Significance
One of the most important life-sustaining metabolisms that results from a net reduction reaction is the conversion of nitrogen gas to ammonia by nitrogenase in a process known as nitrogen fixation. A nitrogenase variant has been described that converts carbon dioxide to methane in vitro. Here, we expressed this variant in an engineered strain of the photosynthetic bacterium Rhodopseudomonas palustris and showed biological light-driven methane production. Its ability to reduce carbon dioxide at ambient temperature and pressure with a single enzyme and energy provided by light makes the methane-producing strain of R. palustris an excellent starting point to understand how a biological system can marshal resources to produce an energy-rich hydrocarbon in one enzymatic step.
Keywords: nitrogenase, Rhodopseudomonas, bioenergy, methane, engineered bacterium
Abstract
Nitrogenase is an ATP-requiring enzyme capable of carrying out multielectron reductions of inert molecules. A purified remodeled nitrogenase containing two amino acid substitutions near the site of its FeMo cofactor was recently described as having the capacity to reduce carbon dioxide (CO2) to methane (CH4). Here, we developed the anoxygenic phototroph, Rhodopseudomonas palustris, as a biocatalyst capable of light-driven CO2 reduction to CH4 in vivo using this remodeled nitrogenase. Conversion of CO2 to CH4 by R. palustris required constitutive expression of nitrogenase, which was achieved by using a variant of the transcription factor NifA that is able to activate expression of nitrogenase under all growth conditions. Also, light was required for generation of ATP by cyclic photophosphorylation. CH4 production by R. palustris could be controlled by manipulating the distribution of electrons and energy available to nitrogenase. This work shows the feasibility of using microbes to generate hydrocarbons from CO2 in one enzymatic step using light energy.
An essential process for life and an important step in the biogeochemical nitrogen cycle is nitrogen fixation by nitrogenase, in which nitrogen gas (N2) is converted to ammonia (NH3) (1). The difficult reduction of N2 to two NH3 occurs at an FeMoS cluster called FeMo cofactor in Mo-dependent nitrogenase in a reaction that requires ATP hydrolysis and dihydrogen production as shown in 1
| [1] |
Nitrogenase deprived of access to N2 but provided with a source of electrons produces H2 exclusively. Also, the ability of nitrogenase to carry out the multielectron reduction of an inert molecule is not limited to reduction of N2. This enzyme can also reduce other molecules with double and triple bonds, including carbon-containing compounds (reviewed in ref. 2). Recently, we found that a remodeled nitrogenase with substitutions in two key amino acids near the FeMo cofactor is capable of reducing carbon dioxide (CO2) to methane (CH4) in vitro. This enzyme did not retain its ability to reduce N2 but was active in H2 production (3). It was unclear if the remodeled nitrogenase gene could confer to bacteria the ability to reduce CO2 to CH4.
Here, we describe a biocatalyst capable of generating the energy-rich hydrocarbon CH4 by reduction of CO2 using a remodeled nitrogenase. Development of this biocatalyst required selection of an appropriate microbial host, because large amounts of cellular reductant and ATP are used by nitrogenase, and as a consequence, this energetically expensive enzyme is repressed by both transcriptional and posttranslational regulatory mechanisms when an alternative nitrogen source, like ammonium, is available (4). Use of nitrogenase to generate a product not used by the organism would require overcoming these regulatory constraints to achieve expression of active enzyme, while at the same time providing cells with ammonium for growth. We reasoned that the anoxygenic photosynthetic bacterium Rhodopseudomonas palustris would be a good chassis for studying the remodeled nitrogenase in the context of a biological system, because it can fix nitrogen, and an activating mutation in nifA, encoding the transcription activator of nitrogenase genes, has been identified that can bypass the regulatory networks that repress nitrogenase (5–7). Also, R. palustris can generate the considerable amount of ATP needed for the activity of a remodeled nitrogenase from light by cyclic photophosphorylation.
We found that R. palustris reduced CO2 to CH4 using a remodeled nitrogenase with NifDV75AH201Q amino acid substitutions when the enzyme was expressed in a genetic background that allows for its constitutive production and cells were incubated in light. We also found that R. palustris is a tractable system that allows control of CH4 production by manipulating the distribution of electrons and energy available to nitrogenase. In nature, the eight-electron reduction of CO2 to CH4 requires a multistep metabolic pathway found only in methanogenic archaea (8). The results presented here describe a one-step route to biological hydrocarbon production.
Results
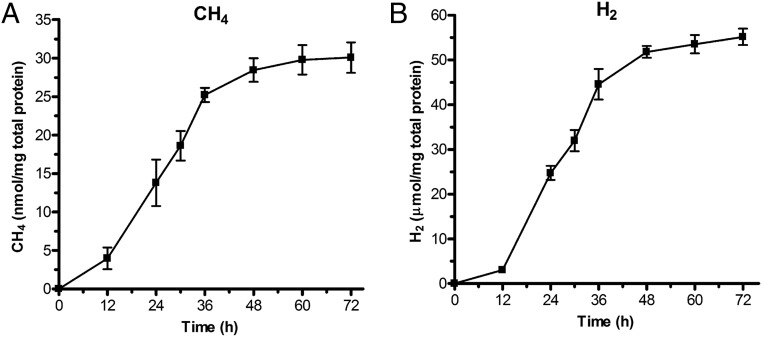
A variant of the FeMo-containing nitrogenase protein, NifD, from Azotobacter vinelandii containing amino acid substitutions V70A and H195Q reduces CO2 to CH4 and also produces H2 in vitro, but it is unable to reduce N2 to NH3 (3). Nucleotide substitutions to generate homologous substitutions of residues in R. palustris NifD (V75A and H201Q) were introduced into the nifD coding sequence by homologous recombination. The R. palustris nifDV75AH201Q allele was integrated into the chromosome of an R. palustris strain CGA009 nifA* mutant that expresses Mo nitrogenase constitutively, even in the presence of ammonium (5, 7). R. palustris can also synthesize alternative V or Fe nitrogenase isozymes, but these isozymes are not expressed in the NifA* strain grown with ammonium (5, 9). The NifA* strain expressing the remodeled nitrogenase produced CH4 when grown with acetate as a carbon source and HCO3− as a source of CO2 (Table 1). The growth rate of this strain was similar to that of its parent strain expressing WT nitrogenase. Both strains also produced comparable levels of H2 (Table 1). R. palustris CGA009 is able to produce H2 only through the activity of its nitrogenase, and thus, H2 production is a measure of nitrogenase activity (10). The nifA* nifDV75AH201Q strain produced almost 2,000 times more H2 than CH4 (Fig. 1 and Table 1). A similarly high ratio of H2 to CH4 produced was observed in vitro with purified remodeled nitrogenase (3).
Table 1.
CH4 and H2 production by R. palustris NifA* strains expressing either a WT nitrogenase or a remodeled nitrogenase
| Genotype | Growth rate (h) | H2 production (μmol/mg total protein) | CH4 production (nmol/mg total protein) |
| nifA* nifDWT | 11.6 (0.1) | 36.2 (6.2) | ND |
| nifA* nifDV75AH201Q | 12.7 (0.9) | 55.1 (3.2) | 30.1 (2.0) |
Data are the average of three or more experiments, with the SDs shown in parentheses. Cells were grown with 20 mM acetate and 10 mM NaHCO3 and incubated in light. ND, not detected.
Fig. 1.
An engineered strain of R. palustris expressing a remodeled nitrogenase produces CH4. R. palustris NifA* cells expressing a remodeled nitrogenase produce (A) CH4 and (B) H2 during growth. These data are the average of three independent experiments, and error bars represent SDs. Cells were grown with 20 mM acetate and 10 mM NaHCO3 and incubated in light.
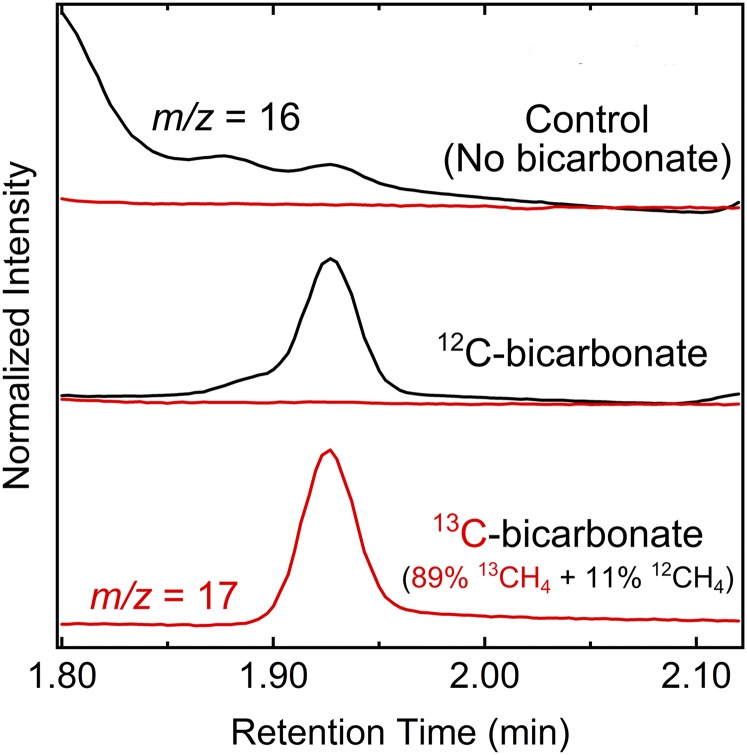
To verify that the remodeled nitrogenase was directly responsible for CH4 production by R. palustris, the product profile of the enzyme was examined. Remodeled enzyme purified from R. palustris converted CO2 to CH4 (1.95 nmol CH4/nmol MoFe protein). No CH4 production was detected for purified WT nitrogenase. In addition, nongrowing cells of the R. palustris nifA* nifDV75AH201Q strain supplied with thiosulfate as an electron donor and H13CO3− as a source of CO2 produced a product that, when analyzed by gas chromatography - mass spectrometry (GC-MS), had an m/z of 17 and a retention time that correlates with 13CH4. A peak with an m/z of 16 and a retention time corresponding to CH4 occurred when H12CO3− was provided. Almost 90% of the CH4 produced was from CO2 reduction (Fig. 2). These data confirm that the CH4 produced by R. palustris cells comes directly from CO2. As expected, the remodeled nitrogenase purified from R. palustris was unable to reduce N2 to NH3, but it reduced acetylene (C2H2) to ethylene (C2H4) (Fig. S1). These results indicate that R. palustris functions to catalyze the production of CH4 and H2 as shown in Fig. 3. Electrons derived from the oxidation of either an organic carbon source (acetate) or an inorganic compound (thiosulfate) are used to reduce CO2 to CH4. HCO3− acquired from outside the cell is converted to intracellular CO2 by carbonic anhydrase (11). Some CO2 is also generated from acetate metabolism.
Fig. 2.
CH4 produced by R. palustris NifA* cells expressing a remodeled nitrogenase is caused by CO2 reduction by the remodeled nitrogenase. GC-MS confirmation of CH4 production from CO2 reduction by R. palustris NifA* cells expressing remodeled nitrogenase. The black traces are the GC-MS data for monitoring 12CH4 (m/z = 16), and the red traces are the data for monitoring 13CH4 (m/z = 17). Thiosulfate was supplied as an electron donor for the reaction.
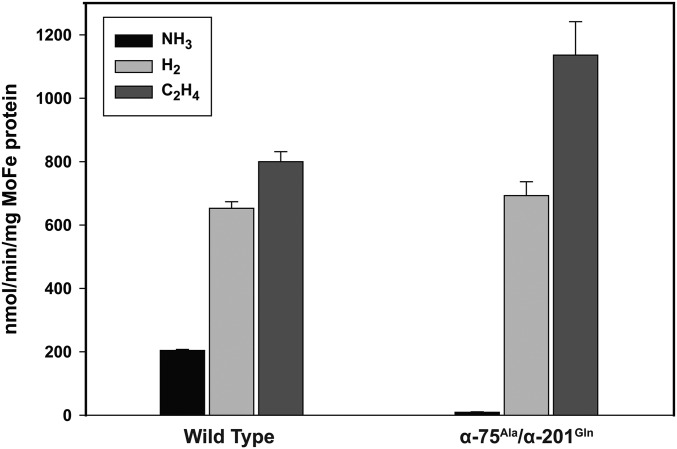
Fig. S1.
Product profiles of WT and remodeled nitrogenase purified from R. palustris NifA*. Both WT and remodeled nitrogenase reduced C2H2 to C2H4, but only the WT nitrogenase reduced N2 to NH3. These data are the average of three independent experiments, and error bars represent SDs.
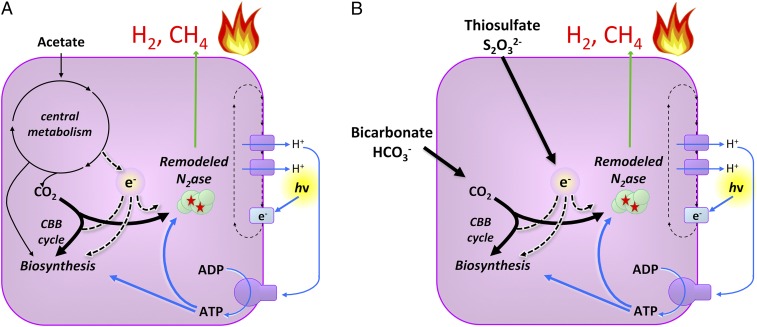
Fig. 3.
Metabolic route of CH4 production by an engineered strain of R. palustris expressing a remodeled nitrogenase. ATP is produced by cyclic photophosphorylation, in which electrons energized by light are cycled through a proton-pumping electron transport chain rather than transferred to a terminal electron acceptor. Electrons are generated by oxidation of (A) organic compounds or (B) inorganic compounds, such as thiosulfate. CO2 from bicarbonate or generated from acetate oxidation is converted by remodeled nitrogenase to CH4 and the CBB cycle to cell material.
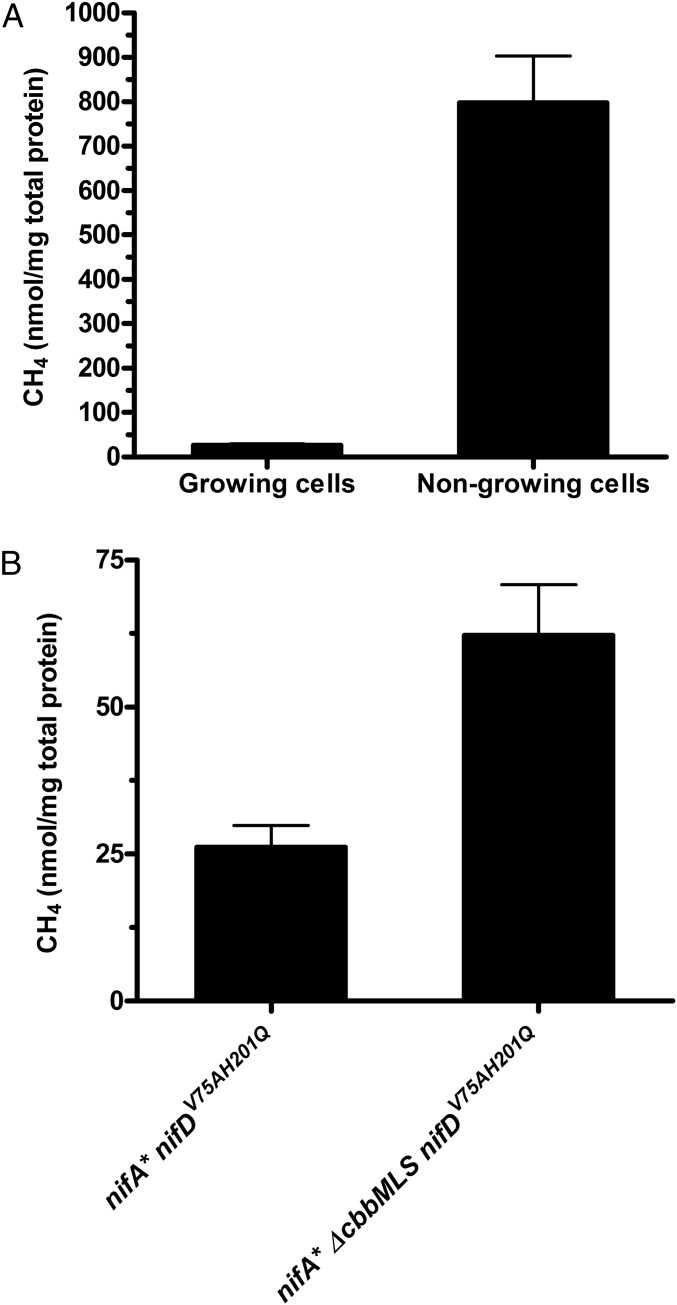
A common method used to divert cellular resources to microbial biocatalysts is to use nongrowing cells. Such cells are spared the imperative of carrying out biosynthetic reactions required for growth, which frees up electrons derived from the oxidation of external electron donors for use in reduction reactions (12). To test if diversion of electrons from biosynthesis leads to more CH4 production by the remodeled nitrogenase, the cells of the R. palustris nifA* nifDV75AH201Q strain grown to midlogarithmic phase were harvested, resuspended in nitrogen-free medium, and tested for their ability to produce CH4. As shown in Fig. 4A, nongrowing cells produced almost 20 times more CH4 than growing cells.
Fig. 4.
Diverting more electrons to the remodeled nitrogenase results in more CH4 production by R. palustris whole cells. Diversion of electrons away from (A) biosynthesis using nongrowing cells or (B) the CBB cycle through genetic mutation (ΔcbbMLS) results in more CH4 production by R. palustris NifA* cells expressing a remodeled nitrogenase. These data are the average of three or more independent experiments, and the error bars represent SDs.
R. palustris has the Calvin Bassham Benson (CBB) cycle for CO2 fixation, and it is known that nitrogenase competes with the CBB cycle for electrons (7, 13). Cells in which CBB cycle activity has been genetically disrupted produce more hydrogen, because more reducing equivalents are available to nitrogenase (7, 13). We found that R. palustris nifA* nifDV75AH201Q ΔcbbMLS cells, which had both sets of Rubisco genes deleted, produced twice as much CH4 as cells with an intact CBB cycle (Fig. 4B).
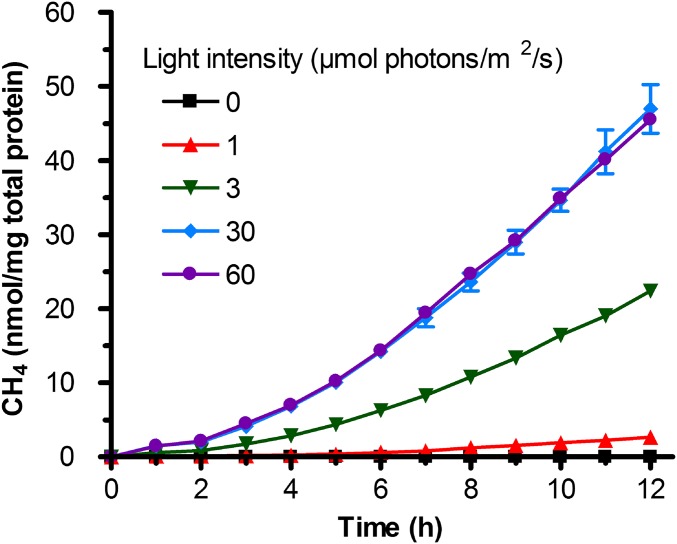
When nongrowing cells of the nifA* nifDV75AH201Q strain were incubated in the dark, no detectable CH4 was produced (Fig. 5). This result was expected, because light is required for ATP production, and ATP is required for nitrogenase activity. As shown in Fig. 5, the amount and rate of CH4 production correlated with light intensity, and more CH4 was produced under high light intensities than under low light intensities. The exception was at the two highest light intensities: 30 and 60 μmol photons/m2 per second, where the amount and rate of CH4 production were not significantly different. This result likely reflects that ATP is not the limiting parameter for CH4 production under higher light intensities.
Fig. 5.
CH4 production by R. palustris NifA* cells expressing a remodeled nitrogenase is a light-driven process that can be controlled by light intensity. Altering the light intensity provided to cells alters the amount of CH4 produced. These data are the average of three or more independent experiments, and the error bars represent SDs.
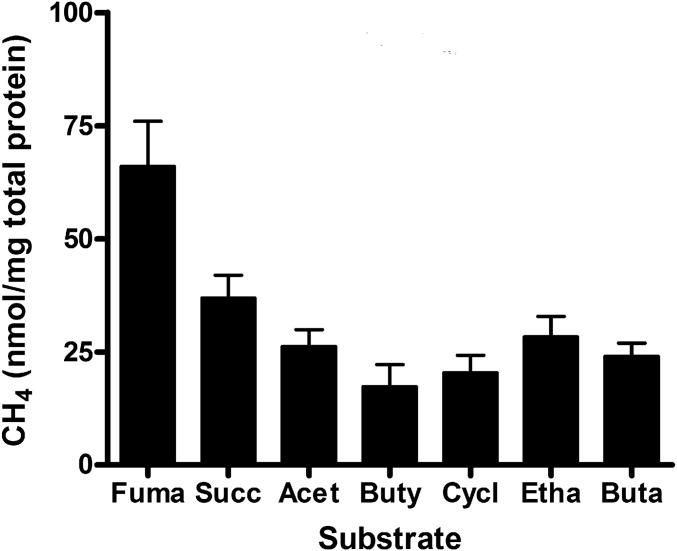
The results shown in Fig. 4 indicate that CH4 production can be modulated by controlling the electron flux to nitrogenase. In R. palustris, electrons are generated during oxidation of organic carbon sources, and we have found that the organic electron donor provided influences the amount of electrons available to nitrogenase (13). The substrate oxidation state, the route that the substrate takes to generate biosynthetic precursors, and the amount of CBB cycle flux that occurs on a given substrate all influence the amount of electrons available to nitrogenase in R. palustris (13). When we grew R. palustris nifA* nifDV75AH201Q cells on different carbon sources to determine how different electron donors may influence CH4 production, we found that, with the exception of fumarate and succinate, the amount of CH4 produced was similar, regardless of the type of organic carbon source used (Fig. 6). R. palustris expressing remodeled nitrogenase produced H2 as well as CH4 under all conditions that we tested. The amount of H2 that cells produced was always on the order of 1,000–7,000 times greater than that of CH4.
Fig. 6.
CH4 produced by R. palustris NifA* cells expressing remodeled nitrogenase is affected by the organic compound supplied as a growth substrate. CH4 production by cells grown on different substrates. Organic acids and alcohols were added at a final concentration of 40 mM carbon. The data shown are the average of five or more independent experiments, and the error bars represent SDs. Acet, acetate; Buta, butanol; Buty, butyrate; Cycl, cyclohexane carboxylate; Etha, ethanol; Fuma, fumarate; Succ, succinate.
Discussion
R. palustris has clear advantages as a system to develop the use of a remodeled nitrogenase in synthetic biology. One is that active remodeled nitrogenase can be produced constitutively. This feature is important, because it allows R. palustris to grow with ammonium as a nitrogen source and use an energetically expensive enzyme to produce a product that it cannot use. The other major advantage to using R. palustris is that it can meet the high ATP requirement for CH4 production by nitrogenase by using cyclic photophosphorylation (Fig. 3). We have shown that the amount and rate of CH4 production are dependent on the light intensity provided (Fig. 5). The simplest explanation for this correlation is that decreased light intensity results in a change in the energy status of the cell, so that less ATP is available for nitrogenase. However, this effect on CH4 production could be more complex, because cellular energy levels may affect posttranslational modification of nitrogenase (14).
CH4 production can be improved by diverting electrons away from biosynthesis or the CBB cycle (Fig. 4), and maximal CH4 production will likely involve manipulating both of these parameters. R. palustris can maintain a nongrowing but metabolically active state for months as long as it has access to light and electrons (15). It can obtain these electrons from both organic and inorganic donors and even use an electric current from an electrode as an electron donor (13, 16, 17). Substrate conversion efficiencies of 86% have been shown using an inorganic electron donor, much higher than the 60% conversion efficiency on an organic electron donor (16). This ability sets up the possibility of exploiting nongrowing R. palustris nifA* nifDV75AH201Q in microbial electrolysis cells (MECs), where electrons are provided by a cathode and used to generate CH4. Generation of a chemical product at the cathode has both environmental and monetary benefits that increase the potential of MECs in biofuel generation (18).
Despite an ability to increase CH4 production by diverting electrons to nitrogenase, maximizing CH4 production using a biocatalyst like R. palustris will ultimately require altering the ratio of CH4 to H2 produced by the remodeled nitrogenase. The nifA* strain used in this study has a defect in its uptake hydrogenase (10). One way to recycle the reductant used to make hydrogen would be to restore the uptake hydrogenase. Additionally, increasing the ratio of the Fe protein to the MoFe protein has been shown to favor CH4 production, resulting in a reduction in the ratio of H2 to CH4 produced (3). A similar approach could be taken by overexpressing the Fe protein in R. palustris nifA* nifDV75AH201Q.
Future studies will focus on manipulating light, electron flux, and nitrogenase itself to improve CO2 reduction to CH4. R. palustris can also synthesize alternative V or Fe nitrogenase isozymes (9). These isozymes could allow for even greater expansion of the catalytic repertoire of nitrogenase and further our understanding of how to produce energy-rich hydrocarbons in one step.
Materials and Methods
Reagents, Bacteria, and Culture Methods.
All reagents were obtained from Sigma-Aldrich or Fisher Scientific and used without additional purification. Gases (argon, dinitrogen, hydrogen, CH4, ethane, C2H2, and C2H4) were purchased from Air Liquide or Gasco. Manipulation of proteins and buffers was done in septum-sealed serum vials under an argon atmosphere or on a Schlenk line. All gas transfers were made using gas-tight syringes. All R. palustris strains were grown on photosynthetic medium (PM) as previously described (5–7, 19). The parent R. palustris strain, CGA009, used in all experiments is defective in expression of its uptake hydrogenase enzyme, and thus, hydrogen production by this strain can be used as a direct measure of nitrogenase activity. All cultures were initially grown anaerobically with 30 μmol photons/m2 per s from a 60-W incandescent light bulb (General Electric) and then, diluted into fresh PM medium. This light intensity supports the maximum growth rate of R. palustris. Where appropriate, organic acids and alcohols were added at a final concentration of 40 mM carbon, and NaHCO3 was added at a final concentration of 10 mM. Light intensity was altered using a 15-W incandescent light bulb controlled by a dimmer switch to provide light intensities of 3 and 1 μmol photons/m2 per second, and light intensity of 60 μmol photons/m2 per second was obtained by using a 200-W incandescent light bulb. We generated suspensions of nongrowing cells by washing midexponential-phase cultures with nitrogen-free PM (NFM) and flushing the headspace with argon gas after degassing the medium. Additional culture methods and growth conditions can be found in SI Materials and Methods.
Genetic Manipulation of R. palustris.
All strains and plasmids used are listed in Table S1. Plasmids were mobilized into R. palustris CGA676 or CGA679 by conjugation with Escherichia coli S17-1, and double–cross-over events for allelic exchange were achieved using a selection and screening strategy as described previously (5). Details of strain and plasmid construction for this study can be found in SI Materials and Methods.
Table S1.
Strains and plasmids used
| Strain or plasmid | Genotype or phenotype | Source |
| R. palustris strains | ||
| CGA009 | WT strain; spontaneous CmR derivative of CGA001 | 19 |
| CGA676 | nifA*; 48-bp deletion encoding Q-linker amino acids 202–217; produces H2 in the presence of NH4+ | 7 |
| CGA676 nifDV75AH201Q | CGA676 in which the nifDV75AH201Q mutation was introduced at its native locus using allelic exchange | This study |
| CGA679 | ΔcbbLS::Kmr ΔcbbM mutant of CGA676 | 7 |
| CGA679 nifDV75AH201Q | CGA679 in which the nifDV75AH201Q mutation was introduced at its native locus using allelic exchange | This study |
| CGA676 his::nifD | CGA676 in which the his::nifD was introduced at its native locus using allelic exchange | This study |
| CGA676 his::nifDV75AH201Q | CGA676 in which the his::nifDV75AH201Q was introduced at its native locus using allelic exchange | This study |
| E. coli strains | ||
| DH5α | F−λ− recA1 Δ(lacZYA-argF)U169 hsdR17 thi-1 gyrA96 supE44 endA1 relA1 Φ80lacZΔM15 | Gibco-BRL |
| S17-1 | thi pro hdsR hdsM+ recA; chromosomal insertion of RP4-2 (Tc::Mu Km::Tn7) | 23 |
| Plasmids | ||
| pJQ200SK | GmR, sacB; mobilizable suicide vector | 24 |
| pJQ-nifD | GmR; nifD coding sequence cloned into the PstI site of pJQ200SK | This study |
| pJQ-nifDV75AH201Q | GmR; nifDV75AH201Q coding sequence cloned into the PstI site of pJQ200SK | This study |
| pJQ-his::nifD | GmR; nifD coding sequence with an his8x tag encoded at the N terminus cloned into the PstI site of pJQ200SK | This study |
| pJQ- his::nifDV75AH201Q | GmR; nifDV75AH201Q coding sequence with an his8x tag encoded at the N terminus cloned into the PstI site of pJQ200SK | This study |
Protein Purification.
Cell extracts from R. palustris cells were prepared by using a French pressure cell operated at 1,500 lb/in2 in a degassed 50 mM Tris⋅HCl buffer (pH 8.0) with 2 mM sodium dithionite under Ar. His-tagged MoFe proteins were purified by an immobilized metal affinity chelation chromatography protocol (20), with minor modifications. Protein concentrations were determined by the Biuret assay using BSA as the standard. The purities of these proteins were determined based on SDS/PAGE analysis with Coommasie staining.
CO2 Reduction Assays.
CO2 reduction assays were conducted in 9.4-mL serum vials containing an assay buffer consisting of an MgATP regeneration system (15 mM MgCl2, 90 mM phosphocreatine, 15 mM ATP, 0.6 mg/mL creatine phosphokinase, 1.2 mg/mL BSA) and 12 mM sodium dithionite in 100 mM 4-morpholinepropanesulfonic acid sodium salt (Mops) buffer at pH 7.0. After solutions were made anaerobic, 0.45 atm CO2 was added, and the gas and liquid phases were allowed to equilibrate for ∼20 min. MoFe protein was then added, the vials were ventilated to atmospheric pressure, and the reaction was initiated by the addition of Fe protein. Reactions were conducted at 30 °C for 60 min and then quenched by the addition of 700 µL 400 mM EDTA (pH 8.0). Quantification of CH4 was done according to a published protocol (3).
Dinitrogen, C2H2, and Proton Reduction Assays.
Reduction assays were conducted in 9.4-mL serum vials containing an assay buffer consisting of an MgATP regeneration system (6.7 mM MgCl2, 30 mM phosphocreatine, 5 mM ATP, 0.2 mg/mL creatine phosphokinase, 1.2 mg/mL BSA) and 12 mM sodium dithionite in 100 mM Mops buffer at pH 7.0. After solutions were made anaerobic, the headspace gases in the reaction vials were adjusted to proper partial pressures of different gases for different substrates (1 atm N2 for N2, 0.1 atm C2H2 and 0.9 atm Ar for C2H2 reduction, and 1 atm Ar for proton reduction). This step was followed by the addition of the MoFe protein. After the vials were ventilated to atmospheric pressure, the reactions were initiated by the addition of the Fe protein. Reactions were conducted at 30 °C for 8 min and then, quenched by the addition of 300 µL 400 mM EDTA (pH 8.0). The products (NH3, H2, and C2H4) from different substrate reduction assays were quantified according to published methods with minor modifications (3, 21, 22).
H2 and CH4 Measurements from R. palustris Cells.
Gas-phase samples (50 μL) were withdrawn with a Hamilton sample lock syringe from the culture vial headspace at intervals, and hydrogen was measured with a Shimadzu GC-2014 Gas Chromatograph as previously described (5, 7, 12, 13). Total protein concentrations were determined using the Bio-Rad Protein Assay Kit. Ethane was used as an internal standard to quantify CH4; 200 μL ethane (0.08% or 0.8%; 1 atm) was placed into a 27-mL anaerobic tube with 10 mL culture and then, shaken gently to distribute the gases uniformly. A 100-μL gas sample was taken from the headspace of the tube for gas chromatography - flame ionization (GC-FID) analysis using a Hamilton Sample Lock Syringe. The separation of CH4 was performed using a stainless steel 80/100 Porapak N Column (1.8 m × 1/8 in × 2.1 mm i.d.) purchased from Sigma-Aldrich. The column temperature was held at 60 °C, and the injector and detector temperatures were set to 85 °C and 150 °C, respectively. Argon was used as the carrier gas at a rate of 35 mL/min.
GC-MS Analysis for in Vivo CO2 Reduction to CH4 by R. palustris.
GC-MS analysis was conducted to confirm the production of CH4 from CO2 reduction using a Shimadzu GC-2010 Gas Chromatograph equipped with a programmed temperature vaporizing injector and a Shimadzu GCMS-QP2010S Mass Spectrometer by using 12/13C-enriched NaHCO3 as the CO2 source for R. palustris V75A/H201Q mutant. Additional details can be found in SI Materials and Methods.
SI Materials and Methods
Bacteria and Growth Conditions.
All Rhodopseudomonas palustris strains were grown aerobically during genetic manipulation on defined mineral medium (PM) agar supplemented with 10 mM succinate at 30 °C. All R. palustris strains were grown anaerobically in PM supplemented with 20 mM acetate, unless otherwise stated, in light at 30 °C. The growth medium was degassed and then, extensively bubbled with N2. The medium was dispensed into culture tubes in an anaerobic glove box, and the tubes were sealed with rubber stoppers. For protein purification, a 1-L culture was used to inoculate 10 L PM medium containing 40 mM acetate and 0.1% yeast extract and incubated at 30 °C under light until it reached an OD660 of ∼0.9. For GC-MS analysis, cells were grown in 1 L PM medium with 20 mM NaHCO3 and 10 mM thiosulfate. Then, the cells were harvested by centrifugation and transferred to NFM medium with 10 mM thiosulfate without bicarbonate or with 10 mM NaH12CO3 or NaH13CO3 (Cambridge Isotope Lab). Escherichia coli S17-1 was grown in LB at 37 °C. When appropriate, R. palustris was grown with gentamicin at 100 μg/mL. E. coli cultures were supplemented with gentamicin at 20 μg/mL.
Genetic Manipulation of R. palustris.
The vector used for allelic exchange of WT nifD for nifDV75AH201Q was constructed by PCR amplification of nifD plus 22 bp upstream of the start codon and 24 bp downstream from the stop codon from genomic DNA purified from R. palustris CGA009 using Phusion High-Fidelity DNA Polymerase (New England Biolabs). The resulting 1.5-kb fragment was incorporated into PstI-digested pJQ200SK using the In-Fusion PCR Cloning System (Clontech). Site-directed mutagenesis of the resulting plasmid using the PCR-based QuikChange Method (Agilent Technologies) was carried out to introduce the V75A and H201Q substitutions into the NifD coding sequence. The vector used for allelic exchange of WT nifD for his::nifD was constructed by PCR amplification of nifD using Phusion High-Fidelity DNA Polymerase and primers that incorporated eight histidines (CAC) after the start codon of nifD, and the resulting product was incorporated into PstI-digested pJQ200SK using the In-Fusion PCR Cloning System. Site-directed mutagenesis of the resulting plasmid using the PCR-based QuikChange Method was carried out to introduce the V75A and H201Q substitutions into the NifD coding sequence. All allelic exchange was verified using PCR amplification and sequencing of the resulting PCR product. To ensure that the his tag did not affect nitrogenase activity, the ability of CGA676 encoding his::nifD to produce hydrogen at equivalent levels as CGA676 was confirmed.
GC-MS Analysis for in Vivo CO2 Reduction to CH4 by R. palustris.
Separation of CH4 was achieved with a GS-CARBONPLOT Column (30 m, 0.32 mm i.d., and 3.0-µm film thickness; Agilent Technologies Inc.). The injector and column temperatures were set to 30 °C. Helium was used as the carrier gas set at a linear velocity of 39.6 cm/s for CH4 separation. For separation of CH4 from other component gases in the reaction vial, 5 µL headspace gases were directly injected onto the column through an on-column injection liner. The mass spectrometer was operated in electron ionization (40 eV) and selected ion monitoring modes by monitoring m/z species of 16 for [12CH4]+ and [13CH3]+ and 17 for [13CH4]+, respectively. The detector voltage of the mass spectrometer was set to 1,500 V to achieve reasonable sensitivity.
The CH4 concentration in each sample was quantified by fitting the molecular ion peak area from GC-MS analysis to a CH4 standard curve with high linearity by varying CH4 concentration and monitoring the m/z = 16 species ([12CH4]+). For 12CH4 concentration determination in controls (no bicarbonate) and samples using 12C-enriched NaHCO3 as a CO2 source, the peak area of m/z = 16 species was applied. For 13CH4 concentration determination in samples using 13C-enriched NaH13CO3 as the 13CO2 source, the peak area of m/z = 17 species was applied. The peak area of m/z = 16 species contributed by 12CH4 in samples using 13C-enriched NaH13CO3 as the 13CO2 source was determined by subtracting the area contributed by the fragmentation ([13CH3]+; m/z = 16) of 13CH4 from the total peak area of m/z = 16 species ([12CH4]+ and [13CH3]+). The fragmentation ratio of [M − 1]+/[M]+ under GC-MS conditions applied in this work was determined by dividing the peak area of m/z = 15 species ([M − 1]+ = [12CH3]+) by that of m/z = 16 species ([M]+ = [12CH4]+) using natural abundant CH4 as the sample gas. Because of the relatively low natural abundances for 13C (1.07%) and 2H (D; 0.0115%), the contribution of naturally abundant 13CH4 and 12CDH3 from other CO2 sources to the m/z = 17 species was neglected in quantification of 13CH4 from 13C samples.
Acknowledgments
We thank the entire Biological and Electron Transfer and Catalysis (BETCy) team for helpful discussions. This work was supported as part of the BETCy Energy Frontier Research Center (EFRC), an EFRC funded by US Department of Energy, Office of Science Grant DE-SC0012518.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611043113/-/DCSupplemental.
References
- 1.Smil V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- 2.Seefeldt LC, Yang Z-Y, Duval S, Dean DR. Nitrogenase reduction of carbon-containing compounds. Biochim Biophys Acta. 2013;1827(8-9):1102–1111. doi: 10.1016/j.bbabio.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z-Y, Moure VR, Dean DR, Seefeldt LC. Carbon dioxide reduction to methane and coupling with acetylene to form propylene catalyzed by remodeled nitrogenase. Proc Natl Acad Sci USA. 2012;109(48):19644–19648. doi: 10.1073/pnas.1213159109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol. 2004;2(8):621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
- 5.Rey FE, Heiniger EK, Harwood CS. Redirection of metabolism for biological hydrogen production. Appl Environ Microbiol. 2007;73(5):1665–1671. doi: 10.1128/AEM.02565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heiniger EK, Oda Y, Samanta SK, Harwood CS. How posttranslational modification of nitrogenase is circumvented in Rhodopseudomonas palustris strains that produce hydrogen gas constitutively. Appl Environ Microbiol. 2012;78(4):1023–1032. doi: 10.1128/AEM.07254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKinlay JB, Harwood CS. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci USA. 2010;107(26):11669–11675. doi: 10.1073/pnas.1006175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa KC, Leigh JA. Metabolic versatility in methanogens. Curr Opin Biotechnol. 2014;29:70–75. doi: 10.1016/j.copbio.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Oda Y, et al. Functional genomic analysis of three nitrogenase isozymes in the photosynthetic bacterium Rhodopseudomonas palustris. J Bacteriol. 2005;187(22):7784–7794. doi: 10.1128/JB.187.22.7784-7794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rey FE, Oda Y, Harwood CS. Regulation of uptake hydrogenase and effects of hydrogen utilization on gene expression in Rhodopseudomonas palustris. J Bacteriol. 2006;188(17):6143–6152. doi: 10.1128/JB.00381-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puskás LG, Inui M, Zahn K, Yukawa H. A periplasmic, alpha-type carbonic anhydrase from Rhodopseudomonas palustris is essential for bicarbonate uptake. Microbiology. 2000;146(Pt 11):2957–2966. doi: 10.1099/00221287-146-11-2957. [DOI] [PubMed] [Google Scholar]
- 12.McKinlay JB, et al. Non-growing Rhodopseudomonas palustris increases the hydrogen gas yield from acetate by shifting from the glyoxylate shunt to the tricarboxylic acid cycle. J Biol Chem. 2014;289(4):1960–1970. doi: 10.1074/jbc.M113.527515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinlay JB, Harwood CS. Calvin cycle flux, pathway constraints, and substrate oxidation state together determine the H2 biofuel yield in photoheterotrophic bacteria. MBio. 2011;2(2):e00323-10. doi: 10.1128/mBio.00323-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huergo LF, et al. PII signal transduction proteins: Pivotal players in post-translational control of nitrogenase activity. Microbiology. 2012;158(Pt 1):176–190. doi: 10.1099/mic.0.049783-0. [DOI] [PubMed] [Google Scholar]
- 15.Gosse JL, Engel BJ, Hui JC-H, Harwood CS, Flickinger MC. Progress toward a biomimetic leaf: 4,000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol Prog. 2010;26(4):907–918. doi: 10.1002/btpr.406. [DOI] [PubMed] [Google Scholar]
- 16.Huang JJ, Heiniger EK, McKinlay JB, Harwood CS. Production of hydrogen gas from light and the inorganic electron donor thiosulfate by Rhodopseudomonas palustris. Appl Environ Microbiol. 2010;76(23):7717–7722. doi: 10.1128/AEM.01143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose A, Gardel EJ, Vidoudez C, Parra EA, Girguis PR. Electron uptake by iron-oxidizing phototrophic bacteria. Nat Commun. 2014;5:3391. doi: 10.1038/ncomms4391. [DOI] [PubMed] [Google Scholar]
- 18.Foley JM, Rozendal RA, Hertle CK, Lant PA, Rabaey K. Life cycle assessment of high-rate anaerobic treatment, microbial fuel cells, and microbial electrolysis cells. Environ Sci Technol. 2010;44(9):3629–3637. doi: 10.1021/es100125h. [DOI] [PubMed] [Google Scholar]
- 19.Kim M-K, Harwood CS. Regulation of benzoate-CoA ligase in Rhodopseudomonas palustris. FEMS Microbiol Lett. 1991;83(2):199–203. [Google Scholar]
- 20.Christiansen J, Goodwin PJ, Lanzilotta WN, Seefeldt LC, Dean DR. Catalytic and biophysical properties of a nitrogenase Apo-MoFe protein produced by a nifB-deletion mutant of Azotobacter vinelandii. Biochemistry. 1998;37(36):12611–12623. doi: 10.1021/bi981165b. [DOI] [PubMed] [Google Scholar]
- 21.Corbin JL. Liquid chromatographic-fluorescence determination of ammonia from nitrogenase reactions: A 2-min assay. Appl Environ Microbiol. 1984;47(5):1027–1030. doi: 10.1128/aem.47.5.1027-1030.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barney BM, Igarashi RY, Dos Santos PC, Dean DR, Seefeldt LC. Substrate interaction at an iron-sulfur face of the FeMo-cofactor during nitrogenase catalysis. J Biol Chem. 2004;279(51):53621–53624. doi: 10.1074/jbc.M410247200. [DOI] [PubMed] [Google Scholar]
- 23.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram-negative bacteria. Biotechnology (NY) 1983;1(9):784–791. [Google Scholar]
- 24.Quandt J, Hynes MF. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene. 1993;127(1):15–21. doi: 10.1016/0378-1119(93)90611-6. [DOI] [PubMed] [Google Scholar]