Significance
Biofilms are surface-associated bacterial communities embedded in an extracellular matrix. Connections between biofilm architectural, material, and mechanical features have never been systematically studied at the individual cell level due to inadequate optical resolution. Here, we develop imaging, experimental, and modeling tools to analyze living, growing bacterial biofilms at single-cell resolution. We discover that Vibrio cholerae biofilms undergo a 2D-to-3D transition as a consequence of directional cell division and anisotropic pressure caused by cell-to-surface adhesion. Moreover, deletion of a single gene responsible for cell-to-cell adhesion changes the biofilm growth mode from directional cell growth to expansion caused by the extracellular matrix. The technology reported here enables future studies of single-cell gene expression in bacterial communities.
Keywords: biofilm, single cell, self-organization, community, biomechanics
Abstract
Biofilms are surface-associated bacterial communities that are crucial in nature and during infection. Despite extensive work to identify biofilm components and to discover how they are regulated, little is known about biofilm structure at the level of individual cells. Here, we use state-of-the-art microscopy techniques to enable live single-cell resolution imaging of a Vibrio cholerae biofilm as it develops from one single founder cell to a mature biofilm of 10,000 cells, and to discover the forces underpinning the architectural evolution. Mutagenesis, matrix labeling, and simulations demonstrate that surface adhesion-mediated compression causes V. cholerae biofilms to transition from a 2D branched morphology to a dense, ordered 3D cluster. We discover that directional proliferation of rod-shaped bacteria plays a dominant role in shaping the biofilm architecture in V. cholerae biofilms, and this growth pattern is controlled by a single gene, rbmA. Competition analyses reveal that the dense growth mode has the advantage of providing the biofilm with superior mechanical properties. Our single-cell technology can broadly link genes to biofilm fine structure and provides a route to assessing cell-to-cell heterogeneity in response to external stimuli.
Biofilms are surface-associated bacterial communities embedded in an extracellular matrix (1–3). Biofilm cells are more resistant to antibiotics than their planktonic counterparts, which is a major problem in the context of chronic infections (4, 5). Also, bacterial biofilms clog networks and filters in industrial settings (6). On the other hand, biofilms can be useful, for example, in waste-water treatment (7). Investigations have focused on the genetic and regulatory features driving biofilm formation and on defining the composition of the extracellular matrix (8). However, still lacking is a fundamental biophysical understanding of how bacteria, in time and space, build these 3D structures that attach to surfaces and resist mechanical and chemical perturbations. One common assumption is that bacteria produce polymeric matrices that expand the volume occupied by cells and carry them into the third dimension, as demonstrated by many computer simulations (9, 10). Matrix proteins, extracellular DNA, lipids, and bacteriophages have also been shown to influence the formation of the overall biofilm structure (8). However, the role the bacterial cells themselves play in shaping the biofilm architecture has not been widely investigated; hence, many basic questions remain. For example, for rod-shaped cells that initially attach parallel to a surface, how is 3D growth possible, given the directional division of the cells? How does the arrangement of individual cells determine the global architecture of the biofilm? The inability to answer these and other basic questions stems from a lack of understanding of the cellular-scale architecture of biofilms, which, in turn, highlights a lack of technology capable of tracking the time evolution of biofilms at the single-cell level, in stark contrast to the established tools available for analogous studies of development in eukaryotic organisms (11). Despite tremendous progress in imaging biofilms (12), bottlenecks remain due to resolution and phototoxicity issues. Thus, our understanding of biofilms primarily comes from high-resolution optical images of immature biofilms that are only a few cell layers thick (13), low-resolution images of the 3D contour (12), or electron microscopy images of processed samples (14), which lack the key spatiotemporal information required to understand the biofilm developmental process at the level of the basic unit: the individual cell.
Here, we succeed in imaging living, growing biofilms with single-cell resolution and use this ability to discover how biological and physical factors combine to drive the construction of bacterial biofilms. We use Vibrio cholerae, the bacterium responsible for the pandemic disease cholera as our model organism. Biofilm formation is a key feature in the V. cholerae pathogenic and environmental lifestyles (15). Earlier studies defined regulatory and matrix components that are crucial for proper V. cholerae biofilm formation (16). In addition to extracellular polysaccharide (Vps), the matrix protein RbmA (rugosity and biofilm structure modulator A) binds mother-daughter cells together at their poles, Bap1 (biofilm-associated protein 1) adheres cells to the surface, and RbmC/Bap1 forms an envelope around cell subclusters in conjunction with Vps (17). The expression of the genes encoding these components is controlled by intracellular cyclic-diguanylate (c-di-GMP) levels and by quorum sensing (18, 19). Beyond these overarching principles, it is not known how V. cholerae builds a biofilm cell by cell. Using live single-cell resolution imaging combined with mutagenesis and in situ matrix labeling, we discover that the directional proliferation of the rod-shaped bacterial cells is the main driving force influencing the overall architecture of the biofilm. We also define how the different matrix proteins contribute distinctly to this process. Finally, we perform fitness and competition analyses to reveal the evolutionary advantage of the dense, final architecture versus other possible architectures.
Results and Discussion
Single-Cell Live Imaging Reveals Ordering.
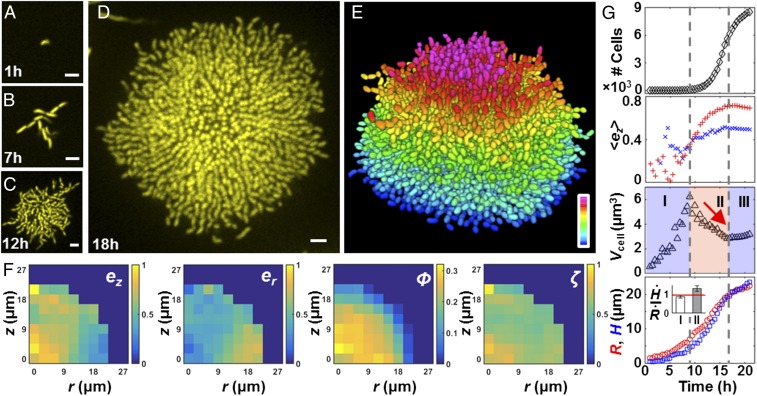
We and others recently reported single-cell resolution imaging of fixed bacterial biofilm samples using staining and ensemble averaging (20, 21). Because these analyses relied on fixed cells, they could not uncover key temporal information about the biofilm developmental process. Therefore, our first goal in the present work was to advance the field by achieving single-cell imaging of living, growing biofilms. To accomplish this goal, we optimized a customized spinning disk confocal microscope, integrated the gene encoding the highly fluorescent and photostable protein mKO as a constitutive reporter into the V. cholerae genome (22), and developed an imaging procedure using minimum laser exposure and adaptive z ranges to reduce phototoxicity to the cells and photobleaching of the chromophores (Materials and Methods). We studied a commonly used rugose variant (denoted Rg) of V. cholerae that forms robust biofilms due to increased production of c-di-GMP (23). We seeded sample chambers at low cell density so that we could follow the development of isolated biofilm clusters from single founder cells to 10,000 cells (Fig. 1 A–D and Movies S1 and S2) at a temporal resolution of one cell-division cycle (≈30 min), using static 96-well chambers. The raw data show that cell clusters initially expand radially in a branched pattern primarily in two dimensions, but subsequently transition into dense 3D domes. Strikingly, in mature biofilms, the central core harbors cells aligned side-by-side oriented vertically to the surface, whereas cells at the periphery align radially and remain horizontal relative to the surface.
Fig. 1.
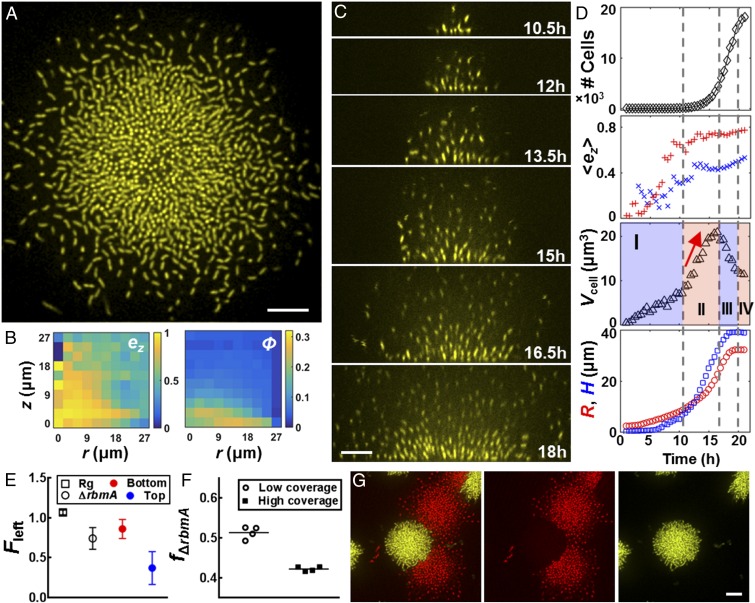
Single-cell imaging of a growing V. cholerae biofilm cluster. Cross-sectional images of the bottom cell layer at 1 h (A), 7 h (B), 12 h (C), and 18 h (D). (Scale bars: 3 μm.) (E) Segmenting the 3D biofilm cluster in D into 7,199 cells, color-coded according to z position (0–21 μm). (F) Spatial distribution of the z and radial components of each cell’s orientation director ez and er, volume fraction ϕ, and alignment order parameter ζ for the 18-h cluster in E. (G) Time evolution of cell number, averaged ez, biovolume per cell Vcell, cluster radius R (red circles), and height H (blue squares). In the ez plot, average values from cells with x–y coordinates that are less and more than R/2 away from the center are shown as red plus signs and blue plus crosses, respectively. We identified three phases, denoted by the vertical dashed lines. Phase II is characterized by a steady decrease of Vcell (red arrow), an increase in , and faster growth of H than R. (Inset) Ratio between growth rates of the cluster height and radius in phases I and II averaged over five samples (error bars correspond to SDs); the red line corresponds to a ratio of 1.
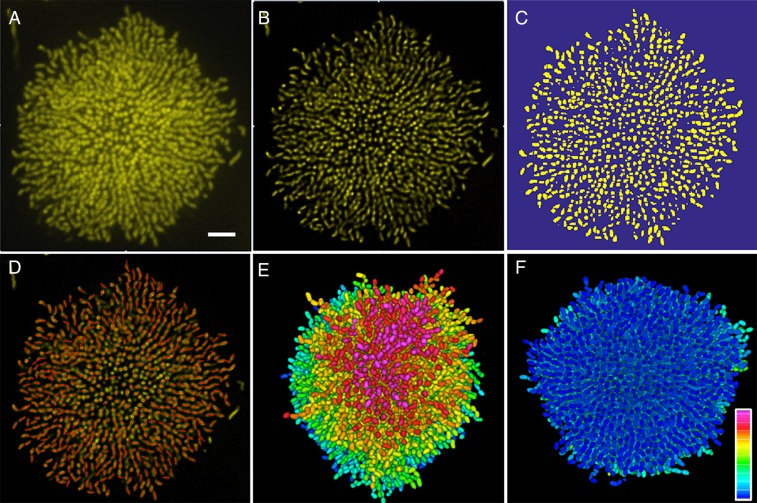
To quantify spatial heterogeneity in cell ordering, we deconvolved the z-stack images and segmented the entire biofilm cluster into individual cells using semiautomated, home-written image analysis codes (Fig. 1E and Fig. S1). We define four parameters (Fig. 1F): ez and er, the z and radial components of each cell’s orientation director, respectively; ϕ, the volume fraction of cells in space; and ζ, the cell-to-cell alignment parameter, defined as the absolute value of the cosine of the angle between neighboring cells, all averaged locally within a given region. The ϕ plot shows that the cluster has a roughly hemispherical shape with a dense core. The term ez captures the vertically aligned central core that begins forming around 14 h after biofilm initiation (Fig. S2 and Movie S3). By contrast, er shows that the cells located at the periphery are radially aligned throughout the entirety of biofilm growth. The ζ plot demonstrates that cells at the center of a mature cluster are aligned parallel to each other, indicating a high nematic order (24).
Fig. S1.
Image analysis procedure. (A) Raw image of the bottom cell layer of an 18-h Rg biofilm cluster. (B) Image following deconvolution. (C) Image following 3D watershed segmentation and thresholding. In this step, we also manually rejected cells that were obviously not members of the original cluster, such as the cell in the upper left corner and the three cells close to the right edge of the image (compare with A and B). (D) Following connection of adjacent voxels, the orientation of each cell was inferred. A red line with a length equal to the cell was drawn to indicate the orientation of each cell. In the projected view, cells lying flat on the substrate show the full length of the red line, whereas cells aligned vertically (primarily at the center of the cluster) appear as red dots. (E and F) Top and bottom views of the segmented cluster, with cells color-coded according to z height. (Scale bar: 5 μm.) The color bar spans from 0 to 21 μm.
Fig. S2.
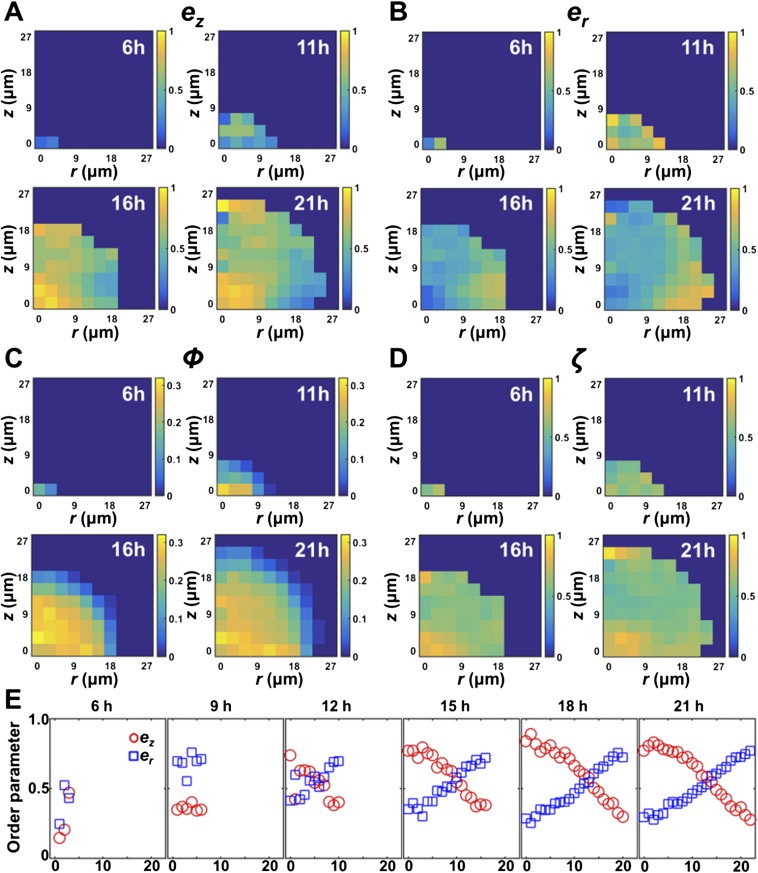
Evolution of spatial structure during the growth of a V. cholerae biofilm cluster. The spatial distribution of ez (A), er (B), ϕ (C), and ζ (D) as a function of height z and radial distance r away from the center of the cluster is shown for four different time points. (E) Time evolution of and as a function of r, averaged over cells that are within a 2-μm distance from the substrate. At t = 9 h, ez begins to increase at the center of the cluster, and the vertically ordered region gradually expands. By contrast, er has a high value for cells at the periphery at early times, and the maximum er value remains approximately constant throughout biofilm growth. These results indicate that the radial expansion of the cells at the rim precedes the vertical ordering of the cells at the center of the cluster.
To define the global features of a growing biofilm cluster during development, we plotted four spatially averaged parameters versus time (Fig. 1G) for a single cluster. The total cell number initially increases exponentially, but subsequently slows before entrance into stationary phase. The growth curve of biofilm cells is similar to the growth curve of their planktonic counterparts. The averaged orientation of the cells in the center of the cluster evolves from a horizontal ( = 0) to vertical orientation ( ∼ 0.7) consistent with observations in Fig. 1 A–D. Vcell, the effective biovolume per cell, characterizes the compactness of the cell cluster and shows three characteristic phases. In phase I, the steady increase in Vcell captures the 2D branched growth pattern. In phase II, Vcell decreases, indicating that the cells proliferate within a confined space in this phase. Notably, this increase in cluster compactness coincides with a steady increase in . Thus, the increase in cell density at the center of the biofilm is tightly coupled to cell reorientation events that vertically align the cells. A faster expansion rate in the cluster height than in the radius occurs immediately after this transition (Fig. 1G, Inset), showing that biofilm development has changed from 2D growth to 3D expansion. Vcell finally plateaus upon entry into stationary phase.
The 2D-to-3D Transition Is Caused by Surface Adhesion.
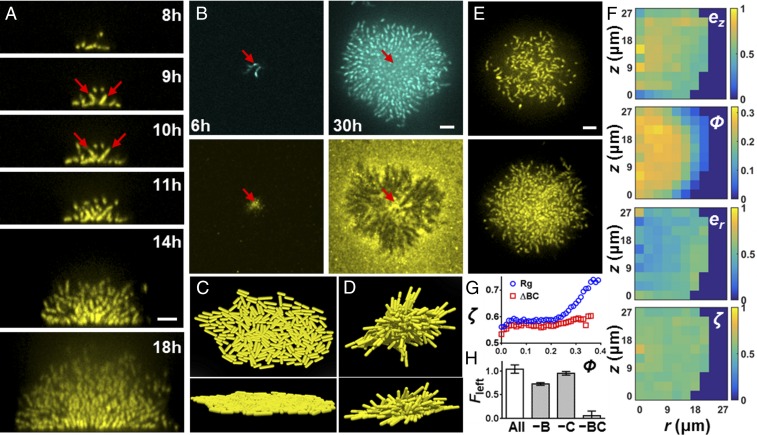
To determine the trigger of the 2D-to-3D biofilm transition and, ultimately, to cell ordering, we inspected the period between phases I and II of the Vcell curve from Fig. 1G. During this transition, individual cells at the center of the biofilm reorient away from their initial configuration parallel to the surface (Fig. 2A). Daughter cells are born from the poles of these reoriented cells and necessarily extend in the third dimension, triggering the formation of the 3D dome. The reorientation event is puzzling, given that V. cholerae cells are adhered to the substrate by the matrix protein Bap1 (25, 26). To investigate this phenomenon, we stained living biofilms made from cells carrying a Bap1-3× FLAG fusion in situ with Cy3-congugated anti-FLAG antibody (Fig. 2B and Movie S4). The fusion caused no changes in biofilm morphology. Consistent with earlier work (17), the founder cells secrete significant Bap1 that diffuses to cover the surrounding surface. Surprisingly, in the mature biofilm, Bap1 remains surface-bound, whereas the cells have been lifted up away from the surface, suggesting that a mechanical force overcomes cell-Bap1 bonds to enable cells to reorient vertically.
Fig. 2.
Cell reorientation and ordering require surface adhesion. (A) Side view of a growing biofilm cluster of V. cholerae at the indicated times. Red arrows indicate the reoriented cells, as well as their daughter cells. (B) Growing V. cholerae cluster (Top) with Bap1 labeled with Cy3 antibody (Bottom) at 6 and 30 h. Arrows indicate the position of the founder cell. (C and D) Top and side views of a simulated biofilm without (C) and with (D) cell-to-surface attachment. (E) Representative biofilm cluster from Δbap1ΔrbmC cells at 18 h at the bottom layer (Top) and 5 μm above the surface (Bottom). (F) Plots of ez, er, ϕ, and ζ for the image shown in E. (G) Local order parameter ζ(ϕ) versus ϕ for the Rg parent strain (blue circles) and Δbap1ΔrbmC (ΔBC, red squares). (H) Restoration of cell-to-surface attachment in Δbap1ΔrbmC biofilms by cell-free conditioned medium lacking the indicated component(s). B denotes Bap1, and C denotes RbmC. Surface attachment is quantified by the fraction of biomass remaining after being subjected to flow (Fleft), averaged over four biological replicates (error bars correspond to SDs). (Scale bars: A, B, and E, 5 μm.)
To represent the biofilm growth process in silico and uncover the forces underlying the cell reorientation events, we developed an agent-based simulation of biofilm formation that incorporates rod-shaped bacteria with and without cell-to-surface bonds (27, 28) (Fig. 2 C and D and Movie S5). We begin the simulation with a founder cell oriented parallel to the surface. Descendent cells lacking surface attachment spread out along the surface but remain in two dimensions. Cells at the periphery tend to orient tangential to the cluster edge due to being pushed by cells inside the cluster (29). However, when surface attachment is added to the simulation, rim cells maintain their radial orientation throughout growth, reproducing the experimental observation. The central cells, by contrast, are forced to tilt into the third dimension when their surface-adhesion bonds at one pole are overpowered by the total mechanical forces exerted by surrounding cells lying flat on the surface. Reoriented cells automatically extend their subsequent descendants further into the vertical dimension. The agent-based simulations reveal the dual role of cell-to-substrate adhesion: It provides a biophysical mechanism for cells first to attach to the surface and second to reorient and undergo 3D growth as cell density increases. The simulation is not intended to reproduce the complete nematic ordering that occurs in mature biofilms, because we have not yet implemented other matrix components (RbmA and Vps).
We hypothesized that the same surface-associated compression that reorients the cells during growth is responsible for the nematic ordering observed in mature biofilms. To test this hypothesis, we generated deletions of the genes encoding the proteins responsible for cell-to-surface adhesion. Single mutants of Δbap1 and ΔrbmC exhibit the same biofilm development pattern as the parent (Fig. S3), consistent with the partially redundant roles of Bap1 and RbmC in mediating cell-to-surface adhesion (17) (Fig. S4). Clusters of the double Δbap1ΔrbmC mutant, however, adopt roughly spherical shapes and float above the surface (Fig. 2E and Movie S6), consistent with surface attachment being eliminated. A remarkable feature of the Δbap1ΔrbmC double-mutant biofilm is that it has also completely lost cell ordering (Fig. 2F): No vertically or radially ordered region can be identified. Indeed, neighboring cell orientation is uncorrelated, even though cells remain connected to one another by RbmA (Fig. S4). The disorder is not simply caused by a reduction in biofilm density: Fig. 2G shows that for the parent strain, higher local volume fraction is correlated with a stronger local alignment, whereas this trend is abolished in the Δbap1ΔrbmC double mutant. Coating the surface with exogenous Bap1 and/or RbmC restores cell-to-surface attachment to the Δbap1ΔrbmC biofilms, as well as partially restoring cell ordering (Fig. 2H and Fig. S5). Therefore, we conclude that the nematic cell ordering inside biofilms is also a surface-associated phenomenon, likely caused by the same growth-induced surface compression that continuously realigns and packs cells in the vertical direction.
Fig. S3.
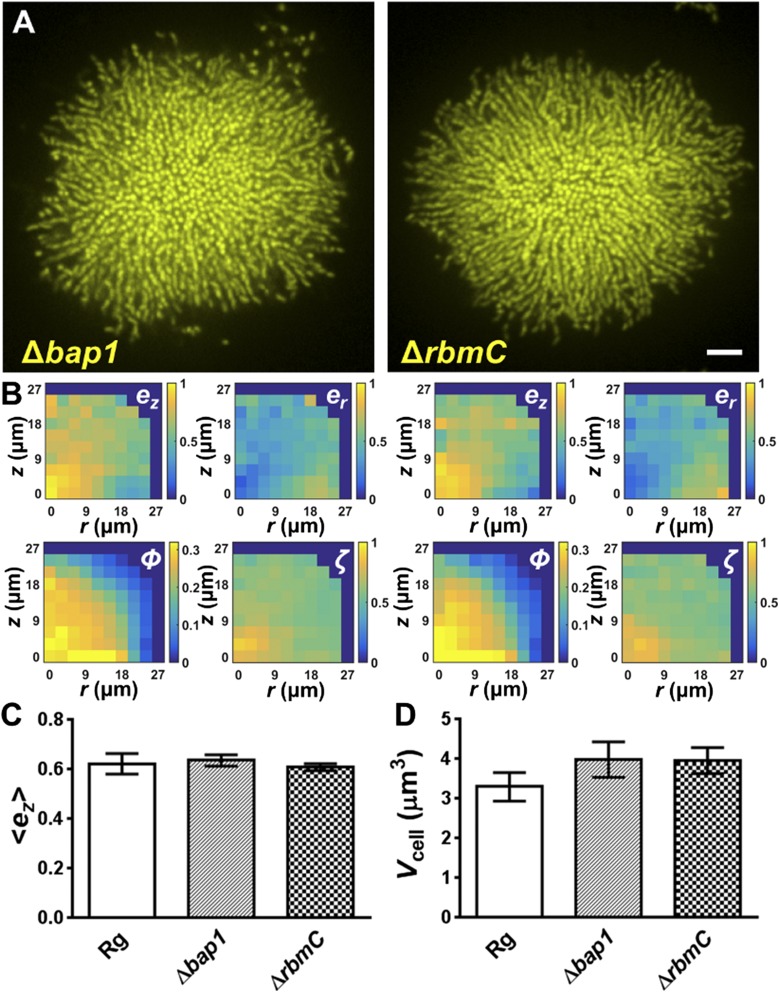
Δbap1 and ΔrbmC single mutants do not exhibit defective biofilm phenotypes. (A) Representative images are shown for a biofilm cluster from the Δbap1 (Left) and the ΔrbmC (Right) single mutants at 21 h. (Scale bar: 5 μm.) (B) Corresponding spatial distributions of ez, er, ϕ, and ζ. No significant difference is apparent compared with the parent strain. (C and D) Spatially averaged vertical alignment parameter and volume per cell Vcell at 21 h averaged over eight clusters for each strain (error bars correspond to SDs). A modest decrease in cluster compactness is evident in the single mutants, presumably due to a looser Vps envelope around the cell clusters that lack Bap1 or RbmC as one of the structural components. Otherwise, no significant difference could be detected in the two single mutants compared with the parent strain.
Fig. S4.
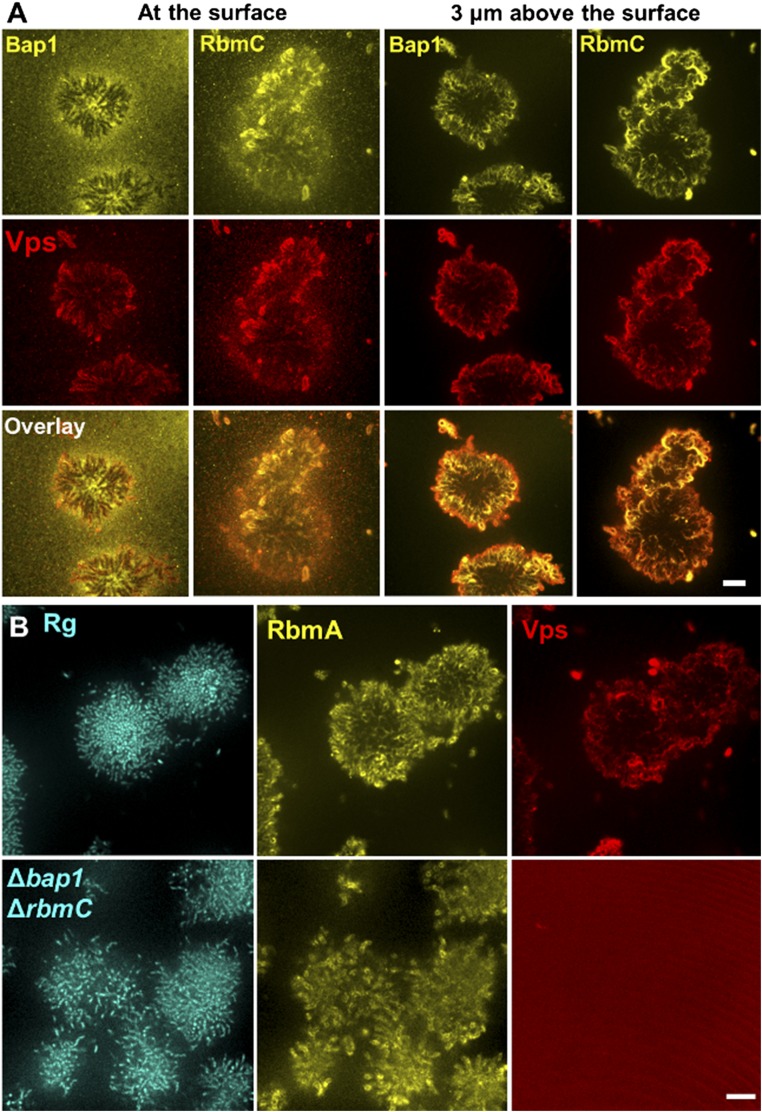
Spatial distributions of matrix components. (A) Biofilm cluster was simultaneously stained with Cy3-conjugated anti-FLAG antibodies and Texas red-conjugated wheat germ agglutinin (WGA) to illustrate the spatial distribution of RbmC and Bap1 (yellow, Top) and compare them with the spatial distribution of Vps (red, Middle). The overlaid images (Bottom). The distributions were followed at the surface (Left) and away from the surface (Right). Away from the surface, both Bap1 and RbmC colocalize to the same extent with Vps, forming an envelope around the biofilm cluster. At the surface, however, the Bap1 signal is more pronounced under the early biofilm-forming cells, whereas the RbmC signal continues to colocalize with Vps. Hence, we suggest that RbmC and Bap1 can each achieve cell-to-surface adhesion independently, but via different mechanisms. Whereas Bap1 directly acts as an adhesive between the cells and the surface, RbmC helps Vps to form an envelope that clamps the entire cluster to the substrate. The partially redundant functions of RbmC and Bap1 are consistent with the high similarity between their sequences (26). The direct adhesion mechanism makes Bap1 more effective than RbmC in mediating cell-to-surface adhesion, as shown by the attachment assay in Fig. 2H. (Scale bar: 10 μm.) (B) RbmA links cells together both in the parent Rg strain and in the Δbap1ΔrbmC mutant. (Left) Strains constitutively expressing mTFP1 from an ectopic chromosomal location. (Center) Distribution of RbmA-3× FLAG stained with Cy3-conjugated anti-FLAG antibodies. (Right) Distribution of Vps stained with Texas red-conjugated WGA. In the biofilm clusters of the Rg parent strain, RbmA permeates throughout the entire cluster and can be imaged on the surfaces of all cells, whereas Vps primarily forms envelopes around cell clusters. In the Δbap1ΔrbmC double mutant, the RbmA signal is still present, but the Vps signal is below the detection limit. This observation suggests that one important function of RbmC and Bap1 is to prevent Vps from leaking away into the medium. (Scale bars: 10 μm.)
Fig. S5.
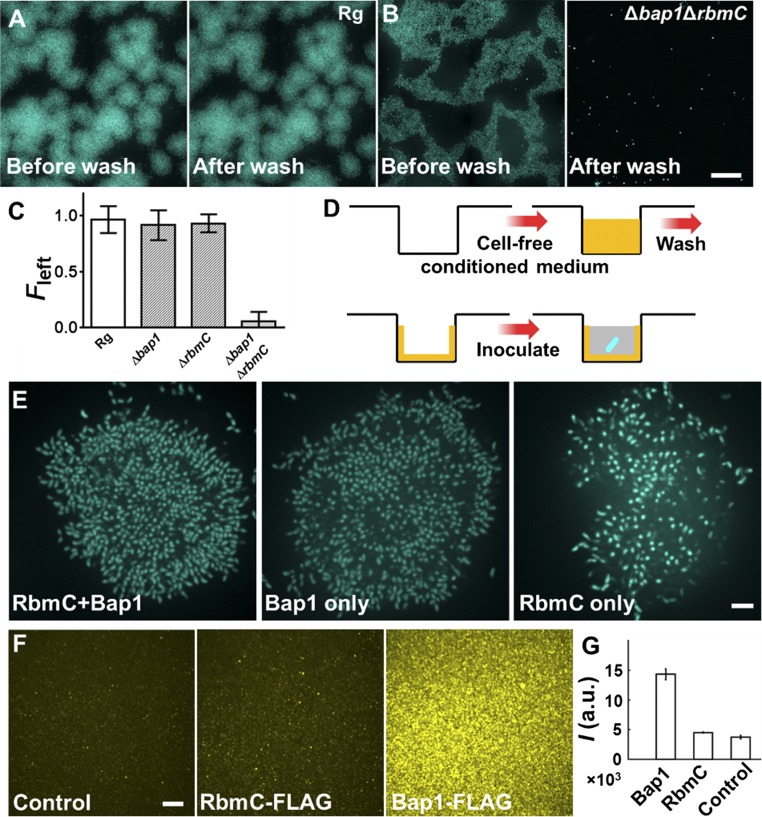
Quantitation of cell-to-substrate adhesion. Representative large-scale images are shown for Rg (A) and Δbap1ΔrbmC double mutant V. cholerae (B) biofilms before (Left) and after (Right) washing. (Scale bar: 40 μm.) Note that under high cell density inoculation conditions, the Δbap1ΔrbmC double-mutant clusters merge. Both strains constitutively express mTFP1 from an ectopic chromosomal location to enable quantification. mTFP1 is less sensitive to oxygen limitation than mKO under high cell density inoculation conditions, which is why this marker was used instead of mKO for this set of experiments. (C) Surface attachment quantified by the fraction of biomass remaining after subjection to flow (Fleft) for different strains. Five biological replicates were examined. Errors bars correspond to SDs. The Δbap1 and ΔrbmC single mutants do not show statistical differences from the Rg parent, whereas the double mutant abolishes the surface attachment. (D) Schematic representation of the surface-coating experiment shown in Fig. 2H. Yellow represents the conditioned medium and the components remaining after washing. Blue represents the V. cholerae cells. Gray represents fresh growth medium. (E) Cross-sectional image of the bottom cell layer for Δbap1ΔrbmC double-mutant clusters grown on surfaces coated with conditioned fluids containing RbmC and Bap1 (Left), Bap1 only (Center), or RbmC only (Right). (Scale bar: 5 μm.) The Δbap1ΔrbmC double-mutant cells attach better to surfaces coated with fluids containing Bap1 than to surfaces with no Bap1. If Bap1 is present, cells display little difference in attachment in the presence or absence of RbmC. This result suggests that Bap1 plays the major role in mediating cell-to-surface adhesion, which is consistent with our demonstration in Fig. 2H that Bap1 is superior to RbmC in rectifying the attachment defect in the Δbap1ΔrbmC double mutant. (F) RbmC and Bap1 spontaneously associate with the surface in the absence of cells. Cell-free conditioned medium from a ΔvpsL V. cholerae strain (control, Left), the ΔvpsL strain carrying FLAG-tagged RbmC (Center), or the ΔvpsL strain carrying FLAG-tagged Bap1 (Right) were used to coat the surfaces. The chambers were washed and subsequently incubated with M9 medium containing Cy3-conjugated anti-FLAG antibody. The Cy3 signal from the surface was imaged. (Scale bar: 10 μm.) Substantial surface-bound Bap1 occurs. This observation suggests that both the Bap1 and RbmC proteins can spontaneously associate with the surface in the absence of cells, with Bap1 adhering more strongly than RbmC, consistent with results in Fig. 2H. (G) Quantitation of surface adsorption of RbmC and Bap1 by integrating Cy3 surface signal intensities I in the images shown in F (n = 3). a.u., arbitrary units. Error bars correspond to SDs.
Cell-to-Cell Adhesion Controls the Biofilm Growth Mode.
We next investigated the role of cell-to-cell adhesion, mediated by the matrix protein RbmA (17, 30, 31), in shaping biofilm architecture. Fig. 3A shows a representative image of the bottom layer of a ΔrbmA mutant biofilm cluster, showing much larger cell-to-cell distances than in the parent. The most striking difference in overall biofilm morphology relative to the parent strain is the much reduced cell density in the structure at increasing distance from the substrate, quantitatively shown in Fig. 3B by the ϕ plot. We note that degradation or down-regulation of RbmA is not responsible for the ordered packing in the parent (Fig. S6). The time evolution of the ΔrbmA mutant biofilm cluster development is shown in Fig. 3C and Movies S7 and S8. Following the reorientation transition in the bottom layer of cells, the ΔrbmA mutant “explodes” into the third dimension insofar as newly born daughter cells immediately move away from the substrate, maintaining their vertical orientation and leaving behind the surface-attached mother cells. The mature ΔrbmA mutant biofilm hence harbors a much larger region of vertically oriented cells than the parent, as characterized by high local ez (Fig. 3 B and D).
Fig. 3.
Deletion of RbmA causes biofilm expansion driven by matrix production. (A) Cross-sectional image of the bottom layer of a biofilm cluster of ΔrbmA cells at 18 h. (B) Corresponding spatial distribution of ez and ϕ. (C) Side view of formation of the ΔrbmA biofilm cluster at indicated times. (D) Time evolution of cell number, , Vcell, R, and H for the ΔrbmA biofilm cluster shown in C and D. Color and symbol designations follow Fig. 1. Vcell shows an additional phase in which Vcell increases sharply (red arrow), characterizing the expansion mode driven by the matrix. (E) Fraction of cells remaining following mechanical perturbation (Fleft) for the Rg parent biofilm (□) and the ΔrbmA biofilm (○) averaged over four biological replicates (error bars correspond to SDs). For ΔrbmA biofilms, we characterize Fleft for the bottom 75% (red) and top 25% (blue) biofilm biomasses, respectively. (F) Final fraction of the ΔrbmA mutant remaining (fΔrbmA) starting from a 1:1 mixture of the Rg parent strain and the ΔrbmA mutant at the surface with low surface coverage (○) and high surface coverage (■). (G) Representative contact configuration between an Rg biofilm cluster (yellow) and a ΔrbmA mutant cluster (red), shown with two channels combined (Left) and separated (Center and Right). (Scale bars: A, C, and G, 10 μm.)
Fig. S6.
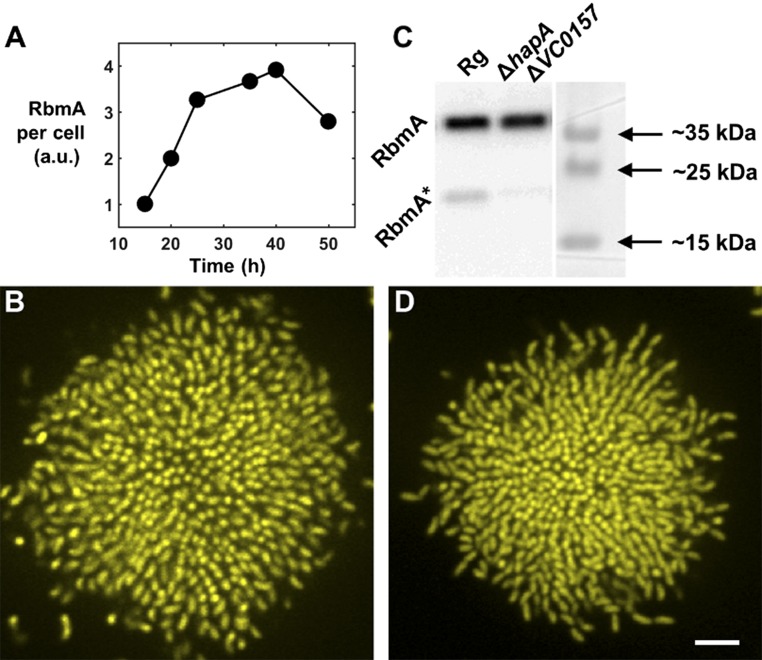
Repression and degradation of RbmA are not responsible for V. cholerae cell ordering in biofilms. (A) Time-dependent Western blot tracking RbmA protein per cell in the biofilm. Signal intensity is normalized to the first time point. RbmA per cell increases for the first 2 d of biofilm growth, consistent with a previous report (40). (B) Cross-sectional image of the bottom cell layer of a V. cholerae biofilm cluster carrying Ptac-rbmA, showing cellular ordering occurs when RbmA is constitutively produced throughout biofilm maturation. It is demonstrated in A and B that altered regulation of rbmA cannot account for the increased cell ordering that occurs in the parent biofilm clusters over time. (C) At high cell density, RbmA is cleaved by several proteases to produce the species denoted RbmA* (40). In a ΔhapAΔVC0157 strain, no RbmA degradation occurs, as shown by the Western blot. (D) Nonetheless, no change in biofilm ordering occurs, suggesting that it is not degradation of RbmA that gives rise to the increased cell ordering that occurs over time in biofilm clusters of the parent strain. (Scale bar: 5 μm.)
We suggest that the absence of cell-to-cell adhesion in the ΔrbmA mutant allows biofilm expansion mediated by the polymer matrix. Specifically, subsequent to the reorientation events at the surface, the polymer matrix is able to fill the spaces between ΔrbmA mother and daughter cells and, in so doing, to carry cells upward, away from the surface. This transport process underpins the dramatic contrast in Vcell between the ΔrbmA mutant and the parent strain. Whereas in the parent Rg strain, the cluster becomes more dense following the reorientation transition, and thus Vcell decreases, in the ΔrbmA mutant, Vcell increases sharply due to the rapid expansion into the third dimension.
Mechanical Advantages of a Dense Biofilm.
We hypothesize that the compact structure of the parent biofilm makes the overall biofilm more resistant to mechanical disruption, perhaps enhancing its stability in the environment. To test this idea, we subjected the different V. cholerae biofilms to mechanical agitation (SI Materials and Methods). The Rg parent biofilm maintained full integrity after vigorous shaking, whereas the ΔrbmA mutant biofilm lost about one-fourth of its original biomass (Fig. 3E). Furthermore, biomass was lost primarily from the top layers, the region in which the ΔrbmA mutant biofilm has low local cell density (Fig. 3B).
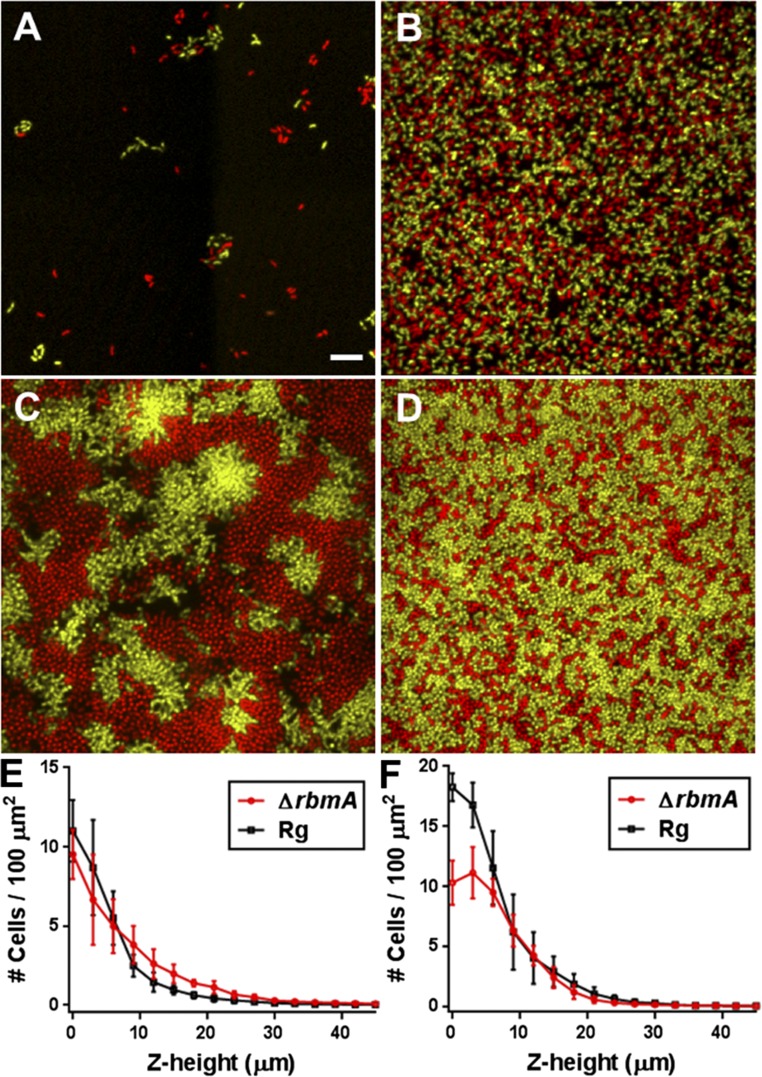
To assess the consequences of the loss of RbmA on biofilm mechanical properties further, we performed a biofilm competition assay between the ΔrbmA mutant and the parent strain (SI Materials and Methods). At a 50:50 starting ratio and at low initial surface coverage (Fig. S7), the two strains had nearly the same fitness (Fig. 3F). However, at high surface coverage, where biofilms of different strains are expected to collide and compete for space (32), the ΔrbmA mutant is at a disadvantage (the final frequency of the ΔrbmA strain, fΔrbmA = 0.42 after 1 d of biofilm growth). Specifically, the ΔrbmA mutant is outcompeted at the bottom layer so much that its access to the third dimension is inhibited (Fig. S7). Although, in principle, the ΔrbmA mutant could extend further into the third dimension and access additional nutrients at later stages of biofilm growth as suggested by simulations (9), we do not observe such a reversal of fitness. Hence, we conclude that, under our conditions, competition for space is the overriding factor that determines the outcome of the competition.
Fig. S7.
Biofilm competition between the ΔrbmA and parent V. cholerae strains. Representative initial conditions for biofilm competition at low surface coverage (A, <5%) and high surface coverage (B, >50%). (Scale bar: 10 μm.) The Rg parent strain constitutively expresses mKO (yellow), and the ΔrbmA strain constitutively express mKate2 (red). Representative images following overnight biofilm growth for low surface coverage (C) and high surface coverage (D), at the bottom cell layer. Biomass distribution as a function of distance away from the surface in the cocultured biofilms, starting from low surface coverage (E) and high surface coverage (F). At low surface coverage, we observe that the ΔrbmA mutant extends further in the z direction, but the overall biomass is similar to the parent strain. At high surface coverage, the ΔrbmA strain is mechanically displaced from the bottom layers by the mechanically stronger parent strain, which prevents the ΔrbmA strain from expanding into the third dimension.
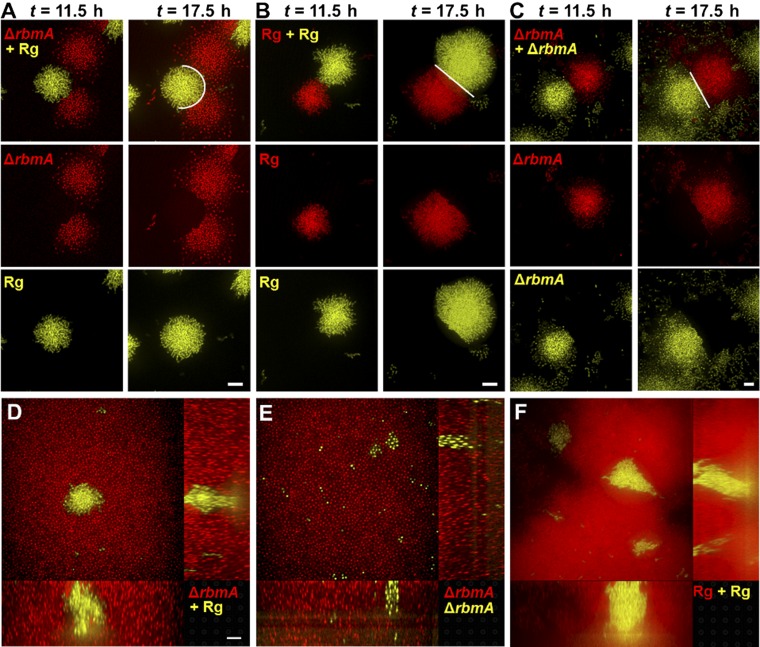
The interface between an Rg parent biofilm cluster and a ΔrbmA mutant cluster exemplifies the mechanical differences between the strains (Fig. 3G). The hemispherical shape of the parent biofilm cluster is minimally perturbed by the presence of the colliding ΔrbmA mutant cluster, whereas the ΔrbmA mutant cluster is deformed by the colliding parent biofilm cluster. By contrast, collisions between two Rg biofilm clusters or two ΔrbmA biofilm clusters result in straight boundaries (Fig. S8). Furthermore, isolated clusters of the parent strain are able to expand laterally even if embedded within ΔrbmA mutant biofilms (Fig. S8). Hence, we conclude that the dense growth mode driven by bacterial proliferation, rather than matrix expansion, endows V. cholerae biofilms with strong mechanical properties, and consequently provides an evolutionary advantage so that they can withstand environmental perturbations, such as shear flow, mechanical shock, or competition with other biofilm-forming species.
Fig. S8.
Biofilm clusters of the ΔrbmA strain are mechanically weaker than the parent strain. (A) After growth of a biofilm mixture containing the Rg and ΔrbmA strains at low seeding density for 10 h, clusters collide with each other. Collision dynamics were monitored over time. Images at t = 11.5 h and t = 17.5 h are shown for the second cell layer, avoiding any contribution from differences in surface attachment abilities. Individual channels (Middle and Bottom) are shown as well as the overlaid images (Top). White lines are guides to the eye for the interfaces. The experiment was repeated for colliding clusters of isogenic strains expressing different fluorescent reporters for the Rg parent strain (B) and for the ΔrbmA strain (C). (D) By seeding the ΔrbmA cells (red) at high surface coverage at 50:1 with the parent cells (yellow), we observe that the mechanically stronger Rg strain is able to expand inside ΔrbmA biofilms (red) by displacing the ΔrbmA cells. Both the xy cross-sectional view (Top left) and two side views (Bottom and Right) are shown. Note that this displacement takes place above the surface, because the two strains have the same cell-to-surface adhesion strength. (E) As a control, we show that the ΔrbmA strain (yellow) is not able to displace isogenic cells (red). (F) The same is true for the parent strain. (Scale bars: 10 μm.)
The V. cholerae Biofilm Development Program.
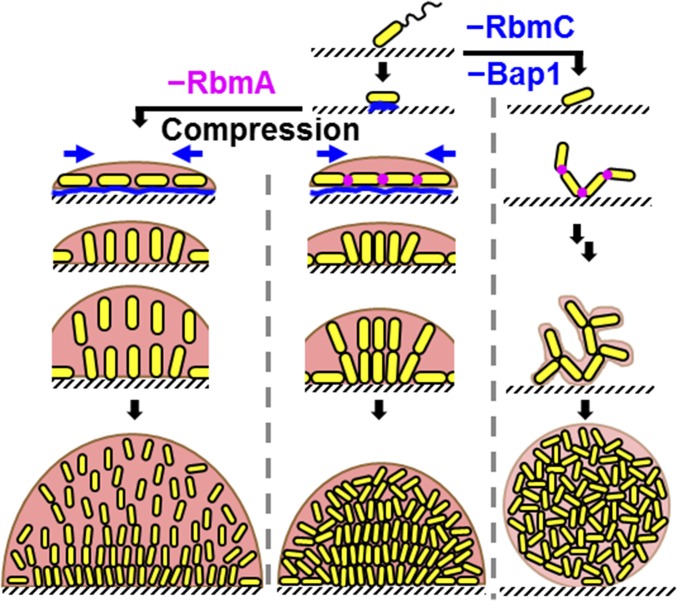
Our results suggest the following model for the V. cholerae biofilm structural development program (Fig. 4). Following attachment to the surface, the founder cell orients horizontally to maximize cell-to-surface adhesion, which is mediated by Bap1/RbmC. Subsequent divisions oriented along the long axes of the rod-shaped cells confine the early descendants to the same plane as the founder cell, resulting in a relatively flat colony. During midexponential growth, cells proliferate rapidly but their expansion in space is restricted by the surface-attached, peripheral cells. The combination of expansion and confinement generates an effective anisotropic stress that overpowers the cell-to-surface adhesion force for cells at the cluster center, causing these cells to realign in the vertical direction and triggering the transition from 2D expansion to 3D growth. The modestly curved shape of V. cholerae might further facilitate such reorientation events. Similar compression-driven reorientation is observed in bacterial colony growth confined between agar and glass (29, 33); in the current context, we show the biological relevance of this transition for natural biofilm growth. The descendants of the reoriented cells remain connected to one another at their poles by RbmA (17), so local proliferation leads to a steady increase in cell density. The increased density translates into an enhanced compression that gradually packs the central core of the cluster into a nematically ordered state with the rod-like cells all oriented perpendicular to the surface, which further amplifies the anisotropic growth of the biofilm in the vertical direction. In this growth mode, biofilm expansion is primarily driven by the directional proliferation of the bacterial cells themselves. The production of extracellular polymer could, in principle, expand the biofilm; however, such expansion is resisted by the cell-to-cell connections. By contrast, in the ΔrbmA mutant, the absence of cell-to-cell linkages switches the biofilm growth mode to one that is mainly driven by matrix expansion, leading to an overall larger, but much looser, biofilm. The absence of cell-to-surface adhesion in the Δbap1ΔrbmC mutant enables the cells to grow away from the surface, but without any surface-mediated compression, the biofilm remains disordered. In essence, V. cholerae has evolved specific matrix proteins with properties that allow it to harness the consequent biophysical processes to achieve a 3D, strong, and ordered biofilm structure that firmly attaches to surfaces. Indeed, underproduction or overproduction of any of these three crucial proteins inhibits proper development of the biofilm structure (Fig. S9). For example, overexpression of Bap1 leads to enhanced attachment of cells to the surface, which impedes the transition into the third dimension.
Fig. 4.
Schematic representation of the V. cholerae biofilm formation process. Yellow cylinders represent the rod-shaped bacterial cells. Blue represents the RbmC/Bap1 matrix proteins that adhere the cells to the surface. Magenta denotes the RbmA protein. The pale peach background represents the Vps matrix, and its transparency in the different panels corresponds to the amount of Vps present in the biofilm. The brown contour at the rim of the biofilms represents the envelope formed by Vps/RbmC/Bap1. Blue arrows denote the spontaneously generated, surface-associated compression that is responsible for cell reorientation and ordering.
Fig. S9.
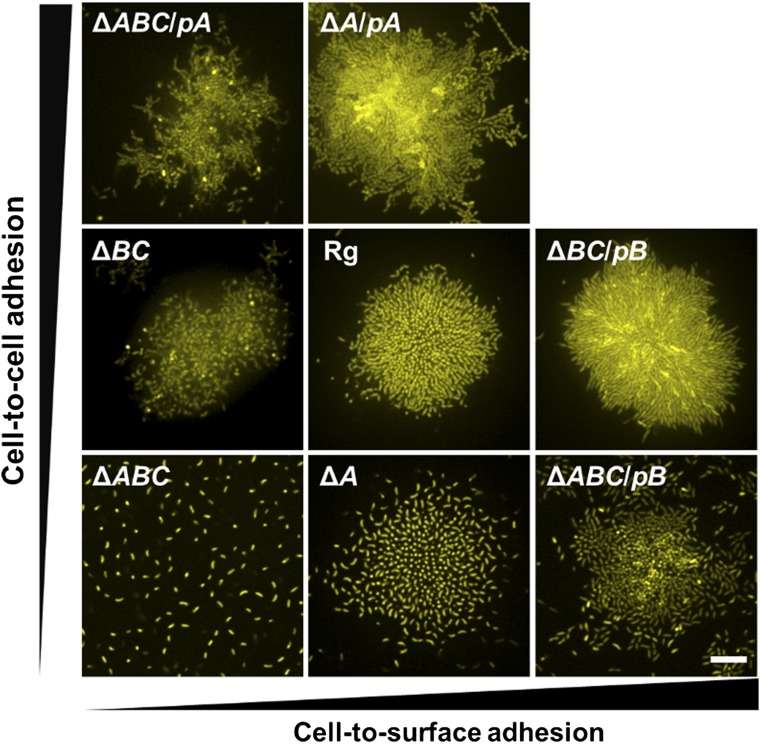
Structure titration with controllable protein production. We alter the biofilm cluster structure by changing RbmA-mediated cell-to-cell adhesion and/or Bap1/RbmC-mediated cell-to-surface adhesion using an arabinose inducible promoter. Shown are cross-sectional images at 5 μm (Left) and 0.5 μm (Center and Right) above the surface. pA and pB denote plasmid production of RbmA and Bap1, respectively. Because Bap1 and RbmC have redundant functions in mediating cell-to-surface adhesion and Bap1 plays the dominant role, we only show the Bap1 control in the ΔrbmAΔbap1ΔrbmC triple mutant (ΔABC). Differences in fluorescence intensity are due to heterogeneity caused by the plasmid. The ΔrbmAΔbap1ΔrbmC triple mutant forms a disorganized sparse web of cells dislodged from the surface. Increasing production of RbmA (going up on the vertical axis) increases the cluster density, but surface adhesion and cell-to-cell alignment do not occur. By contrast, increasing production of Bap1/RbmC (going right on the horizontal axis) causes the cells to adhere to the surface and restores cell-to-cell alignment. Overexpression of Bap1, however, locks the cells to the surface, which prevents the transformation to the 3D structure. Overexpression of RbmA also hinders the cell ordering process because the cells remain locked in a disordered configuration. Hence, V. cholerae possesses the appropriate blend of RbmA and Bap1/RbmC to enable cell-to-surface adhesion so that there is a smooth transition to vertical cellular alignment and formation of a 3D structure that nonetheless remains adhered to the surface. (Scale bar: 10 μm.)
Our findings regarding the V. cholerae biofilm developmental process invite interesting comparisons with other biofilm-forming bacterial species. We provide two here. For species with surface twitching motility, such as Pseudomonas aeruginosa, it is conceivable that surface-associated pressure might be relieved by surface motion, and, indeed, aggregation of motile P. aeruginosa is suggested to initiate biofilm formation rather than clonal growth from a single cell (13). However, in later stages of the process, a nonmotile subset of the P. aeruginosa cells forms the 3D stalk at the base of the biofilm (34). The stalk formation process could nonetheless involve reorientation-extension events similar to those events discovered here. Spherical bacteria, such as Staphylococcus aureus, pose a different challenge for 3D biofilm development. Despite their isotropic shape, cocci divide sequentially in orthogonal planes (35). It remains to be discovered how such directional division drives 3D growth for cocci biofilms, especially in cases of cocci, such as S. aureus, that possess low levels of extracellular polysaccharide. Such systems can now be readily accessed using the technology presented in this study.
In conclusion, with our live, single-cell resolution imaging and analysis protocol, we discovered the key structural transitions in the developmental process of V. cholerae biofilms, as well as the underlying biophysical and genetic principles. Going forward, our single-cell imaging technology enables screening for genes that affect fine features of biofilms not resolvable by traditional assays, such as crystal-violet staining (36), colony morphology descriptions, or overall biomass quantification. Further increasing the spatial and temporal resolution of the technology will enable tracking of cell shape, size, and lineage to address the cell biological processes (37) occurring inside bacterial biofilms. Finally, we envision extending our single-cell biofilm analyses to gene expression to assess cell-to-cell heterogeneity (38, 39) in response to stimuli that include antibiotics, signal molecules, and nutrient limitation.
SI Materials and Methods
Quantification of Surface Adhesion.
To quantify cell-to-surface adhesion, we inoculated Vibrio cholerae strains at OD600 = 0.1 for 1 h to obtain high surface coverage that produced statistically meaningful data. Biofilms were grown at 30 °C for 16–18 h and imaged. The biofilms were washed twice, supplied with fresh medium, and imaged again at the original positions. Custom MATLAB code was used to quantify the biomass before and after the washing steps.
Mechanical Perturbation of Biofilms.
To quantify the mechanical strength of the biofilms, the biofilm growth procedure in the preceding section was conducted. Next, the overnight biofilms were washed twice, and 100 μL of fresh M9 medium without glucose was added to each well before imaging. This step removed cells that were only loosely attached to the biofilm. More importantly, the replacement with medium without nutrient minimized cell growth during the imaging step. The entire 96-well plate was placed on a mechanical vibrator (Union Scientific) at the highest power for 2 min. The washing step was repeated, and the biofilms were imaged again at the original positions. Custom MATLAB code was used to quantify the biomass before and after mechanical perturbation. Four biological replicates were performed for each strain; error bars correspond to SDs. The fraction of cells remaining, Fleft, is slightly larger than 1 for the Rg parent strain due to modest cell growth that occurred during sample preparation and imaging. To define the bottom and top layers, we quantified the biomass for each original biofilm as a function of height and, integrating from the bottom up, set the division plane at the height where the accumulative biomass reached ∼75% of the total.
Surface Coating with Matrix Proteins.
Cultures of a V. cholerae ΔvpsL mutant were grown in 96-well plates as above. The absence of extracellular polysaccharide (Vps) causes this strain to release its matrix proteins into the medium. We collected and prepared cell-free spent culture fluids using syringe filtering with 0.22-μm filters (Millex), and we added 100 μL of the preparations to empty wells of 96-well plates for 90 min. The wells were washed twice, and all liquid was removed before immediate use in biofilm formation assays as above.
Matrix Staining.
We constructed V. cholerae strains constitutively expressing mTFP1 from an ectopic chromosomal locus and carrying a C-terminal 3× FLAG fusion to RbmA, Bap1, or RbmC (17, 26). The genes encoding the matrix protein–FLAG fusions were expressed from their native chromosomal loci. We used the biofilm growth assay procedure described above for biofilm formation, except that the growth medium contained Cy3-conjugated anti-FLAG antibody (1–3 μg/mL; Sigma–Aldrich) and Texas red-conjugated wheat germ agglutinin (1 μg/mL; Thermo Fisher). Three diode lasers (445 nm, 543 nm, and 592 nm) were used sequentially, along with customized dichroic filters (Chroma) for detection. For time course imaging, the time interval was 1 h and the z-step size was 3 μm, which minimized cell damage from the short-wavelength laser. A 60× total internal reflection fluorescence (TIRF) oil objective was used to collect high signal level.
Biofilm Competition Assay.
We competed the parent Rg V. cholerae strain carrying constitutively expressed mKO from an ectopic chromosomal locus and the ΔrbmA strain with constitutively expressed mKate2 from the same locus. Overnight cultures were first grown in the presence of glass beads and vigorously shaken, and then back-diluted 30-fold and grown with shaking in M9 medium, again with glass beads, to OD600 ∼ 0.4 for low surface coverage and to OD600 ∼ 1.0 for high surface coverage. The regrown cultures were transferred to Eppendorf tubes with smaller glass beads (acid-washed, 425–500 μm; Sigma) and vigorously bead-bashed on a vortex mixer. These steps were necessary to disassemble all cell clusters that had formed in the liquid cultures for accurate OD600 measurements and OD600 equalization. The OD600 of each culture was measured, and the culture was back-diluted into fresh M9 medium to a final OD600 of 0.05 for low surface coverage and to 0.5 for high surface coverage for each strain, respectively. One hundred microliters of these mixtures was added to wells of 96-well plates and incubated for 1 h, after which the standard washing step was repeated, and 100 μL of fresh M9 medium was added. The initial condition was imaged under the confocal microscope with two lasers (543 nm and 592 nm) and customized dichroic filters. Customized MATLAB code was used to calculate the initial frequency of each strain. If the initial frequency of one strain was lower than 0.48 or higher than 0.52, the sample was discarded. The surface coverage was also calculated, and samples with initial surface coverages <5% and >50% were used. The cocultured biofilms were grown overnight (∼18–22 h) at 30 °C, washed by the standard procedure, and imaged. Custom MATLAB code was used to calculate the total biomass for each strain in the cocultures, as well as biomasses as a function of height.
To monitor the consequences of collisions between isolated clusters of the ΔrbmA and the Rg parent strain, we first grew biofilms at low density (initial OD600 = 0.001–0.002, inoculation time = 10 min) for 10 h at 30 °C. The sample was transferred onto the microscope stage, and neighboring clusters were imaged. Starting at 10.5 h, images were acquired every 1 h at a z-step size of 1.0 μm for the detected collision pairs. As controls, we repeated this procedure for the ΔrbmA and the Rg parent in isolation, each with two different fluorescent reporters. We also prepared biofilms starting from nonidentical compositions of strains. In this assay, the ΔrbmA strain (mKate2-labeled) was inoculated at OD600 = 0.5, whereas the Rg parent strain (mKO-labeled) was coinoculated at OD600 = 0.01 and incubated for 1 h. This procedure led to clusters of the Rg parent growing embedded a confluent biofilm of the ΔrbmA strain.
Quantification of Protein Production.
Western blots were performed to quantify RbmA protein production using the strain expressing FLAG-tagged RbmA. Biofilms were grown as above, except 35-mm Petri dishes rather than 96-well plates were used to obtain larger quantities of biofilms. At various times, the biofilms were removed from the surface with a pipette tip, resuspended in PBS, and boiled at 95 °C for 10 min. Bradford assays were performed to determine total protein in each sample, and the data were used to normalize the total amount of protein in samples from different time points. The samples were combined with 4× loading dye and heated at 85 °C for 10 min. Western blots were performed, using anti-FLAG peroxidase (HRP) antibody (Sigma–Aldrich) and chemiluminescence detection. The α-subunit of Escherichia coli RNA polymerase was used as the loading control for quantification. Signals were visualized using an ImageQuant LAS-4000 imager (GE Healthcare), and band intensities were quantified using GelQuant software.
Controlled Expression of Genes Encoding Matrix Proteins.
The arabinose-inducible araC-PBAD promoter was cloned upstream of rbmA and bap1 in plasmid pYS149 to generate pCDN013 and pCDN015, respectively. A control plasmid was constructed harboring the araC-PBAD promoter but no downstream gene. These plasmids were conjugated into V. cholerae strains using kanamycin (Kan) selection. Biofilm experiments were performed as above, except that the medium contained 50 μg/mL Kan. In the final biofilm growth medium, either no arabinose or arabinose at 0.05%, 0.1%, 0.5%, 2%, and 5% (wt/wt) was added.
Definition and Analysis of Order Parameters.
The core MATLAB analysis code can be downloaded via https://github.com/yanjing32/Single-Cell-Tracking/releases/tag/v1.0. For each cell i, we recorded the x, y, and z coordinates of its center and its unit orientation director . The vertical component of was extracted by , in which is the unit vector pointing in the z direction. The center of a cluster was extracted based on the coordinates of all constituent cells. Hence, we defined a new position vector for each cell with the center of the coordinate located at the center of the cluster. The term er,i was then defined as , in which is the unit vector of the projection of onto the x–y plane. We binned the space into 3-μm sections in the z direction and 3-μm sections in the radial direction using a cylindrical coordinate system originating at the center of the cluster. Within each bin, we calculated the percentage of pixels belonging to cells, which we defined as the local volume fraction ϕ. The local order parameter ζ was defined as averaged over the particular volume, in which cells i and j are nearest neighbors as extracted by Delaunay triangulation. The average values of ζ, ez, and er for a random configuration of rod-shaped cells are all 0.5. The biovolume of a cluster was defined as the 3D convex hull that enclosed the cluster. The radius of a cluster R was defined at the position at which the radially averaged volume fraction declined below a threshold close to 0 for the 1-μm imaging planes with the largest radii (the bottom plane for the Rg and ΔrbmA clusters and the middle plane for the Δbap1ΔrbmC clusters). Cluster height H was defined as the z height at which the number of cells declined to 0. In cases in which the imaged z range did not encompass the entire cluster, a linear extrapolation was applied to obtain H, and the number of cells was corrected accordingly. To obtain the relative growth rate of cluster radius R and height H, R and H were plotted against the cube root of the cell number. We next applied a linear fit to the two growth phases separated by the time point when Vcell was at its maximum. Five clusters of the Rg V. cholerae were obtained on different days and analyzed. All clusters exhibited consistent characteristics. Two clusters were analyzed for the ΔrbmA single and Δbap1ΔrbmC double mutants. To obtain the ζ(ϕ) versus ϕ plot, we tracked all neighboring cell pairs and calculated as well as the local ϕ. We collapsed all of the data obtained in this way at different time points from different clusters to plot ζ(ϕ) versus ϕ. We obtained roughly 106 neighboring cell pairs for each strain.
Agent-Based Simulation.
Simulations were performed using the individual-based Dynamics of Microbial Communities Simulator (iDynoMiCS) package, version 1.0 (27), with a plug-in for rod-shaped bacteria (28). Each bacterium was represented by two rigidly connected end points. The distance between the two points increased with time until a threshold length was reached, at which time the cell divided into two daughter cells. The shape of the cell was represented by a cylinder capped with two hemispheres. In each simulation step, the length of the cell was updated first. We next checked if cells overlapped. In cases in which cells overlapped, a repulsive force proportional to the overlapping distance was implemented between the two overlapping cells. The system was next allowed to relax until minimum overlap remained. This procedure constituted one time step in the simulation. We modified the source code to model the translational and rotational motions of bacteria correctly due to mechanical interactions with other cells. The pressure field feature of the simulation was not used. Vps was not simulated explicitly to reduce computation time. Cells grow at a fixed exponential rate. Details of the simulation protocol on which we based our work can be found in a study by Álvarez and Aradas (28).
To simulate Bap1/RbmC-mediated cell-to-surface adhesion, we implemented Hookean springs between the cells and the surface. Specifically, at each time step, we imposed a particular probability for each cell end point to establish surface adhesion, represented by a new vertex situated at the present location of the end point. The vertex was coupled to the corresponding end point by a Hookean spring. When this end point moved away from its original position due to the growth, translation, or rotation of the bacterium, the spring exerted a restoring force to move the end point back toward its original position. If the length of the spring became larger than a threshold length or the end point moved beyond a threshold distance from the surface, the spring broke. Over time, each cell end point established new links with the surface and broke old ones, representing the developing and viscoelastic nature of biofilm cell-to-surface adhesion. Each end point was allowed up to a maximum of 10 springs at any given time. During division, each daughter cell inherited springs from the closest end point of the parent cell, which were subsequently distributed equally between each end point of the daughter cell. Noise was introduced via a modest asymmetry in daughter cell lengths. Slight z variation was introduced when assigning each cell-to-surface adhesion vertex to break cell-to-cell height symmetry such that every cell was at a slightly different height from every other cell within a narrow range.
Key parameters used for the simulation are as follows: time step = 36 s, total simulation time = 260 min, domain size = 17 × 17 × 17 μm, specific growth rate = 0.7 h−1, radius of the cell body and the cap = 0.39 μm, threshold length for division = 2 μm, rotational and translational gain = 0.125, maximum spring length = 0.40 μm, cell-to-surface spring constant = 2.5, height at which the cell-to-surface spring is generated = 0.42 μm, height at which the cell-to-surface spring breaks = 0.60 μm, and probability of generating a cell-to-surface spring = 0.6 per time step. The full simulation protocol is available upon request. All other parameters are default settings in the simulation package.
Materials and Methods
Strains and Media.
All V. cholerae strains used in this study are derivatives of the wild-type V. cholerae O1 biovar El Tor strain C6706, harboring a missense mutation in the vpvC gene (VpvC W240R) that elevates c-di-GMP levels, conferring an Rg biofilm phenotype (23). Additional mutations were engineered into this V. cholerae strain using Escherichia coli S17-λpir carrying pKAS32. All strains were grown in LB at 37 °C with shaking. Biofilm experiments were performed in M9 minimal medium, supplemented with 2 mM MgSO4, 100 μM CaCl2, and 0.5% glucose. A detailed strain list is provided in Table S1.
Table S1.
E. coli and V. cholerae strains used in this study
| Strains/plasmid | Relevant features | Source |
| E. coli | ||
| S17 λ-pir | Wild type | |
| V. cholerae | ||
| C6706str2 | El Tor wild type | (41) |
| JY028 | vpvCW240R | This study |
| JY029 | vpvCW240R lacZ:Ptac-mTFP1:lacZ | This study |
| JY030 | vpvCW240R lacZ:Ptac-mKate2:lacZ | This study |
| JY031 | vpvCW240R lacZ:Ptac-mKO:lacZ | This study |
| JY038 | vpvCW240R ΔvpsL | This study |
| JY039 | vpvCW240R ΔvpsL lacZ:Ptac-mTFP1:lacZ | This study |
| JY048 | vpvCW240R ΔrbmA lacZ:Ptac-mKate2:lacZ | This study |
| JY049 | vpvCW240R ΔrbmA lacZ:Ptac-mKO:lacZ | This study |
| JY073 | vpvCW240R ΔrbmC lacZ:Ptac-mKO:lacZ | This study |
| JY076 | vpvCW240R Δbap1 lacZ:Ptac-mKO:lacZ | This study |
| JY085 | vpvCW240R Δbap1ΔrbmC lacZ:Ptac-mKO:lacZ | This study |
| JY088 | vpvCW240R Δbap1ΔrbmC lacZ:Ptac-mTFP1:lacZ | This study |
| JY096 | vpvCW240R ΔrbmAΔbap1ΔrbmC lacZ:Ptac-mKO:lacZ | This study |
| JY097 | vpvCW240R rbmA-3× FLAG | This study |
| JY131 | vpvCW240R bap1-3× FLAG lacZ:Ptac-mTFP1:lacZ | This study |
| JY147 | vpvCW240R rbmA-3× FLAG Δbap1ΔrbmC lacZ:Ptac-mTFP1:lacZ | This study |
| JY159 | vpvCW240R ΔrbmC lacZ:Ptac-mTFP1:lacZ | This study |
| JY160 | vpvCW240R Δbap1 lacZ:Ptac-mTFP1:lacZ | This study |
| JY165 | vpvCW240R bap1-3xFLAG ΔvpsL lacZ:Ptac-mTFP1:lacZ | This study |
| JY167 | vpvCW240R rbmC-3xFLAG ΔvpsL lacZ:Ptac-mTFP1:lacZ | This study |
| JY182 | vpvCW240R ΔvpsL Δbap1 | This study |
| JY183 | vpvCW240R ΔvpsL ΔrbmC | This study |
| JY184 | vpvCW240R ΔvpsL Δbap1 ΔrbmC | This study |
| JY199 | vpvCW240R ΔrbmA lacZ:Ptac-mKO:lacZ pCDN013 | This study |
| JY207 | vpvCW240R Ptac-rbmA lacZ:Ptac-mKO:lacZ | This study |
| JY210 | vpvCW240R Δbap1ΔrbmC lacZ:Ptac-mKO:lacZ pCDN015 | This study |
| JY216 | vpvCW240R ΔrbmAΔbap1ΔrbmC lacZ:Ptac-mKO:lacZ pCDN013 | This study |
| JY222 | vpvCW240R ΔrbmAΔbap1ΔrbmC lacZ:Ptac-mKO:lacZ pCDN015 | This study |
| JY223 | vpvCW240R lacZ:Ptac-mKO:lacZ pJY014 | This study |
| JY224 | vpvCW240R ΔrbmA lacZ:Ptac-mKO:lacZ pJY014 | This study |
| JY225 | vpvCW240R Δbap1ΔrbmC lacZ:Ptac-mKO:lacZ pJY014 | This study |
| JY234 | vpvCW240R ΔhapAΔVC0157 lacZ:Ptac-mKO:lacZ | This study |
| JY243 | vpvCW240R rbmA-3xFLAG ΔhapAΔVC0157 | This study |
| Plasmids | ||
| pKAS32 | Suicide vector, AmpR SmS | (42) |
| pYS249 | araC-PBAD complementation vector, KanR | (43) |
| pNUT144 | Suicide vector, AmpR KanR SmS | (44) |
| pNUT157 | pNUT144 vpvCW240R | (44) |
| pCMW112 | pKAS32 ΔvpsL | (19) |
| pCN003 | pKAS32 lacZ:Ptac-mTFP1:lacZ | (43) |
| pCN004 | pKAS32 lacZ:Ptac-mKate2:lacZ | (43) |
| pCN005 | pKAS32 lacZ:Ptac-mKO:lacZ | (43) |
| pCN007 | pKAS32 ΔrbmA | (43) |
| pCN008 | pKAS32 ΔrbmC | (43) |
| pCN009 | pKAS32 Δbap1 | This study |
| pCN013 | pYS249 araC-PBAD-rbmA | (43) |
| pCN014 | pYS249 araC-PBAD-rbmC | This study |
| pCN015 | pYS249 araC-PBAD-bap1 | This study |
| pCN018 | pKAS32 rbmA-3× FLAG | (43) |
| pCN019 | pKAS32 rbmC-3× FLAG | (43) |
| pCN020 | pKAS32 bap1-3× FLAG | This study |
| pJY006 | pKAS32 ΔhapA | This study |
| pJY011 | pKAS32 Ptac-rbmA | This study |
| pJY014 | pYS249 blunt ligated control vector | This study |
| pJY015 | pKAS32 ΔVC0157 | This study |
Biofilm Growth.
V. cholerae strains were grown overnight at 37 °C in liquid LB with shaking, back-diluted 30-fold, and grown for an additional 2 h with shaking in M9 medium until early exponential phase (OD600 = 0.1–0.2). For time course biofilm imaging, these regrown cultures were diluted to OD600 = 0.001 and 100 μL of the diluted cultures was added to wells of 96-well plates with no. 1.5 coverslip bottoms (MatTek). The cells were allowed to attach for 10 min, after which the wells were washed twice with fresh M9 medium and, subsequently, 100 μL of fresh M9 medium was added. The low initial inoculation density enabled isolated biofilm clusters to form. The locations of the founder cells were identified, and 1 h after inoculation, imaging was begun on the microscope stage at 25 °C. For non-time course experiments, the inoculated plates were incubated at 30 °C for 16–18 h before imaging.
Microscopy.
Images were acquired with a Yokogawa CSU-X1 confocal spinning disk unit mounted on a Nikon Ti-E inverted microscope, using a 60× water objective with a numerical aperture of 1.2, a 543-nm laser (OEM DPSS), and an Andor iXon 897 EMCCD camera. The water objective was crucial to minimize the refractive index mismatch and resulting spherical aberration, which elongates objects, especially for those objects most distant from the surface. To avoid evaporation, immersion oil with a refractive index of 1.3300 ± 0.0002 (Cargille) was used instead of water. To obtain sufficient magnification for automated cell segmentation, a 1.5× lens was placed between the CSU-X1 unit and the Nikon Ti-E side port. The distances between the spinning disk, the tube lens, and the camera sensor were adjusted to provide optimal z resolution. The magnification was 166 nm per pixel in the x–y plane, with a 200-nm step size in the z direction. The point spread function (PSF) of the system was measured under identical conditions (with a 50-nm z-step size) using 200-nm fluorescent polystyrene beads (ex540/em561; Life Technologies). The time difference between each image acquisition was 30 min, and the total acquisition time was 20 h for each experiment. To decrease photodamage to the cells further, an adaptive z range was used: 5 μm for the first 5 h, 15 μm for the next 5 h, and 25 μm for the final 10 h. For the Rg strain, this strategy captured at least 98% of the cells, including those cells in the tallest clusters. For the Δbap1ΔrbmC and ΔrbmA mutants, we increased the z range accordingly. All image acquisitions were automated using Nikon Element software. All experimental images in this work are raw images from this step, rendered by Nikon Element software.
Deconvolution and Image Processing.
The PSF of the system was distilled with Huygens Professional software (SVI). Raw image data were deconvolved using the distilled PSF. To segment the biofilms into individual cells from 3D images, custom code was written in MATLAB (MathWorks). First, a 3D watershed algorithm was applied to the z stacks to separate objects in close proximity to one another. Next, automatic thresholding was independently applied to each layer to account for the decreasing signal with z height. Subsequently, binary objects were connected between the different z planes to obtain 3D volumes for each cell inside the biofilm. The orientation of each cell was next extracted as the first principal component in the principal component analysis of the x, y, and z coordinates for all voxels present in each cell. V. cholerae cells have a slightly curved shape; however, for simplicity, we modeled and tracked the cells as rods. A discussion of the core MATLAB code is available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Knut Drescher for help in image analysis and Dr. Carey Nadell for help with strain construction. We thank them and the members of the B.L.B. laboratory for helpful discussions. We thank Dr. Gary Laevsky and the Confocal Microscopy Facility (a Nikon Center of Excellence) for help with optics. This work was supported by the Howard Hughes Medical Institute, NIH Grant 5R01GM065859, National Science Foundation Grant MCB-0948112 (to B.L.B.), and National Science Foundation Grant MCB-1344191 (to N.S.W., B.L.B., and H.A.S.).
Footnotes
Conflict of interest statement: J.B.X. and B.L.B. were coauthors on a multi-author/multi-institution perspective published in 2012. They have not collaborated scientifically, nor do they have any grants together.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611494113/-/DCSupplemental.
References
- 1.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 2.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 3.Ghannoum M, Parsek M, Whiteley M, Mukherjee PK. Microbial Biofilms. 2nd Ed ASM Press; Washington, DC: 2015. [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Mah TF, et al. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426(6964):306–310. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- 6.Drescher K, Shen Y, Bassler BL, Stone HA. Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proc Natl Acad Sci USA. 2013;110(11):4345–4350. doi: 10.1073/pnas.1300321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nerenberg R. The membrane-biofilm reactor (MBfR) as a counter-diffusional biofilm process. Curr Opin Biotechnol. 2016;38:131–136. doi: 10.1016/j.copbio.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Hobley L, Harkins C, MacPhee CE, Stanley-Wall NR. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol Rev. 2015;39(5):649–669. doi: 10.1093/femsre/fuv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xavier JB, Foster KR. Cooperation and conflict in microbial biofilms. Proc Natl Acad Sci USA. 2007;104(3):876–881. doi: 10.1073/pnas.0607651104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpkvist E, Picioreanu C, van Loosdrecht MCM, Heyden A. Three-dimensional biofilm model with individual cells and continuum EPS matrix. Biotechnol Bioeng. 2006;94(5):961–979. doi: 10.1002/bit.20917. [DOI] [PubMed] [Google Scholar]
- 11.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EHK. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 2008;322(5904):1065–1069. doi: 10.1126/science.1162493. [DOI] [PubMed] [Google Scholar]
- 12.Neu TR, et al. Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol Ecol. 2010;72(1):1–21. doi: 10.1111/j.1574-6941.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K, et al. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature. 2013;497(7449):388–391. doi: 10.1038/nature12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serra DO, Richter AM, Klauck G, Mika F, Hengge R. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. mBio. 2013;4(2):e00103–e00113. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. Cholera transmission: The host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7(10):693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teschler JK, et al. Living in the matrix: Assembly and control of Vibrio cholerae biofilms. Nat Rev Microbiol. 2015;13(5):255–268. doi: 10.1038/nrmicro3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berk V, et al. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337(6091):236–239. doi: 10.1126/science.1222981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krasteva PV, et al. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327(5967):866–868. doi: 10.1126/science.1181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50(1):101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 20.Drescher K, et al. Architectural transitions in Vibrio cholerae biofilms at single-cell resolution. Proc Natl Acad Sci USA. 2016;113(14):E2066–E2072. doi: 10.1073/pnas.1601702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart EJ, Satorius AE, Younger JG, Solomon MJ. Role of environmental and antibiotic stress on Staphylococcus epidermidis biofilm microstructure. Langmuir. 2013;29(23):7017–7024. doi: 10.1021/la401322k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karasawa S, Araki T, Nagai T, Mizuno H, Miyawaki A. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer. Biochem J. 2004;381(Pt 1):307–312. doi: 10.1042/BJ20040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyhan S, Yildiz FH. Smooth to rugose phase variation in Vibrio cholerae can be mediated by a single nucleotide change that targets c-di-GMP signalling pathway. Mol Microbiol. 2007;63(4):995–1007. doi: 10.1111/j.1365-2958.2006.05568.x. [DOI] [PubMed] [Google Scholar]
- 24.Volfson D, Cookson S, Hasty J, Tsimring LS. Biomechanical ordering of dense cell populations. Proc Natl Acad Sci USA. 2008;105(40):15346–15351. doi: 10.1073/pnas.0706805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong JC, Yildiz FH. The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J Bacteriol. 2007;189(6):2319–2330. doi: 10.1128/JB.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Absalon C, Van Dellen K, Watnick PI. A communal bacterial adhesin anchors biofilm and bystander cells to surfaces. PLoS Pathog. 2011;7(8):e1002210. doi: 10.1371/journal.ppat.1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lardon LA, et al. iDynoMiCS: Next-generation individual-based modelling of biofilms. Environ Microbiol. 2011;13(9):2416–2434. doi: 10.1111/j.1462-2920.2011.02414.x. [DOI] [PubMed] [Google Scholar]
- 28.Álvarez JPA, Aradas ARP. 2012 Enhancing iDynoMiCS framework to simulate rod-shape bacterial colonies growth. Available at www.lia.upm.es/index.php/simulators/idynomics. Accessed July 2, 2015.
- 29.Su PT, et al. Bacterial colony from two-dimensional division to three-dimensional development. PLoS One. 2012;7(11):e48098. doi: 10.1371/journal.pone.0048098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fong JC, Karplus K, Schoolnik GK, Yildiz FH. Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J Bacteriol. 2006;188(3):1049–1059. doi: 10.1128/JB.188.3.1049-1059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maestre-Reyna M, Wu WJ, Wang AH. Structural insights into RbmA, a biofilm scaffolding protein of V. cholerae. PLoS One. 2013;8(12):e82458. doi: 10.1371/journal.pone.0082458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schluter J, Nadell CD, Bassler BL, Foster KR. Adhesion as a weapon in microbial competition. ISME J. 2015;9(1):139–149. doi: 10.1038/ismej.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant MA, Wacław B, Allen RJ, Cicuta P. The role of mechanical forces in the planar-to-bulk transition in growing Escherichia coli microcolonies. J R Soc Interface. 2014;11(97):20140400. doi: 10.1098/rsif.2014.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsek MR, Tolker-Nielsen T. Pattern formation in Pseudomonas aeruginosa biofilms. Curr Opin Microbiol. 2008;11(6):560–566. doi: 10.1016/j.mib.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro JM, et al. Cell shape dynamics during the staphylococcal cell cycle. Nat Commun. 2015;6:8055. doi: 10.1038/ncomms9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:e2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3(8):601–610. doi: 10.1038/nrmicro1205. [DOI] [PubMed] [Google Scholar]
- 38.Stewart PS, Franklin MJ. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 2008;6(3):199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 39.Wessel AK, Hmelo L, Parsek MR, Whiteley M. Going local: Technologies for exploring bacterial microenvironments. Nat Rev Microbiol. 2013;11(5):337–348. doi: 10.1038/nrmicro3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DR, et al. In situ proteolysis of the Vibrio cholerae matrix protein RbmA promotes biofilm recruitment. Proc Natl Acad Sci USA. 2015;112(33):10491–10496. doi: 10.1073/pnas.1512424112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64(7):2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skorupski K, Taylor RK. Positive selection vectors for allelic exchange. Gene. 1996;169(1):47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 43.Nadell CD, Drescher K, Wingreen NS, Bassler BL. Extracellular matrix structure governs invasion resistance in bacterial biofilms. ISME J. 2015;9(8):1700–1709. doi: 10.1038/ismej.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL. Solutions to the public goods dilemma in bacterial biofilms. Curr Biol. 2014;24(1):50–55. doi: 10.1016/j.cub.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.