Menghi et al. (1) report a metric to classify tumors into those with and without a tandem duplicator phenotype (TDP), using the frequency of tandem duplications (TDs) in 277 whole-genome sequenced samples. Building on a previous method (2), Menghi et al. (1) identified TDs from SNP array data, and found that the TDP was strongly associated with response to the DNA damaging chemotherapeutic, cisplatin. These findings supplement the growing recognition that genome-wide signatures of mutator phenotypes may prove to be important additions to the companion diagnostic repertoire (3, 4). Although the findings of this report are highly stimulating, accumulating evidence suggests that an elevated abundance of TDs features in not just one but two distinct phenotypes.
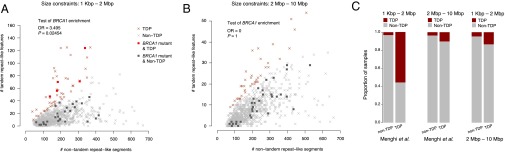
Two of the original studies on the TDP reported a mutual exclusion with breast cancer 1/2 (BRCA1/2) inactivation (2, 5), which conflicts with the enrichment of BRCA1 loss among TDP cancers observed by Menghi et al. (1). Using The Cancer Genome Atlas (TCGA) breast cancer data (6), we established allele-specific copy number profiles using ASCAT (7) before calling TDP status, as described previously (2), using two different size ranges for the TD-like features: (i) between 1 Kbp and 2 Mbp in accordance with the study by Menghi et al. (1); and (ii) between 2 Mbp and 10 Mbp. Five samples with BRCA1 inactivation exhibited the TDP when considering only shorter TDs (Fig. 1A); however, we found no instances of tumors with BRCA1 inactivation among 2- to 10-Mbp TDP cancers (Fig. 1B). Furthermore, although 56% of the Menghi et al. (1) study’s TDP calls were shared with our 1-Kbp to 2-Mbp TDP calls, only 10% of the Menghi et al. study’s TDP calls agreed with our 2- to 10-Mbp TDP calls (Fig. 1C). In addition, we found that although the 1-Kbp to 2-Mbp TDP calls and the Menghi et al. study’s TDP calls were enriched for triple-negative breast cancers (P < 0.001, Fisher’s exact test), the 2- to 10-Mbp TDP calls were not (P = 0.81, Fisher’s exact test). These findings support the notion that the study by Menghi et al. captures one particular TDP distinguishable from a second TDP by length and contrasting relationships with loss of BRCA1 function.
Fig. 1.
Tandem duplication phenotypes in 940 TCGA breast cancers. (A and B) TDP status was determined using genomic segments between 1 Kbp and 2 Mbp (A), or 2 Mbp and 10 Mbp (B), followed by Gaussian mixture modeling of the ratio of TDs to non-TD segments (total number of segments minus double the number of TD segments as per ref. 2). Odds ratio and P value represent Fisher’s exact test of BRCA1 mutation enrichment in the TDP subset of tumors. BRCA1 loss was defined as germ-line or somatic point mutation or deletion. TDP tumors are colored in red and non-TDP tumors in gray. All samples are denoted by an “x,” with the exception of tumors with BRCA1 loss, which are denoted by a square. (C) Bar plots illustrate the overlaps between the different TDP calling methods.
Our results are reinforced by two recent analyses. The first study extracted two TD-enriched rearrangement signatures from 560 whole breast cancer genomes (8). “Signature-1” mostly comprised TDs between 1 and 10 Mbp, whereas “signature-3” mostly comprised TDs ≤ 100 Kbp. Signature-3 was associated with BRCA1 disruption, signatures of homologous recombination deficiency, and was observed in ∼15% of the cohort. In contrast, signature-1 was independent of BRCA1/2 disruptions, exhibited links with mutational signatures of both homologous recombination deficiency and mismatch repair deficiency, and presented in ∼8.5% of the cohort. The second study identified an ovarian and prostate cancer-linked TDP featuring TDs up to 10 Mbp, mutual exclusion with BRCA1/2 inactivation, and enrichment for inactivation of the CDK12 kinase (9).
In conclusion, we propose that there are actually two TDPs, with the study by Menghi et al. (1) providing a comprehensive characterization of the BRCA1 inactivation-linked TDP. The existence of two TDPs has important implications for the robust development of genomic instability-based biomarkers of drug response.
Footnotes
The authors declare no conflict of interest.
References
- 1.Menghi F, et al. The tandem duplicator phenotype as a distinct genomic configuration in cancer. Proc Natl Acad Sci USA. 2016;113(17):E2373–E2382. doi: 10.1073/pnas.1520010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng CKY, et al. The role of tandem duplicator phenotype in tumour evolution in high-grade serous ovarian cancer. J Pathol. 2012;226(5):703–712. doi: 10.1002/path.3980. [DOI] [PubMed] [Google Scholar]
- 3.Schouten PC, et al. Robust BRCA1-like classification of copy number profiles of samples repeated across different datasets and platforms. Mol Oncol. 2015;9(7):1274–1286. doi: 10.1016/j.molonc.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16(3):211. doi: 10.1186/bcr3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride DJ, et al. Tandem duplication of chromosomal segments is common in ovarian and breast cancer genomes. J Pathol. 2012;227(4):446–455. doi: 10.1002/path.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Loo P, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci USA. 2010;107(39):16910–16915. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nik-Zainal S, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popova T, et al. Ovarian cancers harboring inactivating mutations in CDK12 display a distinct genomic instability pattern characterized by large tandem duplications. Cancer Res. 2016;76(7):1882–1891. doi: 10.1158/0008-5472.CAN-15-2128. [DOI] [PubMed] [Google Scholar]