Significance
Our findings identify a previously unidentified role for scaffolding protein ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50; aka NHERF1) in the activation of NADPH oxidases (Nox), a family of professional reactive oxygen species (ROS) producing enzymes implicated in numerous pathologies. We demonstrate that EBP50 is critical for agonist-induced production of ROS superoxide anion (O2•–), and that it directly associates with the Nox organizing subunit p47phox. EBP50 deletion abolishes angiotensin II-induced cellular hypertrophy and resistance artery vasoconstriction. Given the wide array of EBP50 cellular interactions and the ubiquity of Nox, the current findings support a broader, more complex orchestration of Nox regulation than is currently hypothesized. The findings could augment future strategies targeting this interaction in disease involving aberrant ROS, tissue remodeling, and/or smooth muscle constriction.
Keywords: NADPH oxidase, EBP50, smooth muscle, hypertrophy, vascular tone
Abstract
Despite numerous reports implicating NADPH oxidases (Nox) in the pathogenesis of many diseases, precise regulation of this family of professional reactive oxygen species (ROS) producers remains unclear. A unique member of this family, Nox1 oxidase, functions as either a canonical or hybrid system using Nox organizing subunit 1 (NoxO1) or p47phox, respectively, the latter of which is functional in vascular smooth muscle cells (VSMC). In this manuscript, we identify critical requirement of ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50; aka NHERF1) for Nox1 activation and downstream responses. Superoxide (O2•−) production induced by angiotensin II (AngII) was absent in mouse EBP50 KO VSMC vs. WT. Moreover, ex vivo incubation of aortas with AngII showed a significant increase in O2•− in WT but not EBP50 or Nox1 nulls. Similarly, lipopolysaccharide (LPS)-induced oxidative stress was attenuated in femoral arteries from EBP50 KO vs. WT. In silico analyses confirmed by confocal microscopy, immunoprecipitation, proximity ligation assay, FRET, and gain-/loss-of-function mutagenesis revealed binding of EBP50, via its PDZ domains, to a specific motif in p47phox. Functional studies revealed AngII-induced hypertrophy was absent in EBP50 KOs, and in VSMC overexpressing EBP50, Nox1 gene silencing abolished VSMC hypertrophy. Finally, ex vivo measurement of lumen diameter in mouse resistance arteries exhibited attenuated AngII-induced vasoconstriction in EBP50 KO vs. WT. Taken together, our data identify EBP50 as a previously unidentified regulator of Nox1 and support that it promotes Nox1 activity by binding p47phox. This interaction is pivotal for agonist-induced smooth muscle ROS, hypertrophy, and vasoconstriction and has implications for ROS-mediated physiological and pathophysiological processes.
Reactive oxygen species (ROS) and their cellular sources have been the focus of intense study in physiology and disease for more than half a century. Under physiological conditions, ROS participate in key signaling pathways leading to distinct phenotypes including those characterized by cell migration, proliferation, differentiation, tissue remodeling, and apoptosis (1–3). Additionally, ROS mediate cell and tissue oxidative stress and damage at supraphysiological levels (2, 4, 5). The family of NADPH oxidase proteins (Nox) is widely recognized as one of the most robust and critical sources of the ROS superoxide anion (O2•–) (2, 4). Of the 7 Nox isoforms, the Nox1 oxidase is unique in that it comes in two varieties, a canonical and a hybrid system. Canonical Nox1 is composed of Nox1, p22phox, Nox organizing subunit 1 (NoxO1), Nox activating subunit 1 (NoxA1), and the small GTPase Rac1 (2). The hybrid Nox1 system employs the classical organizing subunit p47phox in lieu of its more recently discovered counterpart NoxO1(6–9). Phosphorylation of p47phox confers inducibility to the hybrid Nox1 oxidase, in contrast to the constitutively active canonical Nox1 oxidase (2, 6, 7, 9). Nox1 participates in the many vascular pathologies throughout the body including neointimal formation, smooth muscle hypertrophy, and vascular inflammation, among others (4, 9). However, it is currently unknown how selection for canonical- vs. hybrid-derived ROS occurs at the cellular level. It also remains elusive whether additional modulators of Nox enzymes exist. Thus, a more thorough investigation of factors that regulate Nox1 activity is critical to our fundamental understanding of the complex orchestration of events controlling oxidase function. Such knowledge is also expected to find utility in the development of therapeutics targeting ROS in disease.
Ezrin-radixin-moesin (ERM) binding phosphoprotein 50 (EBP50; aka NHERF1) is a widely expressed PDZ domain-containing scaffolding protein that associates with the actin cytoskeleton and plasma membrane by virtue of its binding to the ERM family of proteins (10–14). Recently, Bisello and coworkers (15–17) showed that EBP50 plays a role in neointimal hyperplasia and contributes to vascular smooth muscle cell (VSMC) phenotype changes. Coincidentally, recent reports have implicated the Nox1 system in these responses (18, 19), therefore suggesting a potential link between EBP50 and Nox1.
Herein, we examine a previously unidentified role for EBP50 in agonist-induced activation of Nox1 and assess its effect on Nox1-mediated VSMC hypertrophy and in vivo oxidative stress. Our data support that EBP50 facilitates Nox1-derived O2•– production and reveal that its permissive function occurs via its binding to p47phox. The data also show that EBP50 plays a significant role in the promotion of angiotensin II (AngII)-induced VSMC hypertrophy and resistance artery vasoconstriction.
Results
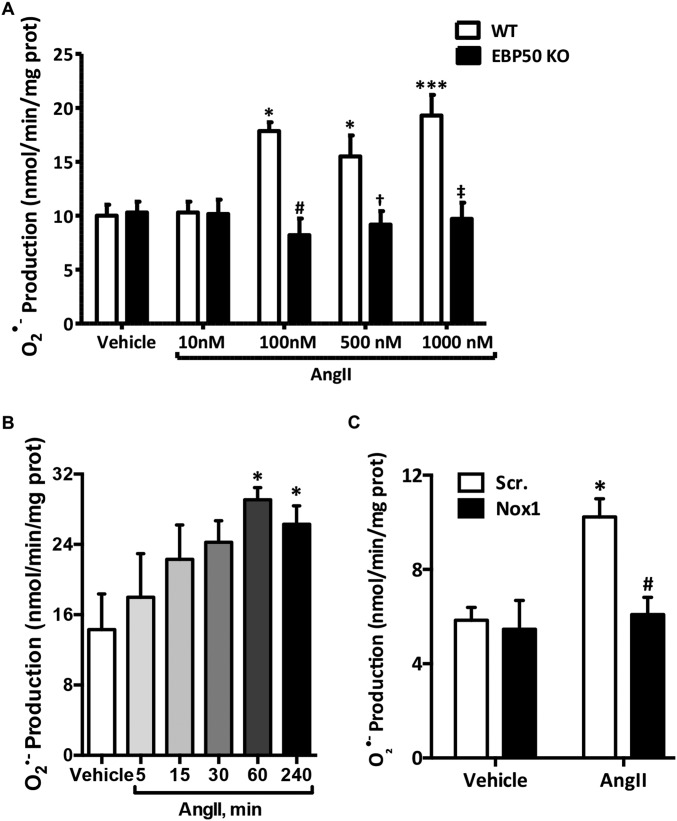
AngII- and H2O2-Induced O2•– Production Is Attenuated in VSMC from EBP50 KO Mice.
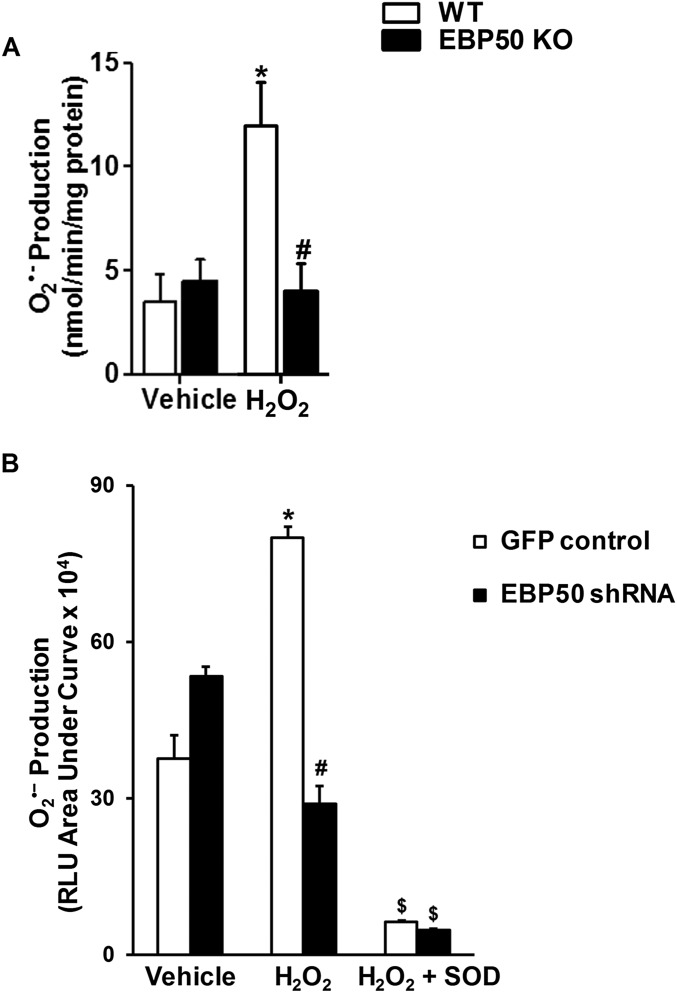
Rodent VSMC express oxidase isoforms Nox1 and 4 (2, 8). We recently reported that H2O2, in addition to AngII, is an efficacious activator of Nox1-derived O2•– production in VSMC (8). To assess the role of scaffolding protein EBP50 in Nox1 activation, O2•– production was investigated in aortic VSMC from EBP50 KO vs. WT mice. It is well established that 100 nM AngII treatment significantly increases O2•– levels, and this increase is Nox1-derived (20–26). We corroborated these findings using concentration and time response experiments and by showing that siRNA against Nox1 abolished the signal (Fig. S1 A–C). Thus, 100 nM AngII exposure for 4 h was selected for the current study to capture posttranslational and transcriptional Nox-derived O2•– production. Treatment of WT cells for 4 h with 100 nM AngII or for 1 h with 50 μM H2O2 resulted in a significant increase in O2•– measured by cytochrome c reduction (11.56 ± 2.14 and 11.92 ± 2.07 vs. 3.49 ± 1.33 nmol O2•–⋅min−1⋅mg−1 membrane fraction protein for AngII- and H2O2- vs. vehicle-treated WT cells, respectively; Fig. 1 A and B and Fig. S2 A and B). This increase in O2•– was almost completely absent in EBP50 KO cells stimulated with AngII or H2O2 (5.04 ± 1.39 and 3.97 ± 1.34 vs. 4.44 ± 1.08 nmol O2•–⋅min−1⋅mg−1 protein for AngII- and H2O2- vs. vehicle-treated KO cells, respectively; Fig. 1 A and B and Fig. S2A). These findings were then confirmed in VSMCs in which EBP50 was gene-silenced by stable transfection with EBP50 shRNA (Fig. S2B) (17). Cells stably expressing EBP50 shRNA-encoding plasmids or control GFP plasmids were treated with 50 μM H2O2 or vehicle for 1 h, and O2•– was measured. Treatment of control cells with H2O2 resulted in doubling O2•– levels compared with vehicle-treated cells, an induction abolished in EBP50 shRNA-expressing cells (Fig. S2B). Treatment of both groups with superoxide dismutase (SOD) completely inhibited the signal, confirming the identity of the ROS detected as O2•–.
Fig. S1.
Concentration- and time-dependent increase in AngII-induced O2•– production is Nox1-derived and absent in EBP50 KO VSMC. (A) Cytochrome c reduction assay was performed on 28,000 × g membrane fractions prepared from lysates of WT or EBP50 KO VSMC treated with 10–1,000 nM AngII. Rate of O2•− production was quantified in nmol/min/mg protein, and data are shown as means ± SEM, n = 3, *P < 0.01 vs. WT vehicle; ***P < 0.001 vs. WT vehicle; #P < 0.01 vs. WT 100 nM AngII; †P < 0.01 vs. WT 500 nM AngII; ‡P < 0.01 vs. WT 1000 nM AngII. (B) Cytochrome c reduction assay was performed as in A on VSMC treated with 100 nM AngII for the indicated times. Data are shown as means ± SEM, n = 4, *P < 0.05 vs. vehicle. (C) Cytochrome c reduction assay was performed as in A on VSMC transfected with scrambled (Scr.) or Nox1 siRNA. Data are shown as means ± SEM, n = 4, *P < 0.05 vs. Scr. vehicle; #P < 0.05 vs. Scr. AngII.
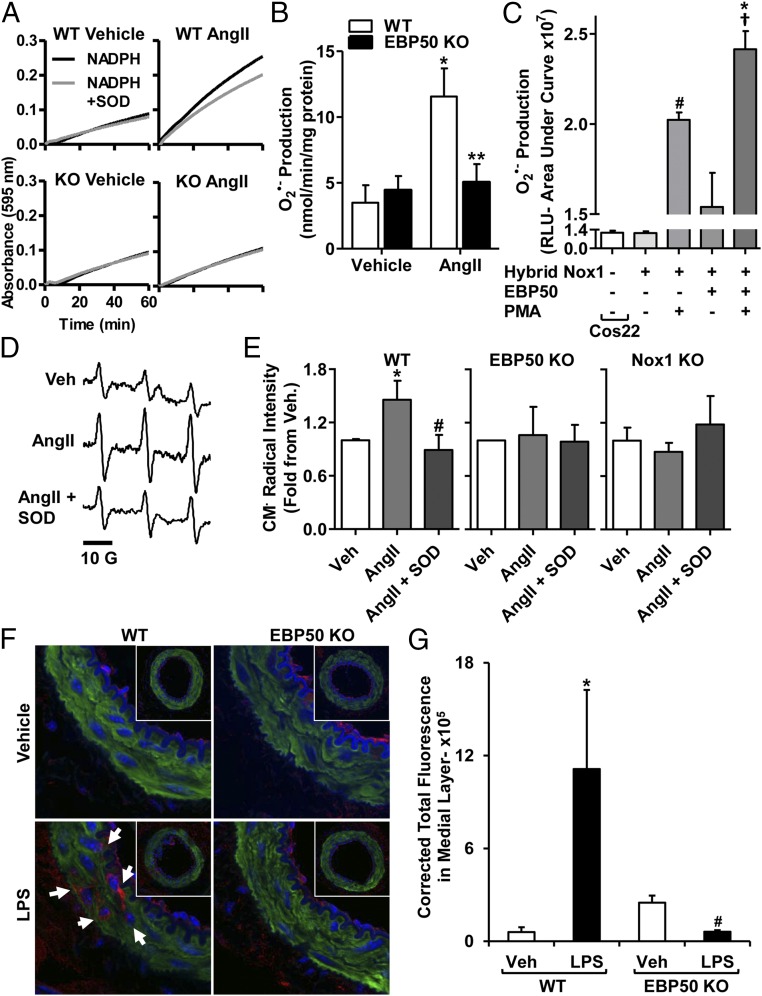
Fig. 1.
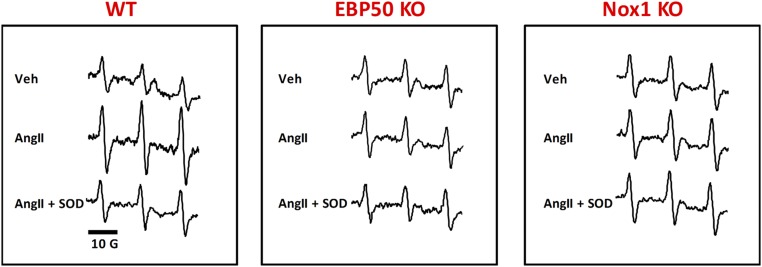
EBP50 is involved in agonist-induced O2•− production. Representative cytochrome c reduction plots in the presence or absence of SOD (A) and bar graphs showing summary data of cytochrome c reduction assays (B) performed on 28,000 × g membrane fractions prepared from lysates of WT or EBP50 KO VSMC treated with 100 nM AngII. AngII induced a significant increase in O2•− production in WT, which was absent in EBP50 KO VSMC. Rate of O2•− production was quantified in nmol⋅min−1⋅mg−1 protein, and data are shown in B as means ± SEM, n = 6–13, *P < 0.01 vs. WT vehicle; **P < 0.01 vs. WT AngII. (C) L-012 assay on Cos22 cells stably expressing p22phox transfected with other components of the hybrid Nox1 system (Nox1, NoxA1, and p47phox) or the hybrid Nox1 system plus EBP50 and treated with vehicle or 5 μM PMA. Data demonstrate that EBP50 augments PMA-induced O2•– production by the hybrid Nox1 oxidase system in Cos22 cells. White bar represents basal O2•− production in Cos22 cells. Data are shown as means ± SEM, n = 24, #P < 0.001 vs. vehicle-treated hybrid Nox1; †P < 0.001 vs. vehicle-treated hybrid Nox1 + EBP50; *P < 0.05 vs. PMA-treated hybrid Nox1. EPR sample spectra from mouse WT aortic rings (D) and quantification of EPR signal amplitude (E) from WT, EBP50 KO, or Nox1 KO mice aortic rings subjected to vehicle or AngII (100 nM) incubation in the presence or absence of SOD (200 U/mL) using the spin probe CMH (500 μM). AngII significantly increased CM•-radical intensity (indicative of O2•− production) that was inhibited by SOD. This increase was absent in EBP50 KO and Nox1 KO rings. Data are shown as means ± SEM, n = 5, *P < 0.05 vs. vehicle; #P < 0.05 vs. AngII. (F) Representative confocal images of femoral artery tissue cross-sections from LPS- or vehicle-treated WT or EBP50 KO mice stained with 4-HNE antibody (red), phalloidin (F-actin; green), and Hoechst (nuclei; blue). Autofluorescence of the internal and external elastic laminae is shown in blue. Arrows indicate locations of 4-HNE–positive fluorescence within the medial layer. (G) Quantification of 4-HNE fluorescence in the medial layer of femoral arteries in A shows that LPS induces a significant increase in tissue oxidation in WT but not EBP50 KO arteries. Data are shown as means ± SEM of corrected total fluorescence values, n = 3–4 animals, *P < 0.05 vs. vehicle WT; #P < 0.05 vs. LPS WT.
Fig. S2.
Absence or knockdown of EBP50 in VSMC reduces H2O2-induced O2•– production. (A) Cytochrome c reduction assay was performed as in Fig. S1 on WT and EBP50 KO VSMC treated with 50 μM H2O2. Data are shown as means ± SEM n = 3. *P < 0.05 vs. WT vehicle; #P < 0.05 vs. WT H2O2. (B) L-012 assay on VSMC stably transfected to express EBP50 shRNA or GFP control and treated with 50 μM H2O2 with or without 200 U/mL SOD. Data are shown as means ± SEM n = 3. *P < 0.05 vs. GFP vehicle; #P < 0.05 vs. GFP H2O2; $P < 0.05 vs. respective H2O2.
To investigate whether cellular EBP50 expression facilitates hybrid Nox1 activation (prevalent in VSMC), we recapitulated both EBP50 and the oxidase in Cos7 cells by transient transfection. Fig. 1C shows that a classical Nox activator, phorbol ester (PMA), resulted in significantly higher O2•– compared with vehicle-treated Nox1-transfected cells as well as untransfected cells (2.0 ± 0.05 vs. 1.1 ± 0.14 and 1.2 ± 0.16 × 107 RLU, respectively). PMA also increased O2•– in the Nox1 + EBP50 group. The increase in O2•– was greater in cells transfected with Nox1 + EBP50 vs. Nox1 alone (2.4 ± 0.10 vs. 2.0 ± 0.05 × 107 RLU). There appeared to be a rise in O2•– in cells transfected with both EBP50 and the hybrid Nox1 in the absence of PMA stimulation (1.5 ± 0.19 × 107 vs. 1.1 ± 0.14 × 107 RLU for vehicle-treated Nox1-transfected cells). This, however, did not reach statistical significance.
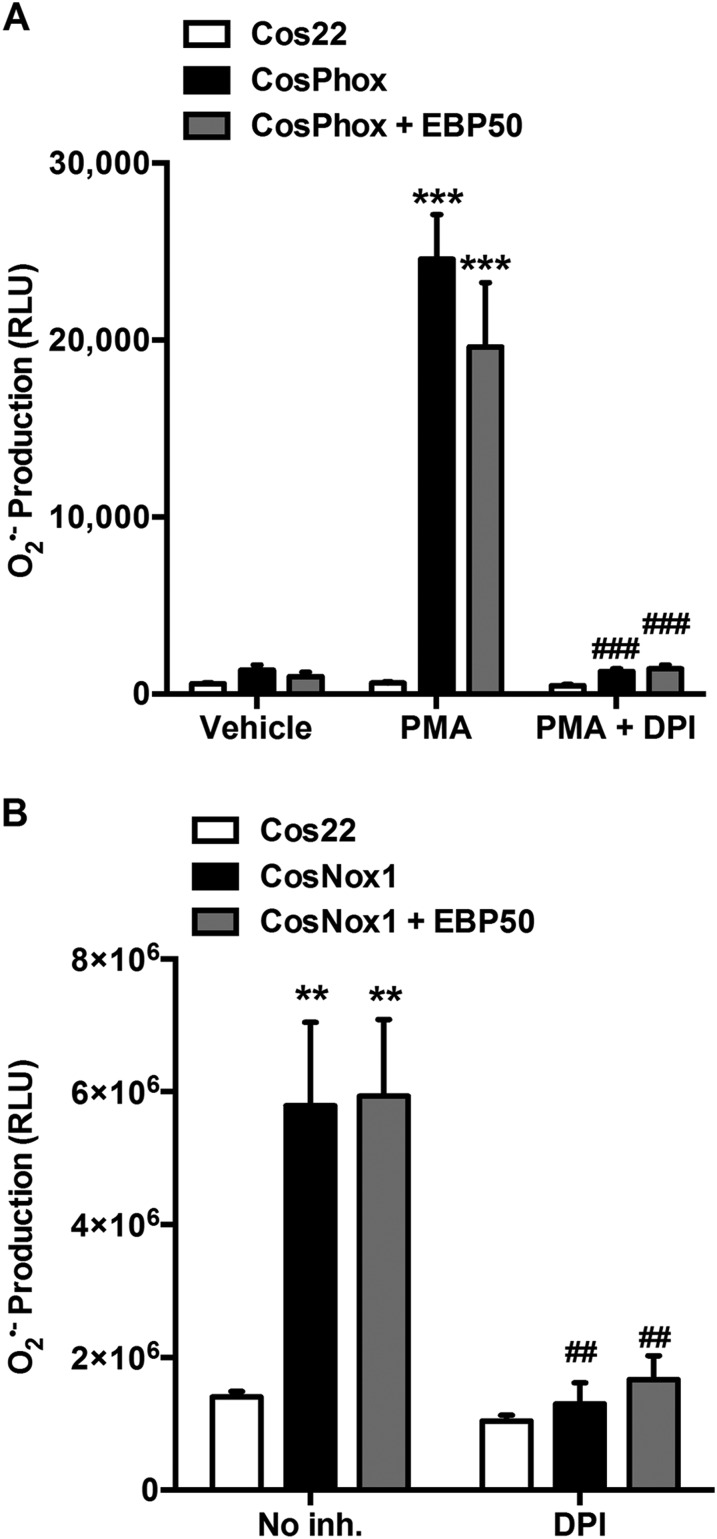
We next tested whether this observation is unique to the Nox1 hybrid system. First, Cos7 cells expressing the canonical Nox2 system (CosPhox cells) were tested for enhanced ROS production following EBP50 overexpression (CosPhox + EBP50). Fig. S3A demonstrates that PMA treatment results in a robust production of O2•– that is inhibited by diphenylene iodonium (DPI), a flavoprotein enzyme inhibitor that inhibits Nox proteins. Overexpression of EBP50 in these cells did not enhance the O2•– signal (Fig. S3A). Second, to test the canonical Nox1 system, using NoxO1, the constitutively active homolog of p47phox, Cos7 cells were transfected with the canonical Nox1 system in the absence (CosNox1) or the presence of EBP50 overexpression (CosNox1 + EBP50). Expressing the canonical Nox1 system in Cos22 cells caused a robust, DPI-inhibitable increase in O2•– (Fig. S3B). The presence of EBP50, however, did not enhance O2•– production by the canonical Nox1 system (Fig. S3B). Taken together, these data support a unique role for EBP50 on the hybrid Nox1 system using p47phox as its organizing subunit.
Fig. S3.
EBP50 does not enhance ROS production by canonical Nox2 or Nox1 systems. (A) L-012 assay on Cos22, CosPhox (stably expressing the canonical Nox2 system: Nox2, p67phox, and p47phox), or CosPhox transfected with EBP50 (CosPhox + EBP50) and treated with vehicle or 5 μM PMA in the presence or absence of DPI. Data are shown as means ± SEM, n = 3, ***P < 0.001 vs. vehicle; ###P < 0.001 vs. PMA. (B) L-012 assay on Cos22 cells transfected with other components of the canonical Nox1 system (CosNox1: Nox1, p22phox, NoxA1, and NoxO1) or the canonical Nox1 system plus EBP50 (CosNox1 + EBP50) and treated with vehicle (No inh.) or DPI. Data are shown as means ± SEM, n = 3, **P < 0.01 vs. Cos22; ##P < 0.01 vs. No inh.
To test whether EBP50 is essential for tissue ROS production, fresh aortic rings isolated from WT and EBP50 KO mice were incubated ex vivo with AngII in the presence or absence of SOD and subjected to electron paramagnetic resonance (EPR) and the spin trap CMH. As seen in Fig. 1 D and E, AngII induced a significant, SOD-inhibitable signal, indicative of an increase in extracellular O2•– production compared with vehicle. This induction was completely absent in aortas isolated from EBP50 KO mice, supporting that this protein is essential for O2•– generation in intact tissue. Moreover, when aortic rings from Nox1 KO mice were incubated with AngII, elevation in O2•– was not observed (Fig. 1 D and E and Fig. S4). These findings support a link between EBP50 and Nox1 that is essential for AngII-induced ROS. To explore whether EBP50-dependent ROS production is functional in the arterial media composed of VSMC, we contrasted tissue oxidation in femoral arteries from WT and EBP50 KO mice. Mice were subjected to vehicle or lipopolysaccharide (LPS) (10 mg⋅kg−1, i.p.; 16 h), another well-established activator of ROS and Nox activity (27–29), including Nox1 (30–33), the major Nox in the media of conduit arteries (1, 2, 22). Femoral arteries from these mice were isolated, sectioned, and stained for 4-hydroxynonenal (4-HNE, a footprint marker of lipid oxidation and oxidative stress). Arterial sections from LPS-treated WT animals showed significant medial 4-HNE staining (red) compared with vehicle treatment where no apparent staining was detected (Fig. 1F). Arteries from LPS-treated EBP50 KO mice showed no detectable 4-HNE fluorescence in the media. F-actin (phalloidin; green) and the internal and external elastic laminae (blue) demarcated the media for 4-HNE quantification. Fig. 1G reveals an 18-fold increase in the oxidation marker in LPS-treated WT mice vs. vehicle control (11.13 ± 5.11 vs. 0.60 ± 0.31 ×105 CFT units, respectively). This signal was absent in femoral arteries from LPS-treated KO mice (0.61 ± 0.11 × 105 CFT units).
Fig. S4.
Representative EPR spectra. EPR sample spectra from mouse WT, EBP50 KO, and Nox1 KO aortic rings subjected to vehicle or AngII (100 nM) incubation in the presence or absence of SOD (200 U/mL) using the spin probe CMH (500 μM).
EBP50 Regulates Nox1 Activity by Binding to p47phox.
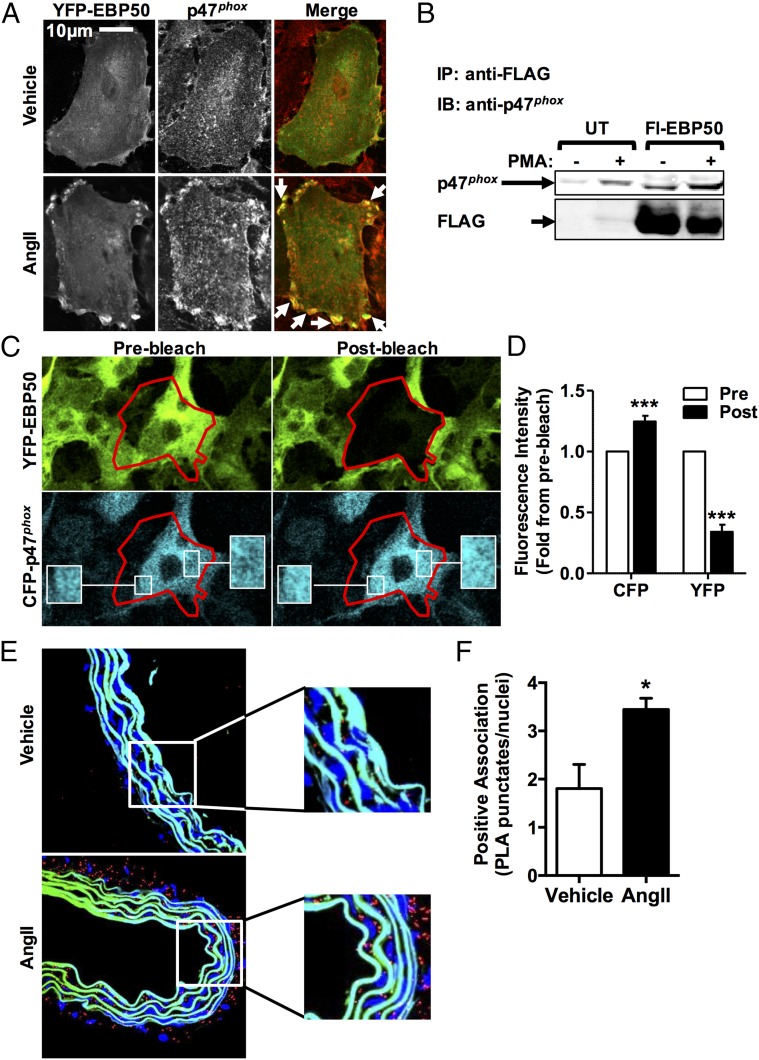
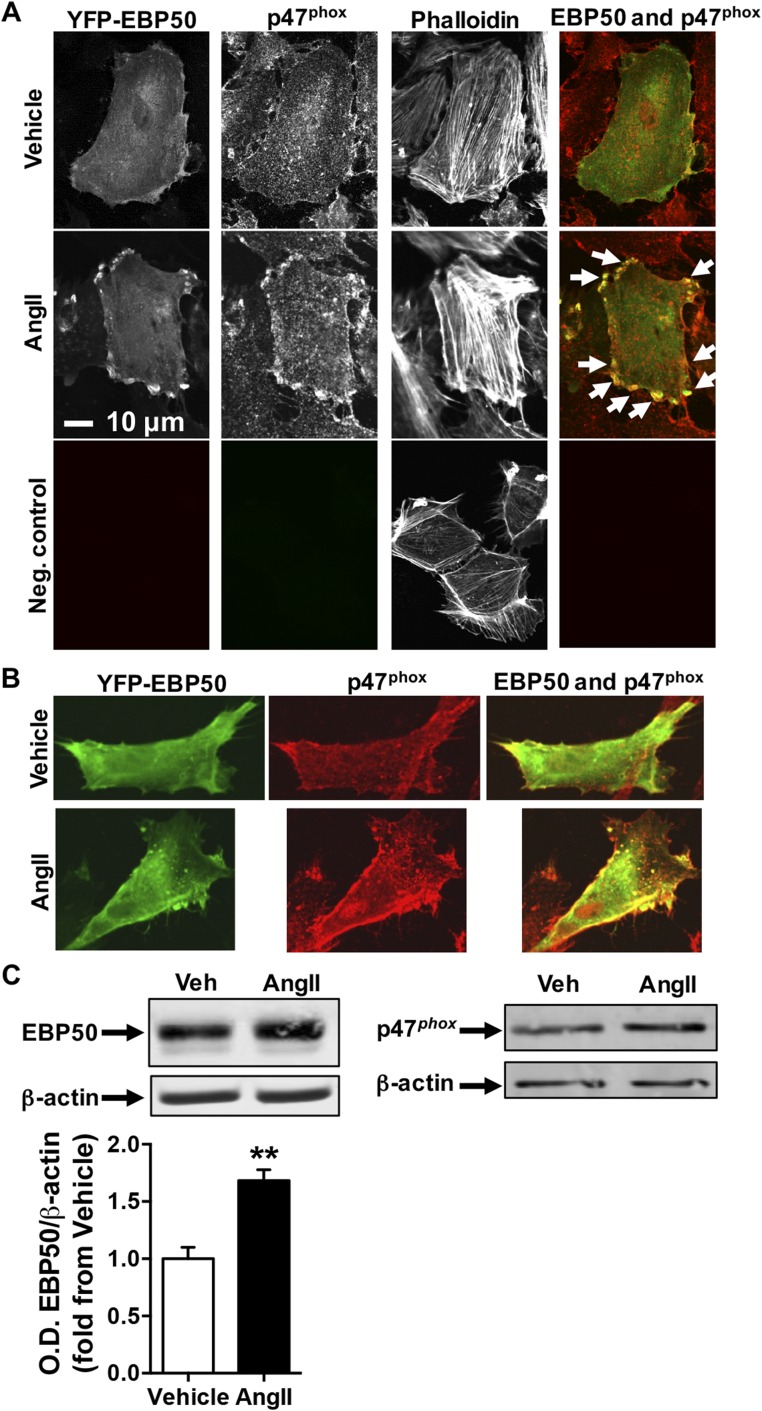
Next, we explored a potential link between EBP50 and p47phox. Colocalization of EBP50 and p47phox was investigated by confocal microscopy of rat aortic VSMC (rASMC) transfected with YFP-labeled EBP50 (green pseudocolor in the merged image) and treated with AngII. Fig. 2A and Fig. S5 A and B show that cells treated with vehicle followed by immunostaining for p47phox using Cy3-conjugated secondary antibody (red pseudocolor in the merged image and in Fig. S5B) revealed ubiquitous distribution of EBP50 and p47phox throughout the cytosol. As indicated by minimal yellow fluorescence in the merged image, EBP50 and p47phox display little colocalization under untreated conditions. In contrast, upon AngII treatment, increased presence of both proteins is observed, as well as enhanced presence of both proteins in or near the plasma membrane. Moreover, in the merged image, intense yellow fluorescence suggests colocalization (arrows, Fig. 2A). There was no autofluorescence or nonspecific secondary antibody signal in the negative control (Fig. S5A). To test whether there was an associated increase in expression of EBP50 and p47phox in addition to cellular redistribution, Western blots were performed on lysates from these cells treated with vehicle or AngII. Fig. S5C demonstrates that AngII treatment results in a significant increase in EBP50 protein expression. On the other hand, our data did not support a strong increase in p47phox expression in these cells.
Fig. 2.
EBP50 associates with p47phox. (A) Confocal fluorescent images of VSMC transfected with YFP-labeled EBP50 (green in merge), treated with vehicle or 100 nM AngII, and stained with p47phox antibody (red in merge) showing increased colocalization between EBP50 and p47phox following AngII treatment. Representative of three independent experiments. Arrows indicate colocalization areas. (B) Immunoprecipitation with anti-FLAG antibody followed by Western blot with anti-p47phox or anti-FLAG antibodies on lysates from Cos7 cells stably expressing p47phox without (UT) or with FLAG-EBP50 (Fl-EBP50) transfection and treated with vehicle (−) or PMA (+). PMA treatment increases p47phox association with EBP50. Representative of three independent experiments. Representative images (C) and fluorescence intensity quantification (D) of FRET experiments with confocal spectral imaging using Cos7 cells transfected simultaneously CFP-p47phox and YFP-EBP50 and treated with PMA as above. Stimulation of CFP (457 nm) resulted in a YFP spectral emission (Prebleach). Acceptor (YFP) photobleaching (514 nm, 50% laser power, four cycles of 5 s) resulted in an increase in donor (CFP) fluorescence (Postbleach). These data support direct association of EBP50 and p47phox. Red tracing, bleach area; white box, zoom area; Pre, prephotobleaching; Post, postphotobleacing. Data are shown as means ± SEM of fluorescence spectral emission intensity for each fluorophore, n = 9, ***P < 0.001 vs. prephotobleach. (E) Representative images of PLA experiments imaged by confocal using a 60× objective on WT mice aortic rings subjected to vehicle or AngII (100 nM) showing increased EBP50-p47phox association (positive PLA; red punctates). Blue, nuclei (Hoechst); autofluorescence of the internal and external elastic laminae is shown in blue/green; white box, zoom area. (F) Bar graph shows quantification of punctates normalized to number of nuclei. Data and means ± SEM, n = 3, *P < 0.05.
Fig. S5.
Representative confocal colocalization images. (A) Confocal fluorescent images as in Fig. 2A of VSMC transfected with YFP-labeled EBP50 (green in merge), treated with vehicle or 100 nM AngII, and stained with p47phox antibody and Cy3-labeled secondary antibodies (red in merge) followed by Alexa Fluor 647-phalloidin staining. Representative of three independent experiments. Arrows indicate colocalization areas. Neg. control indicates imaging of untransfected VSMC that were stained only with Cy3-labeled secondary antibody and Alexa Fluor 647-phalloidin. (B) A second representative set of confocal images of the experiments in A. Green, YFP-EBP50; red, p47phox. (C) Western blot on lysates of VSMC treated with vehicle or 100 nM AngII and probed for EBP50 (Left) or p47phox (Right) and β-actin as a loading control. Representative of three independent experiments. Bar graph shows O.D. quantification of EBP50 band relative to β-actin shown as fold from vehicle. Data are means ± SEM, n = 3, **P < 0.01.
To investigate whether binding between EBP50 and p47phox could explain their colocalization, cosedimentation experiments were performed using lysates of Cos22 cells transfected with a FLAG-tagged EBP50 and p47phox and stimulated with vehicle or PMA. PMA was used in these experiments because Cos7 cells do not express AngII receptors (34). Data represented in Fig. 2B are consistent with the association of p47phox and EBP50. That is, when lysates from Cos7 cells (expressing both p47phox and EBP50 and treated with 20 μM PMA) were immunoprecipitated with an antibody against FLAG and immunoblotted for p47phox, a distinct band appeared consistent with the molecular weight of the organizing subunit p47phox. Cells treated with vehicle exhibited a faint band, indicating weaker binding in the absence of stimulus.
To further explore binding of EBP50 to p47phox, Cos7 cells were cotransfected with a CFP-p47phox fusion protein and YFP-EBP50 (Fig. 2 C and D). FRET experiments with confocal spectral imaging demonstrated that fluorescent specific excitation of CFP (457 nm) resulted in spectral emission from YFP, indicating energy transfer from CFP to YFP (i.e., FRET). As can be seen in Fig. 2C and the fluorescence quantification in Fig. 2D, acceptor photobleaching of YFP-EBP50 resulted in an increase in donor CFP-p47phox emission fluorescence intensity due to unquenching. FRET efficiency was calculated to be 18.99 ± 2.96%. Taken together, these results confirm a direct association between EBP50 and p47phox.
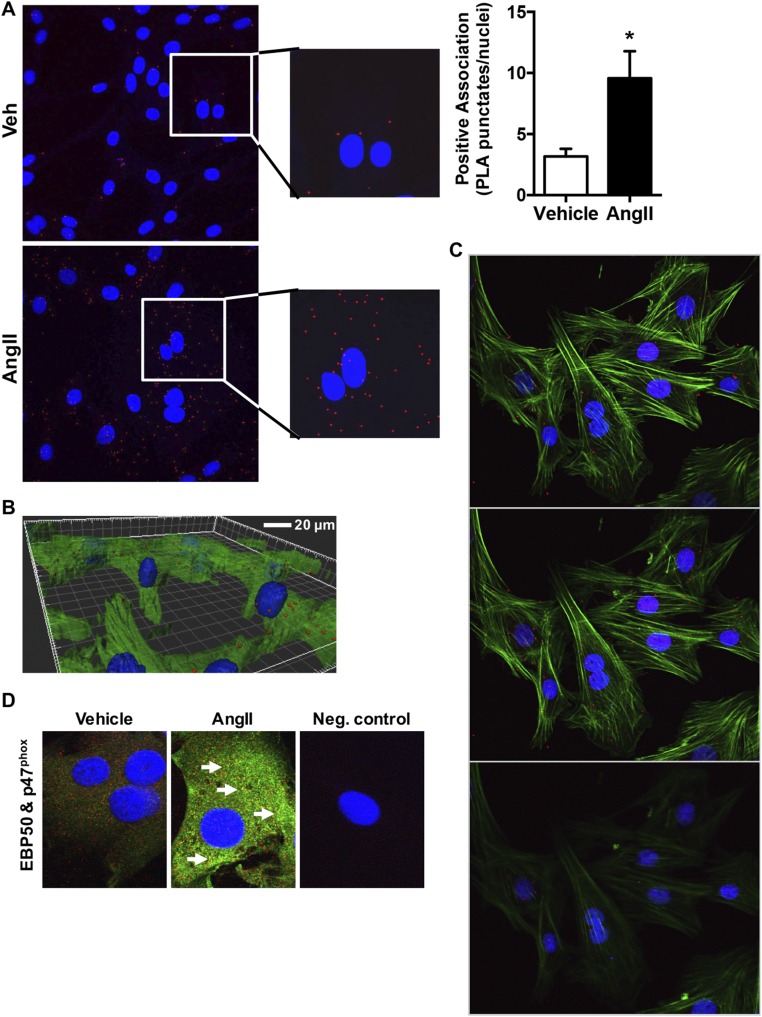
Because initial experiments were performed in overexpression systems, a question remained as to whether EBP50 and p47phox interacted under endogenous physiologic or pathophysiologic conditions. To test this, the proximity ligation assay (PLA), which detects tissue and cell protein–protein interaction with a resolution of 30–40 nm, was used (35). Positive proximity ligation is indicated by the appearance of punctates which localize interaction of the proteins. Fresh aortic rings isolated from WT and EBP50 KO mice were incubated ex vivo with AngII and subjected to PLA. Fig. 2 E and F demonstrate that AngII treatment resulted in an increase in PLA punctates in the aortic medial layer, indicating a direct association between EBP50 and p47phox in these tissues. These data were supported by PLA experiments in rASMC cells treated with vehicle or AngII, where AngII resulted in a significant increase in punctates indicative of EBP50–p47phox interaction in these cells (Fig. S6 A–C). Colocalization of endogenously expressed EBP50 and p47phox corroborated these results (Fig. S6D). The 3D projection and serial z-stack visualization of AngII-treated cells confirmed membrane-proximal EBP50/p47phox association and revealed cytosolic; perinuclear; and to a lesser extent, nuclear localization as well. Taken together, these data are consistent with a direct endogenous interaction between EBP50 and p47phox, supporting physiological relevance.
Fig. S6.
Proximity ligation assay (PLA) and confocal colocalization in VSMC treated with vehicle or AngII. (A) VSMC were treated with vehicle or 100 nM AngII and imaged for PLA by confocal using a 60× objective. Images were generated as maximum intensity composites (maximum intensity z projections) of z-stacks at 10 planes (optical slices). Red punctates, positive PLA; blue, nuclei (Hoechst). Insets show zoom view. Bar graph shows quantification of punctates normalized to number of nuclei. Data are means ± SEM, n = 3, *P < 0.05. (B) PLA was performed on VSMC as in A above with an additional staining for F-actin (Phalloidin; green) and a 3D reconstruction of the z-stacks of AngII-treated cells shown (representative of three experiments). (C) Representative optical slices (3 out of 10: planes z2–4) of a z-stack of AngII-treated VSMC imaged as in B. (D) Representative confocal fluorescent images of VSMC treated with vehicle or 100 nM AngII and stained with EBP50 (green) and p47phox (red) antibodies and Alexa Fluor 488- or Cy3-labeled secondary antibodies, respectively, followed by nuclei staining (blue; Hoechst). n = 3. Arrows indicate colocalization areas. Neg. control indicates imaging of VSMC stained only with secondary antibodies and Hoechst.
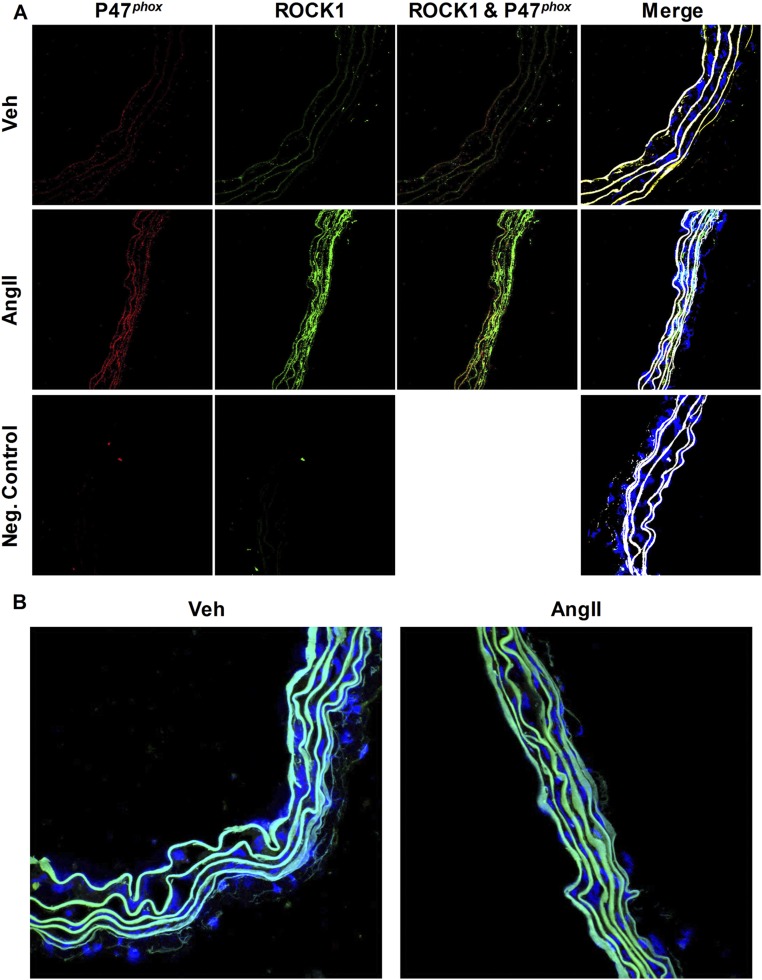
Rho kinase 1 (ROCK1) has been indirectly linked to EBP50 because it has been shown that ERM family proteins, which associate with EBP50, are substrates of ROCK1 (11, 36). Because p47phox possesses phosphorylation sites that regulate its activity, and given our present findings demonstrating p47phox association with EBP50, we tested whether ROCK1 kinase associates with p47phox in vascular tissues. Fresh aortic rings incubated with AngII were subjected to confocal colocalization as well as PLA. Fig. S7A demonstrates enhanced expression in response to AngII of ROCK1 and p47phox, in contrast to Western blot data in cells (Fig. S5C). No interaction between the two proteins was detected (Fig. S7 A and B).
Fig. S7.
ROCK1 does not associate with p47phox in aortic tissue. (A) Representative confocal images of cross-sections of aortic rings subjected to vehicle or AngII (100 nM) stained with ROCK1 antibody (green), p47phox antibody (red), and Hoechst (nuclei; blue). Autofluorescence of the internal and external elastic laminae is shown in white in the merged images. Neg. control indicates imaging of sections stained only with secondary antibodies. (B) Representative images of PLA experiments on WT mice aortic rings subjected to vehicle or AngII (100 nM) investigating association of ROCK1 to p47phox (positive PLA; red punctates). Blue, nuclei (Hoechst); autofluorescence of the internal and external elastic laminae is shown in blue/green. Representative of n = 3 animals. Images were obtained using a 40× objective.
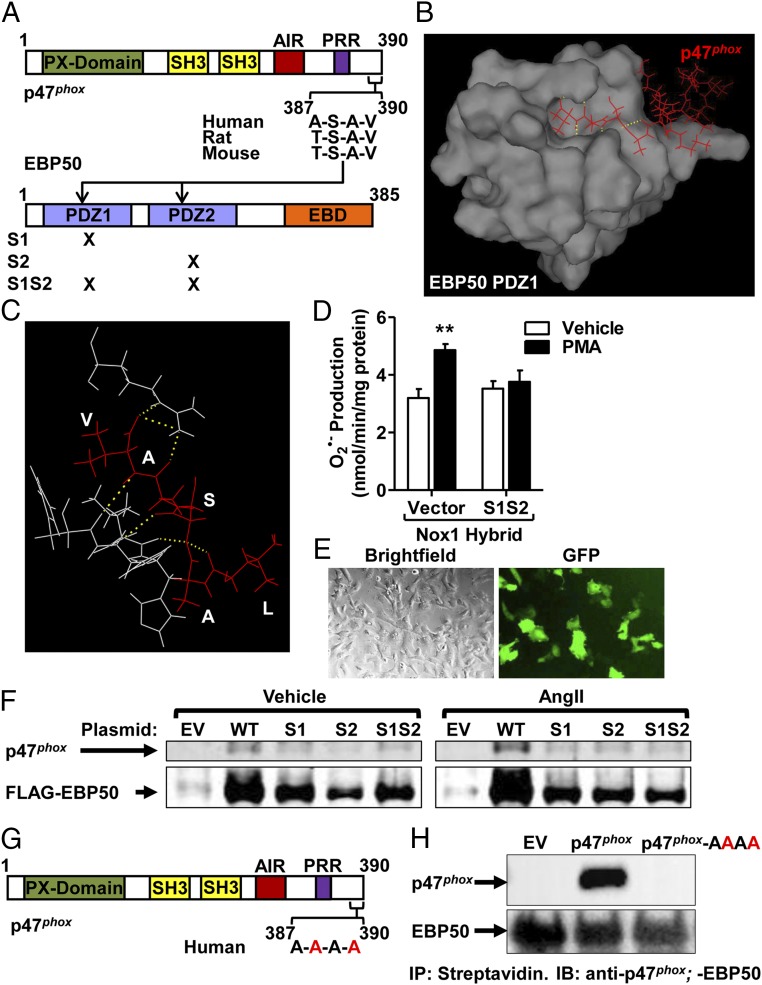
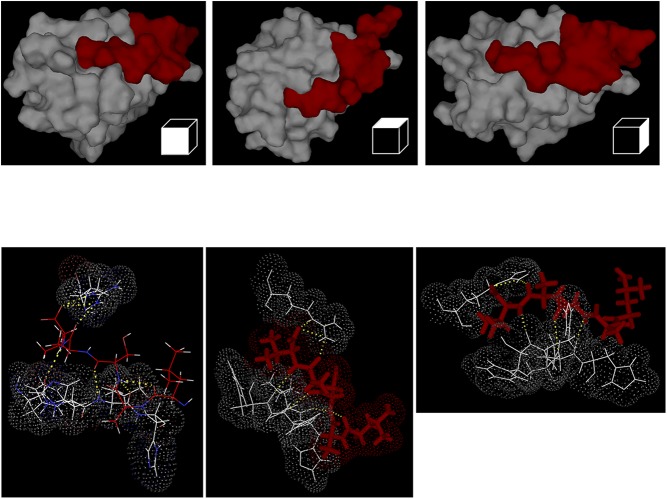
Because EBP50 reportedly associates with its binding partners via hallmark PDZ domains (PDZ1 and PDZ2) (10, 11, 17), we searched for potential PDZ-binding motifs in p47phox. Fig. 3A shows that a conserved PDZ-binding motif, A/T-S-A-V, exists on the C terminus of p47phox. This structure falls under the class I PDZ ligands (C-terminal motif with a hydrophobic amino acid in the C-terminal residue given position number 0, any residue in position −1, a Ser/Thr in position −2, and any residue in position −3) (37–39). To model a potential interaction between this motif in p47phox and a PDZ1 domain from EBP50, we used the NMR solution structure of the C-terminal tail of p47phox (PDB code: 1K4U) (40) and the PDZ1 domain of EBP50 (PDB code: 1GQ4) (41). The analysis revealed that docking of the C-terminal 4 residues motif of p47phox within the ligand pocket of PDZ1 of EBP50 is favorable (Fig. 3 B and C and Fig. S8). That is, the C-terminal Val at position 0 was predicted to be buried deep within a hydrophobic pocket of PDZ1 formed by the side chains of Gly23, Tyr24, Gly25, Phe26 (GYGF loop), Leu28, Val76, and Ile79 (42, 43). Another conserved interaction typical for the class I PDZ-binding motifs is observed between the −OH group of Ser at position −2 of p47phox and the imidazole group of His72 in EBP50 PDZ (42, 43). Moreover, the presence of two lysine residues at positions −5 and −7 within the C-terminal of p47phox, which possess positively charged side chains, could be attracted to the negatively charged chain of Glu31 of EBP50, further stabilizing the interaction.
Fig. 3.
The p47phox-EBP50 association occurs at the PDZ domain of EBP50. (A) Outline of EBP50 and p47phox sequence structures highlighting the PDZ domains of EBP50 and the potential PDZ-binding motif on p47phox. S1, S2, and S1S2 are functional mutants of PDZ1, PDZ2, and both. (B and C) In silico dynamic modeling and docking analysis using crystal PDZ1 structure of EBP50 (PDB code 1G9O) and p47phox C-terminal tail (aa 359–390; PDB code 1k4u) graphically represented using PyMol Software to show surface representation (B) and a magnified view of the interacting residues (C). Analysis reveals docking of C-terminal residues A-S-A-V of p47phox in binding pocket of EBP50 PDZ1 domain and formation of hydrogen bonds. Red, p47phox; white, EBP50; yellow dotted lines, hydrogen bonds. (D) Cytochrome c reduction assay on lysates of Cos22 cells transfected with Nox1 hybrid system plus empty vector or the EBP50 functional PDZ1 and 2 mutant (S1S2) and treated with vehicle or 5 μM PMA. PMA-induced O2•− production was abolished by expression of the functional EBP50 mutant. Rate of O2•− production was quantified in nmol/min/mg protein, and data are shown as means ± SEM, n = 3, **P < 0.01 vs. vehicle. (E) Representative brightfield and GFP fluorescence images of EBP50 KO VSMC demonstrating 30–40% transfection efficiency. (F) Immunoprecipitation with anti-FLAG antibody followed by Western blot with anti-p47phox or anti-FLAG antibodies on lysates from EBP50 KO VSMC transfected with empty vector (EV), FLAG-WT EBP50 (WT), FLAG-mutant PDZ1 EBP50 (S1), FLAG-mutant PDZ2 EBP50 (S2), or dual mutant PDZ1 and PDZ2 EBP50 (S1S2) and treated with vehicle or AngII (100 nM). AngII treatment increases p47phox association with EBP50 in KO cells in which WT EBP50 was reconstituted but not in cells expressing the functional mutants of EBP50. Representative experiment. (G) Outline of p47phox sequence structures highlighting the mutation (in red) of C-terminal PDZ-binding motif on p47phox. (H) Immunoprecipitation with streptavidin followed by Western blot with anti-p47phox or anti-EBP50 antibodies on lysates from Hek293 GnTi cells stability expressing TAP-EBP50 and transfected with empty vector (EV), WT p47phox (p47phox) or p47phox-AAAA mutant. Interruption of the ASAV C-terminal motif of p47phox disrupts its association to EBP50. Representative experiment of n = 3.
Fig. S8.
Representative in silico dynamic modeling and docking analysis as in Fig. 3. Images with different views of docking and zoom in representations using crystal PDZ1 structure of EBP50 (PDB code 1G9O) and p47phox C-terminal tail (aa 359–390; PDB code 1k4u) graphically represented using PyMol Software.
To verify empirically whether the interaction between EBP50 and p47phox involves PDZ domains, we used mutant EBP50 plasmids lacking active PDZ1, PDZ2, or both (Fig. 3A). First, we tested whether PDZ interruption is associated with a reduction in ROS production. Cos7 cells were transfected with the Nox1 hybrid system in the presence or absence of the dual PDZ mutant S1S2, treated with PMA and O2•– production assessed by SOD-inhibitable cytochrome c reduction. Fig. 3D shows that PMA resulted in an increase in O2•– production that was completely absent in cells transfected with the PDZ1/2 EBP50 mutant. Next, the EBP50 mutant constructs were transfected into EBP50 KO VSMC and contrasted for p47phox immunoprecipitation with WT constructs, following vehicle or AngII treatment. Transfection of GFP plasmids into these cells demonstrated an approximately 30–40% transfection efficiency as assessed by fluorescence microscopy (Fig. 3E). Recapitulation of WT EBP50 into EBP50 KO VSMC resulted in sedimentation of p47phox as seen by coimmunoprecipitation experiments (Fig. 3F). This signal was increased when cells were subjected to AngII treatment. Sedimentation of p47phox was markedly reduced in KO cells in which the PDZ1, PDZ2, or both mutants were transfected, both under vehicle and AngII treatment conditions. Thus, these data are consistent with a functional association of EBP50 with p47phox at the PDZ domains of EBP50 that is essential for hybrid Nox1 activity.
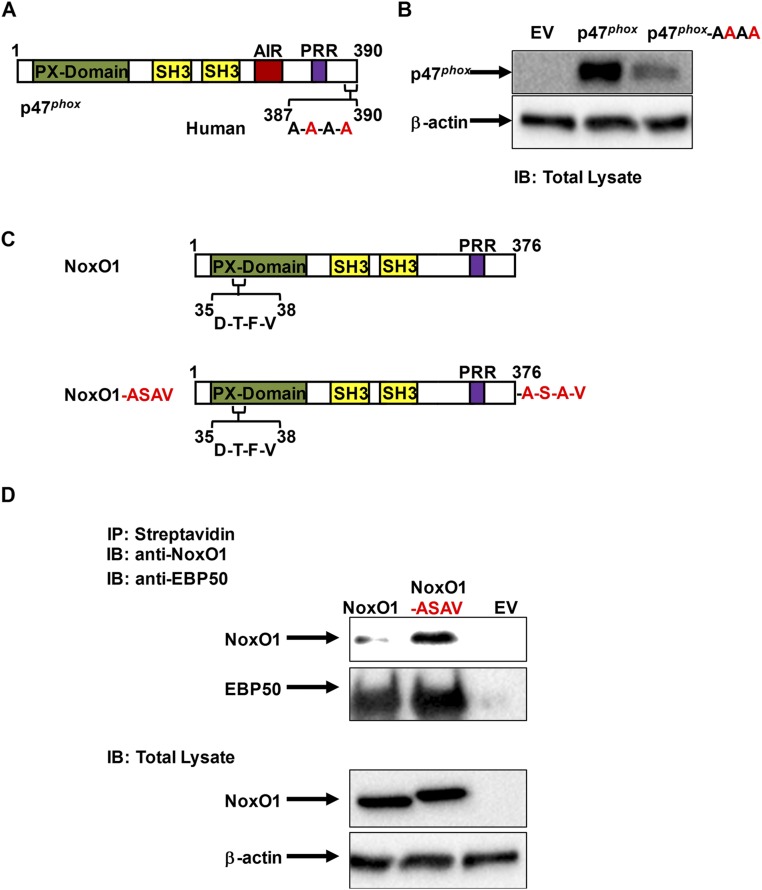
We next tested whether the C-terminal A-S-A-V motif of human p47phox is important for association with EBP50. We generated a mutant p47phox in which serine 388 and valine 390 were mutated to alanine (p47phox-AAAA; Fig. 3G). This mutant protein was contrasted to WT p47phox in a heterologous system in which EBP50 was also expressed. Fig. 3H demonstrates that disruption of the A-S-A-V motif on p47phox abolishes coimmunoprecipitation with EBP50. We observed a somewhat lower protein expression of p47phox-AAAA compared with WT p47phox, which could be indicative of the importance of this motif, and/or its association with EBP50, in posttranslational stability of p47phox (Fig. S9 A and B).
Fig. S9.
Recapitulation of p47phox C-terminal A-S-A-V motif into NoxO1 enhances association to EBP50. (A) Outline of p47phox sequence structures highlighting the mutation of C-terminal PDZ-binding motif on p47phox. (B) Representative Western blot of total lysates from HEK293 cells stably expressing EBP50 and transfected with empty vector (EV), WT p47phox (p47phox), or p47phox-AAAA mutant probed with antibodies for p47phox and β-actin as a loading control. (C) Outline of NoxO1 sequence structures highlighting the putative PX-domain PDZ motif (native NoxO1) as well as the insertion mutation of C-terminal PDZ-binding motif of p47phox (A-S-A-V) to the C-terminal of NoxO1 (NoxO1-ASAV). (D) Immunoprecipitation with streptavidin followed by Western blot with anti-NoxO1 or anti-EBP50 antibodies on lysates from HEK293 cells stably expressing EBP50 and transfected with empty vector (EV), WT NoxO1 (NoxO1), or NoxO1-ASAV insertion mutant. Lower panel shows an immunoblot of the total cell lysates from this experiment probed with anti-NoxO1 and β-actin. Representative of three independent experiments.
NoxO1 in its native form lacks the A-S-A-V motif. To test whether this motif is sufficient for EBP50 interaction, we generated a NoxO1 mutant wherein the C-terminal A-S-A-V motif was appended (NoxO1-ASAV; Fig. S9C). WT NoxO1 displayed a weak association with EBP50 (Fig. S9D), likely owing to a rare and unusual potential PDZ-binding motif embedded deep within its sequence and close to the N terminus (D-T-F-V; Fig. S9C). In contrast, association with EBP50 was substantially augmented in cells expressing NoxO1-ASAV (Fig. S9D). Collectively, these data demonstrate that the A-S-A-V motif in the C-terminal of p47phox is critical and sufficient for EBP50 binding.
EBP50 Is Essential for VSMC Hypertrophy.
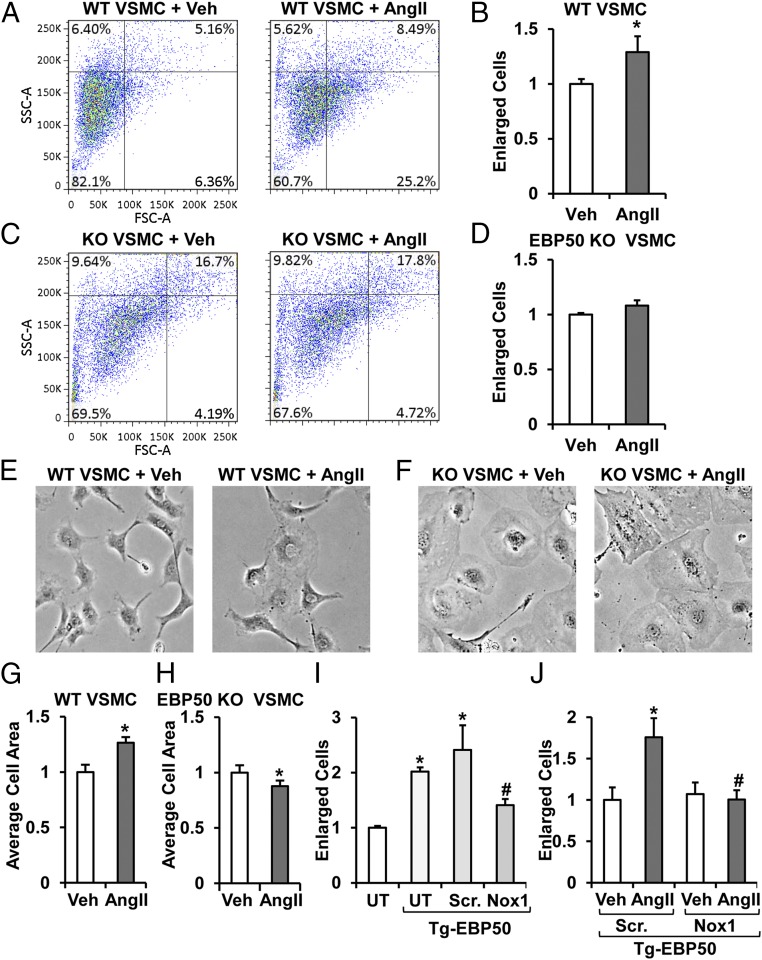
To assess whether the EBP50-modulated Nox1 leads to a change in cell phenotype, AngII-induced VSMC hypertrophy in EBP50 KO vs. WT VSMC was measured. Cells were treated with 100 nM AngII for 24 h and evaluated by FACS for the change in percentage of enlarged cells vs. vehicle controls. Treatment of WT VSMC with AngII resulted in a 30% increase in the number of enlarged cells (Fig. 4 A and B). In contrast, Fig. 4 C and D show that EBP50 KO VSMC displayed no difference in enlarged cells (vehicle vs. AngII). To confirm these findings, WT and EBP50 KO cells were treated with AngII and imaged 24 h posttreatment followed by quantification of individual cell sizes. Consistent with Fig. 4A, treatment of WT VSMC with AngII also resulted in a similar increase in cell size (Fig. 4 E and G). Moreover, AngII treatment of EBP50 KO VSMC resulted in no increase in cell size (Fig. 4 F and H).
Fig. 4.
AngII-induced VSMC hypertrophy is abolished in EBP50 KO cells. Representative experiment (A and C) and summary data (B and D) of FACS analysis of percent enlarged cells of total (upper right quadrant) of WT (A and B) and EBP50 KO (C and D) VSMC treated with vehicle or 100 nM AngII expressed as fold from vehicle. Data shown as means ± SEM, n = 5–6, *P < 0.05 vs. vehicle. Representative cell images (E and F) and averaged cell size quantification expressed as fold from vehicle (G and H) of WT (E and G) and EBP50 KO (F and H) VSMC treated with vehicle or 100 nM AngII. Data are shown as means ± SEM, n = 3 (area of 36 cells were measured per condition from three independent experiments), *P < 0.05 vs. vehicle. (I) FACS analysis of percent enlarged cells of total expressed as fold from untransfected control group (UT; no siRNA or Tg-EBP50) of rASMC transfected with WT EBP50 alone (UT, Tg-EBP50), WT EBP50 plus scrambled siRNA (Scr., Tg-EBP50), or WT EBP50 plus Nox1 siRNA (Nox1, Tg-EBP50). Overexpression of EBP50 significantly increased cell size in a Nox1-dependent mechanism. Data are shown as means ± SEM, n = 3. *P < 0.05 vs. UT. #P < 0.05 vs. Scr. + EBP50. (J) FACS analysis of percent enlarged cells of total expressed as fold from scrambled siRNA vehicle-treated (Veh, Scr., Tg-EBP50) of rASMC transfected with WT EBP50 plus scrambled (Scr.) or Nox1 siRNA and treated with vehicle or 100 nM AngII. Exacerbated AngII-induced hypertrophy in EBP50 overexpressing cells was attenuated by cotransfection with Nox1 siRNA. Data are shown as means ± SEM, n = 3. *P < 0.05 vs. Scr. + EBP50 + vehicle. #P < 0.05 vs. Scr. + EBP50 + AngII.
To ascertain a causal link between EBP50 and Nox1, EBP50 was overexpressed (Tg-EBP50) in the presence or absence of Nox1 siRNA vs. scrambled control (8). Fig. 4I illustrates by FACS an approximately 100% increase in the percentage of enlarged Tg-EBP50 cells vs. untransfected controls. A similar increase was observed in Tg-EBP50, scrambled siRNA-treated cells. On the contrary, hypertrophy was significantly attenuated by cotransfection with Nox1 suppression (Fig. 4I). This influence of EBP50 and Nox1 was extended to modulation of AngII-induced VSMC hypertrophy. That is, FACS analysis shows ablation of the AngII response in Tg-EBP50 cells treated with Nox1 siRNA (Fig. 4J). These data support that EBP50, via modulation of Nox1 activity, regulates VSMC hypertrophy and has thus important implications in vascular disease.
EBP50 Is Essential for Vascular Tone Regulation of Resistance Arteries.
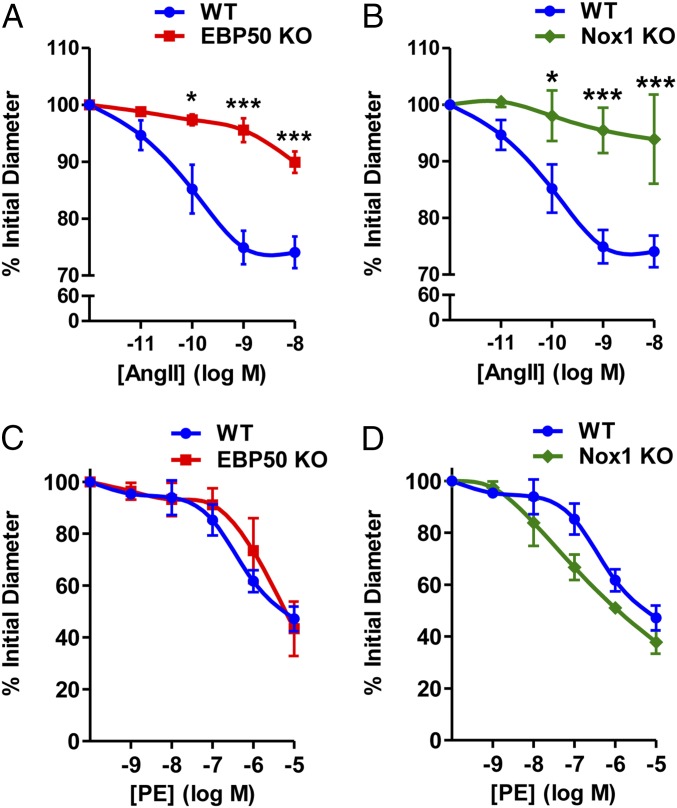
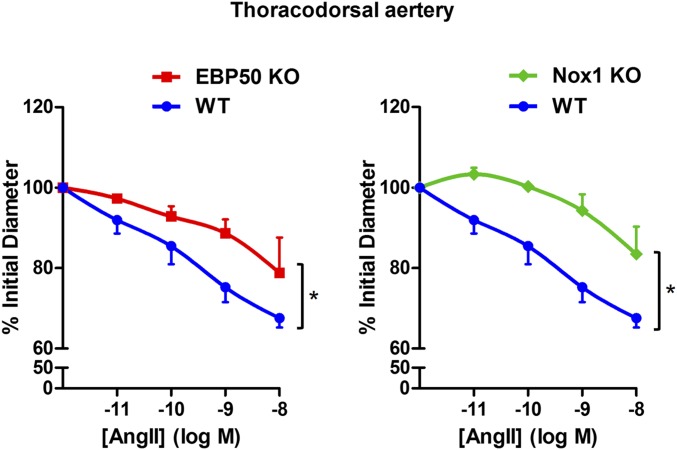
To further extend our functional findings, we explored whether EBP50 modulates resistance vessel tone. In third-order isolated, perfused mesenteric arteries, concentration-dependent vasoconstriction in response to AngII was significantly attenuated in EBP50 KO arteries vs. WT (Fig. 5A). A similarly attenuated response was observed in resistance vessels from Nox1 KO mice (Fig. 5B). These findings were supported by data in thoracodorsal arteries (Fig. S10). The response was agonist specific because vessels from both Nox1 and EBP50 KO mice exhibited no attenuation in phenylephrine (PE)-mediated vasoconstriction relative to WT (Fig. 5 C and D).
Fig. 5.
EBP50 mediates resistance vessel tone. Pressure myography on isolated perfused third-order mesenteric arteries from WT and EBP50 KO (A and C) and WT and Nox1 KO (B and D) mice treated with the indicated concentrations of AngII (A and B) or phenylephrine (PE; C and D) and assessed for vasoconstriction represented as percent of initial diameter. Data shown are means ± SEM, n = 3–5. *P < 0.05 vs. WT, ***P < 0.001 vs. WT.
Fig. S10.
EBP50 mediates thoracodorsal artery tone. Pressure myography on isolated perfused thoracodorsal arteries from WT, EBP50 KO, and Nox1 KO mice treated with the indicated concentrations of AngII and assessed for vasoconstriction represented as percent of initial diameter. Data shown are means ± SEM, n = 3–5. *P < 0.05 vs. WT.
Discussion
It is well established that Nox-derived ROS are critical components of myriad cellular pathways and drive vital responses such as host defense and inflammation. Nox-derived ROS, on the other hand, are established malefactors in aging, cancer, and neurodegenerative and cardiovascular diseases (2, 9). Despite the seminal importance of the Nox family of oxidases, little progress has been made over the past decade to delineate the mechanisms of Nox activation and regulation. This is particularly true of the Nox1 oxidase, of which two basic systems have been described (canonical vs. hybrid) and which was shown to be a central player in cardiovascular diseases (8, 9, 44, 45). In the current study we identify the scaffolding protein EBP50 as a previously unidentified binding partner and regulator of the Nox1 oxidase. We demonstrate the importance of EBP50 for ROS production in vascular cells and tissues in vitro, ex vivo, and in vivo under different stimuli and using a number of techniques. With a focus on the hybrid Nox1 system, which drives pathophysiological responses in disease, we demonstrate that EBP50, via its PDZ domains, associates with the Nox organizer subunit p47phox at a unique PDZ-binding C-terminal motif. This interaction, we posit, is the means by which EBP50 regulates Nox1 activity. To investigate whether our findings carry relevance to pathophysiologic phenomena, we demonstrate, in a Nox1-associated manner, EBP50’s involvement in two responses concomitant with cardiovascular disease: smooth muscle hypertrophy and resistance vessel constriction.
Initial experiments demonstrated a robust role of EBP50 in ROS production. VSMC deficient in EBP50 were incapable of producing ROS in response to AngII and H2O2, both potent stimuli of O2•– production (8, 20, 22–24, 46, 47). Ex vivo EPR studies on aortic rings also showed absence of an O2•– signal in response to AngII in EBP50 KO, correlating with findings in Nox1 KO. Moreover, in vivo data demonstrated that in response to another robust inducer of Nox-derived ROS, LPS (27–33), vascular medial smooth muscle oxidation was absent in EBP50 KO mice. Because Nox1 is a major source of O2•– in the vasculature, especially in VSMC (2, 8), we focused our attention on this Nox isoform. A unique hybrid Nox1 system exists in VSMC, which depends on p47phox as its organizer subunit in lieu of NoxO1 (6–9). Prior reports drew our attention to a possible molecular interaction between EBP50 and p47phox. First, EBP50’s interaction with actin cytoskeletal elements (11, 48, 49) piqued our interest in that p47phox is known to bind actin as it translocates to and activates the Nox catalytic core (2, 50–53). Moreover, the modulatory role of protein kinase C (PKC) at key sites on both molecules suggested a possible common pathway (2, 9, 54). Indeed, recapitulating the hybrid Nox1 oxidase in a heterologous Cos7 system in concert with an overexpression of EBP50 significantly augmented PMA-stimulated O2•– generation compared with cells basally expressing EBP50. This observation was unique to the Nox1 hybrid system because EBP50 did not enhance ROS generation by either the canonical Nox1 system or the canonical Nox2 system, which also employs p47phox. This supports a unique role for EBP50 in regulating the hybrid Nox1 system, and it is tempting to speculate whether this occurs as a result of heretofore unknown binding partners of the EBP50–p47phox complex and/or a modification of p47phox mediated by EBP50 that channels it to Nox1 instead of Nox2.
Direct association between EBP50 and p47phox was demonstrated via four distinct lines of evidence. Confocal imaging experiments in VSMC confirmed that EBP50 colocalizes with p47phox and indicated strong membrane localization of both proteins after AngII treatment in VSMC. Coimmunoprecipitation experiments in lysates of Cos7 cells expressing both proteins further supported a positive association that is stronger after PMA stimulation, consistent with disinhibition of p47phox enhancing binding (2, 9). FRET experiments in Cos7 cells corroborate a direct association between EBP50 and p47phox. Finally, PLA experiments demonstrated this binding occurs at the endogenous protein level in vascular tissues and cells.
ROCK1 has been implicated to mediate association of ERM family proteins to EBP50 by directly phosphorylating them (11). This raised the possibility that ROCK1 may interact with p47phox to play a role in facilitating the latter’s association with EBP50 and, henceforth, cytoskeletal elements. Our data, however, revealed that ROCK1 does not colocalize or associate with p47phox. Nonetheless, ROCK may still have a yet-to-be-determined effect on components of the hybrid Nox1 system affecting their trafficking, activation, or both.
Given the protein structure of EBP50, we surmised that the interaction between EBP50 and p47phox could take place on EBP50’s PDZ domains. Sequence analysis of the C terminus of p47phox identified a conserved potential PDZ-binding motif (A-S-A-V) and in silico docking analyses revealed that this domain can favorably dock within the hydrophobic pocket of PDZ1 domain in EBP50. Coimmunoprecipitation studies recapitulating wild-type EBP50 or functional PDZ domain mutants into EBP50 KO VSMC confirmed that EBP50’s PDZ domains are critical for this interaction. Moreover, ROS detection assays showed that the PDZ domains are essential for hybrid Nox1-derived O2•– production. To further investigate whether the interaction involves the A-S-A-V motif in human p47phox, gain-of-function/loss-of-function experiments manipulating this C-terminal A-S-A-V motif were performed. Interruption of this domain in human p47phox abolished EBP50 association and addition of this domain to the C-terminal of NoxO1 substantially augmented EBP50 association. These data demonstrated that the A-S-A-V motif is both critical and sufficient for EBP50 association of p47phox.
Functional significance of this interaction was deduced from smooth muscle hypertrophy and resistance vessel vasoconstriction experiments. Medial hypertrophy is causally associated with hypertension and heart failure, and Nox-derived ROS are key players in this process (55–58). In contrast to WT cells, EBP50 KO VSMC did not exhibit a hypertrophic response following treatment with AngII, a potent activator of Nox1 and inducer of VSMC hypertrophy (8, 20, 22–24, 44, 45). Overexpression of EBP50 augmented AngII-induced hypertrophy in a Nox1-dependent manner. These data demonstrate that both EBP50 and Nox1 propagate vascular hypertrophy and support a mechanism by which EBP50 regulates Nox activity leading to cellular phenotypic responses in disease.
Because vascular diseases involve both vascular tone dysfunction as well as remodeling, we investigated whether EBP50 is involved in mediating agonist-induced resistance vessel constriction. In both third-order mesenteric arteries and thoracodorsal arteries we observed a significant attenuation in AngII-induced constriction in EBP50 KO vessels. This response was virtually superimposable with the response in Nox1 KO vessels, indicative of a collaboration of the two proteins in mediating this response. The vasoconstriction response was agonist-specific because vessels from Nox1 or EBP50 KO mice exhibited a response akin to WT in response to phenylephrine. These data support that AngII and phenylephrine contractile responses diverge at the level of EBP50 and Nox1, supporting a specific ligand-receptor targeted response.
In summary, the current findings point to a mechanism of Nox regulation whereby EBP50 binds p47phox and activates Nox1-derived ROS. The data support that through this binding, EBP50 promotes p47phox recruitment to Nox1, bringing about oxidase assembly and activation. This interaction has functional consequences, and our results support its necessity for both medial smooth muscle hypertrophy and resistance vessel constriction. Targeting this interaction, therefore, presents a viable future therapeutic strategy to combat cardiovascular diseases. Moreover, this previously unidentified interaction has profound implications in myriad other diseases owing to the ubiquitous tissue expression of EBP50, Nox1, and p47phox.
Materials and Methods
Please consult SI Materials and Methods for detailed methods.
Materials.
A complete list of materials is provided in SI Materials and Methods.
Cell Culture.
WT and EBP50 KO VSMC were isolated from abdominal aortas of 8- to 10-wk-old male C57BL/6 WT and KO littermates as previously described (15, 17). Rat aortic smooth muscle cells (rASMC) were purchased from Lonza Cologne GmbH. Cos7 cells stably expressing the p22phox subunit (Cos22) were a kind gift from Mary C. Dinauer (Washington University School of Medicine, St. Louis, MO). Hek293 GnTi, which lack endogenous EBP50, stably expressing a TAP-domain–containing EBP50 were a kind gift from Peter Friedman (University of Pittsburgh, Pittsburgh, PA). Lentiviral shRNA expression was performed as previously described (17).
O2•– Detection by L-012 Chemiluminescence.
L-012 (400 µM) chemiluminescence was performed as previously described (8). O2•– production was quantified as relative light units (RLU) area under the curve vs. time.
O2•– Detection by Cytochrome c Reduction Assay.
Cytochrome c reduction assay was conducted as previously described (8). O2•– was measured in the presence of 0.2 mM cytochrome c and 1,000 U/mL catalase. The initial rate following NADPH (180 μM) addition of SOD-inhibitable cytochrome c reduction was quantified at 550 nm, and the extinction coefficient 21.1 mmol− 1•L cm− 1 was used to calculate O2•–. Because this assay measures O2•– in broken cells poststimulation and in the presence of the Nox substrate NADPH, it encompasses Nox activity that is due both to assembly of active complexes as well as to removal of repression on assembled inactive complexes.
O2•– Detection by Electron Paramagnetic Resonance Spectroscopy.
The EPR spin probe CMH (500 μM) was used to examine O2•– production by EPR as previously described (8, 59). Briefly, 2 mm thoracic aorta rings freshly isolated from WT, EBP50 KO, and Nox1 KO mice were treated with vehicle or AngII (100 nM, 4 h) in the presence or absence of SOD (200 U/mL). Data were quantified using the amplitude of the first spectral peak as fold from vehicle control after background subtraction.
Plasmid Transfection.
Plasmids encoding full-length human cDNAs for Nox1 (pcDNA3.1-hNox1), NoxA1 (pCMVsport 6-hNoxA1), and p47phox (pCMV-Tag4A-hp47) were kindly provided by David Lambeth (Emory University, Atlanta, GA). Plasmids encoding N-terminal FLAG-human WT EBP50, PDZ1 (S1), PDZ2 (S2), and PDZ1&2 (S1S2) functional mutants, as well as YFP-human WT EBP50 were a kind gift from Peter Friedman (University of Pittsburgh, Pittsburgh PA). Plasmids encoding CFP-p47phox were purchased from OriGene Technologies, Inc. Site-directed mutagenesis was performed to generate the p47phox serine 388 to alanine and valine 390 to alanine double mutant (p47phox-AAAA). NoxO1–ASAV fusion was generated by fusing the alanine–serine–alanine–valine C-terminal motif of p47phox onto the C-terminal end of NoxO1. Cos22 cells were simultaneously transfected with empty vector or plasmids for Nox1, NoxA1, and p47phox; Nox1, NoxA1, p47phox, and EBP50 or EBP50 S1S2 mutant; Nox1, NoxA1, and NoxO1; or Nox1, NoxA1, NoxO1, and EBP50. CosPhox cells were transfected with empty vector or EBP50. For FRET, Cos22 cells were transfected with YFP-EBP50, CFP-p47phox, or YFP-EBP50 and CFP-p47phox. rASMC were transfected with YFP-EBP50. For immunoprecipitation, Cos7 cells stably expressing p47phox were transfected with FLAG-EBP50, and EBP50 KO VSMC were transfected with GFP control or FLAG-WT, FLAG-S1, FLAG-S2, or FLAG-S1S2 EBP50. GnTi Hek cells stably expressing TAP-EBP50 were transfected with p47phox, p47phox-AAAA, NoxO1, or NoxO1-ASAV.
siRNA Transfection to Suppress Nox1 in rASMC.
Cells were cotransfected with EBP50 plasmid and scrambled or Nox1 siRNA (5 pmol) using Lipofectamine 2000.
Confocal 4-HNE in Vivo Immunofluorescence.
C57BL/6 WT or EBP50 KO mice were injected with 10 mg⋅kg−1 i.p. LPS for 16 h. Femoral arteries were harvested, fixed, and sectioned. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC). Sections were incubated with anti-4-HNE antibody (Alpha Diagnostics) and Cy3-conjugated secondary antibody (Jackson ImmunoResearch) in combination with Alexa Fluor 647-conjugated phalloidin (Invitrogen). Nuclei were stained with Hoeschst dye. A total of three to four animals were used per treatment group. For each animal, three confocal images from three separate sections were captured.
Confocal in Vitro Colocalization.
rASMC transfected with YFP-EBP50 on coverslips were treated with 100 nM AngII for 4 h, fixed, and permeabilized. After blocking and incubation with anti-p47phox antibody and Cy3-conjugated secondary antibody, coverslips were stained with Alexa Fluor 647-phalloidin. For each experiment, five to eight confocal images per treatment group were captured. Three independent experiments were performed. No spectral overlap occurred between the different fluorescence emission channels. For endogenous protein experiments, EBP50 was assessed by an EBP50 antibody followed by Alexa Fluor 488-conjugated secondary antibody. For tissue, sections were mounted on slides, fixed, permeabilized, and blocked followed with primary antibodies for EBP50 and p47phox or ROCK1 and p47phox followed by secondary antibodies. Hoechst was added, and coverslips were applied.
Fluorescence Resonance Energy Transfer.
Cos7 cells seeded on coverslips were transfected separately or simultaneously with CFP-p47phox and YFP-EBP50, treated with PMA, and fixed. FRET experiments were performed with confocal spectral imaging using a Nikon A1 system (Nikon Instruments Inc.) as previously described with modifications (60). Emission spectra for YFP and CFP were obtained separately using spectral scanning and the excitation wavelengths of 514 or 457 nm, respectively, and used for spectral unmixing. Emission spectra on cells cotransfected with both proteins and excited with 457 nm (CFP) were unmixed. Acceptor (YFP) photobleaching was performed at 514 nm, 50% laser power, and four cycles of 5 s each. Fluorescence intensities were calculated as fold from prebleach. FRET efficiency was calculated as
Proximity Ligation Assay.
The proximity ligation assay (PLA) was performed according to the manufacturer’s protocol (Sigma). Primary antibodies for EBP50 and p47phox or p47phox and ROCK1 were used. Confocal imaging was performed by capturing z-stacks in 10 planes for each sample and generation of maximum intensity composite z projections. Data were quantified as ratio of PLA punctates to number of nuclei per sample. In a subset of experiments, phalloidin was added as above. For 3D reconstruction, stacks were processed using the surface and dots features for the cell area and PLA punctates, respectively.
Immunoprecipitation and Western Blot.
Cells were transfected as above. Cos7 cells were treated with vehicle or PMA (20 μM). EBP50 KO VSMC were treated with vehicle or AngII (100 nM). One hundred micrograms of cell lysates were incubated with anti-FLAG M2 resin mouse antibody overnight at 4 °C. For immunoprecipitation using Hek GnTi, cells were transfected as detailed above and lysed, and supernatants were incubated with streptavidin beads to pull down TAP-EBP50. Samples were prepared for Western blot, resolved using SDS–PAGE, and transferred onto Trans Blot nitrocellulose membranes (Bio-Rad). Membranes were blocked with the Odyssey Blocking Buffer (Li-Cor Biosciences) and incubated with primary antibodies followed by secondary antibodies (Li-Cor). Digital imaging was obtained using an Odyssey Infra-Red Imaging system (Li-Cor).
FACS Analysis for Quantification of Hypertrophy.
Flow cytometry evaluation of cell hypertrophy was performed as previously described (8). Briefly, cells grown to 80% confluence and treated with vehicle or 100 nM AngII for 24 h at 37 °C were resuspended in PBS (37 °C; 1 × 106 cells per mL). Forward scatter (FSC) and side scatter (SSC) were measured recording 20,000 events per sample using a BD LSR Fortessa (BD Biosciences). Quantification was performed using FloJo (Tree Star Inc.). Density plots were gated to exclude debris and quadrant parameters were selected and assigned across all measurements based on the size of 80–85% of cells from control groups. The percentage of individual cells in quadrant 2 (FSC+, SSC+) were quantified for each group and taken as a ratio over quadrant 2 cell percentage in control groups.
Cell Size Measurement for Quantification of Hypertrophy.
These experiments were carried out as previously described (8). Briefly, three to six brightfield cell images were captured (10× objective) per treatment group per experiment. The areas of four individual cells selected at random per image were quantified using Image J (NIH) and calculated as a fold from control.
Docking Analysis.
In silico association analysis was performed using published crystal structures of relevant regions of p47phox and EBP50: C-terminal tail of p47phox (aa 359–390) complexed to C-terminal SH3 domain of p67phox (PDB code: 1k4u) (40), and EBP50 PDZ1 in complex with C-terminal fragment of β2– adrenergic receptor (AR; PDB code: 1GQ4) (41), respectively. To model the C-terminal motif of p47phox (LASAV sequence), the NDSLL sequence of C-terminal fragment of β2–AR was modified as follows: the C-terminal Leu0 was replaced by Val, Leu−1 by Ala, Asp−3 by Ala, and Asn−4 by Leu. The generated structure includes the five residues immediately flanking the C terminus of p47phox (386–390 aa) and EBP50 PDZ1 residues Leu11–Glu94. Visual modeling of predicted interaction was generated using PyMOL.
Pressure Myography.
Third-order mesenteric arteries were freshly isolated from WT, EBP50 KO, and Nox1 KO mice, cannulated, pressurized (75 mm Hg), and contractile responses studied using cumulative concentrations of AngII (10−11 M to 10−7 M) or phenylephrine (PE; 10−9 M to 10−4 M). Acetylcholine (10−5 M) was used to verify endothelial response, and potassium chloride (40 mM) to verify vessel contraction. Maximum diameter was measured by incubating vessels in a Krebs calcium-free, ethylene glycol tetraacetic acid (EGTA, 1 mM) and sodium nitroprusside (SNP 10 μM) solution. Vessel diameter was quantified using DMT vessel acquisition software and data expressed as percent of initial diameter.
Statistical Analyses.
Data are presented as means ± SEM. Data comparisons were performed with a Student’s t test or a one- or two-way ANOVA followed by the Bonferroni post hoc analysis. Differences were deemed statistically significant at a P < 0.05.
SI Materials and Methods
Materials.
Hydrogen peroxide (H2O2), cytochrome c, collagenase, elastase, angiotensin II (AngII), phorbol ester (PMA), polyvinyl alcohol, glycerol, sodium azide, catalase, and superoxide dismutase (SOD) were purchased from Sigma (Sigma-Aldrich). L-012 was purchased from Wako Chemicals (Wako Chemicals USA Inc.). The 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH), diethyl-dithiocarbamate, and deferoxamine were purchased from Noxygen Science Transfer. Trypsin and DMEM were purchased from Mediatech (Mediatech Inc.). Optimem, Lipofectamine 2000, Lipofectamine LTX, Nox1 and scrambled Stealth siRNA, and FBS were purchased from Invitrogen. Rabbit anti-p47phox and anti-Nox1 were purchased from Santa Cruz Biotechnology. Rabbit anti-NoxO1 antibody was purchased from Rockland Immunochemicals Inc. Rabbit anti-EBP50 antibody was purchased from Thermo Fisher Scientific. Rabbit anti-ROCK1 was purchased from Cell Signaling Technology. Anti-FLAG M2 resin and rabbit anti-FLAG antibodies were purchased from Sigma. The Duolink In Situ PLA assay was purchased from Sigma.
Cell Culture.
WT and EBP50 KO VSMC were isolated from thoracic aortas of 8- to 10-wk-old male C57BL/6 WT and KO littermates as described previously (15, 17). All animal studies have been approved by the University of Pittsburgh IACUC. rASMC were purchased from Lonza Cologne GmbH. Cos7 cells stably expressing the p22phox subunit (Cos22) or the canonical Nox2 system (CosPhox; expressive Nox2, p22phox, p47phox, and p67phox), were a kind gift from Mary C. Dinauer (Washington University School of Medicine, St. Louis, MO). Hek293 GnTi, which lack endogenous EBP50, stably expressing a TAP-domain–containing EBP50 were also used (a kind gift from Peter Friedman, University of Pittsburgh, Pittsburgh, PA). Cells were maintained in DMEM supplemented with 10% (vol/vol) FBS and containing 100 units per mL penicillin and 100 µg⋅mL−1 streptomycin (Invitrogen). Cells were cultured at 37 °C with 5% CO2. VSMC between passages 2 and 15 were used. Lentiviral shRNA expression was performed as previously described (17). Briefly, lentiviral shRNA vectors specific to mouse EBP50 and GFP expression control lentivirus were prepared by the Lentiviral Facility at the University of Pittsburgh Cancer Institute. Aliquots of virus were used to infect exponentially growing cells. Cells were used 72 h after infection.
O2•– Detection by L-012 Chemiluminescence.
L-012 chemiluminescence was performed as previously described (8). In experiments involving VSMC, cells were grown to 90% confluence on white clear-bottom 96-well culture plates and serum starved for 18–24 h. Cells were stimulated with 50 µM H2O2 for 1 h. The media was replaced with PBS (pH 7.4) supplemented with CaCl2 (0.9 mM) and MgSO4 (0.49 mM), and luminol derivative L-012 (400 µM) was added followed by chemiluminescence detection in a BioTek Synergy 4 Hybrid Multi-Mode Microplate Reader (BioTek) for at least 30 min poststimulation. In a subset of experiments, SOD (300 U⋅mL−1) was added immediately before L-012 additions. In experiments performed on Cos22 cells, cells were transfected as detailed below. Cells were then resuspended in Optimem media, and cell suspensions of 50,000 cells per well were added to 96-well plates followed by 400 µM L-012 addition and chemiluminescence measurement. After 4 min of baseline measurement, 5 µM PMA was added, and measurement resumed for 1.5 h. O2•– production was quantified as relative light units (RLU) area under the curve vs. time.
O2•– Detection by Cytochrome C Reduction Assay.
Cytochrome c reduction assay was conducted as described previously (8). Briefly, cells were grown to 90% confluence, serum starved, and treated with 10–1,000 nM AngII for 5 min to 4 h, 50 μmol⋅L−1 H2O2 for 1 h, or 5 µM PMA for 1 h. Cells were washed twice with ice cold PBS and 400 μL of ice cold disruption buffer (8 mM potassium, sodium phosphate buffer, pH 7.0, 131 mM NaCl, 340 mM sucrose, 2 mM NaN3, 5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, and protease inhibitor mixture) was added. Cells were then scraped and subjected to five freeze/thaw cycles followed by five passages through a 30-gauge needle to lyse them. Lysates were centrifuged at 1,000 × g for 10 min at 4 °C, and the supernatant was collected and further centrifuged at 28,000 × g for 20 min at 4 °C to collect the membrane fraction pellet, which was resuspended in 100 μL disruption buffer. Membrane fractions (5–10 μg per well) were added to cytochrome c-containing oxidase assay buffer (65 mM sodium phosphate buffer, pH 7.0, 1 mM EGTA, 10 μM FAD, 1 mM MgCl2, 2 mM NaN3, 0.2 mM cytochrome c, and 1,000 U⋅mL−1 catalase), and O2•– was measured as the initial rate of SOD-inhibitable cytochrome c reduction quantified at 550 nm using the extinction coefficient 21.1 mmol−1⋅L⋅cm−1. For siRNA studies, total cell lysates were used. After a 5-min baseline measurement, NADPH (180 μM) was added, and O2•– production was calculated from the initial linear rate (first 10 min) of cytochrome c reduction.
O2•– Detection by Electron Paramagnetic Resonance Spectroscopy.
The EPR spin probe CMH was used to examine O2•– production using a Bruker eScan Table-Top EPR spectrometer (Bruker Biospin) as performed previously (8, 59). Briefly, thoracic aortas were freshly isolated from WT, EBP50 KO, and Nox1 KO mice; cleaned; and cut into rings 2 mm in length. Aortic rings were suspended in PSS buffer (mM: NaCl 130, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, NaHCO3 14.9, glucose 5.5, CaCl2 1.6, and EDTA 0.026, pH 7.4), preincubated at 37 °C for 30 min, then treated with vehicle or AngII (100 nM, 4 h) in the presence or absence of SOD (200 U⋅mL−1). CMH (500 μM) was added to the solution followed by 15 min incubation at 37 °C. This duration limits background nonspecific oxidation and allows for detection of the tissues’ capacity to produce O2•– following stimulation (as a result of increased active Nox complexes, for example) rather than measurement of accumulated signal during the stimulation period. Supernatants were transferred to an EPR capillary tube, and EPR spectra were obtained (field sweep: 50 G; microwave frequency: 9.78 GHz; microwave power: 20 mW; modulation amplitude: 2 G; conversion time: 327 ms; time constant: 655 ms; receiver gain: 1 × 105). To minimize the deleterious effects of contaminating metals, the buffers were treated with Chelex resin and contained 25 μM of the iron chelator deferoxamine and 5 μM diethyl-dithiocarbamate. Data were quantified using the amplitude of the first spectral peak taken as fold from each experiment’s vehicle control after background subtraction (buffer + CMH).
Plasmid Transfection.
Plasmids encoding full-length human cDNAs for Nox1 (pcDNA3.1-hNox1), NoxA1 (pCMVsport 6-hNoxA1), and p47phox (pCMV-Tag4A-hp47) were kindly provided by David Lambeth (Emory University, Atlanta, GA). Plasmids encoding N-terminal FLAG-human WT EBP50, PDZ1 (S1), PDZ2 (S2), and PDZ1&2 (S1S2) functional mutants, as well as YFP-human WT EBP50 were a kind gift from Peter Friedman (University of Pittsburgh, Pittsburgh PA). Plasmids encoding CFP-p47phox were purchased from OriGene Technologies, Inc. Sited-directed mutagenesis was performed to generate the p47phox serine 388 to alanine and valine 390 to alanine double mutant (p47phox-AAAA). NoxO1–ASAV fusion was generated by fusing the alanine–serine–alanine–valine C-terminal motif of p47phox onto the C-terminal end of NoxO1. Plasmids were transformed and amplified into Escherichia coli strain TOP10 (Invitrogen). Plasmids were purified using a QIAfilter plasmid purification kit (Qiagen). For transfections in Cos22 (Cos7 parental line stably expressing p22phox), cells were grown to 50–60% confluence on 100-mm plates and simultaneously transfected with empty vector or plasmids for Nox1, NoxA1, and p47phox; Nox1, NoxA1, p47phox, and EBP50 or EBP50 S1S2 mutant; Nox1, NoxA1, and NoxO1; or Nox1, NoxA1, NoxO1, and EBP50 using the transfection reagent Lipofectamine LTX with PLUS reagent according to the manufacturer’s protocol. CosPhox Cells were transfected with empty vector or EBP50. Cos7 cells possess endogenous Rac and thus do not require further transfection of this protein for Nox activity (61). Cells were assayed 24 h later. For FRET studies, Cos22 cells were transfected with YFP-EBP50, CFP-p47phox, or YFP-EBP50 and CFP-p47phox using the same protocol. For confocal microscopy studies, rASMC were transfected with YFP-EBP50. For immunoprecipitation studies, Cos7 cells stably expressing p47phox were transfected with FLAG-tagged EBP50, and EBP50 KO VSMC were transfected with GFP control or FLAG-WT, FLAG-S1, FLAG-S2, or FLAG-S1S2 EBP50. GnTi Hek cells stably expressing TAP-EBP50 were transfected with p47phox, p47phox-AAAA, NoxO1, or NoxO1-ASAV.
siRNA Transfection to Suppress Nox1 in rASMC.
Cells were transfected with Nox1 siRNA as previously described (8). Briefly, cells grown to 50% confluence on six-well plates and were cotransfected with EBP50 plasmid and scrambled siRNA or Nox1 siRNA (5 pmol) using the transfection reagent Lipofectamine 2000 according to the manufacturer’s protocol with modifications to optimize cotransfection. Cells were assayed 48 h later. This protocol yields a knockdown of ∼70% (8).
Confocal 4-HNE in Vivo Immunofluorescence.
C57BL/6 WT or EBP50 KO mice were injected with 10 mg⋅kg−1 i.p. LPS for 16 h before sacrifice. Femoral arteries were harvested and fixed with 4% (vol/vol) paraformaldehyde before embedding in OCT Tissue-Tek (Sakura Finetek) and cryostat sectioning at 10 μm. All animal procedures were approved by the University of Pittsburgh IACUC. Cryostat sections of femoral arteries were washed three times with PBS, followed by three washes with 0.5% BSA solution. Sections were blocked with 5% (vol/vol) goat serum in BSA solution and incubated with anti-4-HNE antibody (Alpha Diagnostics). After washing, slides were incubated with Cy3-conjugated secondary antibody (Jackson ImmunoResearch) in combination with F-actin specific Alexa Fluor 647-conjugated phalloidin (Invitrogen). Nuclei were stained with Hoeschst dye (bisbenzamide 1 mg per 100 mL water) for 30 s. After three rinses with PBS, coverslips were adhered to slides with gelvatol mounting media (polyvinylalcohol, glycerol, H2O, sodium azide, and Tris, pH 8.5). Fluorescent images were captured with an Olympus Fluoview 1000 confocal microscope. A total of three to four animals were used per treatment group. For each animal, three images from three separate sections were captured.
Confocal Fluorescence Microscopy.
Transfection of rASMC grown on coverslips with YFP-EBP50 was performed as indicated above. Cells were serum starved, treated with 100 nM AngII for 4 h, fixed in 2% (vol/vol) paraformaldehyde, and permeabilized with 0.1% Triton X-100. After extensive washing, coverslips were blocked with 2% (wt/vol) BSA solution followed by incubation with anti-p47phox antibody and subsequent incubation with Cy3-conjugated secondary antibody (Jackson ImmunoResearch). Coverslips were stained with Alexa Fluor 647-conjugated phalloidin (Invitrogen) and adhered to glass slides using gelvatol mounting media. Confocal images were captured on an Olympus Fluoview 1000 confocal microscope (Olympus America Inc.) using an confocal aperture size (C.A.) of 106 μm. The following wavelengths (nm) for excitation and emission, respectively, were used: 559 and 572 (Cy3), 649 and 666 (Alexa Fluor 647), and 488 and 520 (YFP). For each experiment, five to eight images per treatment group were captured. Three independent experiments were performed. No spectral overlap occurred between the different fluorescence emission channels used in these studies eliminating the possibility of false positive colocalization. For endogenous protein confocal experiments, rASMC were processed as above without transfection. EBP50 was instead assessed by an EBP50 antibody followed by Alexa Fluor 488-conjugated secondary antibody.
Tissue sections were mounted on slides. Slides were submersed in ice cold acetone, washed with PBS, submersed in 0.1% Triton X-100, and then washed in PBS. For immunofluorescence staining, slides were blocked with fish skin gelatin in PBS containing 10% (vol/vol) horse serum for 30 min. Tissues were incubated with primary antibodies for EBP50 and p47phox or ROCK1 and p47phox. Slides were washed in PBS containing 0.1% Tween-20 and then incubated with secondary antibodies. After washing slides, DAPI was applied for 30 min. Slides were washed and coverslipped with Vectashield antifade reagent (Vector Laboratories).
Fluorescence Resonance Energy Transfer.
Cos7 cells seeded on coverslips were transfected separately or simultaneously with CFP-p47phox and YFP-EBP50 and treated with PMA as above. Cells were washed and fixed in 2% (vol/vol) paraformaldehyde. FRET experiments were performed with confocal spectral imaging using a Nikon A1 microscope system (Nikon Instruments Inc.) as previously with modifications (60). Emission spectra for YFP and CFP were obtained separately for each fluorophore using spectral scanning on cells transfected with either YFP-EBP50 or CFP-p47phox and the excitation wavelengths of 514 or 457 nm, respectively. These spectra were later used for spectral unmixing of emission spectra obtained by spectral scanning on cells cotransfected with both proteins and excited with 457 nm (CFP). Acceptor (YFP) photobleaching was performed at 514 nm, 50% laser power, and four cycles of 5 s each. Fluorescence intensities were calculated after spectral unmixing and represented as fold from prebleach for each fluorophore. FRET efficiency was calculated as
Proximity Ligation Assay.
For the proximity ligation assay, the manufacturer’s protocol for the Duolink system was followed (Sigma). Cells or tissues were prepared on microscope slides as detailed for confocal microscopy above. Primary antibodies for EBP50 and p47phox or p47phox and ROCK1 were allowed to incubate overnight followed by incubation with secondary antibodies. Confocal imaging was conducted as above capturing z-stacks in 10 planes for each sample and compressed using Image J software into maximum intensity composite z projections. Data are quantified as the ratio of PLA punctates to the number of nuclei per sample. In a subset of experiments, phalloidin was added as described above. For 3D reconstruction, Imaris software was used to process the stacks using the surface and dots features for the cell area and PLA punctates, respectively.
Immunoprecipitation and Western Blot.
Cells were transfected as indicated above. Cos7 cells were treated with vehicle or PMA (20 μM). EBP50 KO VSMC were treated with vehicle or AngII (100 nM). Cells were washed with ice cold PBS (1×) and lysed in RIPA buffer supplemented with protease and phosphatase inhibitors (Roche Diagnostics GmbH). Cells were centrifuged at 1000 × g for 10 min at 4 °C, and the supernatant was collected. Protein concentration was measured using the Bradford method (Thermo Scientific). One hundred micrograms of cell lysates were mixed with anti-FLAG M2 resin mouse antibody overnight at 4 °C. Samples were centrifuged at 3000 × g for 10 min at 4 °C and pellet washed extensively then resuspended in Tris-Glycine SDS sample buffer, boiled, and centrifuged at 1,000 × g for 10 min. For immunoprecipitation using Hek GnTi cells transfected as detailed above, cells were lysed, and supernatants were incubated with streptavidin beads to pull down TAP-EBP50. After extensive washing, samples were prepared for Western blot.
For Western blot experiments, samples were resolved with SDS–PAGE and transferred onto Trans Blot nitrocellulose membranes (Bio-Rad). Membranes were blocked with the Odyssey Blocking Buffer (Li-Cor Biosciences) and incubated with primary antibodies. Membranes were probed with secondary antibodies (Li-Cor Biosciences). Digital imaging was obtained using the Odyssey Infra-Red Imaging system (Li-Cor Biosciences).
FACS Analysis for Quantification of Hypertrophy.
We recently established flow cytometry criteria for assessment of cell hypertrophy and validated these against two other methods (protein:DNA ratio and measurement of cell area using microscopy) (8). In the current study flow cytometry was performed in accordance with these recently described protocols (8). Briefly, rASMC transfected as indicated above or WT or EBP50 KO VSMC were grown to 80% confluence then serum starved as indicated above and treated with vehicle or 100 nM AngII for 24 h at 37 °C. Cells were trypsinized and resuspended in warm PBS (37 °C) at a concentration of 1 × 106 cells per mL. Forward scatter (FSC) and side scatter (SSC) were measured recording 20,000 events per sample using a BD LSR Fortessa (BD Biosciences) flow cytometer. Quantification was then performed using FloJo software (Tree Star Inc.). Density plots were gated to exclude debris and then quadrant parameters were selected and assigned across all measurements based on the size of 80–85% of cells from control groups. The percentage of individual cells (events) recorded in quadrant 2 (FSC+, SSC+) were quantified for each group and taken as a ratio over quadrant 2 events percentage in control groups.
Cell Size Measurement for Quantification of Hypertrophy.
These experiments were carried out as described previously (8). Briefly, WT or EBP50 KO VSMC were treated as above, and images were captured at an objective lens magnification of 10×. Three to six images were taken per treatment group per experiment. The areas of four individual cells selected at random per image were quantified using Image J (NIH), calculated as a fold from control and averaged.
Docking Analysis.
In silico association analysis was performed using the published crystal structures of the relevant regions of EBP50 and p47phox. For p47phox the NMR crystal solution structure of the C-terminal tail region of p47phox (aa 359–390) complexed to the C-terminal SH3 domain of p67phox was used (PDB code: 1k4u) (40). For EBP50, the X-ray crystal structure of PDZ1 domain in complex with the C-terminal fragment of β2–adrenergic receptor (AR; PDB code: 1GQ4) (41) was used. To model the C-terminal motif of p47phox (L-A-S-A-V sequence), the −NDSLL sequence of the carboxyl-terminal fragment of β2–AR was modified as follows: the C-terminal Leu0 was replaced by Val, Leu−1 was replaced by Ala, Asp−3 was replaced by Ala, and Asn−4 was replaced by Leu. By convention, the carboxyl-terminal residue is numbered starting at zero with upstream residues designated as −1, −2, −3, −4, etc. The generated structure includes the five residues immediately flanking the C terminus of p47phox (386-390 aa) and EBP50 PDZ1 residues Leu11–Glu94. Visual modeling of this predicted interaction was generated using the software PyMOL.
Pressure Myography.
Third-order mesenteric arteries were freshly isolated from WT, EBP50 KO, and Nox1 KO mice. Vessels were cannulated, pressurized (75 mm Hg), and equilibrated (Krebs–Hepes, 30 min, 37 °C), and contractile responses were studied using cumulative concentrations of AngII (10−11 M to 10−7 M) or phenylephrine (PE; 10−9 M and 10−4 M). Acetylcholine (10−5 M) was administered to verify endothelial response, and potassium chloride (40 mM) was administered to verify vessel contraction. Maximum diameter was measured by incubating vessels in a Krebs calcium-free, ethylene glycol tetraacetic acid (EGTA, 1 mM) and sodium nitroprusside (SNP 10 μM) solution. Vessel diameter was quantified using DMT vessel acquisition software, and data were expressed as percent of initial diameter.
Statistical Analyses.
Data are presented as means ± SEM. Data comparisons were performed with a Student’s t test or a one- or two-way ANOVA followed by the Bonferroni post hoc analysis. Differences were deemed statistically significant at P < 0.05.
Acknowledgments
We thank Drs. John F. McDyer and Iulia D. Popescu for assistance in FACS protocols. We thank Dr. Guillermo Romero for critical feedback. We also thank the University of Pittsburgh Center for Biologic Imaging and Drs. Claudette M. St Croix and Simon Watkins, as well as Mark Ross, Mackenzie Mosher, and Morgan Nelson for confocal microscopy resources, assistance, and expert advice. We thank Joseph Whatley, Adam Henry, and Christina Goldbach for technical assistance. Finally, we thank Drs. Yao Li and Daniel Simoes de Jesus for assistance with some of the biochemical assays. I.A.G. received support from the American Heart Association (15SDG24910003). P.J.P. received support from the NIH (R01HL079207, R01HL112914, and P01HL103455-01). P.A.F. received support from the NIH (DK105811-01A1). Access to Center for Biologic Imaging resources was made possible in part by NIH (1S10OD019973) (S. Watkins). All Vascular Medicine Institute investigators received support from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514161113/-/DCSupplemental.
References
- 1.Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110(10):1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Ghouleh I, et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51(7):1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takac I, Schröder K, Brandes RP. The Nox family of NADPH oxidases: Friend or foe of the vascular system? Curr Hypertens Rep. 2012;14(1):70–78. doi: 10.1007/s11906-011-0238-3. [DOI] [PubMed] [Google Scholar]
- 4.Csányi G, Taylor WR, Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47(9):1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Autréaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 6.Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278(6):3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 7.Brandes RP, Schröder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med. 2008;18(1):15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Al Ghouleh I, et al. Aquaporin 1, Nox1 and Ask1 mediate oxidant-induced smooth muscle cell hypertrophy. Cardiovasc Res. 2013;97(1):134–142. doi: 10.1093/cvr/cvs295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Reczek D, Berryman M, Bretscher A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139(1):169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeyens N, Horman S, Vertommen D, Rider M, Morel N. Identification and functional implication of a Rho kinase-dependent moesin-EBP50 interaction in noradrenaline-stimulated artery. Am J Physiol Cell Physiol. 2010;299(6):C1530–C1540. doi: 10.1152/ajpcell.00175.2010. [DOI] [PubMed] [Google Scholar]
- 12.Naren AP, et al. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA. 2003;100(1):342–346. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troncoso M, et al. Expression of NHERF1 in colonic tumors induced by 1,2-dimethylhydrazine in rats is independent of plasma ovarian steroids. Horm Cancer. 2011;2(4):214–223. doi: 10.1007/s12672-011-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi Y, Molina JR, Hamilton SR, Georgescu MM. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia. 2010;12(12):1013–1022. doi: 10.1593/neo.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song GJ, et al. EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells. J Mol Cell Cardiol. 2010;49(6):1012–1021. doi: 10.1016/j.yjmcc.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song GJ, Fiaschi-Taesch N, Bisello A. Endogenous parathyroid hormone-related protein regulates the expression of PTH type 1 receptor and proliferation of vascular smooth muscle cells. Mol Endocrinol. 2009;23(10):1681–1690. doi: 10.1210/me.2009-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song GJ, et al. The scaffolding protein EBP50 promotes vascular smooth muscle cell proliferation and neointima formation by regulating Skp2 and p21(cip1) Arterioscler Thromb Vasc Biol. 2012;32(1):33–41. doi: 10.1161/ATVBAHA.111.235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MY, et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29(4):480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S, et al. Increased expression of Nox1 in neointimal smooth muscle cells promotes activation of matrix metalloproteinase-9. J Vasc Res. 2012;49(3):242–248. doi: 10.1159/000332958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74(6):1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 21.Nickenig G, et al. Negative feedback regulation of reactive oxygen species on AT1 receptor gene expression. Br J Pharmacol. 2000;131(4):795–803. doi: 10.1038/sj.bjp.0703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassègue B, et al. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88(9):888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 23.Dikalova A, et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112(17):2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 24.Matsuno K, et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation. 2005;112(17):2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 25.Dikalov SI, et al. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45(9):1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basset O, et al. NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin II-receptor localization in vascular smooth muscle. Antioxid Redox Signal. 2009;11(10):2371–2384. doi: 10.1089/ars.2009.2584. [DOI] [PubMed] [Google Scholar]
- 27.Al Ghouleh I, Magder S. Nicotinamide adenine dinucleotide phosphate (reduced form) oxidase is important for LPS-induced endothelial cell activation. Shock. 2008;29(5):553–559. doi: 10.1097/SHK.0b013e318157ebc8. [DOI] [PubMed] [Google Scholar]
- 28.Sastre E, Blanco-Rivero J, Caracuel L, Lahera V, Balfagón G. Effects of lipopolysaccharide on the neuronal control of mesenteric vascular tone in rats: Mechanisms involved. Shock. 2012;38(3):328–334. doi: 10.1097/SHK.0b013e31826240ba. [DOI] [PubMed] [Google Scholar]
- 29.Wang XP, et al. Arginase I attenuates inflammatory cytokine secretion induced by lipopolysaccharide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31(8):1853–1860. doi: 10.1161/ATVBAHA.111.229302. [DOI] [PubMed] [Google Scholar]
- 30.Matsuno K, et al. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free Radic Biol Med. 2012;53(9):1718–1728. doi: 10.1016/j.freeradbiomed.2012.08.590. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi T, et al. The role of endothelial interleukin-8/NADPH oxidase 1 axis in sepsis. Immunology. 2010;131(3):331–339. doi: 10.1111/j.1365-2567.2010.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chéret C, et al. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28(46):12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusumoto K, et al. Ecabet sodium inhibits Helicobacter pylori lipopolysaccharide-induced activation of NADPH oxidase 1 or apoptosis of guinea pig gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol. 2005;288(2):G300–G307. doi: 10.1152/ajpgi.00274.2004. [DOI] [PubMed] [Google Scholar]
- 34.Wolf G, et al. Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors. Kidney Int. 2002;61(6):1986–1995. doi: 10.1046/j.1523-1755.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 35.Straub AC, et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491(7424):473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeyens N, de Meester C, Yerna X, Morel N. EBP50 is involved in the regulation of vascular smooth muscle cell migration and cytokinesis. J Cell Biochem. 2011;112(9):2574–2584. doi: 10.1002/jcb.23183. [DOI] [PubMed] [Google Scholar]
- 37.Harris BZ, Lim WA. Mechanism and role of PDZ domains in signaling complex assembly. J Cell Sci. 2001;114(Pt 18):3219–3231. doi: 10.1242/jcs.114.18.3219. [DOI] [PubMed] [Google Scholar]
- 38.Doyle DA, et al. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85(7):1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 39.Songyang Z, et al. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275(5296):73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 40.Kami K, Takeya R, Sumimoto H, Kohda D. Diverse recognition of non-PxxP peptide ligands by the SH3 domains from p67(phox), Grb2 and Pex13p. EMBO J. 2002;21(16):4268–4276. doi: 10.1093/emboj/cdf428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karthikeyan S, Leung T, Ladias JAA. Structural basis of the Na+/H+ exchanger regulatory factor PDZ1 interaction with the carboxyl-terminal region of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2001;276(23):19683–19686. doi: 10.1074/jbc.C100154200. [DOI] [PubMed] [Google Scholar]
- 42.Mamonova T, Kurnikova M, Friedman PA. Structural basis for NHERF1 PDZ domain binding. Biochemistry. 2012;51(14):3110–3120. doi: 10.1021/bi201213w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamonova T, et al. Canonical and noncanonical sites determine NPT2A binding selectivity to NHERF1 PDZ1. PLoS One. 2015;10(6):e0129554. doi: 10.1371/journal.pone.0129554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem. 1998;273(24):15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 45.Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22(6):1141–1149. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Li WG, et al. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276(31):29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boulden BM, et al. Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med. 2006;41(5):810–817. doi: 10.1016/j.freeradbiomed.2006.05.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- 49.Voltz JW, Weinman EJ, Shenolikar S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene. 2001;20(44):6309–6314. doi: 10.1038/sj.onc.1204774. [DOI] [PubMed] [Google Scholar]
- 50.Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: Role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25(3):512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 51.Touyz RM, Yao G, Schiffrin EL. Role of the actin cytoskeleton in angiotensin II signaling in human vascular smooth muscle cells. Can J Physiol Pharmacol. 2005;83(1):91–97. doi: 10.1139/y05-006. [DOI] [PubMed] [Google Scholar]
- 52.Tamura M, Itoh K, Akita H, Takano K, Oku S. Identification of an actin-binding site in p47phox an organizer protein of NADPH oxidase. FEBS Lett. 2006;580(1):261–267. doi: 10.1016/j.febslet.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 53.Tamura M, Kai T, Tsunawaki S, Lambeth JD, Kameda K. Direct interaction of actin with p47(phox) of neutrophil NADPH oxidase. Biochem Biophys Res Commun. 2000;276(3):1186–1190. doi: 10.1006/bbrc.2000.3598. [DOI] [PubMed] [Google Scholar]
- 54.Fouassier L, et al. Protein kinase C regulates the phosphorylation and oligomerization of ERM binding phosphoprotein 50. Exp Cell Res. 2005;306(1):264–273. doi: 10.1016/j.yexcr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Parker SB, Wade SS, Prewitt RL. Pressure mediates angiotensin II-induced arterial hypertrophy and PDGF-A expression. Hypertension. 1998;32(3):452–458. doi: 10.1161/01.hyp.32.3.452. [DOI] [PubMed] [Google Scholar]
- 56.Griffin SA, et al. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension. 1991;17(5):626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- 57.Kamal MF, Campbell AC. Medial hypertrophy in the small intestinal arteries in systemic hypertension, renal and essential. J Pathol. 1979;129(2):99–110. doi: 10.1002/path.1711290208. [DOI] [PubMed] [Google Scholar]
- 58.Dzau VJ, Gibbons GH. Cell biology of vascular hypertrophy in systemic hypertension. Am J Cardiol. 1988;62(11):30G–35G. doi: 10.1016/0002-9149(88)90029-x. [DOI] [PubMed] [Google Scholar]
- 59.Csányi G, et al. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol. 2012;32(12):2966–2973. doi: 10.1161/ATVBAHA.112.300031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ranayhossaini DJ, et al. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J Biol Chem. 2013;288(51):36437–36450. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price MO, et al. Creation of a genetic system for analysis of the phagocyte respiratory burst: High-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood. 2002;99(8):2653–2661. doi: 10.1182/blood.v99.8.2653. [DOI] [PubMed] [Google Scholar]