Significance
Mucosal-associated invariant T (MAIT) cells are innate-like T lymphocytes with potent antibacterial reactivity. In this study, we investigated whether MAIT cells also contribute to immunity against influenza A viruses. Compared with those who succumbed, hospitalized patients who recovered from severe avian H7N9 influenza infection had higher numbers of MAIT cells. Subsequent in vitro analysis established that MAIT cells from healthy donors are indirectly activated by influenza infection via an IL-18–dependent (but not IL-12–dependent) mechanism requiring the involvement of CD14+ monocytes. Our findings highlight the potential for MAIT cells to promote protective immunity in human influenza.
Keywords: MAIT cells, influenza virus, H7N9, IL-18, monocytes
Abstract
Mucosal-associated invariant T (MAIT) cells are innate-like T lymphocytes known to elicit potent immunity to a broad range of bacteria, mainly via the rapid production of inflammatory cytokines. Whether MAIT cells contribute to antiviral immunity is less clear. Here we asked whether MAIT cells produce cytokines/chemokines during severe human influenza virus infection. Our analysis in patients hospitalized with avian H7N9 influenza pneumonia showed that individuals who recovered had higher numbers of CD161+Vα7.2+ MAIT cells in peripheral blood compared with those who succumbed, suggesting a possible protective role for this lymphocyte population. To understand the mechanism underlying MAIT cell activation during influenza, we cocultured influenza A virus (IAV)-infected human lung epithelial cells (A549) and human peripheral blood mononuclear cells in vitro, then assayed them by intracellular cytokine staining. Comparison of influenza-induced MAIT cell activation with the profile for natural killer cells (CD56+CD3−) showed robust up-regulation of IFNγ for both cell populations and granzyme B in MAIT cells, although the individual responses varied among healthy donors. However, in contrast to the requirement for cell-associated factors to promote NK cell activation, the induction of MAIT cell cytokine production was dependent on IL-18 (but not IL-12) production by IAV-exposed CD14+ monocytes. Overall, this evidence for IAV activation via an indirect, IL-18–dependent mechanism indicates that MAIT cells are protective in influenza, and also possibly in any human disease process in which inflammation and IL-18 production occur.
Human mucosal associated invariant T (MAIT) cells are innate-like T lymphocytes characterized by a semi-invariant T-cell receptor (TCR), Vα7.2 (TRAV 1–2), paired with variable β-chains (1, 2). In adult blood, MAIT cells are further defined by constitutive, high-level expression of the IL-18 receptor (IL-18R), the promyelocytic leukemia zinc finger (PLZF) transcription factor, and the CD161 C-type lectin (3, 4). Potently reactive to metabolites of vitamin B2 biosynthetic intermediates (5, 6) that bind to the nonclassical MHC-1–related (MR1) proteins, MAIT cells are robustly activated to produce cytokines and chemokines [IFNγ, TNF, granzyme B (GzmB), IL-17A, and IL-22] following exposure to microbes (Salmonella, Escherichia coli, and Candida albicans) that possess riboflavin pathways (4, 7). Given the prominence of MAIT cells in human peripheral blood (1–10%) and in tissue sites of inflammation, including the gut and lung (3, 8, 9), there is a need to define their role in immunity, with the situation for viral infections remaining relatively unclear.
During HIV-1 infection, numbers of MAIT cells, as defined by coexpression of CD161 and Vα7.2, are reduced (10–12), and their numbers in the periphery are not restored after antiretroviral drug treatment, although it is thought that microbial translocation may drive chronic MAIT cell activation (10, 11). Moreover, with the advent of MR1 tetramers to detect MAIT cells, more recent studies have confirmed the loss of MAIT during chronic HIV infection (1, 12). To date, only murine studies in Vα19Vβ6 transgenics have demonstrated that MAIT cells, as measured by CD69 up-regulation in vitro, are not activated by coculture with antigen-presenting cells infected with a range of viruses, including Sendai virus, Newcastle disease virus, and herpes simplex virus (8). Stimulation with IL-12 and IL-18 can, however, induce IFNγ production in human MAIT cells in an MR1-independent manner (13, 14).
Because MAIT cells are both proinflammatory and potentially activated by IL-12 and IL-18, the possibility that they play a positive role in respiratory virus infections seems plausible. In a recent report of a hospitalized patient cohort, we established that a spectrum of innate and antigen-specific responder cells is recruited during the course of severe H7N9 influenza pneumonia (15). Our further analysis from this study of the CD161+Vα7.2+ MAIT cells recovered ex vivo from these patients showed that the numbers were 2.5-fold greater in those who survived, suggesting that MAIT cells may indeed contribute to protection.
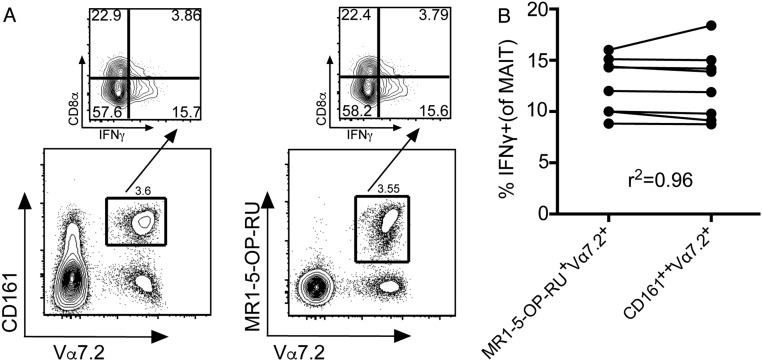
To understand how MAIT cells are activated during early IAV infection, we established an in vitro coculture system with IAV-infected human lung epithelial cells and normal human peripheral blood mononuclear cells (PBMCs). Using a 12–14 parameter flow cytometry panel to measure innate and adaptive responses, which included a MR1 tetramer loaded with 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (MR1-5-OP-RU), we found that IAV infection of epithelial cells induced robust, early IFNγ production in MAIT cells, defined as MR1-5-OP-RU tetramer+Vα7.2+ or by the surrogate MAIT cell markers CD161+Vα7.2+. Antibody blocking indicated that this effect was mediated by IL-18, but not by IL-12. Furthermore, CD14+ monocytes were also required for MAIT cell activation during coculture, most likely as a source of inflammatory mediators. The present analysis indicates that MAIT cells may indeed help protect against the most severe consequences of human influenza virus infection.
Results
Reduced Numbers of MAIT Cells in Fatal Influenza.
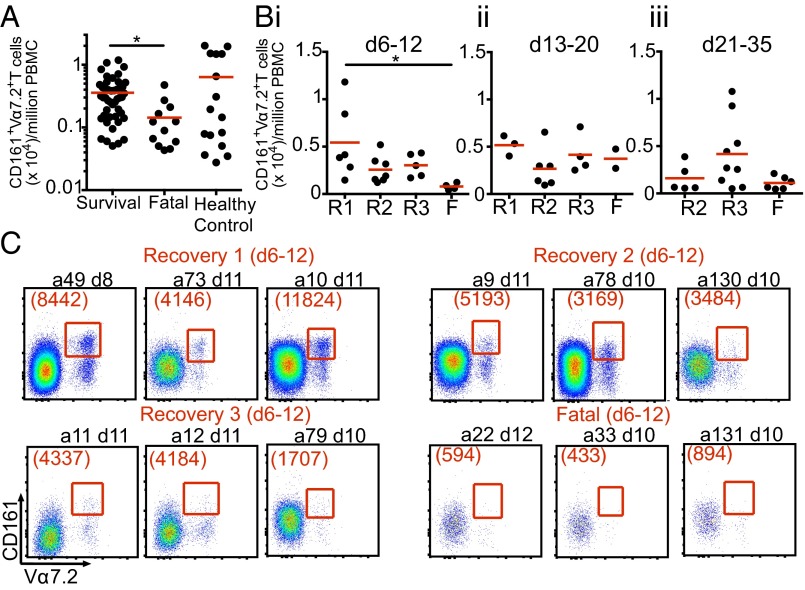
We recently investigated the population dynamics of CD8+ T-cell responses in hospitalized H7N9 patients with severe influenza pneumonia (15). Given that the CD8+ Vα7.2+ subset is likely to include both MR1-restricted and -nonrestricted MAIT cells (1), we performed a retrospective comprehensive analyses using CD161 in combination with TCR Vα7.2 to determine whether the MAIT cell response varies in hospitalized patients with different clinical outcomes. Comparing the numbers of CD161+Vα7.2+ T cells in stored PBMCs between patients who recovered and those with a fatal outcome across all available time points after disease onset (n = 16 patients; days 6–34) revealed significantly lower numbers of MAIT cells in the fatal cases (P = 0.014, nonparametric one-way ANOVA) (Fig. 1A). However, the comparable peripheral blood counts for CD161+Vα7.2+ MAIT cells in the survivors (mean age, 68 y) and in healthy controls (mean age, 70 y) indicates that MAIT cell activation may be markedly reduced in fatal influenza infection. Another possibility is that the MAIT cells are recruited to, and perhaps destroyed at, the site of pathology.
Fig. 1.
Reduced numbers of CD161+Vα7.2+ MAIT cells in patients hospitalized with fatal H7N9 disease. (A) Comparison of MAIT cell numbers during the course of H7N9 infection in surviving patients (n = 47–81; median, 67.5), fatal patients (n = 56–88; median, 68.5), and age-matched controls (n = 52–79; median, 72). (B) MAIT cell numbers across different patient recovery groups (R1: recovered days 14–18; R2: days 21–27; R3: days 31–35) and the fatal group (F) during (i) the first week of hospitalization (days 6–12 after disease onset), (ii) days 13–20, and (iii) days 21–35. (i) *P = 0.014; (ii and iii) *P = 0.012, Kruskal–Wallis test. (C) Representative FACS plots showing CD161+Vα7.2+ MAIT cells during the first week of hospitalization across different recovery or fatal groups. MAIT cells were gated on live singlets CD3+CD14−CD19−CD161+Vα7.2+. The number of MAIT cells per million PBMCs was calculated as MAIT count × Freq CD3+ × Freq of live lymphocytes × 1 × 106 PBMCs.
To gain insight into the dynamics of MAIT cell involvement during the course of severe influenza disease, we analyzed CD161+Vα7.2+ T cells at multiple time points after disease onset: days 6–12, days 13–20, and days 21–35 (Fig. 1B). As in our previous study (15), we subdivided patients hospitalized at ∼days 6–8 after the onset of symptoms according to their recovery rates, ranging from early (R1: days 14–18 after disease onset) to delayed (R2: days 21–27, R3: days 31–35), vs. a fatal outcome. Our data show that during the first week of hospitalization (days 6–12), only the patients who recovered early from H7N9 disease (R1) had higher MAIT cell numbers than seen in the fatal group (P < 0.05) (Fig. 1 B, i and C). Interestingly, there was a trend (albeit insignificant) toward higher numbers of MAIT cells in delayed recovery group R3 during the last week of hospitalization (days 21–35) (Fig. 1 B, iii), which was not observed in group R2, which had a shorter hospital stay. This finding might be linked to the kinetics of MAIT cells before the recovery during prolonged hospitalization. Further experiments with a larger patient cohort and more frequent assessment time points are needed to better understand the kinetics of MAIT cells during prolonged severe influenza disease. Our present findings suggest that MAIT cells may contribute to recovery from influenza, and highlight the importance of understanding MAIT cell dynamics in patients with severe viral pneumonia, which of course are influenced by the cells’ profiles of migration to (and from) the site of inflammatory pathology.
IAV Infection in PBMC Cocultures Induces IFNγ Production by MAIT Cells.
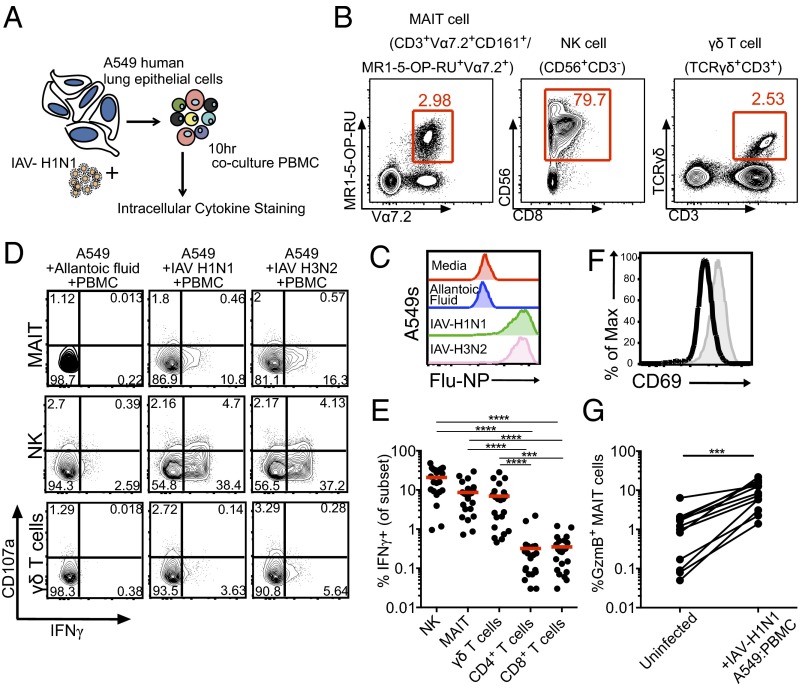
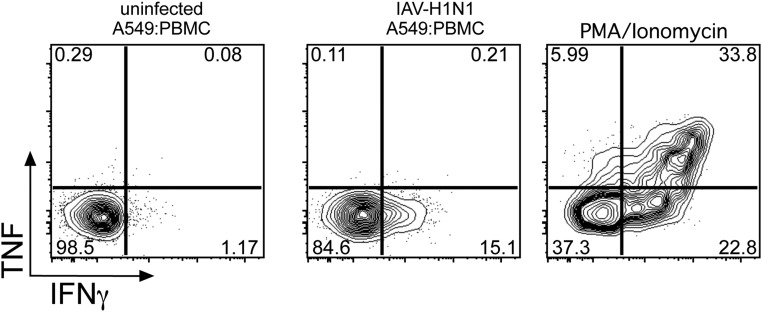
The lung epithelial cells that are the primary sites of productive IAV infection make inflammatory mediators that promote the influx of innate and adaptive immune cells at the site of infection (16). To understand how MAIT cells are activated during IAV infection, we established a coculture system in which A549 cells (a human lung type 2 epithelial cell line) were infected with the H1N1 and H3N2 IAVs (Fig. 2A). These cells (>80% infected at 10 h; Fig. 2C) were then cocultured with human PBMCs for 10 h in the presence of the protein export inhibitor brefeldin A (last 6 h of culture), allowing the production (and intracellular retention) of virus-induced soluble or cell-associated factors (Fig. 2A). An analysis of intracellular cytokine staining (ICS) for IFNγ in the MAIT, natural killer (NK), and γδ T-cell subsets (Fig. 2B and Fig. S1) from PBMCs incubated with control (previously incubated in allantoic fluid) and H1N1/H3N2-infected A549 cells established that the IAV exposure induced IFNγ production in the MAIT cells (8.6 ± 7.5%; n = 22), at levels (Fig. 2 D and E) comparable to those found for γδ T cells (7 ± 7.5%; n = 22) and NK cells (20.8 ± 13.5%; n = 22). Indeed, significantly higher up-regulation of IFNγ in MAIT, NK, and γδ T cells was found compared with classical MHC-restricted “helper” and “killer” T cells (P ≤ 0.001; Fig. 2E), reflecting that IFNγ production in the conventional CD4+ or CD8+ T-cell subsets is antigen-specific.
Fig. 2.
MAIT cells are activated during coculture with IAV-infected lung epithelial cells and PBMCs. (A) Diagram of the coculture system. (B) Human lung epithelial cells were infected with IAV-H1N1 at an MOI of 10–30 for 1 h, washed and trypsinized, and cocultured with PBMCs for 10 h, followed by intracellular cytokine staining for different immune subsets and activation markers. (C) IAV nucleoprotein staining in A549 cells. (D) Representative FACS plots of IFNγ and CD107a expression in NK, MAIT, and γδ T cells after PBMC coculture with allantoic fluid (mock) (Left), IAV-H1N1–infected A549 cells (Middle), or IAV-H3N2–infected A549 cells. (E) Summary of IFNγ+ production after IAV coculture in NK, MAIT, γδ T, CD4+, and CD8+cells. n = 22. ***P < 0.001, ****P < 0.0001, Kruskal–Wallis test. (F and G) Surface CD69 expression (F) and intracellular GzmB (G) are up-regulated in MAIT cells after coculture with IAV-infected A549 cells. ***P < 0.001, Wilcoxon rank-sum test.
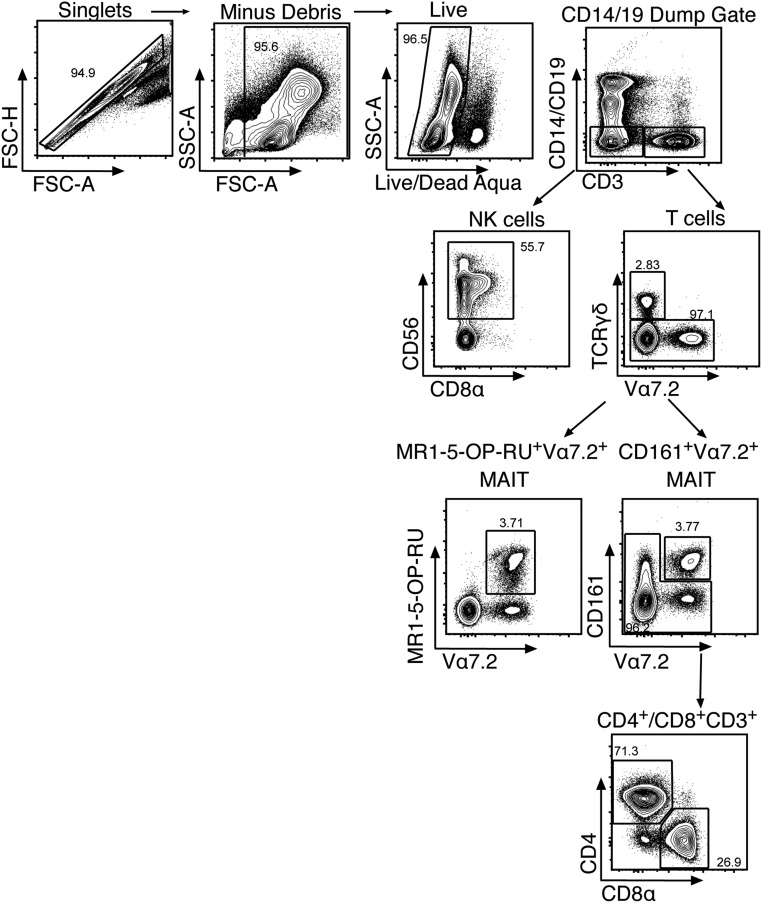
Fig. S1.
The gating strategy for MAIT, NK, γδ, CD4+, and CD8+ T cells using 12–14 parameter flow cytometery.
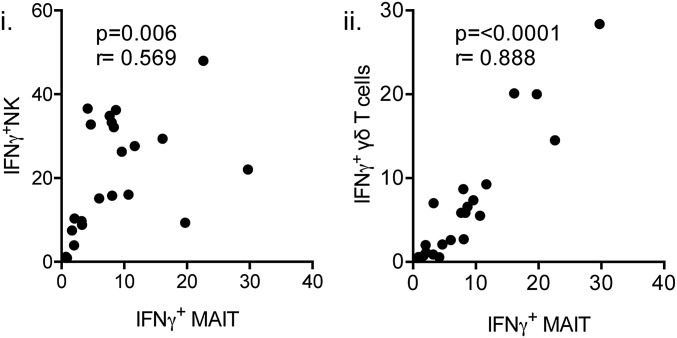
Given the robust IFNγ production by MAIT, NK, and γδ T cells, we next evaluated for any correlation between the high frequency of MAIT cells producing IFNγ during influenza infection and IFNγ production in NK or γδ T cells within the same donor. Indeed, we observed strong correlations in IFNγ production between MAIT/NK (P < 0.01; r = 0.569, Spearman rank correlation) and MAIT/γδ T cells (P < 0.0001; r = 0.888) (Fig. S2), suggesting that overall, these three subsets respond mutually during IAV infection. This does not imply that MAIT cells are dependent on NK or γδ T cells to produce IFNγ, however.
Fig. S2.
IFNγ production by MAIT cells is highly correlated with NK cell (i) and γδ T cell (ii) IFNγ up-regulation during in vitro coculture of PBMCs with IAV-infected A549 cells. Spearman rank correlation. n = 22.
Coculture of PBMCs with IAV-infected A549 cells did not result in significant expression of CD107a (minimally on NK cells), a known marker of degranulation for NK, MAIT, and γδ T cells (Fig. 2D), or TNF production (Fig. S3). However, the MAIT cells, and not the conventional CD8+/CD4+ T-cell subsets, up-regulated CD69 surface expression during coculture with IAV-infected A549 cells (Fig. 2F). Significant up-regulation of intracellular GzmB was also observed (***P < 0.001; n = 12). We further suggest that GzmB is an early marker of MAIT cell activation (Fig. 2G), and also that the surrogate MAIT cell markers CD161 and Vα7.2 identify the IFNγ-producing MR1-5-OP-RU-Tet+Vα7.2+ after early IAV infection (Fig. S4).
Fig. S3.
MAIT cells do not up-regulate TNF production during coculture with IAV-infected lung epithelial cells and PBMCs.
Fig. S4.
(A) CD161+Vα7.2+ or MR1-5-OP-RU tetramer+ populations (gated on Live CD3+ cells) identify the same IFNγ-producing MAIT cells after PBMC coculture with IAV-infected A549 cells. (B) In matched donors, the Pearson correlation coefficient was calculated for IFNγ production in MAIT cells, as defined by either the MR1-5-OP-RU tetramer+ or CD161+Vα7.2+ (n = 8).
MAIT Cell Activation Is Not Abrogated by MR1-Blocking Antibody.
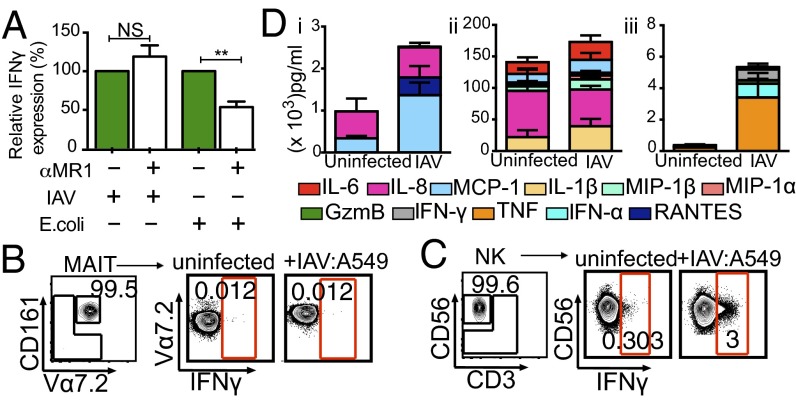
To understand MAIT cell activation during IAV infection, we first asked whether MAIT cell IFNγ production after exposure to IAV-infected epithelial cells is MR1-dependent. Several riboflavin derivatives from microbial species, including Escherichia coli, are presented by MR1 (5, 6, 17, 18); however, the addition of α-MR1–blocking monoclonal antibody (clone 26.5) to the IAV coculture system did not reduce the relative expression levels of IFNγ compared with coculture of PBMCs with 1% paraformaldehyde-fixed E. coli, where α-MR1 is known to inhibit cytokine production in MAIT cells (by approximately twofold) (Fig. 3A). This suggests that the activation of MAIT cells during IAV coculture is MR1-independent.
Fig. 3.
Activation of MAIT cells during coculture is not abrogated by MR1-blocking. (A) αMR1 antibody was added during PBMC coculture with IAV-infected epithelial cells or PBMC coculture with 1% paraformaldehyde-fixed E. coli (MOI 0.1) (10). **P < 0.01, paired t test. n = 4. (B and C) Sort-purified NK cells, but not MAIT cells, can directly respond to IAV-infected human lung epithelial cells. IFNγ and CD107a were measured by ICS after 10 h of culture with IAV-infected A549 cells. (D) Inflammatory molecule analyses by 17-plex CBA. (i) Supernatants from IAV-infected A549 cells alone). (ii and iii) Supernatants from PBMC cocultures with IAV-infected A549 cells. Brefeldin-A was added to cell cultures at 3 h after coculture. Total coculture time was 10 h. n = 3.
In the absence of other PBMC subsets, FACS-purified CD161+Vα7.2+CD3+ MAIT cells cultured with IAV-infected A549 cells for 10 h in the presence of BFA did not make IFNγ (Fig. 3B). However, comparably enriched CD56+CD3− NK cells up-regulated IFNγ production above background (uninfected A549) during direct coculture with IAV-infected A549 cells (Fig. 3C). Taken together, our results indicate that the activation of MAIT cells during IAV infection requires soluble inflammatory factors and/or accessory cells found in the PBMC pool.
Soluble Factors in Culture Supernatants and Their Role in MAIT Cell Activation.
To understand whether MAIT cells are activated via the IAV-induced inflammatory milieu, we assayed for cytokines/chemokines in the culture supernatants by a cytometric bead array (CBA) analysis. After IAV infection, but in the absence of PBMCs, the A549 cells up-regulated chemotactic molecules for monocytes and lymphocytes, namely MCP-1 and RANTES (Fig. 3 D, i). Elevated levels of IL-6, MCP-1, MIP1α/β, and IL-1β were observed during IAV infection, but these were present in uninfected controls as well (Fig. 3 D, ii). Type I IFN (IFNα) production was, as might be expected, limited to the IAV-exposed cells. Moreover, when both IAV-infected A549 cells and PBMCs were present, TNF, IFNγ, and GzmB were detected early in the culture supernatants (Fig. 3 D, iii).
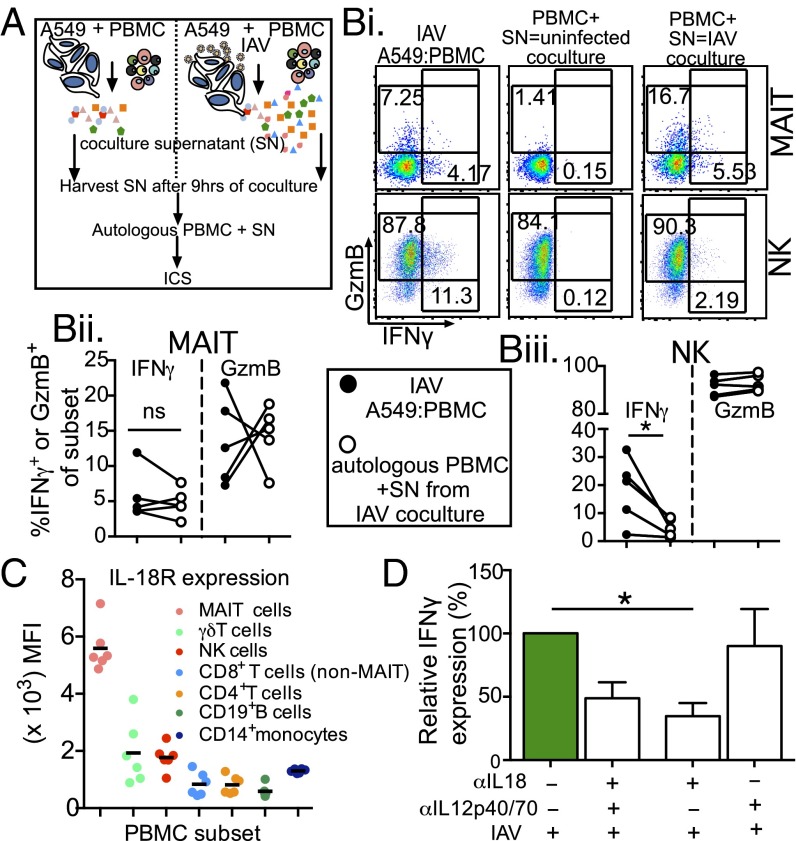
To examine whether influenza-induced soluble mediators can directly activate MAIT cells to produce IFNγ, we transferred supernatants from uninfected and IAV-infected cocultures to autologous PBMCs and measured IFNγ responses in the various lymphocyte subsets by ICS (Fig. 4A). We found that supernatants from uninfected A549-PBMC cocultures contained inflammatory mediators (Fig. 3D) that did not activate MAIT cells (Fig. 4 B, i, Middle). In contrast, when the supernatants from the IAV cocultures were added to autologous PBMCs, they induced robust production of IFNγ in MAIT cells (Fig. 4 B, i and ii), although MAIT cell GzmB expression was affected variably (Fig. 4 B, ii). Conversely, the up-regulation of IFNγ in NK cells was significantly reduced when cocultured with the IAV supernatants, indicating that NK cells require both accessory cells and inflammatory mediators for optimal induction during IAV infection (Fig. 4 B, iii). Taken together, these data suggest that MAIT cells are activated by soluble inflammatory mediator(s) induced during IAV infection.
Fig. 4.
MAIT cells are activated by soluble mediators during IAV cocultures. (A) Schematic of supernatant (SN) transfers. (B) (i) Representative FACS plots of IFNγ and GzmB staining after IAV A549:PBMC coculture (Left) or after transfer of SN from uninfected (Middle) or infected (Right) to autologous PBMCs in MAIT (Top) or NK (Bottom) cells. (ii and iii) Summary of MAIT (ii) and NK (iii) cell SN transfer. *P < 0.05, Student’s t test. n = 5. (C) IL-18R median fluorescence staining in different immune subsets. n = 6. (D) αIL-18 and/or αIL-12p40/70 antibodies were added during PBMC coculture with IAV-infected epithelial cells. Relative IFNγ expression in MAIT cells after coculture is shown. *P < 0.05, one-way ANOVA.
IL-18–Dependent Activation of MAIT Cells During IAV Infection.
Earlier studies (14, 19) have shown that MAIT cells respond robustly to cytokine-driven stimulation (IL-18, IL-12, and IL-7) and constitutively express high surface levels of the IL-18 and IL-12 receptors (14). Given our findings indicating that MAIT cells make IFNγ and GzmB when stimulated in IAV A549-PBMC cocultures (Fig. 4 A and B), we assessed whether IL-12 and IL-18 are the soluble inflammatory mediators driving this process. Indeed, compared with other PBMC subsets, the MAIT cells expressed higher surface levels of the IL-18R (Fig. 4C). Subsequent addition of both αIL-18 and αIL-12 to the IAV A549-PBMC cocultures led to an ∼50% reduction in IFNγ production (48.5 ± 12.5%; n = 4) by MAIT cells (Fig. 4D, second column). This drop in IFNγ secretion is attributed to IL-18 rather than to IL-12 (Fig. 4D, third column; *P < 0.05), because robust IFNγ production was retained for cultures containing only the IL-12 p40/70 blocking antibody (Fig. 4D, fourth column). Even so, given that we did not observe a complete reduction in IFNγ production, our data suggest that in addition to IL-18, other inflammatory mediators also may contribute to the up-regulation of IFNγ production during early IAV infection.
CD14+ monocytes are required for MAIT cell activation during IAV coculture.
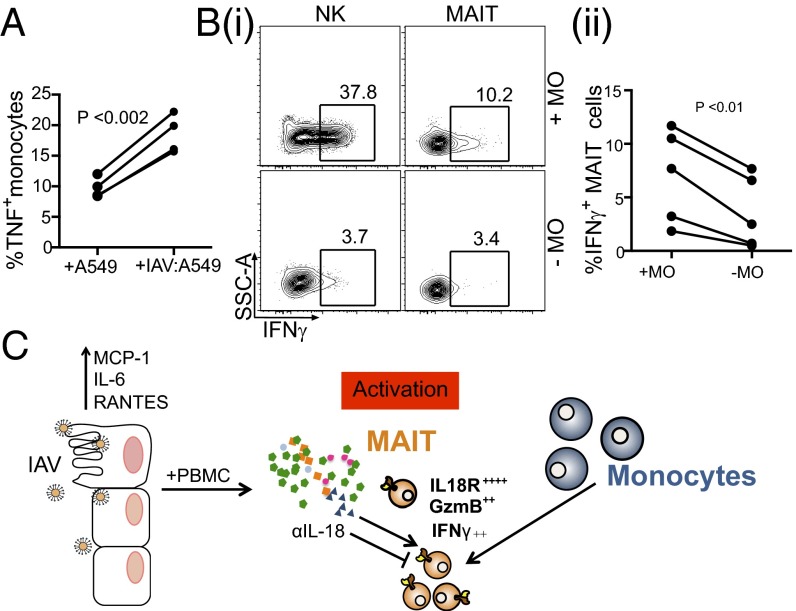
It is clear that IAV infection triggers the recruitment and differentiation of monocytes (Fig. 3D) via chemotactic cytokines/chemokines (20). Activated monocytes can then serve as a source of proinflammatory and antiviral mediators during IAV infection (reviewed in ref. 16). When CD14+ monocytes were cocultured with IAV-infected lung epithelial cells, we observed a significant increase in TNF production compared with that found after exposure to uninfected epithelial cells (Fig. 5A; **P < 0.002). This suggests that monocytes are directly activated by exposure to IAV-infected epithelium, and in turn contribute to the induction of MAIT cells during influenza.
Fig. 5.
Monocytes are required for IFNγ up-regulation in MAIT cells after IAV infection. (A) TNF production by magnetic bead-enriched CD14+ monocytes was measured by ICS after 10 h of coculture with IAV-infected A549 cells. **P = 0.0015, Student’s t test. n = 4. (B) (i) Monocyte depletion from PBMCs results in abrogated NK and MAIT IFNγ responses during IAV-infected A549 coculture. (ii) IFNγ production as measured by ICS in NK and MAIT cells after coculture with monocyte-depleted or -nondepleted PBMCs + IAV-infected lung epithelial cells. **P = 0.007, Student’s t test. n = 5. (C) A model for MAIT cell activation during IAV infection.
To examine this possibility, we depleted the CD14+ monocytes (>99%) from PBMCs using magnetic beads. In the absence of the CD14+ sets, IFNγ production in MAIT cells was significantly, although not completely, reduced (Fig. 5 B, i and ii; **P < 0.01), although IFNγ in the NK cells was reduced to background levels (Fig. 5 B, i). This indicates that CD14+ monocytes contribute to the activation of MAIT cells during coculture, and may provide a source of IL-18 for activation.
Discussion
Influenza contributes significantly to morbidity and mortality in high-risk groups, including elderly persons, young children, pregnant women, and immunocompromised individuals (21, 22). Although B cells producing strain-specific neutralizing antibodies are the gold standard of sterilizing immunity, they provide little or no protection against newly emerging pandemic IAV strains. Consequently, if we are to deal effectively with the threat posed by a novel IAV subtype (or some other emerging pathogen), we first need to understand the roles of other arms of innate and adaptive immunity, then investigate whether it is possible to manipulate such defense mechanisms. These potential mediators of “universal” immunity include nonneutralizing antibodies, cross-reactive IAV-specific T cells, neutrophils, macrophages, NK cells, and other “innate-like” T cells (23, 24). Although it is well appreciated that classical innate cell types, such as neutrophils, macrophages, and dendritic cells, play essential roles in the influenza-specific host response (16), there is a paucity of data on whether, and if so how, MAIT cells contribute to antiviral immunity during influenza and other viral infections.
The present study shows that in the absence of preexisting neutralizing antibodies, the absolute numbers of CD161+Vα7.2+ MAIT cells in H7N9-infected patients are higher in patients with severe but nonfatal influenza than in those who succumb to this disease. This situation is in contrast to chronic viral infections such as HIV, where an ongoing inflammatory process is not characterized by any recovery in MAIT cell numbers, even after effective antiretroviral therapy (10, 12).
Having established a possible correlation with MAIT cell numbers in hospitalized patients with H7N9-induced IAV pneumonia of varying severity, we next asked how MAIT cells are activated during early influenza infection, using an in vitro model of IAV-infected human lung epithelial cells cocultured with PBMCs. Our findings led to the proposal that robust MAIT cell up-regulation of IFNγ and GzmB follows such IAV exposure and is dependent on soluble inflammatory mediators, particularly IL-18 (Fig. 5C). Although previous studies with Vα19Vβ6-Tg mice indicated that a range of virus-infected (but not including IAV) macrophages failed to activate MAIT cells in vitro (7), we have shown that CD14+ monocytes are required for the optimal induction of the MAIT cell response in IAV pneumonia. Furthermore, our findings suggest that GzmB is a useful ex vivo marker for MAIT cell activation during early IAV infection.
It is well established that T and NK cells make IFNγ when cultured in the presence of IL-18, a potent proinflammatory cytokine. Indeed, our data demonstrate that IFNγ production in NK cells is modulated in part by IL-18, although we suggest that additional cell-associated factors are needed for complete activation. The mechanisms leading to MAIT activation (IFNγ and GzmB up-regulation) are indirect, with the MAIT cells induced predominantly by soluble mediators produced during early IAV infection. Earlier work in human PBMCs has shown that IL-18, acting synergistically with IL-12, is important for the TLR-driven activation of MAIT cells (14, 25, 26); however, our present analysis demonstrates that blocking soluble IL-18 (but not IL-12 singly) abrogates IFNγ production during IAV infection. Similarly, previous studies using murine models of IAV infection have demonstrated that IL-18 alone is required for optimal IFNγ production in CD8+ T cells (27). Although IL-12 was not detected in our CBA analyses, the physiological concentration may be below the limit of detection. Furthermore, Guo et al. (28) reported that IL-18 (but not IL-12) was readily found in the serum of hospitalized H7N9 patients. Indeed, the fact that IL-18 is present at significant levels in the peripheral circulation suggests that a “global” activation of MAIT cells may occur in multiple peripheral sites in addition to the infected lung.
Considering that complete abrogation of IFNγ production was not found consequent to IL-18 blocking, it is possible that other inflammatory mediators (e.g., MCP-1, TNF, type 1 IFN) in supernatants from the IAV-infected cocultures may further activate MAIT cells. These inflammatory mediators are characteristic of the “cytokine storm” that can be detected in both bronchiolar lavage fluid and serum from hospitalized H7N9 patients (20). Given such potent hypercytokinemia, it could prove informative to look more closely at MAIT cells in severe, debilitating viral infections, both in patients and in animal models.
We speculate that monocytes are the source of IL-18 during in vitro coculture, given that depletion of this subset from the PBMC pool resulted in a significant abrogation in MAIT IFNγ up-regulation. Recent studies showing TCR-independent MAIT cell induction for PBMCs cultured in vitro with IL-15 have demonstrated that this activation is dependent on the presence of IL-18–producing monocytes (25). Previous studies also have suggested that live IAV infection can promote the differentiation of monocytes into dendritic cells (29, 30). Thus, the cytokine/chemokine milieu generated during in vitro coculture may promote first monocyte differentiation, then MAIT cell activation during IAV infection. In addition, lung epithelial cells express the IL-18 precursor protein (31). Although cleavage to the mature form of IL-18 in response to IAV remains unclear, these cells may serve as a potential source of IL-18. Further experiments using sensitive detection methods for the active form of IL-18 are needed.
When it comes to functional activity and possible protection, it is clear that, following TCR-mediated stimulation and subsequent up-regulation of perforin and GzmB, MAIT cells can kill bacterially infected targets. Furthermore, the expression of CD107a, a surrogate marker of degranulation, suggests that this indeed operates via a cytotoxic mechanism (32). Our data further suggest that during influenza infection, the inflammatory environment alone can be sufficient for the early up-regulation of GzmB, increasing the cytotoxic potential of MAIT cells. Similarly, another study of in vitro culture analysis using PBMCs from healthy adults demonstrated that IL-7 cytokine signaling alone can induce GzmB and elevate perforin and granzyme A levels in MAIT cells, enhancing their cytolytic effector function before bacterial exposure (33). Indeed, another cohort of H7N9 patients analyzed by Guo et al. (28) exhibited significantly elevated serum IL-7 levels. Further studies are warranted to address the killing potential of MAIT cells during influenza infection.
Overall, our data show that MAIT cells potently up-regulate antiviral effector molecules, including IFNγ and GzmB, during early IAV infection. This activation is independent of MR1 and is driven by soluble inflammatory mediators, including IL-18 produced during influenza infection. This raises the possibility that MAIT cells can provide a measure of influenza immunity, even to newly emerging influenza viruses such as H7N9, where the host lacks preexisting antigen-specific (T cell and antibody) immunity. Perhaps of even more importance, severe influenza disease and lung pathology are associated with an increase in secondary or cobacterial infections with respiratory pathogens, including Streptococcus pneumoniae, Hemophilus influenzae, Staphylococcus aureus, and, more recently, Mycobacterium tuberculosis, identified by surveillance during the 2009 pH1N1 epidemic (34, 35). Several of these bacteria use riboflavin pathways, which provide biosynthetic intermediates that are metabolized to ligands for MR1 presentation and activation of MAIT cells in a TCR-dependent manner. Thus, in the setting of concurrent or secondary bacterial infections, MAIT cells activated by IAV infection may mediate a potent, “prearmed” antibacterial effector function, providing a possible mechanism for promoting protection (or limiting infection) against concurrent bacterial infection in severely debilitated patients with pneumonia.
Materials and Methods
PBMC Isolation.
After informed consent was obtained, heparinized blood was collected from >30 healthy donors (age 20–75 y), and PBMCs were isolated by Ficoll-Paque PLUS density-gradient centrifugation. Experiments were performed according to the National Health and Medical Research’s code of practice and approved by the University of Melbourne Human Ethics Committee (1443389). PBMCs from 16 H7N9-infected patients were obtained from Shanghai Public Health Clinic Center, with ethics approval by the center’s Ethics Committee (15).
IAV Infection of A549 Lung Epithelial Cells.
A549 human lung epithelial cells (American Type Culture Collection) were seeded at 5 × 106 into T75 flasks for 24 h before IAV infection. Infection was performed for 1 h with PR8; H1N1 at a multiplicity of infection (MOI) of 10–30. Cells were washed and trypsinized before coculture with PBMCs. In selected experiments, CD14+ monocytes were enriched from PBMCs using CD14 magnetic bead enrichment (Miltenyi Biotec).
Coculture of IAV-Infected A549 Cells with PBMCs.
Here 1 × 106 PBMCs were cocultured with 2 × 105 IAV-infected or uninfected A549 cells in a 96-well U-bottomed plate and incubated at 37 °C for 10 h, with the addition of brefeldin A at 3 h. In selected experiments, α-MR1 (clone 26.5) at 20 μg/mL or αIL-18 and/or αIL-12 blocking antibodies at 5 μg/mL were added at the start of the coculture. Cells were centrifuged, and supernatants were removed and stored at −20 °C for CBA analyses. Surface-stained MAIT cells (see below) were fixed with Cytofix/Cytoperm (BD Biosciences), washed with Perm/Wash Buffer (BD Biosciences), and then incubated with anti–IFN-γ-AF700 (clone B27), anti–TNF-Alexa Fluor 700 (clone MAb11), or anti-GzmB (clone GB11, AF700; BD Pharmingen) for 30 min at 4 °C. Cells were washed and then analyzed by flow cytometry.
MAIT Cell Analysis.
Antibodies were obtained from BD Biosciences unless indicated otherwise. MAIT cells were defined by the MR1-5-OP-RU tetramer conjugated to SA-Brilliant Violet 421 (BV421) or phycoethryin (PE) (1, 5), anti–Vα7.2-allophycocyanin (APC) or PE (3C10; Biolegend), anti-CD161 (BV605; Biolegend), anti–CD3-PECF594, or Alexa Fluor 700 (UCHT1). B cells/monocytes were excluded based on anti-CD14 APC-H7 (MφP9) and anti-CD19 APC-H7 (HIB19). NK cells and γδ T cells were identified by anti-CD56 (NCAM16.2 PE-Cy7) and TCR γδ (2F11, FITC). CD4+ (OKT4 BV650; Biolegend) and CD8+ (SK1 PerCP-Cy5.5) cells were included. Dead cells were excluded with Live/Dead cell marker (Aqua or Near-Infared; Thermo Fisher Scientific). Then 1 × 106 cells were stained in MACS wash buffer (0.5% BSA, 2 mM EDTA) for 30 min, then washed and fixed in Cytofix/Cytoperm (BD Biosciences). Samples were acquired on an LSRFortessa cell analyzer (BD Biosciences) and analyzed using FlowJo software.
CBA.
Culture supernatants were analyzed with a multiplex CBA (BD Biosciences) of 17 analytes: IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p40, TNF, MCP-1, MIP-1α, MIP-1β, RANTES, GzmB, IFNα, IFNγ, IL-17A, and FasL.
Statistical Analyses.
Statistical comparisons among the paired samples were performed using a paired t test, with a confidence level of 95%. P < 0.05 was considered significant.
Note Added in Proof.
An additional manuscript on the role of MAIT cells in viral infections has been published while our manuscript was in revision (36).
Acknowledgments
We thank Andrew Brooks for insightful discussions; Ted Hansen for the α-MR1-26.5 blocking reagent; and Thakshila Amarasena, Sheilajen Alcantara, and Bernie McCudden for collecting blood. We thank all donors for donating blood for this study. This work was supported by National Health and Medical Research Council Program (NHMRC) Grant 1071916 (to P.C.D. and K.K.). L. Loh and S.J. were supported by an NHMRC C. J. Martin fellowship. Z.W. was supported by an NHMRC Australia–China exchange fellowship. K.K. is an NHMRC Senior Research Fellow. J.R. is an NHMRC Australia Fellow (AF50). D.P.F. is an NHMRC Senior Principal Research Fellow (1027369). S.S. is supported by a Victoria–India Doctoral Scholarship and a Melbourne International Fee Remission Scholarship (MIFRS). M.K. is supported by a Melbourne International Research Scholarship and an MIFRS.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610750113/-/DCSupplemental.
References
- 1.Reantragoon R, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210(11):2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treiner E, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 3.Martin E, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7(3):e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold MC, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8(6):e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett AJ, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509(7500):361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 6.Kjer-Nielsen L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 7.Le Bourhis L, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 8.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 9.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5:3143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeansyah E, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121(7):1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosgrove C, et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood. 2013;121(6):951–961. doi: 10.1182/blood-2012-06-436436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez CS, et al. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol. 2015;93(2):177–188. doi: 10.1038/icb.2014.91. [DOI] [PubMed] [Google Scholar]
- 13.Fergusson JR, et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Reports. 2014;9(3):1075–1088. doi: 10.1016/j.celrep.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ussher JE, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8⁺ T cells. Nat Commun. 2015;6:6833. doi: 10.1038/ncomms7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tripathi S, White MR, Hartshorn KL. The amazing innate immune response to influenza A virus infection. Innate Immun. 2015;21(1):73–98. doi: 10.1177/1753425913508992. [DOI] [PubMed] [Google Scholar]
- 17.Patel O, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- 18.Eckle SB, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med. 2014;211(8):1585–1600. doi: 10.1084/jem.20140484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang XZ, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190(7):3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, et al. Early hypercytokinemia is associated with interferon-induced transmembrane protein-3 dysfunction and predictive of fatal H7N9 infection. Proc Natl Acad Sci USA. 2014;111(2):769–774. doi: 10.1073/pnas.1321748111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiñones-Parra S, et al. Preexisting CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc Natl Acad Sci USA. 2014;111(3):1049–1054. doi: 10.1073/pnas.1322229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiore AE, et al. Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 23.Quiñones-Parra S, Loh L, Brown LE, Kedzierska K, Valkenburg SA. Universal immunity to influenza must outwit immune evasion. Front Microbiol. 2014;5:285. doi: 10.3389/fmicb.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Loh L, Kedzierski L, Kedzierska K. Avian influenza viruses, inflammation, and CD8(+) T cell immunity. Front Immunol. 2016;7:60. doi: 10.3389/fimmu.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattler A, Dang-Heine C, Reinke P, Babel N. IL-15-dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur J Immunol. 2015;45(8):2286–2298. doi: 10.1002/eji.201445313. [DOI] [PubMed] [Google Scholar]
- 26.Jo J, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10(6):e1004210. doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denton AE, Doherty PC, Turner SJ, La Gruta NL. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol. 2007;37(2):368–375. doi: 10.1002/eji.200636766. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, et al. The serum profile of hypercytokinemia factors identified in H7N9-infected patients can predict fatal outcomes. Sci Rep. 2015;5:10942. doi: 10.1038/srep10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao W, et al. Rapid differentiation of monocytes into type I IFN-producing myeloid dendritic cells as an antiviral strategy against influenza virus infection. J Immunol. 2012;189(5):2257–2265. doi: 10.4049/jimmunol.1200168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou W, et al. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119(13):3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H, Shen C, Brunham RC. Chlamydia trachomatis infection of epithelial cells induces the activation of caspase-1 and release of mature IL-18. J Immunol. 2000;165(3):1463–1469. doi: 10.4049/jimmunol.165.3.1463. [DOI] [PubMed] [Google Scholar]
- 32.Kurioka A, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8(2):429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeansyah E, et al. Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 infection. PLoS Pathog. 2015;11(8):e1005072. doi: 10.1371/journal.ppat.1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Archer B, et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: Epidemiology and factors associated with fatal cases. Euro Surveill. 2009;14(42):19369. doi: 10.2807/ese.14.42.19369-en. [DOI] [PubMed] [Google Scholar]
- 35.Metersky ML, Masterton RG, Lode H, File TM, Jr, Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16(5):e321–e331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 36.van Wilgenburg B, et al. MAIT cells are activated during human viral infections. Nat Commun. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]