, so we chose this polypeptide for a crystallographic analysis of the complex with Sec23.
Molecular Recognition of TANGO1 by Sec23.
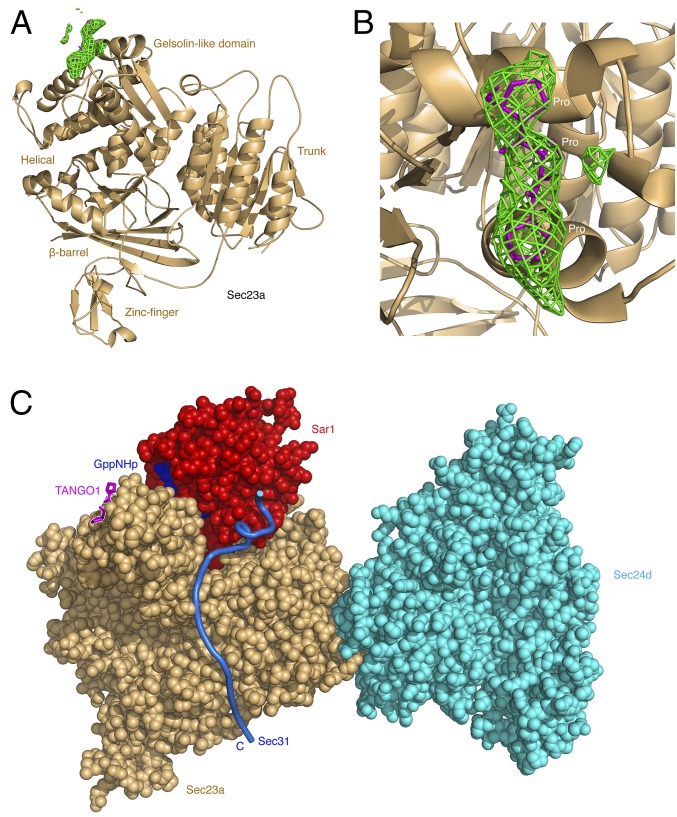
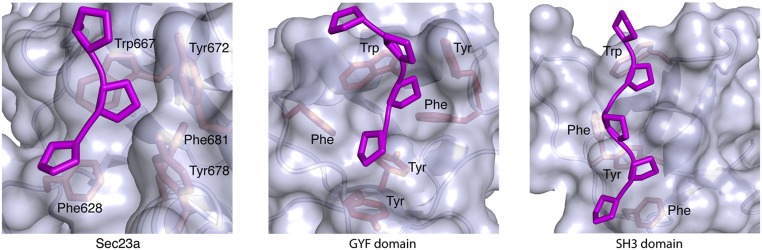
The complex comprising full-length human Sec23a and TANGO1 residues 1780–1840 was cocrystallized, and the structure was determined and refined to 2.6 Å resolution (Table S1). Despite the fact that 61 residues of TANGO1 are present in the crystals, difference electron density reveals just a three-residue peptide element bound to a site on the gelsolin-like domain of Sec23a (Fig. 2 A and B) [Sec23 domain nomenclature was defined previously (18)]. The tripeptide can be best modeled as three proline residues in the polyproline type II (PPII) helical conformation. The central proline in particular is positioned between Sec23a residues Trp667 and Tyr678 (Fig. S1, Left). Indeed, the entire binding site is formed from aromatic residues, and a striking similarity is observed with the polyproline-binding sites of evolutionarily unrelated domains. Fig. S1 compares Sec23 to the SH3 and GYF domains (WW and EVH1 domains offer additional examples). The domain topologies are unrelated, but all of the polyproline-binding sites are formed from aromatic residues positioned to form a series of ridges and grooves that accommodate the multiple proline side chains (19, 20).
Table S1.
Data collection and refinement statistics
| COPII protein | Sec23a | Sec23a/24d | Sec23a/24d | Sec23a/24d | Sec23a/24d |
| TANGO1 peptide | 1780–1840 | QLCGPFGPRP | GPRPLPPP | LPPPFGPGM | GMRPPLGLRE |
| Protein Data Bank ID | 5KYN | 5KYX | 5KYU | 5KYW | 5KYY |
| Space group | C2 | P212121 | P212121 | P212121 | P212121 |
| Cell parameters a, b, c (Å) | 133.5, 65.9, 230.0 | 102.5, 141.5, 152.1 | 103.3, 140.4, 150.4 | 101.6, 141.3, 151.6 | 103.4, 142.3, 152.7 |
| α, β, γ (°) | 90, 97.4, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 |
| Data processing | |||||
| Resolution (Å) | 47–2.6 | 50–3.4 | 50–3.5 | 50–3.3 | 50–3.2 |
| Rmerge* (%) | 10.3 (36.7)* | 8.9 (43.4) | 7.4 (39.2) | 11.2 (41.6) | 8.2 (38.0) |
| I/σ | 18.1 (2.2) | 12.3 (1.4) | 8.4 (2.5) | 10.6 (2.3) | 13.7 (2.3) |
| Completeness (%) | 99.8 (98.2) | 99.7 (91.8) | 97.8 (95.3) | 99.9 (99.7) | 99.8 (92.6) |
| Redundancy | 2.9 (2.1) | 2.9 (2.4) | 4.1 (3.8) | 3.2 (3.1) | 3.5 (3.4) |
| Refinement statistics | |||||
| Data range (Å) | 47–2.6 | 50–3.4 | 50–3.5 | 50–3.3 | 50–3.2 |
| Nonhydrogen atoms | 11,047 | 11,724 | 11,724 | 11,724 | 11,724 |
| Water molecules | 36 | ||||
| Rms ∆ bonds† (Å) | 0.008 | 0.009 | 0.003 | 0.009 | 0.004 |
| Rms ∆ angles† (°) | 1.2 | 1.2 | 0.7 | 1.3 | 0.7 |
| R factor‡ (%) | 18.7 | 18.3 | 18.5 | 19.1 | 18.3 |
| Rfree‡,§ (%) | 25.4 | 25.7 | 25.8 | 25.7 | 25.1 |
Highest resolution shell is shown in parentheses.
Rmerge = 100 × ∑h∑i | Ii(h) − <I(h)> |/∑h<I(h)>, where Ii(h) is the ith measurement and <I(h)> is the weighted mean of all measurement of I(h) for Miller indices h.
Rmsd (rms ∆) from target geometries.
R factor = 100 × ∑|FP – FP(calc)|/∑ FP.
Rfree was calculated with 5% of the data.
Fig. 2.
TANGO1 binding site located by X-ray crystallography. (A) Crystal structure of Sec23a bound to TANGO1 (residues 1780–1840) is drawn in ribbon representation. Green contour lines show residual electron density (Fo-Fc map; 2.6 Å resolution, 2.3 σ contour level) following refinement of Sec23a coordinates, in the vicinity of the gelsolin-like domain. (B) Close-up view of the difference electron density for the bound TANGO1 peptide, with the density calculated as in A. A tripeptide PPP sequence is modeled in a PPII helical conformation. (C) Composite model to indicate the location of the TANGO1-binding site in relation to the complete COPII inner coat complex. This is a view toward the membrane-distal surface of the complex. Sec23a (colored gold) and TANGO1 PPP motif (magenta) are drawn in the same orientation as A. In addition, we have included the structure of Sec24d, colored cyan (data from ref. 21; PDB ID code 3EG9), as well as Sar1 (colored red with bound GppNHp nucleotide in blue) and the 50-residue binding element of Sec31, drawn as a dark blue worm (from the yeast crystal structure of Sec23•Sar1 complexed with Sec31, PDB ID code 2QTV) (22).
Fig. S1.
Polyproline recognition by Sec23a, GYF domain, and SH3 domain: the convergent binding modes for polyproline motifs on Sec23a and two other evolutionarily unrelated protein domains. The hydrophobic binding sites are lined with Trp, Phe, and Tyr side chains. These aromatic residues form a series of ridges and grooves on the protein surface into which proline residues of the PPII helix pack. Note that the SH3 domain has additional ridges and grooves to recognize a longer polyproline motif. The GYF domain structure is from the protein CD2BP2 in complex with a peptide SHRPPPPGHRV derived from CD2 (PDB ID code 1L2Z), and the SH3 domain is from Sem-5 complexed with the peptide PPPVPPRRR from mSos (PDB ID code 1SEM).
The unexpected crystallographic result of a minimal PPP sequence of TANGO1 bound to Sec23a suggests a model of TANGO1 binding to Sec23 via a 3-aa motif that repeats through the PRD, thus providing a plausible explanation for our inability to pinpoint the binding sequence of TANGO1 in biochemical experiments: Seven copies of the PPP motif are present in the human TANGO1 PRD (cTAGE5 PRD contains five PPP motifs), and the crystallized fragment of TANGO1 (1780–1840) has three PPP motifs (Fig. 1A). However, the crystallographic result does not definitively assign the binding motif as being uniquely PPP, because the electron density is almost certainly a composite of multiple proline-rich tripeptide sequences from TANGO1 residues 1780–1840 and may include XPP or PPX sequences. Thus, we proceeded to test the preference of Sec23 for PPP and related motifs, and also to verify that the gelsolin-like domain is the binding site for TANGO1.
Individual PPP Motifs Bind to Sec23.
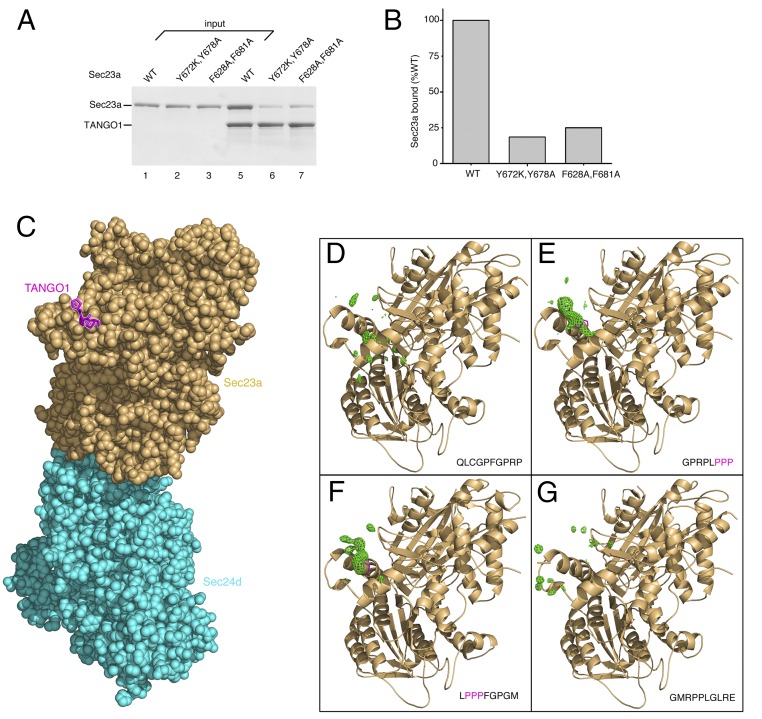
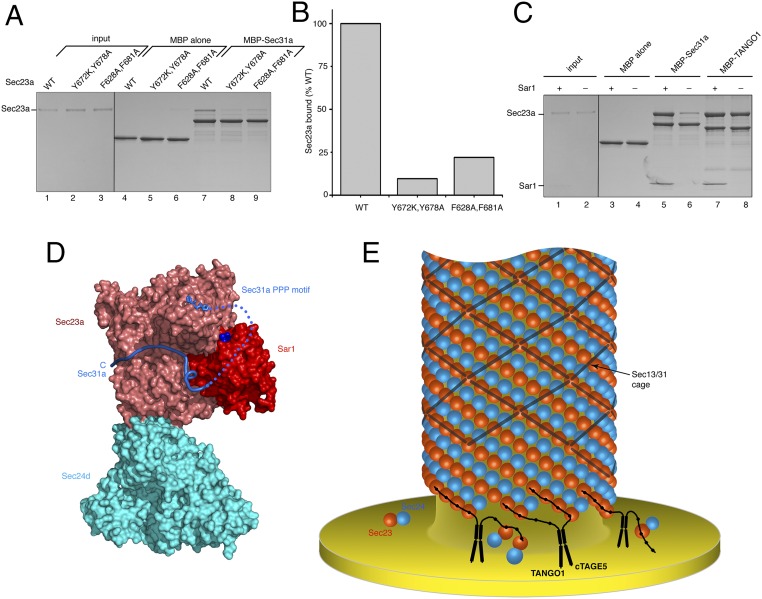
To confirm that TANGO1 binds to the gelsolin-like domain of Sec23, we generated two Sec23a mutants (a double mutant Y672K/Y678A and a double mutant F628A/F681A), thus altering key aromatic residues (Fig. S1, Left). Consistent with the crystallographic observation, both mutants showed impaired ability to bind TANGO1 (Fig. 3 A and B). The tyrosine double-mutant Sec23a lost rather more affinity, possibly because of the critical role of residue Tyr678 in sandwiching the central proline residue of PPP (Fig. S1).
Fig. 3.
Examination of the TANGO1-binding site on Sec23a, and of the Sec23-binding motif on TANGO1. (A) Mutations to Sec23a were introduced into the binding site defined crystallographically, and these are found to substantially reduce binding to TANGO1 PRD residues 1751–1907. Two mutants of Sec23a were tested, both double mutants (Y672K/Y678A and F628A/F681A); the location of these residues is shown in Fig. S1. (B) The reduction in binding to TANGO1 caused by mutation of Sec23a was quantified by densitometry of bands in A. (C) All-atom representation of the Sec23a/24d crystal structure with bound TANGO PPP peptide (peptide sequence GPRPLPPP) (Table S1). (D) Residual electron density in the vicinity of the gelsolin-like domain, following refinement of Sec23a/Sec24d crystals soaked with the TANGO1 peptide 1790QLCGPFGPRP1799 (green contour lines show Fo–Fc difference electron density at the 2.3 σ contour level, calculated at 3.4 Å resolution). Note that the picture is oriented as in C. The crystallographic result indicates no binding for this peptide. (E) Residual electron density in the vicinity of the gelsolin-like domain indicates bound TANGO1 peptide 1796GPRPLPPP1803 (Fo–Fc map; 3.5 Å resolution, 2.3 σ contour level). (F) Electron density shows bound TANGO1 peptide 1800LPPPFGPGM1808 (Fo–Fc map; 3.3 Å resolution, 2.3 σ contour level). (G) Absence of bound peptide at the gelsolin-like domain in crystals soaked with TANGO1 peptide 1807GMRPPLGLRE1816 (Fo–Fc map; 3.2 Å resolution, 2.3 σ contour level).
Next, we tested the binding of individual PPP motifs to Sec23a. A single PPP motif binds to Sec23 too weakly to measure by using conventional methods (e.g., isothermal calorimetry), because the binding signal cannot be distinguished from background with confidence. Our solution to this problem was to use crystallography to provide spatial information that confirms specific binding. We grew a crystal form of Sec23a/24d (described in ref. 21) in which the gelsolin-binding domain is unobstructed by crystal contacts, so that short proline-rich peptides could be soaked into, and equilibrated with, the binding site. Residual electron density at the gelsolin-binding domain was then assessed for evidence of specific binding. Four TANGO1 peptides, 8–10 residues in length, were synthesized. The sequences are partially overlapping (encompassing TANGO1 residues 1790–1816) and were chosen such that two peptides contain a PPP motif, one peptide has a PP motif, and the final peptide contains only three isolated proline residues (Fig. 3 D–G). Peptides were soaked at high concentrations (6 mM) into crystals of Sec23a/24d. The crystals diffract to only medium resolution (3.2–3.5 Å) (Table S1); nevertheless, specific binding could be established straightforwardly by the appearance of residual electron density. TANGO1 peptides GPRPLPPP and LPPPFGPGM (Fig. 3 E and F) show clear binding to Sec23a (compare the electron density with the location of TANGO1 PPP in Fig. 3C, which is oriented similarly). By contrast, TANGO1 peptides lacking the PPP motif—QLCGPFGPRP and GMRPPLGLRE in Fig. 3 D and G—do not bind to Sec23a.
These data indicate that TANGO1 binds to the gelsolin-like domain of Sec23a via a PPP motif that repeats through the TANGO1 and cTAGE5 cytosolic regions. In Fig. 2C, we summarize these findings in a composite atomic model to indicate the location of the bound PPP motif on the COPII inner coat complex, near to Sar1-GTP and in proximity to the “active fragment” (the Sec23•Sar1-binding element) of Sec31.
Repeated PPP Motifs in TANGO1 and cTAGE5.
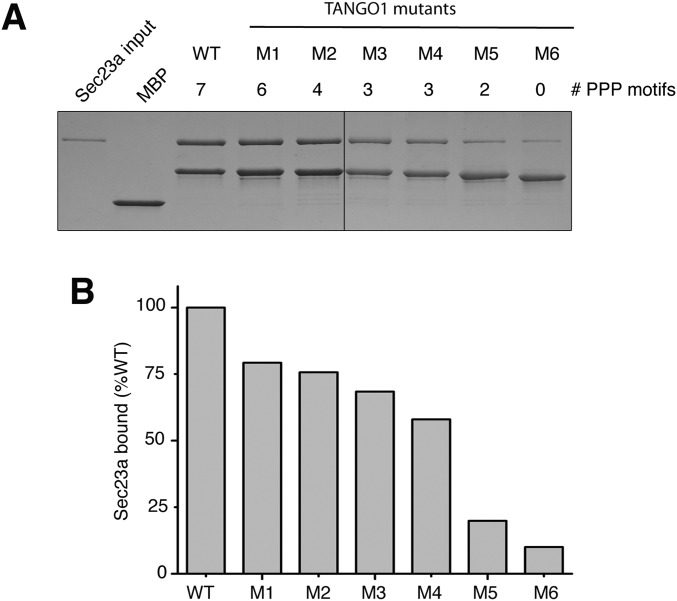
A region of the TANGO1 PRD containing seven PPP motifs, residues 1751–1907, seems to be responsible for all of the Sec23a-binding affinity, and forms of this polypeptide lacking the full complement of motifs bind less Sec23a (Fig. 1B). These results conform to our model of TANGO1 binding to Sec23 through an array of distributed PPP motifs; however, the experiment involves TANGO1 constructs of diverse sizes and compositions, so binding cannot easily be compared quantitatively. We measured, on a more systematic basis, how changes in the number of PPP repeats affect binding of TANGO1, using a series of TANGO1 polypeptides (residues 1751–1907) in which sets of proline residues were mutated sequentially to glycine or serine (Fig. S2; see Fig. S3 for sequences of the mutants, named M1 to M6).
Fig. S2.
Increasing the number of PPP repeats increases binding to Sec23a. (A) A series of TANGO1 constructs encompassing PRD residues 1751–1907 was tested for interaction with Sec23a. In the TANGO1 mutant proteins, labeled M1 to M6, sets of proline residues were changed sequentially to glycine or serine (see Fig. S3 for details of mutant sequences). TANGO1 polypeptide 1751–1907 contains 46 proline residues, so in each mutant seven or eight additional prolines were mutated. Mutant M6 contains no proline residues. The number of PPP motifs in each construct is indicated. (B) Binding of Sec23a to TANGO1 mutants was quantified by densitometry of Sec23a bands (normalized to MBP-TANGO1 bands and expressed as a percentage of binding to wild-type TANGO1).
Fig. S3.
Sequences of wild type and mutant TANGO1 polypeptides. The figure shows the amino acid sequence of wild-type human TANGO1 encompassing residues 1751–1907, together with six mutant TANGO1 sequences—named M1 to M6—in which sets of proline residues (outlined in green) are changed sequentially to (alternating) glycine and serine residues (changes outlined in yellow). These sequences, fused at their N termini to maltose-binding protein were used in the binding experiments shown in Fig. S1. Note the number of PPP motifs present in each TANGO1 sequence: in wild type, seven PPP motifs; in mutant M1, six PPP motifs; in M2, four PPP motifs; in M3, three PPP motifs; in M4, three PPP motifs; in M5, two PPP motifs; and in M6, zero PPP motifs.
A form of TANGO1 1751–1907 with all 46 of its proline residues mutated retains only residual affinity for Sec23a (mutant M6 in Fig. S2). Weak binding is observed between Sec23a and TANGO1 mutant M5, which has just two PPP motifs. Inclusion of an additional repeat (mutant M4 with three PPP motifs) increases binding further; each addition of PPP motifs increments the binding to Sec23a. These results show a clear correlation between the number of PPP repeats and the amount of Sec23a bound.
PPP Motifs in the Sec31 Outer Coat Protein Bind to Sec23.
The COPII outer coat protein Sec31 contains an intrinsically disordered PRD that includes the active fragment for binding Sec23•Sar1 (22). Besides the repeated PPP motifs in TANGO1 and cTAGE5, we also noted the presence of conserved PPP motifs in this strategically important region of the Sec31 polypeptide. Specifically, in human Sec31a, PPP motifs are located at residues 838PPPP841 and 944PPPP947; the active fragment is located at residues 980–1015.
To test whether PPP motifs on Sec31 can bind to the gelsolin-like domain of Sec23, we generated a form of human Sec31a containing both PPP motifs plus the active fragment (in total, residues 835–1026), and expressed this as a C-terminal fusion to maltose-binding protein. Sec31a bound to Sec23a (Fig. 4A, lane 7); binding is weak because Sec31a has only two PPP motifs (compare, for example, with mutant M5 in Fig. S2). Importantly, Sec31a did not bind the Sec23a mutants Y672K/Y678A or F628A/F681A, establishing that the interaction was via the gelsolin-like domain (Fig. 4 A and B). The background binding to the tyrosine double mutant Sec23a was low, in concordance with the binding behavior of the two mutants toward TANGO1 (compare Fig. 4B with Fig. 3B).
Fig. 4.
Binding sites for Sec31 and TANGO1 on Sec23a•Sar1. (A) The polypeptide region of human Sec31 encompassing two PPP motifs and the active fragment (in total, residues 835–1026) binds to the PPP-binding site on Sec23a. Binding observed between Sec31 and wild-type Sec23a (lane 7) is substantially reduced by mutation of the PPP-binding site (lanes 8 and 9). (B) The reduction in binding to Sec31 caused by mutation to Sec23a was quantified by densitometry of bands in A. This figure can be usefully compared with Fig. 3B. (C) Binding of proteins to Sec23a was tested in the presence of Sar1 (human Sar1a, residues 25–198) complexed with the nonhydrolyzable GTP analog, GppNHp. Tight binding of Sec31 (residues 835–1026) to Sec23a requires Sar1-GppNHp (compare lanes 5 and 6). By contrast, TANGO1 (residues 1751–1907) binding to Sec23a is independent of Sar1 (lanes 7 and 8). (D) Surface representation of a COPII complex in which we model the bivalent interaction of Sec31 to Sec23a, as indicated by the results of binding analysis. Residues 945PPP947 of human Sec31a are bound to the gelsolin-like domain of Sec23a, followed by a 32-residue linker (dotted line) and the active fragment (residues 980–1015). This is a composite model of Sec23a/24d/PPP taken from the present analysis, plus Sar1-GppNHp and the active fragment of Sec31 (from the yeast crystal structure of Sec23•Sar1 complexed with Sec31 active fragment; PDB ID code 2QTV). Coloring is the same as Fig. 2C. (E) Model showing how the TANGO1/cTAGE5 protein might promote the growth of the Sec23/24•Sar1 helical lattice on a COPII tubule. The dimensions and symmetry of the COPII-coated tubule are replicated from the published tomography images in ref. 17. The locations of the repeat PPP motifs on the TANGO1 and cTAGE5 molecules are indicated by black dots. In actuality, TANGO1 has seven PPP motifs and cTAGE5 five PPP motifs, but the spacing of motifs suggests that both proteins could bind to four copies of Sec23/24•Sar1 in array. This assumes a general PPII helical character for the bound PRD regions, induced by the ∼30% proline content.
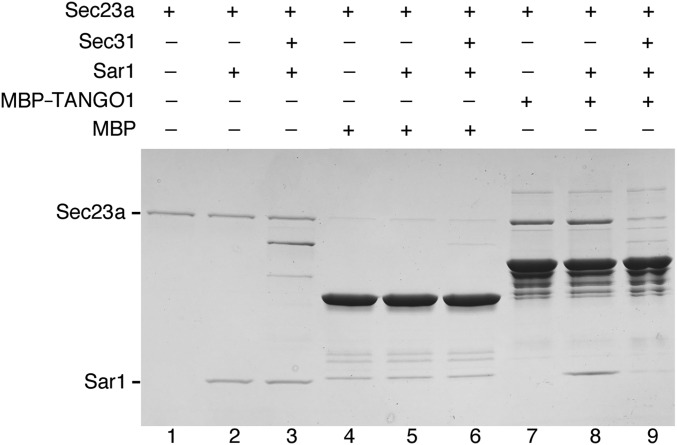
It is important to note that, in this experiment, there is no evidence for Sec31a binding to Sec23a via the active fragment; this interaction depends on the presence of GTP-bound Sar1 (22). We verified this point by testing the binding of Sec31a (residues 835–1026) to Sec23a in the presence of Sar1 complexed with the nonhydrolyzable GTP analog GppNHp (we used soluble human Sar1a, residues 25–198, lacking the N-terminal membrane anchor). Indeed, the interaction highly depended on Sar1-GppNHp, which increased ∼fivefold the quantity of Sec23a bound to Sec31a (Fig. 4C, lanes 5 and 6). By contrast, binding of Sec23a to TANGO1 is independent of Sar1-GppNHp (lanes 7 and 8). Note that Sec23a binding to TANGO1 is appreciable in this experiment because we used construct 1751–1907 containing seven PPP motifs, whereas the Sec31 polypeptide has just two PPP motifs. If we adopt the data in Fig. S2 as a metric, it is clear that the interaction of Sec31a with Sec23a•Sar1 is significantly tighter than the binding of a single PPP motif to Sec23a.
Finally, we tested whether Sec31 can compete with a TANGO1 polypeptide for binding to Sec23 in vitro (Fig. S4). A TANGO1 polypeptide containing three PPP motifs (residues 1800–1907, fused to MBP) was immobilized on amylose resin beads and probed for binding to Sec23a in the presence (Fig. S4, lane 9) and absence (lane 8) of Sec31a (residues 835–1026) (SI Materials and Methods). A twofold molar excess of Sec31a relative to Sec23a is sufficient to markedly reduce the binding of Sec23a to TANGO1. Note the reduction in binding of both the Sar1-GppNHp and Sec23a proteins. The data support our model of a competition between the TANGO1 and Sec31 polypeptides for binding to Sec23.
Fig. S4.
Competition between Sec31 and TANGO1 for binding to Sec23. Binding observed between TANGO1 (residues 1800–1907, fused to MBP and immobilized on amylose beads) and Sec23a•Sar1 (lane 8) is substantially reduced in the presence of a Sec31a polypeptide (lane 9) containing the active fragment and two upstream PPP motifs (residues 835–1026). The stoichiometry of input proteins in lanes 3, 6, and 9 is 1:2:2 for Sec23a:Sar1:Sec31a. For this experiment, a Sec31a construct lacking MBP was used (Sec31a was fused instead to His6-Smt3 and gluthathione S-transferase; see SI Materials and Methods). Note the reduction in binding of both the Sec23a and Sar1-GppNHp proteins in lane 9.
These results, regarding Sec31 interaction with Sec23•Sar1, are summarized in the composite molecular model in Fig. 4D.
SI Materials and Methods
Protein and Peptide Production.
The expression of wild-type and mutant COPII proteins was carried out in baculovirus-infected insect cells, using in all cases pFastbac-HTB vectors (Invitrogen Bac-to-Bac system), which included Homo sapiens proteins Sec23a, Sec23b, Sec24c (lacking residues 1–299), heterodimeric Sec23a/Sec24d (Sec24d lacking residues 1–266), and mutants Sec23a (Y672K, Y678A) and Sec23a (F628A, F681A). In all cases, Hi-5 insect cells were infected with viruses for 48 h and then collected by centrifugation. The proteins were purified initially by Ni2+-IMAC chromatography, and subsequently on Q-Sepharose and Superdex 200 columns.
DNA constructs encoding the various cytosolic fragments of TANGO1 (residues 1444–1907, 1617–1757, 1751–1907, 1800–1907, 1751–1852, 1770–1852, 1780–1852, 1780–1840, and 1790–1840), cTAGE5 (residues 300–804, 651–804, 700–804, and 651–751), and Sec31a (residues 835–1026) were cloned into an expression vector to encode an N-terminal MBP and C-terminal His6 tag. For all of the constructs, protein expression in Escherichia coli BL21(DE3) cells was induced with 0.2 mM IPTG at 37 °C for 3 h. Proteins were purified first by Ni2+-IMAC chromatography, then on an amylose resin column (New England Biolabs). Purified proteins were dialyzed overnight against 20 mM Tris⋅HCl pH 7.5, 150 mM NaCl, and 5 mM DTT (buffer A).
The fragment of human Sec31a used in experiments reported in Fig. 4 (Sec31a residues 835–1026 containing two PPP motifs plus the active fragment) was expressed as a fusion protein with an N-terminal MBP and C-terminal His6 tag, and was purified in the same manner as MBP-TANGO1 fragments. For the experiment shown in Fig. S4, we used the same Sec31a fragment (residues 835–1026) but fused in a different manner to avoid the MBP-binding function; instead, Sec31a was fused to an N-terminal His6-Smt3 tag and C-terminal gluthathione S-transferase. The protein was purified by Ni2+-IMAC chromatography, followed by a glutathione-Sepharose column.
The constructs comprising TANGO1 residues 1751–1907, in which proline residues were mutated alternately to glycine and serine, originated as synthetic DNAs (purchased from GENEWIZ) (Fig. S3). The proteins were expressed, as before, with N-terminal MBP and C-terminal His6 tags, and purified as described for the other TANGO1 proteins.
The TANGO1 construct used for crystallization (residues 1780–1840) was produced by a dual-tagging approach, with an N-terminal His6-Smt3 tag and C-terminal gluthathione S-transferase tag. Protein was purified first by Ni2+-IMAC chromatography, then on a glutathione-Sepharose column. The terminal tags were removed by using Ulp1 and thrombin proteases, in sequential reactions, followed by additional purification on Q-Sepharose and Superdex 200 columns. Protein in buffer A was concentrated, aliquoted, and flash frozen.
Synthetic TANGO1 peptides for crystal soaking experiments (sequences QLCGPFGPRP, GPRPLPPP, LPPPFGPGM, and GMRPPLFLRE) were >95% purity and were purchased from the Tufts University Core Facility (Table S1).
Human Sar1a (lacking the membrane anchor residues 1–25) was cloned into pGEX-4T-1 vector for expression with an N-terminal GST tag. GST-Sar1a was purified on a glutathione Sepharose column, the GST tag was removed by thrombin cleavage, and Sar1a was purified further by Q-Sepharose chromatography. Bound nucleotides were replaced with GppNHp (guanosine-5′-[β,γ-imido]-triphosphate) by mixing 2 mM Sar1a, 10 mM GppNHp, and 2,000 units of calf intestinal phosphatase (New England Biolabs), and dialyzing this mixture at 4 °C overnight against 20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 5 mM dithithreitol, and 2 mM MgCl2. Finally, Sar1a-GppNHp was separated from unbound nucleotides and remaining contaminants by using Superdex 75 size exclusion chromatography. Protein was concentrated to 50 mg/mL, flash frozen, and stored at −80 °C.
Binding Assays.
For pull-down experiments, a saturating amount of MBP-tagged proteins (generally 300–400 μg) was incubated with 16 μL of a 50% (vol/vol) slurry of amylose resin (New England Biolabs) at 4 °C for 30 min. Beads were washed once with 600 μL of buffer B (containing 50 mM Tris⋅HCl pH 7.5, 5 mM DTT, 150 mM NaCl, 0.2% (vol/vol) Triton X-100). Beads were then mixed with 80 μg of Sec23a in 100-μL assay volume of buffer B. The binding mixture was incubated at 4 °C for 30 min, then beads were washed twice with 100 μL of buffer B. Bound proteins were eluted by using SDS sample buffer and analyzed by 4–20% gradient SDS/PAGE and Coomassie blue staining. Protein bands were measured by densitometry, and the extent of Sec23a binding was assessed as (ODSec23a – ODBackground). All pull-down experiments that tested TANGO1, cTAGE5, and Sec31 protein binding to COPII (wild-type and mutant) proteins were carried out by using this protocol. Additionally, for tests involving the ternary Sec23a•Sar1/Sec31 complex, pull-down experiments used Sec31a (835–1026) and 80 μg of Sec23a, as before, plus an additional 21 μg of Sar1–GppNHp and 1 mM MgCl2.
Crystallization and Structure Determination.
For crystallization of the complex of Sec23a and TANGO1, approximately equimolar amounts of Sec23a and TANGO1 (residues 1780–1840) were mixed to a final protein concentration of 10.9 mg/mL in 20 mM Tris⋅HCl pH 7.5, 0.5 M NaCl, 5 mM DTT. Crystals were grown by the hanging drop method. Protein solution (1 μL) was mixed with an equal volume of crystallization buffer, comprising 100 mM Hepes pH 7.5, 6% (vol/vol) isopropanol, and 300 mM sodium citrate. Crystals formed in 1 wk at 4 °C. Crystals were cryoprotected by transfer into a buffer comprising crystallization buffer plus 26% glycerol; such crystals diffracted synchrotron X-rays beyond 2.6 Å resolution.
To test for binding of short TANGO1 peptides to Sec23a, crystals of Sec23a/24d were grown and then soaked in peptide solutions. Specifically, 1 μL of purified Sec23a/24d (Sec24d lacking residues 1–266) was mixed with an equal volume of 100 mM Hepes pH 7.5, 5.5% (wt/vol) PEG 4000, 100 mM MgSO4, and crystals were grown by using the hanging drop approach at 22 °C. Crystals were then harvested and transferred to “peptide soak solution” comprising 100 mM Hepes pH 7.5, 5.5% (wt/vol) PEG 4000, 100 mM MgSO4, plus 6 mM TANGO1 peptide (see Table S1 for peptide sequences). Crystals were soaked overnight at 22 °C, then cryoprotected by transferring into peptide soak solution containing an additional 32% ethylene glycol; these crystals diffracted synchrotron X-rays to ∼3.3 Å resolution.
X-ray diffraction data were collected from single frozen crystals at Northeast Collaborative Access Team beamlines 24ID-C and 24ID-E of the Advanced Photon Source at the Argonne National Laboratory. Data from all of the crystals were collected by using an X-ray wavelength of 0.9875 Å. Data were processed by using the HKL2000 suite of programs (33). The structures were determined by molecular replacement approaches with the program Phaser within the Phenix suite (34), using either Sec23a or Sec23a/24d heterodimer as the search model (PDB ID code 3EG9). Atomic models were refined by using Phenix, initially by rigid-body, then by all-atom and restrained B-factor refinement. The model for Sec23a/TANGO1 (these crystals contain two copies of Sec23a in the asymmetric unit) were refined to an R factor of 25.3% (Rfree = 28.1%) for data between 47 and 2.6 Å resolution. The final model comprises 11,047 nonhydrogen atoms. The following residues have been omitted from both noncrystallographic symmetry-related copies of Sec23a: residues 1–2, 211–224, and 718–741. Additionally, the PPP-binding domain (gelsolin-like domain) is partially disordered in one copy of Sec23a in the asymmetric unit, and residues 659–686 are omitted from this region because of disorder and correspondingly poor electron density. The X-ray datasets for Sec23a/Sec24d crystals soaked with TANGO1 peptides were all refined to final models comprising 11,724 nonhydrogen atoms, with the following residues of Sec23a missing because of poor electron density: 1–2, 211–225, 465–474, and 728–745. As noted above, residues 1–266 were absent from the Sec24d protein construct. The final refined R factors and data resolution ranges for the four Sec23a/Sec24d structures are listed in Table S1.
Discussion
In this study, we have characterized the association between COPII coat proteins and the TANGO1/cTAGE5 receptor for procollagen packaging. We report that an unexpectedly short PPP motif in the TANGO1 polypeptide binds to Sec23a, and that TANGO1 and cTAGE5 contain repeated PPP motifs distributed across their flexible cytosolic PRD regions. This unusual arrangement may enable TANGO1/cTAGE5 to engage multiple copies of Sec23/24•Sar1 at ER exit sites, leading to the speculative model that the role of the receptor is to propagate the COPII inner coat lattice of a growing tubular carrier.
Architecture of the COPII Inner Coat Complex.
The picture in Fig. 2C is a view toward the membrane-distal surface of the inner coat complex, to which the PPP motif and the Sec31 active fragment bind. The gelsolin-like domain of Sec23 extends radially away from the membrane, such that the PPP motif will reside ∼45 Å from the bilayer surface (estimated from the tomography images presented in ref. 17).
The observation that TANGO1 and Sec31 both contain a PRD led to the suggestion that TANGO1 may mimic the binding mode of the Sec31 active fragment and, thereby, stall Sec13/31-catalyzed GTP hydrolysis on Sar1 (9). Although our data indicate interplay between the protein molecules (Fig. 4 A–C), the TANGO1 PPP motif clearly binds to a unique site on Sec23a. The idea that rapid GTP hydrolysis will mediate against assembly of large COPII coats is countered by the alternative model that Sec12 (ER-localized Sar1 nucleotide exchange factor) activity maintains sufficient steady-state levels of Sar1-GTP, and that the COPII coat remains stably bound to membrane regardless of dynamic Sar1 cycling (23, 24).
A Consideration of COPII Lattice Adaptability.
Structural studies have established that the adaptability of the COPII coat (i.e., the capacity to adopt vesicular and tubular forms) depends on two molecular features: variable angles at the vertex, and centrally along the edge, of the Sec13/31 cage; and a flexible (unstructured) polypeptide connection between the inner and outer coat proteins (17, 22, 25). The variable angles enable the Sec13/31 assembly unit to adapt to cages of different curvature (in two dimensions), and the flexible link between Sec23/24•Sar1 and Sec13/31 allows for variation in the geometric relationship between the inner and outer coat proteins (17).
Implications for the Role of TANGO1/cTAGE5 in COPII Coat Assembly.
Through these mechanisms the COPII coat is inherently adaptable, but what switches coat assembly from small spherical vesicles to large tubular carriers? The essential difference between the COPII coat on vesicles and tubules is the helical lattice of Sec23/24•Sar1 observed on the latter, which self-assembles to a close-packed structure, to yield a coat that contains supernumerary inner complexes (i.e., the stoichiometry of the coat layers is 2:1 of Sec23/24:Sec13/31, such that only half of all of the Sec23/24 proteins bind a Sec13/31 partner). The helical symmetry is incompatible with an isometric structure, thus the Sec23/24•Sar1 array is absent from COPII-coated vesicles, which have a more open and random arrangement of inner coat protein (17). In view of this finding, we propose that TANGO1/cTAGE5 stimulates the growth of COPII-coated tubules by promoting the addition of assembly units to the helical Sec23/24•Sar1 array (Fig. 4E). The repeat distance between gelsolin-like domains on a COPII-coated tubule (78 Å along the helical lattice line) (see, for example, ref. 17) and the distances between PPP motifs in the PRDs would allow TANGO1 and cTAGE5 to bridge as many as four copies of Sec23/24•Sar1 (Fig. 4E). Although TANGO1/cTAGE5 PRD sequences are rather divergent across species, the high proline content and the PPP motifs are present throughout (note that we cannot rule out the possibility that XPP or PPX are binding motifs, where X is a residue that favors the PPII helical conformation, such as glycine or alanine). According to our model, the arrangement of multiple weak-binding motifs spread across the inherently flexible PRDs will allow the two arms of TANGO1/cTAGE5 to be mobile and easily displaced from individual binding sites on Sec23/24•Sar1, enabling the receptor to adopt multiple binding modes and alternately stabilize the Sec23/24•Sar1 lattice and capture additional Sec23/24•Sar1 assembly units (Fig. 4E).
The model that we have proposed has significant parallel with the mechanism of action of tau protein on the microtubule helical lattice. Four repeated binding motifs in tau are separated by flexible linker sequences, and the binding of tau to multiple tubulin units stabilizes and enhances microtubule lattice polymerization without impeding microtubule dynamics (26, 27).
Stepwise COPII Coat Assembly and Compartmentation of TANGO1/cTAGE5 to the ER Membrane.
Puzzlingly, TANGO1 does not depart with cargo in the COPII-coated carrier (9). The finding that PPP motifs are present in Sec31 suggests that the recruitment of the Sec13/31 outer coat could displace TANGO1/cTAGE5 and confine it the ER membrane at the base of the growing tubule. COPII-coat assembly on membranes occurs stepwise—Sar1-GTP recruits Sec23/24, which recruits Sec13/31—because the binding site for Sec31 comprises a composite surface of the Sec23 and Sar1-GTP molecules (1, 22) (Fig. 4C, lanes 5 and 6). Thus, the compartmentation of TANGO1/cTAGE5 to the base of the growing tubule may be a spatial consequence of the stepwise assembly process.
Finally, the model we have proposed for TANGO1/cTAGE5 function envisages two self-assembly reactions competing to influence the shape of the COPII carrier: Sec23/24•Sar1 lattice formation to propagate the tubule, and Sec13/31 lattice formation to impose an isometric cage or to terminate a tubule. Accordingly, TANGO1/cTAGE5 would bias coat assembly toward tubulation by increasing the local concentration of Sec23/24•Sar1 assembly units, and stabilizing the lattice, at the base of the tubule (Fig. 4E). Likewise, other protein factors could influence the shape of the forming COPII carrier by regulating the availability of inner or outer COPII proteins at ER exit sites. Candidates for such a function include the Sedlin component of the TRAPP complex (28), the ubiquitin ligase CUL3-KLHL12 (29), and Ca2+/ALG-2 (30, 31), which binds to a site on Sec31 PRD that includes one of the two PPP motifs, residues 838PPP841 (32). The biochemical analysis and the mechanism that we have presented for TANGO1/cTAGE5 interplay with COPII should provide a basis on which to further explore the role of these protein factors in ER-to-Golgi transport.
Materials and Methods
Protein and Production and X-Ray Crystallography.
COPII coat proteins were expressed in baculovirus-infected insect cells. Sar1, TANGO1, and Sec31 polypeptides were overproduced in Escherichia coli. Proteins were purified by affinity and size-exclusion chromatography. Crystals of Sec23a bound to TANGO1 (residues 1780–1840) were obtained by vapor diffusion using a crystallization solution comprising 100 mM Hepes pH 7.5, 6% (vol/vol) isopropanol, and 300 mM sodium citrate. The crystal structure was determined by molecular replacement and refined to 2.6 Å resolution. Details are provided in SI Materials and Methods.
Other Methods.
Binding experiments are described in SI Materials and Methods.
Acknowledgments
We thank staff of the Northeast Collaborative Access Team beamlines at the Advanced Photon Source of the Argonne National Laboratory for access to synchrotron facilities. This research was supported in part by the Memorial Sloan Kettering Cancer Center Core Grant P30-CA008748.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.J.O. is a Guest Editor invited by the Editorial Board.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5KYN, 5KYX, 5KYU, 5KYW, and 5KYY).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605916113/-/DCSupplemental.
References
- 1.Matsuoka K, et al. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93(2):263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 2.Barlowe C, et al. COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77(6):895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 3.Stagg SM, et al. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439(7073):234–238. doi: 10.1038/nature04339. [DOI] [PubMed] [Google Scholar]
- 4.Zeuschner D, et al. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol. 2006;8(4):377–383. doi: 10.1038/ncb1371. [DOI] [PubMed] [Google Scholar]
- 5.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 6.Boyadjiev SA, et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet. 2006;38(10):1192–1197. doi: 10.1038/ng1876. [DOI] [PubMed] [Google Scholar]
- 7.Garbes L, et al. Mutations in SEC24D, encoding a component of the COPII machinery, cause a syndromic form of osteogenesis imperfecta. Am J Hum Genet. 2015;96(3):432–439. doi: 10.1016/j.ajhg.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townley AK, et al. Efficient coupling of Sec23-Sec24 to Sec13-Sec31 drives COPII-dependent collagen secretion and is essential for normal craniofacial development. J Cell Sci. 2008;121(Pt 18):3025–3034. doi: 10.1242/jcs.031070. [DOI] [PubMed] [Google Scholar]
- 9.Saito K, et al. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136(5):891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Saito K, et al. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22(13):2301–2308. doi: 10.1091/mbc.E11-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson DG, et al. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol. 2011;193(5):935–951. doi: 10.1083/jcb.201007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nogueira C, et al. SLY1 and Syntaxin 18 specify a distinct pathway for procollagen VII export from the endoplasmic reticulum. eLife. 2014;3:e02784. doi: 10.7554/eLife.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos AJ, Raote I, Scarpa M, Brouwers N, Malhotra B. TANGO1 recruites ERGIC membranes to the endoplasmic reticulum for procollagen export. eLife. 2015;4:e10982. doi: 10.7554/eLife.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135(1):19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson P, Stephens DJ. ER-to-Golgi transport: Form and formation of vesicular and tubular carriers. Biochim Biophys Acta. 2005;1744(3):304–315. doi: 10.1016/j.bbamcr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Bacia K, et al. Multibudded tubules formed by COPII on artificial liposomes. Sci Rep. 2011;1:17. doi: 10.1038/srep00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanetti G, et al. The structure of the COPII transport-vesicle coat assembled on membranes. eLife. 2013;2:e00951. doi: 10.7554/eLife.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi X, Corpina RA, Goldberg J. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature. 2002;419(6904):271–277. doi: 10.1038/nature01040. [DOI] [PubMed] [Google Scholar]
- 19.Peterson FC, Volkman BF. Diversity of polyproline recognition by EVH1 domains. Front Biosci (Landmark Ed) 2009;14:833–846. doi: 10.2741/3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarrinpar A, Lim WA. Converging on proline: The mechanism of WW domain peptide recognition. Nat Struct Biol. 2000;7(8):611–613. doi: 10.1038/77891. [DOI] [PubMed] [Google Scholar]
- 21.Mancias JD, Goldberg J. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 2008;27(21):2918–2928. doi: 10.1038/emboj.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi X, Mancias JD, Goldberg J. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev Cell. 2007;13(5):635–645. doi: 10.1016/j.devcel.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futai E, Hamamoto S, Orci L, Schekman R. GTP/GDP exchange by Sec12p enables COPII vesicle bud formation on synthetic liposomes. EMBO J. 2004;23(21):4146–4155. doi: 10.1038/sj.emboj.7600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato K, Nakano A. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol. 2005;12(2):167–174. doi: 10.1038/nsmb893. [DOI] [PubMed] [Google Scholar]
- 25.Noble AJ, et al. A pseudoatomic model of the COPII cage obtained from cryo-electron microscopy and mass spectrometry. Nat Struct Mol Biol. 2013;20(2):167–173. doi: 10.1038/nsmb.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115(3):717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venditti R, et al. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 2012;337(6102):1668–1672. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin L, et al. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482(7386):495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helm JR, et al. Apoptosis-linked gene-2 (ALG-2)/Sec31 interactions regulate endoplasmic reticulum (ER)-to-Golgi transport: A potential effector pathway for luminal calcium. J Biol Chem. 2014;289(34):23609–23628. doi: 10.1074/jbc.M114.561829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.la Cour JM, Schindler AJ, Berchtold MW, Schekman R. ALG-2 attenuates COPII budding in vitro and stabilizes the Sec23/Sec31A complex. PLoS One. 2013;8(9):e75309. doi: 10.1371/journal.pone.0075309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi T, et al. Structural analysis of the complex between penta-EF-hand ALG-2 protein and Sec31A peptide reveals a novel target recognition mechanism of ALG-2. Int J Mol Sci. 2015;16(2):3677–3699. doi: 10.3390/ijms16023677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otwinowski W, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276(Part A):307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 34.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]