Significance
Long-term depression (LTD), a unique type of synaptic plasticity exhibited by cerebellar Purkinje cells, has been postulated to play a key role in motor learning. This LTD hypothesis of motor learning has been supported by observations of the coincidence of inactivation of LTD by genetic or pharmacological manipulation with the impairment of motor learning. A controversy, however, has recently arisen because of the observation that in certain mutant mice, this coincidence did not occur; motor learning normally proceeded even when LTD was not induced. We reexamined these mutant mice and found that LTD was inducible even in these mutant mice when stimulation conditions were adequately modified. The present observations support the LTD hypothesis of motor learning.
Keywords: cerebellum, LTD, Marr–Albus–Ito hypothesis, motor-learning, slice
Abstract
Long-term depression (LTD) of synaptic transmission from parallel fibers (PFs) to a Purkinje cell (PC) in the cerebellum has been considered to be a core mechanism of motor learning. Recently, however, discrepancies between LTD and motor learning have been reported in mice with a mutation that targeted the expression of PF–PC LTD by blocking AMPA-subtype glutamate receptor internalization regulated via the phosphorylation of AMPA receptors. In these mice, motor learning behavior was normal, but no PF–PC LTD was observed. We reexamined slices obtained from these GluA2 K882A and GluA2 Δ7 knockin mutants at 3–6 mo of age. The conventional protocols of stimulation did not induce LTD in these mutant mice, as previously reported, but surprisingly, LTD was induced using certain modified protocols. Such modifications involved increases in the number of PF stimulation (from one to two or five), replacement of climbing fiber stimulation with somatic depolarization (50 ms), filling a patch pipette with a Cs+-based solution, or extension of the duration of conjunction. We also found that intracellular infusion of a selective PKCα inhibitor (Gö6976) blocked LTD induction in the mutants, as in WT, suggesting that functional compensation occurred downstream of PKCα. The possibility that LTD in the mutants was caused by changes in membrane resistance, access resistance, or presynaptic property was excluded. The present results demonstrate that LTD is inducible by intensified conjunctive stimulations even in K882A and Δ7 mutants, indicating no contradiction against the LTD hypothesis of motor learning.
In the efforts to decipher the elaborate neural network in the cerebellum (1), Marr (2) and Albus (3) focused on the dual innervation of Purkinje cells (PCs) by two types of excitatory input, climbing fibers (CFs) and parallel fibers (PFs), and interpreted it as a unique memory device. Incorporation of this memory device into the cerebellar cortical network leads to the formation of a learning machine, which is now called the Marr–Albus model. The postulated memory device was assumed to operate in such a way that conjunctive stimulation (Cj) of a CF and PFs induces plasticity changes at PF–PC synapses. A decade later, it became clear that, as recorded from a PC, repeated Cjs of a bundle of PFs and the CF that specifically innervate the PC indeed induces long-term depression (LTD) for the transmission efficacy in the PF–PC synapses (4–8). In the subsequent explorations of signal transduction pathways underlying LTD, various inhibitors and genetically manipulated mice have become available, which provided effective means to block LTD. The close coincidence between the induced LTD blockade and impairment of behavioral motor learning has been taken as evidence for LTD playing a key role in the mechanisms of motor learning (9). These views are collectively called the LTD hypothesis of motor learning, or the Marr–Albus–Ito hypothesis (10–12).
This hypothesis has recently been challenged by Schonewille et al. (12). These authors reported that in three types of gene-manipulated mouse they tested, motor learning remained normal and no LTD was induced in cerebellar slices obtained from these mice. Because these findings are incompatible with the Marr–Albus–Ito hypothesis, Schonewille et al. (12) claimed that the hypothesis should be reconsidered. We received from Richard Huganir of Johns Hopkins University, in Baltimore, MD, two of the three mouse mutant genotypes. One is the GluA2 K882A knockin (KI; hereafter, K882A) genotype containing the mutated form of GluA2 with a single lysine mutation incorporated in the consensus recognition motif for the protein kinase C α-subtype (PKCα). Normally, PKCα phosphorylates Ser880 and thereby detaches the AMPA-subtype glutamate receptors (AMPARs) from a synaptic scaffold protein, GRIP1/2; consequently, AMPARs are internalized. On the other hand, PDZ ligands and phosphorylation by other kinases remain functionally intact (13–17). The other is the GluA2 Δ7 KI (hereafter, Δ7) genotype, which lacks the last 7 amino acids of the C-terminal tail of GluA2. The region lacking the 7 amino acids is essential for the interaction of GluA2 with PICK1 and GRIP1/2 on the synaptic membrane (15–17). Because of the mutations, these mice are believed to be devoid of LTD; however, we found in this study that LTD is still inducible under certain stimulation conditions.
Results
The two types of mutant, GluA2 K882A and Δ7, examined in this study showed seemingly normal daily behavior. Acute slices were obtained from them at 3–6 mo of age, at which motor-learning capability was fully developed (12). Whole-cell recording from PCs under the voltage or current clamp condition showed virtually normal features of membrane resistance and configurations of PF–excitatory postsynaptic current (EPSC), simple spikes, and complex spikes in the mutant slices compared with the WT. Cj was usually followed by short-term depression, which decayed in 5–10 min, and followed by slow development of LTD (Fig. 1B). LTD continued for more than 1 h, but for convenience the magnitude of LTD was estimated by measuring the amplitude of the depressed PF–EPSC peak at 25–29 min after the onset of Cj, relative to the mean amplitude measured during the 5-min preconjunction period.
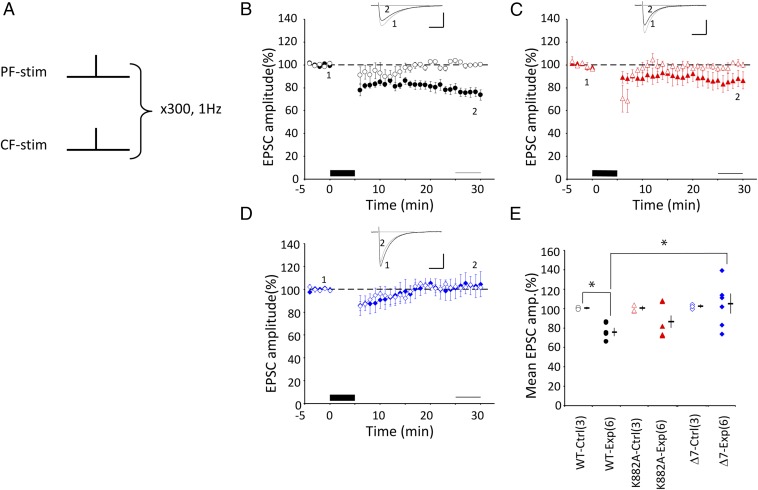
Fig. 1.
LTD induction by protocol-1 Cj. (A) Schematic illustration of protocol-1 Cj. 1PF and 1CF stimuli were applied simultaneously at 1 Hz for 5 min under a current clamp condition. (B) Mean PF–EPSC amplitude recorded from WT PCs before and after protocol-1 Cj (black column at bottom). The horizontal thin line (bottom) indicates the period of 25–29 min after Cj onset, where the amplitudes of PF–EPSCs were compared with estimate LTD. PF–EPSC amplitude was normalized by those recorded before Cj. Filled symbols indicate the mean amplitude of the experimental group. Open symbols indicate the PF–EPSC amplitude of the control group, in which only CF-stimulation was applied in place of Cj. Error bars denote SEM. (Inset) Superposed PF–EPSC traces (Upper) recorded before (marked 1) and 25–29 min after Cj-stimulation onset (marked 2). Each trace represents the average of 6 records. (Scale bars: 100 ms, 150 pA.) (C) Similar to B but for K882A PCs. (D) Similar to B, but for Δ7 PCs. (E) Summary plot of mean PF–EPSC amplitude recorded during 25–29 min after onset of Cj in WT, K882A, or Δ7 mice. Filled symbols are plots for the test with protocol-1, whereas open symbols are plots for the control measurement where Cj was replaced by 1CF stimulation. The mean and SEM of each group are indicated by a horizontal rod and vertical bar, respectively. Numbers in parentheses represent cell number. *P < 0.05. Intragroup comparison, t test. Intergroup comparison, ANOVA and post hoc Tukey–Kramer test.
LTD Induction in PCs.
Four different Cj protocols were used. First, a single shock stimulus (0.1 ms in duration) was applied to PFs in combination with a single stimulus simultaneously applied to CF and repeated at 1 Hz for 5 min (300 pulses) (Fig. 1A). (PF + CF) Cj (hereafter, protoco1-1) was used previously for effectively inducing LTD (12, 18). In the present study, protocol-1 was effective in inducing LTD in WT PCs (75.6 ± 4.0%, n = 6, P = 0.002, t test), but not in Δ7 PCs (103.9 ± 9.6, n = 6, P = 0.90) (Fig. 1 B and D). In Fig. 1C, K882A PCs may appear to exhibit a modest LTD, the magnitude of which, however, is not statistically significant (86.0 ± 7.2, n = 6, P = 0.23). Thus, protocol-1 was effective in inducing LTD only in WT, but not in either K882A or Δ7 PCs (Fig. 1E), which is in agreement with the report by Schoneville et al. (12).
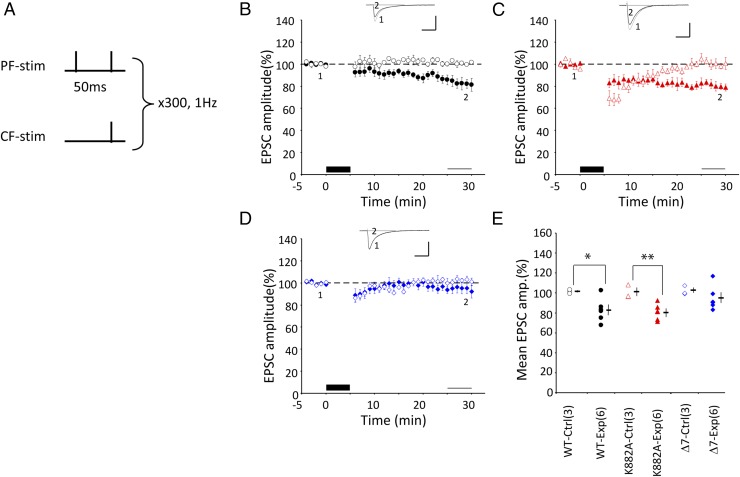
Second, PFs were stimulated twice at an interval of 50 ms and the second PF stimulus was synchronized with a single CF stimulus (Fig. 2A). This (2PF + CF) Cj (protocol-2) induced significant LTD in K882A PCs (81.0 ± 3.4, n = 6, P = 0.008) (Fig. 2C), comparable to that in WT PCs (82.8 ± 4.95%, n = 6, P = 0.036) (Fig. 2B), which showed no statistically significant difference between them (P = 0.957, Tukey–Kramer test). However, no LTD was induced in Δ7 PCs (94.8 ± 5.1, n = 6, P = 0.35) (Fig. 2D). Thus, protocol-2 was effective in inducing LTD in both WT and K882A, but not in Δ7 PCs (Fig. 2E).
Fig. 2.
LTD induction by protocol-2 Cj. (A–E) Similar to Fig. 1, but for conjunction protocol-2, where 2PF and 1CF stimulations were applied conjunctively at 1 Hz for 5 min under current clamp conditions. In E: *P < 0.05, **P < 0.01, t -test.
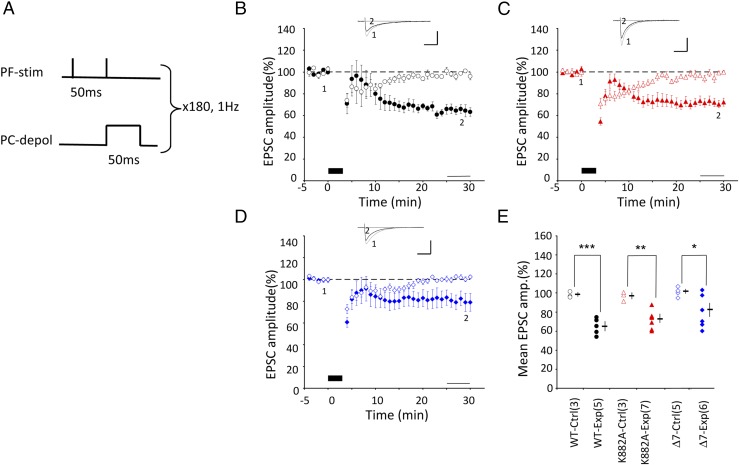
Third, the CF stimulus was replaced with a single depolarizing pulse (50 ms) applied through a patch pipette (Fig. 3A) (19, 20). The patch pipette was filled with a Cs+-based internal solution to improve space clamping along PC dendrites by blocking K+ channels (15, 21). The mean Rm (membrane resistance in MΩ, measured by passing square currents through the patch pipette) in cells recorded with the pipette containing the Cs+-based internal solution was three- to four-times larger than those recorded with a pipette containing a K+-based solution (Table S1). This maneuver is expected to ensure efficient activation of voltage-dependent Ca2+ channels located at dendritic regions, where PFs and CFs form synapses on PCs. A pulse was applied 50 ms after the first PF shock stimulus, and repeated at 1 Hz for 3 min (180 pulses). With this (2PF + depolarization) Cj (protocol-3), LTD was induced in WT PCs (64.9 ± 4.3%, n = 5, P = 0.0007), K882A PCs (72.0 ± 3.8, n = 7, P = 0.003), and Δ7 PCs (80.0 ± 7.2, n = 6, P = 0.026) (Fig. 3 B–D). No significant difference was found between these three groups (P = 0.15, one-way ANOVA) (Fig. 3E).
Fig. 3.
LTD induction by protocol-3 Cj. (A–E) Similar to Fig. 1, but for conjunction protocol-3, where 2PF stimulation and one depolarizing pulse from a holding potential to a voltage between 0 and +95 mV were applied conjunctively at 1 Hz for 3 min under voltage clamp conditions. In E: *P < 0.05, **P < 0.01, ***P < 0.001, t test.
Table S1.
Relation between membrane resistance and LTD
| Protocol | Genotype | Rm before Cj (b) | Rm after Cj (a) | n | a/b | P (t test) | P (ANOVA) before Cj | P (ANOVA) after Cj |
| 1 | WT | 53.7 ± 4.4 | 54.7 ± 6.3 | (6) | 1.02 | 0.78 | ||
| K882A | 48.2 ± 2.9 | 51.7 ± 3.0 | (6) | 1.07 | 0.10 | 0.62 | 0.93 | |
| Δ7 | 51.2 ± 8.1 | 52.5 ± 7.2 | (6) | 1.02 | 0.60 | |||
| 2 | WT | 49.3 ± 1.7 | 50.0 ± 5.1 | (6) | 1.01 | 0.88 | ||
| K882A | 50.1 ± 6.2 | 47.2 ± 8.2 | (6) | 0.94 | 0.47 | 0.93 | 0.63 | |
| Δ7 | 52.0 ± 7.3 | 48.4 ± 7.7 | (6) | 0.93 | 0.14 | |||
| 3 | WT | 162.7 ± 40.2 | 146.0 ± 26.7 | (5) | 0.90 | 0.43 | ||
| K882A | 164.3 ± 55.3 | 167.5 ± 64.6 | (7) | 1.02 | 0.81 | 0.94 | 0.81 | |
| Δ7 | 182.5 ± 15.6 | 191.3 ± 13.7 | (6) | 1.05 | 0.35 | |||
| 4 | WT | 209.8 ± 13.1 | 199.8 ± 16.1 | (3) | 0.95 | 0.09 | ||
| K882A | 223.0 ± 10.0 | 243.7 ± 22.7 | (4) | 1.09 | 0.26 | 0.11 | 0.10 | |
| Δ7 | 185.9 ± 7.1 | 180.5 ± 4.5 | (3) | 0.97 | 0.53 |
No significant difference was detected between Rm values measured with four Cj protocol and three genotypes. Data are shown as mean ± SEM (n). Note that changes in a/b ratio were less than 0.1.
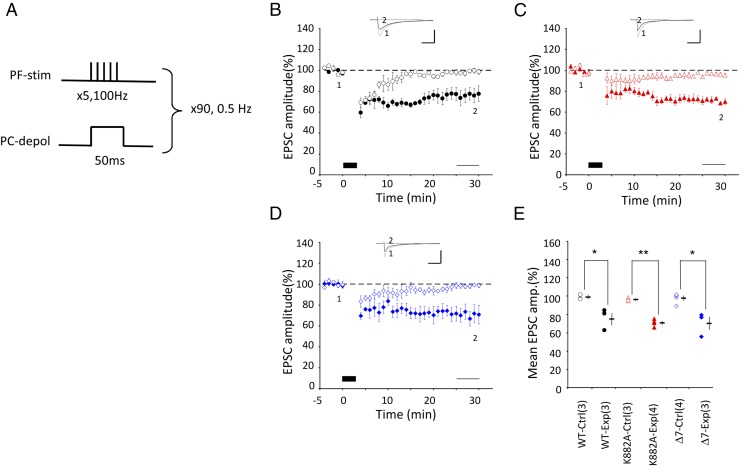
Fourth, using the Cs+-based internal solution, we also combined five PF stimuli and somatic depolarization (Fig. 4A). This protocol was originally used by Steinberg et al. (15), with five PF stimulations at 100 Hz concomitant with somatic depolarization (100-ms duration) applied at 2-s intervals for 1 min. This protocol induced LTD in slices obtained from young mice [postnatal day (P) 20–25] in WT, but in neither K882A nor Δ7 PCs (15). In the present study, however, when applied to mature WT mice (3–6 mo), the original protocol of Steinberg et al. (15) was ineffective for inducing LTD (98.0 ± 5.7%, n = 4, P = 0.78, paired t test). After some trials and errors, however, we found that an effective protocol consists of five PF stimulations at 100 Hz concomitant with somatic depolarization (−70 to 0 mV; duration, 50 ms) repeated at 2-s intervals for 3 min. This (5PF + depolarization) Cj (protocol-4) was effective for inducing LTD in WT PCs (76.0 ± 6.8%, n = 3, P = 0.031), K882A PCs (71.0 ± 2.0%, n = 4, P = 0.00017), and Δ7 PCs (70.7 ± 7.4%, n = 3, P = 0.013) (Fig. 4 B–D). No statistically significant difference was found among the three groups (P = 0.749, one-way ANOVA) (Fig. 4E).
Fig. 4.
LTD induction by protocol-4 Cj. (A–E) Similar to Fig. 1, but for conjunction protocol-4, where 5PF stimulation and one depolarizing pulse were applied conjunctively at 0.5 Hz for 3 min under voltage clamp conditions. In E: *P < 0.05, **P < 0.01, t test.
Cj Dependence of LTD Induction.
To ensure that protocol-2–evoked LTD is specifically induced by Cj of PF and CF and not by stimulation of PF alone or that of CF alone, we tested the effect of applying CF stimuli alone in place of PF–CF Cj. No significant change in EPSC amplitude during 25–29 min was observed in any of the three PC groups: WT PCs (101.1 ± 1.5%, n = 3, P = 0.41, t test), K882A PCs (100.5 ± 3.9%, n = 3, P = 0.90), and Δ7 PCs (102.2 ±2.8, n = 3, P = 0.48), as plotted in Figs. 1 B–D and 2 B–D as control for Cj. Stimulation of PF alone in place of PF–CF Cj was previously shown to induce no LTD in WT, K882A, or Δ7 PCs (12). In this study, we also observed that PF or 2PF induced no LTD, but often LTP, as has been known (12, 22).
With protocol-3 and protocol-4, CF stimulation was replaced with depolarizing pulses that caused Ca2+ entry, which was similar to that evoked by CF stimulation (15, 23). In a previous control study using a K+-based internal solution, the Cj dependence of LTD induction was confirmed by the absence of LTD after the separate application of depolarizing pulses (20). In this study, we applied 50-ms depolarizing pulses at 1 Hz for 3 min separately from PF stimuli. These depolarizing pulses induced short-term depression lasting for 20 min but did not cause LTD in any of the three PC groups (Figs. 3 and 4).
PKCα Inhibitor Sensitivity.
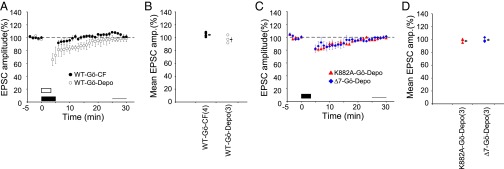
It may be questioned whether the LTD induced by replacing CF stimuli with depolarization in protocol-3 or protocol-4 shares a common signal transduction mechanism with the LTD induced by PF–CF Cj in protocol-1 and protocol-2. To test this, we chose Gö6976, a potent PKCα inhibitor that has been shown to play a crucial role in LTD induction (17). When Gö6976 (0.3 μM) was included in the internal solution, the EPSC amplitude after Cj failed to exhibit LTD in WT PCs either with protocol-2 (105.0 ± 1.7%, n = 4, P = 0.06, t test) or protocol-3 (97.7 ± 3.6%, n = 3, P = 0.61) (Fig. 5 A and B). Hence, as far as the blocking effect of Gö6976 is concerned, the LTD induced by (2PF + depolarization) Cj is indistinguishable from that induced by (2PF + CF) Cj.
Fig. 5.
Effects of PKCα blocker on LTD induction. (A) Plots are similar to Fig. 2B, but obtained under infusion of Gö6976 with protocols-2 (filled symbols) and 3 (open symbols) in WT mice. (B) Summary plot of mean PF–EPSC amplitude recorded during 25–29 min after onset of Cj in WT mice. (C and D) Similar to A and B, obtained with protocol-3 under infusion of Gö6976 in K882A or Δ7 PCs. In B and D, no significant difference was found in the mean EPSC amplitude between before Cj and after Cj (P > 0.05, paired t test).
As tested with protocol-3, LTD was also blocked by Gö6976 in K882A PCs (98.5 ± 1.6%, n = 3, P = 0.41) and Δ7 PCs (99.7 ± 1.7%, n = 3, P = 0.79) (Fig. 5 C and D). Hence, PKCα appears to play an essential role in LTD induction also in mutant PCs.
Presynaptic Release Mechanism.
This mechanism is an important factor that might affect PF–EPSCs. To examine whether the presynaptic release properties were affected by Cj, we compared the paired-pulse ratio (PPR) of PF to PC–EPSC measured before and after Cj. PPR was calculated as the mean of the second EPSC amplitude divided by the mean of the first (24). There was no significant change in PPR between before and after Cj with protocols 1–4 in each genotype (Table S2). Thus, a possibility that LTD induced in the K882A and Δ7 mutant mice was mediated by a presynaptic mechanism could be excluded.
Table S2.
Values of PPR of PF–EPSC
| Protocol | Genotype | PPR (before Cj) | PPR (after Cj) | n | P (t test) | P (ANOVA) before Cj | P (ANOVA) after Cj |
| 1 | WT | 1.49 ± 0.04 | 1.50 ± 0.04 | (3) | 0.924 | ||
| K882A | 1.42 ± 0.05 | 1.42 ± 0.04 | (4) | 0.992 | 0.575 | 0.341 | |
| Δ7 | 1.44 ± 0.03 | 1.47 ± 0.01 | (3) | 0.331 | |||
| 2 | WT | 1.36 ± 0.02 | 1.38 ± 0.01 | (6) | 0.250 | ||
| K882A | 1.39 ± 0.03 | 1.39 ± 0.01 | (3) | 0.707 | 0.136 | 0.390 | |
| Δ7 | 1.48 ± 0.06 | 1.43 ± 0.01 | (3) | 0.466 | |||
| 3 | WT | 1.39 ± 0.03 | 1.39 ± 0.02 | (3) | 0.720 | ||
| K882A | 1.34 ± 0.04 | 1.34 ± 0.03 | (3) | 0.888 | 0.208 | 0.504 | |
| Δ7 | 1.48 ± 0.07 | 1.46 ± 0.10 | (3) | 0.485 | |||
| 4 | WT | 1.37 ± 0.03 | 1.39 ± 0.03 | (3) | 0.963 | ||
| K882A | 1.34 ± 0.03 | 1.36 ± 0.02 | (3) | 0.391 | 0.253 | 0.362 | |
| Δ7 | 1.41 ± 0.01 | 1.42 ± 0.01 | (3) | 0.204 |
PPR was calculated as the mean of the second EPSC-amplitude divided by the mean of the first and EPSC. PPR was compared between before and after Cj, between WT, K882A and Δ7 PCs, and between protocols one and four. No significant difference was detected, suggesting that Cj of any protocol did not affect presynaptic release probability in any of WT, K882A, and Δ7 group. Also no difference was detected in PPR among three genotypes before or after Cj stimulation. Data are shown as mean ± SEM (n).
Access Resistance and Rm.
In the whole-cell recording in the voltage-clamp mode, as used in the present study, the recorded EPSCs might decrease when access resistance (Rs) increases during Cj. This undesirable effect was avoided by removing all of the data showing a change of more than 15% in Rs between before and after Cj.
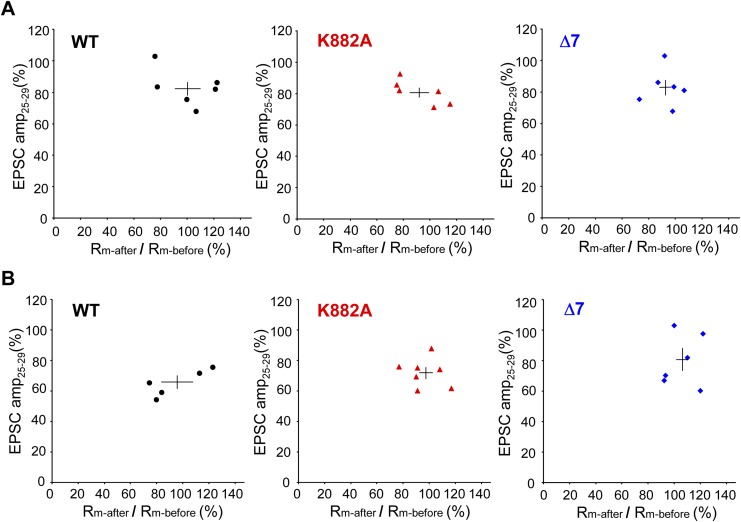
An incidental decrease in Rm might cause a decrease in the recorded PF–EPSCs. The mean change in Rm before and after Cj was less than 10% in each genotype for all Cj protocols (Table S1). Rm of each cell varied within the range of ±25% between before and after Cj, but this variation did not correlate with LTD magnitude (Fig. S1).
Fig. S1.
Relation between LTD and changes of Rm. Ordinates, EPSC-amplitude during 25−29 min after induction of LTD by Cj. Abscissae, Rm-after/Rm-before LTD induction. (A) With (2PF + CF) Cj (protocol-2). (B) Similar to A, but with (2PF + depolariziation) Cj (protocol-3). Left plots, for WT mice. Center plots, for K882A mice. Right plots, for Δ7 mice. In A, correlation coefficient for WT, K882A or Δ7 was −0.46 (P = 0.36), −0.77 (P = 0.07), or 0.04 (P = 0.93), respectively. In B, correlation coefficient for WT, K882A, or Δ7 was 0.84 (P = 0.08), −0.15 (P = 0.75), or 0.15 (P = 0.78), respectively. No significant correlation was found between Rm-after/Rm-before ratio and LTD in any group. Horizontal and vertical bars indicate mean and SEM, respectively.
Discussion
It has been believed that K882A and Δ7 mutant PCs are devoid of LTD because of impairment of AMPAR internalization (13, 15). However, the present study demonstrated that, whereas the previously used protocol-1 failed to induce LTD in K882A and Δ7 mutants, LTD was still induced in these mutants when tested with three modified Cj protocols (protocols-2, -3, and -4). To induce LTD in K882A PCs, Cj requires 2PF (300 pulses) (Fig. 2C), whereas 1PF (300 pulses) was sufficient in WT PCs (Fig. 1B). In Δ7, even 2PF was insufficient to induce LTD (Fig. 2D), but replacing CF with depolarizing pulses and filling the patch pipette with the Cs+-based internal solution, LTD was successfully induced in all of WT, K882A, and Δ7 PCs (Figs. 3 and 4). Thus, the intensity of Cj required for LTD induction gradually increases in the order of WT < K882A < Δ7. What determines this order is presently unknown.
The present study disclosed that LTD induced in K882A and Δ7 KI mice was dependent on PKCα activity as in WT mice, because the selective PKCα inhibitor Gö6976 effectively blocked LTD induction in these mutant mice and WT mice (Fig. 5). It may be that the stronger activation of PKCα drives the downstream molecular machinery that may be modified by functional compensation for the mutations.
The mechanism of such compensation is yet to be explored (25), but the involvement of GluA3 may be discussed here as a likely possibility. Note that AMPAR at the PF–PC synapse consists of GluA2 and GluA3 subtypes (26), and that the GluA3 expression level is normal even in K882A and Δ7 mice (15). Note also that the GluA3 C terminus can bind to GRIP/ABP and PICK1 (27). Suppression of binding between the GluA3 C terminus and GRIP/ABP through phosphorylation of Ser at the C terminus by PKCα seems plausible because the 5-amino acid sequence at the C terminus of GluA3 is identical to that of GluA2, including the Ser site (see ref. 28). It is interesting to determine whether phosphorylation of Ser at the C terminus of GluA3 in these mutant mice requires a higher concentration of activated PKCα, just as LTD requires a stronger Cj in these mutant mice. However, because in cultured PCs even a strong Cj did not induce LTD in cells from K882A or Δ7 mice (15), the capability of developing compensation by GluA3 might be lost in cultured conditions, or compensation by GluA3 might depend on age, because PCs in cerebellar slices from young K882A and Δ7 mice (P20–25) showed no LTD, even after conjunction of high-frequency PF stimulation and somatic depolarization (0.5 Hz, 1 min) using a Cs+-containing electrode (15). However, a similar but longer Cj (3 min) effectively induced LTD in older mutant mice (3–6 mo) (Fig. 4).
It has been shown that there is a second Cj–LTD pathway involving cytosolic phospholipase A2α, cyclooxygenase-2, and prostaglandin D2/E2 (20). This pathway might also mediate LTD induction under stronger Cj. However, the possibility is unlikely because prostaglandin D2/E2 cannot rescue the PKCα-inhibitor–induced LTD blockade.
Compensation in K882A and Δ7 mutant mice might involve scaffold molecules in addition to GRIP1/2 for maintaining GluA2 at the postsynaptic density. Transmembrane AMPAR regulatory proteins (TARPs) are candidate molecules that deserve attention (29). TARP γ-2, which is highly phosphorylated in its basal state, was dephosphorylated during chemically induced LTD in cerebellar slices (30). Furthermore, PC-specific conditional deletion of TARP γ-2 with a γ-7 KO background impaired motor behaviors (31). Both GRIP1/2 and TARPs might be involved in stabilization of GluA2/3-containing AMPAR, and dissociation of GluA2/3 from them through direct or indirect action of PKCα might underlie LTD. However, the link between PKCα activation and the dissociation of GluA2/3 from TARP is missing.
Even though the molecular mechanism of compensation for LTD attained in K882A and Δ7 mice has yet to be explored, an important conclusion from the present results is that LTD remains inducible in the mutants as long as the intensity of Cj is sufficiently increased. Hence, there is no reason to assume that LTD does not occur when these mutants perform motor learning. In this sense, the present results support the Marr–Albus–Ito hypothesis.
Materials and Methods
Animals.
The KI mice (GluR2K882A and GluR2Δ7), which were gifts from R. L. Huganir of Johns Hopkins University, Baltimore, MD, were described previously (15). Sperms were obtained from both KI mouse genotypes by ectomy and used for in vitro insemination of oocytes from C57BL/6 mice. The offspring from the fertilized oocytes was proliferated by back-crossing to C57BL/6 mice, and then by cross-breeding to obtain homozygous KI mice. Genotypes were determined by PCR amplification of tail DNA samples followed by digestion with restriction enzymes for which unique sites had been engineered (GluR2Δ7, BglII; GluR2K882A, BslI) (15). The primers used were Glu L (5′-AACGAATGAAGGTGGCAAAG-3′) and Glu R (5′-AAGAGCTTAAGGACGCGACA-3′). Genotypes were confirmed by second-round genotyping on genomic DNA purified from mouse brain slices used for electrophysiological recording. Mice were kept in the animal facility of RIKEN Brain Science Institute under well-controlled living conditions.
Electrophysiological Studies.
All procedures involving animals were approved by the RIKEN Committee on the Care and Use of Animals in experiments. To prepare cerebellar slices, adult mice were decapitated under halothane inhalation. The cerebellum was rapidly isolated and immersed in ice-cold ACSF containing the following (21): 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 20 mM glucose. The cerebellum was bubbled with 95% (vol/vol) O2 and 5% CO2. Sagittal slices of the cerebellar vermis of 300-μm thickness were prepared in artificial cerebrospinal fluid (ACSF) containing 1 μM tetrodotoxin using a microslicer (PRO7, Dosaka) and kept in normal ACSF at room temperature (25 °C) for at least 1 h. The recording chamber was perfused with oxygenated ACSF containing 100 μM picrotoxin (Sigma-Aldrich) at a rate of 2 mL/min and maintained at 29.0 ± 1.0 °C. The volume of the perfusion bath was 0.5 mL. Whole-cell slice-patch recordings of PCs were performed under an upright microscope (Eclipse E600FN, Nikon) with a 40× water-immersion objective. Patch pipettes were pulled from borosilicate tubings and filled with a solution containing the following (resistance, 2–4 MΩ). For the K+-based internal solution: 60 mM KCl, 60 mM K-gluconate, 0.3 mM EGTA, 4 mM MgCl2, 4 mM ATP, 0.4 mM GTP, and 30 mM Hepes (pH 7.2). For the Cs+-based internal solution: 60 mM CsCl, 40 mM d-gluconate, 20 mM TEA-Cl, 0.3 mM EGTA, 4, MgCl2, 4 mM ATP; 0.4 mM GTP, and 30 mM Hepes (pH 7.2, adjusted using CsOH). Whole-cell recordings were performed using a patch-clamp amplifier (MultiClamp 700A, Molecular Devices). Recorded signals were filtered at 3 KHz and digitized at 10 KHz. Stimulation and on-line data acquisition were performed using pClamp 9 software (Molecular Devices). Access resistance and input resistance were constantly monitored by applying a small hyperpolarizing voltage step (2 mV, 100 ms). PFs in the molecular layer or CFs in the granule cell layer or molecular cell layer were focally stimulated by applying pulses (duration, 0.1 ms) to a slice through a glass pipette (tip diameter, 5–10 μm) positioned on the surface of a cerebellar slice. The membrane potential was held at −65 or −75 mV, and a PF–EPSC was evoked at a frequency of 0.1 Hz as a test response.
In protocol-1 or -2, after switching to the current-clamp mode, LTD was induced by a protocol composed of 300 single or double PF stimuli in conjunction with CF stimuli. In the double PF stimuli protocol (protocol-2), the first PF stimulus was followed by a CF stimulus and the second PF stimulus at 50-ms intervals. During the PF–CF conjunctive stimulation, no DC current was applied except when there was the need to prevent spontaneous firing. The resting membrane potential was varied between −50 and −60 mV. Under the voltage-clamp condition using a recording electrode containing the Cs+-based internal solution, 2 (protocol-3) or 5 (protocol-4) PF stimuli were applied repeatedly at 1 or 0.5 Hz for 3 min, respectively, coupled with a depolarizing pulse for 50 ms from a holding potential of −75 mV to a potential (0 to +95 mV) at which a few inward current surges were observed, probably representing the generation of dendritic Ca2+ spikes.
Statistics.
To evaluate statistical significances of differences in data between the experimental group and the control group, the t test was used. On the other hand, the statistical significance of intergroup differences among the three genotypes was tested by one-way ANOVA and the Tukey–Kramer post hoc test. Data are represented as mean ± SEM (cell number of tested PCs) (Table S2).
Acknowledgments
We thank A. Oba for her technical assistance; the staff of the Research Resource Center of the Brain Science Institute for artificial insemination and the first step of the mice proliferation; and R. L. Huganir of Johns Hopkins Medical School for kindly providing us with GluR2K882A and GluR2Δ7 knockin mice. This work was supported in part by RIKEN Designated Donations Fund Grant No. G30-81000.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609957113/-/DCSupplemental.
References
- 1.Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Springer; New York: 1967. [Google Scholar]
- 2.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albus JS. Theory of cerebellar function. Math Biosci. 1971;10(1):25–61. [Google Scholar]
- 4.Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33(3):253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 6.Ekerot C-F, Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985;342(2):357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol. 1987;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 9.Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886(1-2):237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- 10.De Schutter E, Maex R. The cerebellum: Cortical processing and theory. Curr Opin Neurobiol. 1996;6(6):759–764. doi: 10.1016/s0959-4388(96)80025-0. [DOI] [PubMed] [Google Scholar]
- 11.Manto MU. Physiology of the cerebellum. In: Manto MU, editor. Cerebellar Disorders: A Practical Approach to Diagnosis and Management. Cambridge Univ Press; Cambridge, UK: 2010. pp. 23–35. [Google Scholar]
- 12.Schonewille M, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70(1):43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300(5626):1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- 14.Kemp BE, Pearson RB. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg JP, et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49(6):845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25(3):635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 17.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28(2):499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 18.Karachot L, Kado RT, Ito M. Stimulus parameters for induction of long-term depression in in vitro rat Purkinje cells. Neurosci Res. 1994;21(2):161–168. doi: 10.1016/0168-0102(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 19.Miyata M, Okada D, Hashimoto K, Kano M, Ito M. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999;22(4):763–775. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 20.Le TD, et al. Lipid signaling in cytosolic phospholipase A2α-cyclooxygenase-2 cascade mediates cerebellar long-term depression and motor learning. Proc Natl Acad Sci USA. 2010;107(7):3198–3203. doi: 10.1073/pnas.0915020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koekkoek SKE, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47(3):339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci USA. 2002;99(12):8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda H, Ellis-Davies GC, Ito K, Miyashita Y, Kasai H. Supralinear Ca2+ signaling by cooperative and mobile Ca2+ buffering in Purkinje neurons. Neuron. 1999;24(4):989–1002. doi: 10.1016/s0896-6273(00)81045-4. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21(24):9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M, Yamaguchi K, Nagao S, Yamazaki T. Long-term depression as a model of cerebellar plasticity. Prog Brain Res. 2014;210:1–30. doi: 10.1016/B978-0-444-63356-9.00001-7. [DOI] [PubMed] [Google Scholar]
- 26.Douyard J, Shen L, Huganir RL, Rubio ME. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J Comp Neurol. 2007;502(1):141–156. doi: 10.1002/cne.21294. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava S, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21(3):581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- 28.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40(2):361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki M, et al. TARPs γ-2 and γ-7 are essential for AMPA receptor expression in the cerebellum. Eur J Neurosci. 2010;31(12):2204–2220. doi: 10.1111/j.1460-9568.2010.07254.x. [DOI] [PubMed] [Google Scholar]
- 30.Nomura T, et al. Cerebellar long-term depression requires dephosphorylation of TARP in Purkinje cells. Eur J Neurosci. 2012;35(3):402–410. doi: 10.1111/j.1460-9568.2011.07963.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki M, et al. Relative contribution of TARPs γ-2 and γ-7 to cerebellar excitatory synaptic transmission and motor behavior. Proc Natl Acad Sci USA. 2015;112(4):E371–E379. doi: 10.1073/pnas.1423670112. [DOI] [PMC free article] [PubMed] [Google Scholar]