Abstract
In typical development there is a bias to orient visual attention to social information. Children with ASD do not reliably demonstrate this bias, and the role of attention orienting has not been well studied. We examined attention orienting via the inhibition of return (IOR) mechanism in a spatial cueing task using social-emotional cues; we studied 8- to 17-year-old children with ASD (n=41) and typically developing controls (TDC) (n=25). The ASD group exhibited a significantly stronger IOR effect than the TDC group, and the IOR effect correlated positively with social impairments, but was unrelated to co-occurring ADHD or anxiety symptoms. These results provide evidence of an early visual attention mechanism that is directly related to core social deficits in ASD.
Keywords: visual attention, orienting, inhibition of return, children, autism spectrum disorder, comorbidities
Introduction
Visual attention can prioritize social signals. For example, preferential looking to social stimuli is present minutes after birth, with newborns attending to upright faces relative to scrambled faces (Johnson, Dziurawiec, Ellis, & Morton, 1991). This preference to faces is also apparent later in life across free viewing (Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Nakano et al., 2010; Rice, Moriuchi, Jones, & Klin, 2012) and various visual search tasks (Langton, Law, Burton, & Schweinberger, 2008; Riby, Brown, Jones, & Hanley, 2012; Yerys et al., 2012). Autism spectrum disorder (ASD) is characterized by social impairment, and significant evidence suggests that atypical patterns of visual attention may have positive or negative effects depending on the domain. For example, enhanced attention to visual details may lead to strengths in certain tasks, and even to developing specialized abilities (Happé, 1994; Mottron, Burack, Iarocci, Belleville, & Enns, 2003), Alternatively, reduced attention to social information may limit social learning opportunities, leading to negative downstream effects on social cognition and skill (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Dawson & Lewy, 1989; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Keehn, Müller, & Townsend, 2013).
While multiple processes comprise visual attention, the orienting process, which allows us to disengage, shift, and reengage attention, has a strong link to how we search our environment (Posner & Petersen, 1990). Disruptions in the orienting process have consistently been found in ASD. Deficits in spontaneous shifts to faces have been observed early in life in naturalistic settings (Swettenham et al., 1998). Children with ASD and infants at high-risk for developing ASD also have difficulties disengaging their visual attention compared to controls during spatial attention tasks (Elsabbagh et al., 2009; Kikuchi et al., 2011; Landry & Bryson, 2004; Sacrey, Bryson, & Zwaigenbaum, 2013; Zwaigenbaum et al., 2005). It is not yet known if this attention impairment transcends across other facets of orienting, such as the mechanism of inhibition of return (IOR).
The mechanism of IOR reflects a bias in visual attention orienting. This orienting bias discounts previously inspected spatial locations in favor of unexplored regions and facilitates effective visual search. Disruptions to this mechanism could result in repetitive foraging of already inspected areas (Itti & Koch, 2001; Klein, 2000; Tipper, Weaver, & Watson, 1996; Wang & Klein, 2010). The IOR mechanism can be evoked and measured using a spatial cueing task in which participants are presented with a cue (e.g. picture of a face to the left or right of center) followed by a target (e.g., an asterisk ‘*’) that appears on the same (Valid) or opposite side (Invalid) of the cue’s location. When the interval between the cue and target is longer than 300 ms, individuals make a slower manual response to validly cued trials than invalidly cued trials; this difference in manual response time is known as the IOR effect (IOR effect=Valid-Invalid response time; Posner, Walker, Friedrich, & Rafal, 1984). There is evidence to support that schematic drawings of angry facial expressions diminish the IOR effect in young adults (Fox, Russo, & Dutton, 2002), and that anxiety and worry traits interact with this effect (Verkuil, Brosschot, Putman, & Thayer, 2009). Together, these findings suggest that threat-related cues are harder to disengage from and capture attention to a greater degree. Thus, using social-emotional cue stimuli in a spatial cueing paradigm provides an opportunity to test how social information may be prioritized in the orienting component of visual attention.
To our knowledge, only two small sample studies have previously examined the IOR effect in individuals with ASD, and neither used social-emotional stimuli. The first study tested the IOR effect using a non-social cue stimulus in children with autism (without intellectual disability), Asperger’s syndrome, and typically developing controls (Rinehart, Bradshaw, Moss, Brereton, & Tonge, 2008). The autism and control groups exhibited similar IOR effects, but the Asperger’s syndrome group trended towards a more pronounced IOR effect compared to the control group. The second study found similar IOR effects in Asperger’s syndrome and control groups (Marotta et al., 2013). These studies are likely limited by small sample sizes (n’s<15) to detect group differences in the IOR effect. Furthermore, neither study evaluated the IOR effect using social-emotional cues with ASD; it is unknown if social-emotional cues would enhance potential group differences. Thus, it remains an open question as to whether the IOR effect is a sensitive index of altered orienting of visual attention in children with ASD.
Atypical visual attention is also observed in youth with ADHD and adults with high anxiety traits (Fox et al., 2002; Shaw, Stringaris, Nigg, & Leibenluft, 2014; Waters, Nitz, Craske, & Johnson, 2007). Symptoms of these two disorders occur frequently in individuals with ASD. For example, children with ADHD display a delayed response time and poor behavior performance in spatial cuing tasks (Ortega, López, Carrasco, Anllo-Vento, & Aboitiz, 2013), and demonstrate a trend toward a diminished IOR (Li, Chang, & Lin, 2003; White, 2007)(Li, Chang, Lin, 2002; White, 2007). For anxiety symptoms, there is a relationship between anxiety and diminished IOR in conditions with emotional face stimuli, reflecting the attentional capture of emotional faces (Fox et al. 2002, Verkuil et al. 2009, Perez-Dueñas et al, 2009, 2014). Thus, it is possible that differences in the IOR may not be related to ASD symptoms, but to the presence of these co-occurring symptoms.
We investigated the IOR effect using neutral and angry facial expressions as cues in 8–17 year-old children with ASD compared to an age-, IQ-, and sex-ratio matched typically developing cohort. Based on the prior literature, we predicted that both groups would respond slower in Valid than Invalid conditions, reflecting the IOR effect. One of the preliminary studies suggests that children with ASD may have a stronger IOR effect (Rinehart et al., 2008). Thus, we predicted a Group-by-Cue interaction where the ASD group would have a stronger IOR effect than the control group. We also hypothesized a Group-by-Cue-by-Emotion interaction, where the TDC group would have a weaker IOR effect in the angry emotion condition, but the ASD group’s IOR effect would not differ between neutral and angry emotion conditions. This would reflect a deficiency in prioritizing social emotional information in children with ASD. We predicted that current ASD symptom severity would correlate with the IOR effect. There is now an appreciation for co-occurring anxiety and ADHD symptoms influencing performance on attention, executive function, and social processing tasks in youth with ASD (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Herrington, Miller, Pandey, & Schultz, 2015; Hollocks et al., 2014; Pugliese et al., 2015; Sinzig, Bruning, Morsch, & Lehmkuhl, 2008; Yerys, Kenworthy, Jankowski, Strang, & Wallace, 2013). Thus, we also tested whether differences in the IOR effect are correlated with severity of co-occurring anxiety or ADHD symptoms. Predictions included that more ADHD symptoms in ASD would correlate negatively with the IOR effects overall, while more anxiety symptoms would correlate negatively with the IOR effect in the Emotional facial expression condition (emotional IOR=Angry Valid-Angry Invalid response time), but no correlation with the IOR effect in the Neutral facial expression condition.
Method
Participants
A total of 78 children participated in the study; this included 49 ASD (without intellectual disability) and 29 TDC. The groups were matched on chronological age, sex-ratio, and General Conceptual Ability (GCA) as measured by the Differential Ability Scales – Second Edition (Elliott, 2007). See Table 1 for group characteristics. Children in the ASD group met the DSM-IV-TR criteria for autism, Asperger’s syndrome, or pervasive developmental disorder – not otherwise specified (American Psychiatric Association, 2000), and this was confirmed with the autism diagnostic observation schedule – 2nd edition (Lord et al., 2012) and the autism diagnostic interview – revised (Lord, Rutter, & Le Couteur, 1994). Children with ASD were screened and excluded if GCA<70, if parents reported any known genetic, current mood or psychotic disorder, neurological disorder, premature birth (gestational age<37 weeks), or other significant medical condition that affects functioning. Children prescribed atypical antipsychotics were excluded, but children prescribed stimulant medication were asked to withhold on the day of the study (n=6). TDC participants were screened and excluded if parents reported any known genetic, language, learning, neurological, or psychiatric disorder, premature birth, or first- or second-degree relative with ASD. TDC children were also excluded if parents reported elevated symptoms on the Child and Adolescent Symptom Inventory (CASI-4R; Gadow & Sprafkin, 2000, 2010) or ADHD Rating Scale (DuPaul, Power, Anastopoulos, & Reid, 2016). Three TDC participants were excluded for the following: significant sleep disturbances (n=1), premature birth (n=1), and computer error (n=1). Five ASD participants were excluded for the following: GCA standard score below 70 (n=1), congenital visual problems (n=2), brain abnormality (n=1), and computer error (n=1). Four additional children were dropped after completing the task (see Data Processing and Analysis Plan)
Table 1.
Participant characteristics by diagnostic group.
| TDC n=25 |
ASD n=41 |
p-value | |
|---|---|---|---|
| Age (years) M(SD) | 13.27 (2.26) | 12.73 (2.30) | 0.35 |
| Range | 10.08–17.17 | 8.17–17.58 | |
| GCA (SS) M(SD) | 110.44 (17.26) | 106.10 (17.91) | 0.33 |
| Range | 92–149 | 79–154 | |
| Sex (M:F) | 19:6 | 33:8 | 0.67 |
| ADI Soc.* | -- | 19.18 (5.14) | -- |
| Range | 3–27 | ||
| ADI Verbal Comm.* | -- | 15.20 (4.34) | -- |
| Range | 6–24 | ||
| ADI RRB* | -- | 6.23 (1.97) | -- |
| Range | 3–10 | ||
| ADOS-2 Social Affect | -- | 9.22 (3.57) | -- |
| Range | 3–16 | ||
| ADOS-2 RRB | -- | 2.76 (1.59) | -- |
| Range | 0–6 | ||
| ADOS-2 CCS | -- | 6.95 (3.25) | -- |
| Range | 3–10 | ||
| ADHD Total Raw | 3.48 (3.24) | 21.44 (10.41) | <0.001 |
| Range | 0–11 | 5–45 | |
| CASI Anxiety Raw | 1.88 (2.15) | 10.95 (7.26) | <0.001 |
| Range | 0–7 | 0–32 |
CCS=Calibrated Comparison Score
GCA=Global Composite Ability
RRB=Restricted and Repetitive Behaviors
SS=Standard Score (M=100; SD=15)
n=40
As shown in Table 1, there were no differences in age, sex-ratio or GCA standard score across groups; the ASD group had higher ADHD rating scale scores than TDCs.
Stimulus and Materials
The Social-Emotional Inhibition of Return (IOR) Task displayed stimuli on two white rectangular boxes to the left and right of the center of the screen over a light gray background, on a 17-inch laptop using E-Prime version 2.0 (Psychology Software Tools Inc., Pittsburg, PA). Seven female and six male actors were used from the NimStim photoset (Tottenham et al., 2009) to create 20 neutral and 20 angry cues. The cues included open and closed mouths for both emotions. Disparities in numbers between male and female actors were a result from matching sex and ethnicity in each emotion condition. This was done to prevent sex or ethnicity biases toward one emotion condition.
Participants were seated approximately 50 cm from the laptop and were instructed to keep their eyes on the plus sign in the middle of the screen and press the corresponding ‘L’ or ‘R' labeled key on the side the red “star” (i.e. asterisk ‘*’) appeared as quickly and as accurately as possible. They were also told that other images would appear on either side, but would not be predictive of where the red star appears.
The IOR task was adapted from Fox, Russo, & Dutton, (2002). The task consisted of 200 trials divided into 4 blocks. The first two blocks included only neutral faces and the last two blocks included only angry faces. This was done to minimize potential cumulative effects of viewing angry faces (Compton et al., 2003; Dalgleish, 1995; Herrington et al., 2005; Holle, Neely, & Heimberg, 1997; Koven, Heller, Banich, & Miller, 2003). Participants were presented with a screen that stated ‘Hit the spacebar when you are ready to begin’ in the beginning of each block. This was done so that they could take a self-paced break in between blocks. Each trial began with a fixation cross for 200 ms. A face cue was presented in one of the peripheral boxes for 300 ms, blank peripheral boxes were presented for 200 ms, followed by 300 ms of a darkened fixation cross. The initial fixation was presented for 160 ms, followed by the red asterisk target in the upper half of the right or left white box until the child responded or 2000 ms elapsed. Each block ended with 1000 ms of a fixation cross. The cue-target onset asynchrony was a total of 960 ms and the intertrial interval was 1000 ms.
The IOR task consisted of three types of trials. See Figure 1. Of the 200 total trials, 160 consisted of target trials which varied in the facial expression and side of the cue stimulus; half of these target trials were Valid, in which the cue and target appeared on the same side, and the other half were Invalid, where the cue and target appeared on opposite sides. Forty catch trials were included; twenty were right-side cues without a target asterisk, and twenty were left-side cues without a target asterisk and were interspersed through the blocks. The catch trials were included to ‘catch’ children with a bias to responding impulsively without attending to the task.
Figure 1.
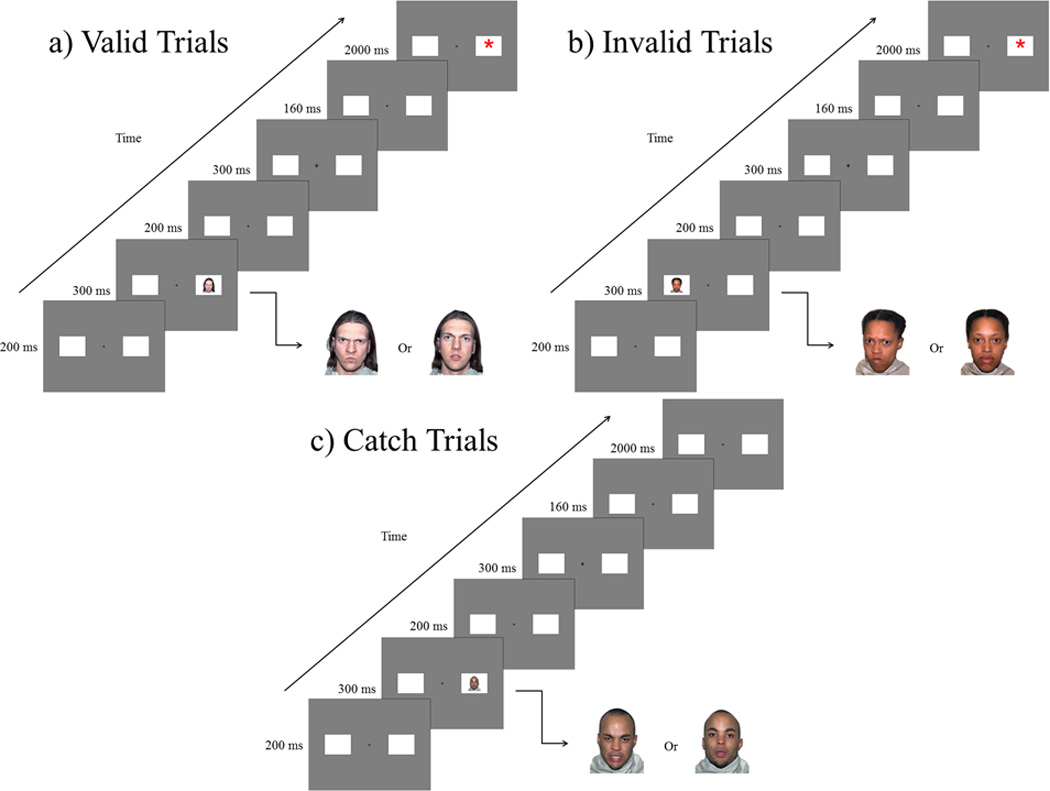
The IOR task. a) Valid trials in which the cue and the target were presented on the same side. b) Invalid trials in which the cue and target were presented on opposite sides. c) Catch trials where no target was presented after a cue.
The Autism Diagnostic Observation Schedule-2 (ADOS-2) is a semi-structured, standardized diagnostic measure designed to assess the domains of Social Affect, and Restricted and Repetitive Behaviors (Lord et al., 2012). Clinicians observe and code these behaviors, which are converted into algorithm scores for each domain. Each domain raw score and the combined raw score total can be converted into a Calibrated Comparison Score that takes age into account for the Module. Scores range from 1–10, and higher scores indicate greater severity (Gotham, Pickles, & Lord, 2009; Hus, Gotham, & Lord, 2012; Hus & Lord, 2014). The present study used the raw scores for Social Affect as an individual domain and the overall Calibrated Comparison Score that combines Social Affect and Restricted Repetitive Behaviors.
The ADHD Rating Scale IV (DuPaul et al., 2016) screens for severity in inattention and hyperactivity/impulsivity symptoms. This 18-question scale yields two domains: inattention and hyperactivity/impulsivity. For each question, parents use a 0–3 scale to rate the participant. A higher score indicates greater symptom severity.
The Child and Adolescent Symptom Inventory-Fourth Edition Revised is a 142-item questionnaire that screens for childhood psychopathology in children ages 5 to 18. For the purposes of the present study, we used a subset of the Anxiety scale – the CASI 20 - developed by Sukhodolsky and colleagues (2008) to reduce measurement confounding when assessing anxiety in the presence of ASD. All individual items are scored on a scale of 0–3, with higher scores indicating greater symptom severity.
Procedures
This study was conducted at the Center for Autism Research at The Children's Hospital of Philadelphia. All participants and their guardians completed a battery of tests examining the neuropsychological, neural, and genetic basis of cognitive control in ASD. If participants were re-recruited from prior studies within one year, diagnostic and cognitive tests were not readministered. The hospital’s Institutional Review Board approved the research protocol. Prior to participation, consent was obtained from all legal guardians and assent was obtained from all children. Parents completed the ADHD Rating Scale and Child and Adolescent Symptom Inventory either prior to the visit or the same day.
Data Processing and Analysis Plan
Accuracy and response time (RT) for accurate trials were calculated for each participant. Outlier trials were defined within each participant as an RT of two standard deviations above or below their mean; RTs defined as outliers were dropped from RT analyses, but correct outlier trials were still counted in accuracy analyses. After dropping outlier trials, we calculated the accuracy and average RT for each trial type (i.e. Neutral Valid, Neutral Invalid, Angry Valid, Angry Invalid) and percentage of catch trial errors. Additionally, we dropped one participant in the TDC group that had an average RT two standard deviations from the group mean, two participants in the ASD group that had an overall accuracy of <50%, and one participant in the ASD group with >50% Catch trial errors. Thus, after removing children who met exclusion criteria described above and those whose task performance were significant outliers the final sample included 41 children in the ASD group, and 25 in the TDC group.
A 2 (Cue: Valid, Invalid) × 2 (Emotion: Neutral, Angry) × 2 (Group: TDC, ASD) repeated measures ANOVA was used. Effect sizes are reported with p-values for significant main effects and interactions in ANOVAs (eta squared, η2), and Welch’s t-tests (Cohen’s d). The directionality of interaction effects revealed by the omnibus ANOVA (F) is determined with an independent Welch’s t-test (unequal variance). We also conducted a Pearson’s r correlation to examine the relationships between the IOR effect and the ADHD rating scale, and CASI 20, and a Spearman’s rho correlation to examine relationships between the IOR effect and ADOS-2 (Sears et al., 1999).
Results
Accuracy
There was no main effect of Group for accuracy, F(1, 64)=2.07, p=0.16, η2=0.01. There were no main effects for Emotion, F(1, 64)=0.89, p=0.35, η2<0.01, or Cue, F(1, 64)=0.06, p=0.81, η2<0.001. There were no significant interactions (all Fs<2.35, all ps>0.12, all η2<0.01). There was a significant difference in catch trial errors between groups, t(47.94)=362, p<0.001, d=0.74, with the ASD group exhibiting more errors.
Response Time (RT)
There was no significant main effect of Group, F(1, 64)=0.79, p=0.38, η2=0.01, or Emotion, F(1, 64)=0.54, p=0.47, η2<0.001 (See Table 2 for RT means). There was a significant main effect of Cue, F(1, 64)=69.82, p<0.001, η2=0.02, with faster RT in Invalid trials than in Valid trials, reflecting the IOR effect. There was a significant Group-by-Cue interaction for RT, F(1, 64)=5.74, p=0.02, η2<0.01 (See Figure 2). There was no significant group difference for Valid trials, t(59.84)=1.3, p=0.19, d=0.32, or Invalid trials, t(56.86)=0.54, p=0.59, d=0.13. No other interactions were significant (all Fs<1.14, all ps>0.29, all η2<0.001). To follow up the Group-by-Cue interaction, we ran an independent samples t-test for an Overall IOR effect which collapsed emotion conditions (([Angry Valid RT − Angry Invalid RT] + [Neutral Valid RT − Neutral Invalid RT])/2), t(51.70)=2.41, p=0.02, d=0.61.
Table 2.
Task performance by diagnostic group
| TDC M (SD) |
ASD M (SD) |
|
|---|---|---|
| Neutral-Valid | ||
| Accuracy (%) | 98.40 (2.38) | 97.87 (2.66) |
| RT (ms) | 398 (56) | 415 (71) |
| Neutral-Invalid | ||
| Accuracy (%) | 99.10 (1.59) | 97.80 (2.92) |
| RT (ms) | 387 (56) | 392 (67) |
| Anger-Valid | ||
| Accuracy (%) | 98.20 (3.50) | 98.60 (1.86) |
| RT (ms) | 398 (59) | 423 (76) |
| Anger-Invalid | ||
| Accuracy (%) | 98.90 (2.05) | 98.05 (2.34) |
| RT (ms) | 384 (68) | 396 (81) |
| Catch Trial Errors | ||
| Errors (%) | 0.20 (0.69) | 1.83 (2.74) |
| Overall | ||
| Accuracy (%) | 98.65 (1.58) | 98.08 (1.55) |
| RT (ms) | 392 (58) | 406 (70) |
| Overall Valid | ||
| RT(ms) | 398 (56) | 419 (71) |
| Overall Invalid | ||
| RT(ms) | 385 (61) | 394 (71) |
| Overall IOR | ||
| RT (ms) | 12.97 (19.66) | 25.08 (20.08) |
Note: Bold variables indicate significant difference between groups (p<0.05)
Figure 2.
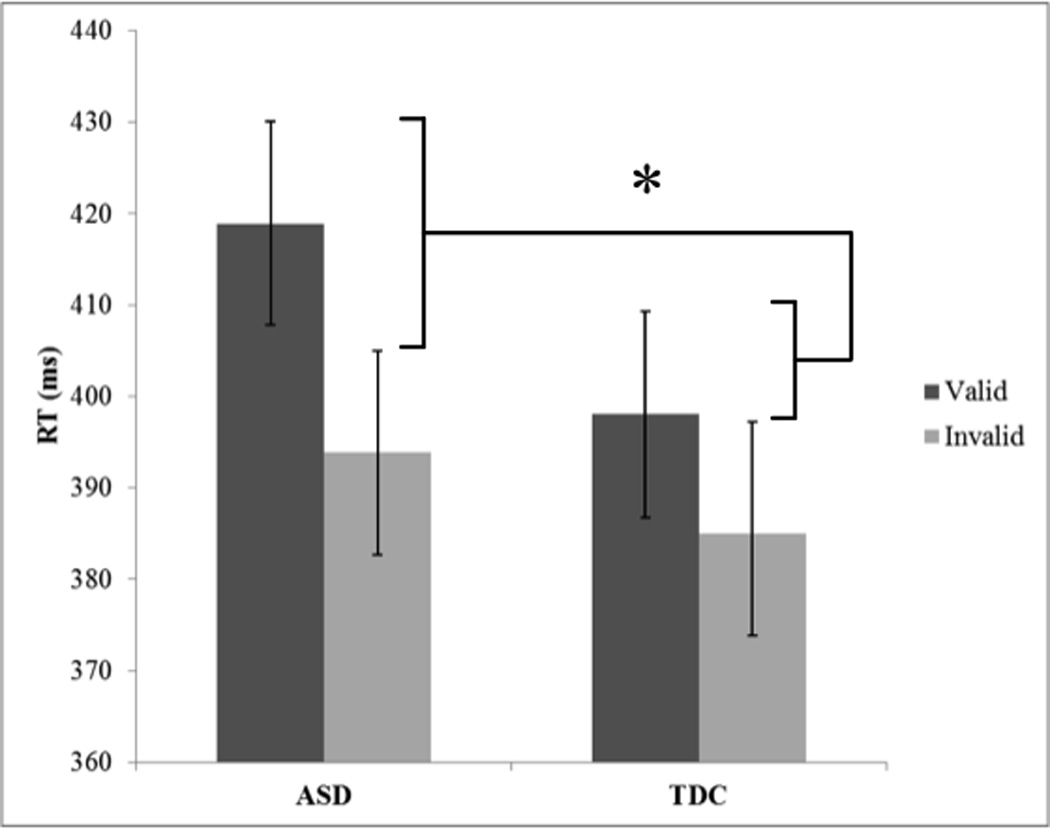
Bar graph displaying the post-hoc analysis for Group-by-Cue interaction.
Correlations with Overall and Emotional IOR
Within the ASD group, the Overall IOR correlated positively with the ADOS-2 Social Affect (rho=0.36, p=0.02), and ADOS-2 Total Calibrated Comparison score (rho=0.40, p=0.01; Figure 3). However, within ASD and TDC groups there was no significant relationship between the Overall IOR effect and the total raw score from the ADHD Rating Scale (all rs<0.16, all ps>0.32) or the CASI 20 anxiety scale (all rs<0.13, all ps>0.55). Taken together, these correlations suggest the stronger IOR effect is related to ASD symptoms (notably social), but is not significantly related to co-occurring anxiety or ADHD symptoms. Contradictory to our hypotheses, symptoms of anxiety were not related to the emotional IOR in either group (all rs<0.13, all ps>0.45).
Figure 3.
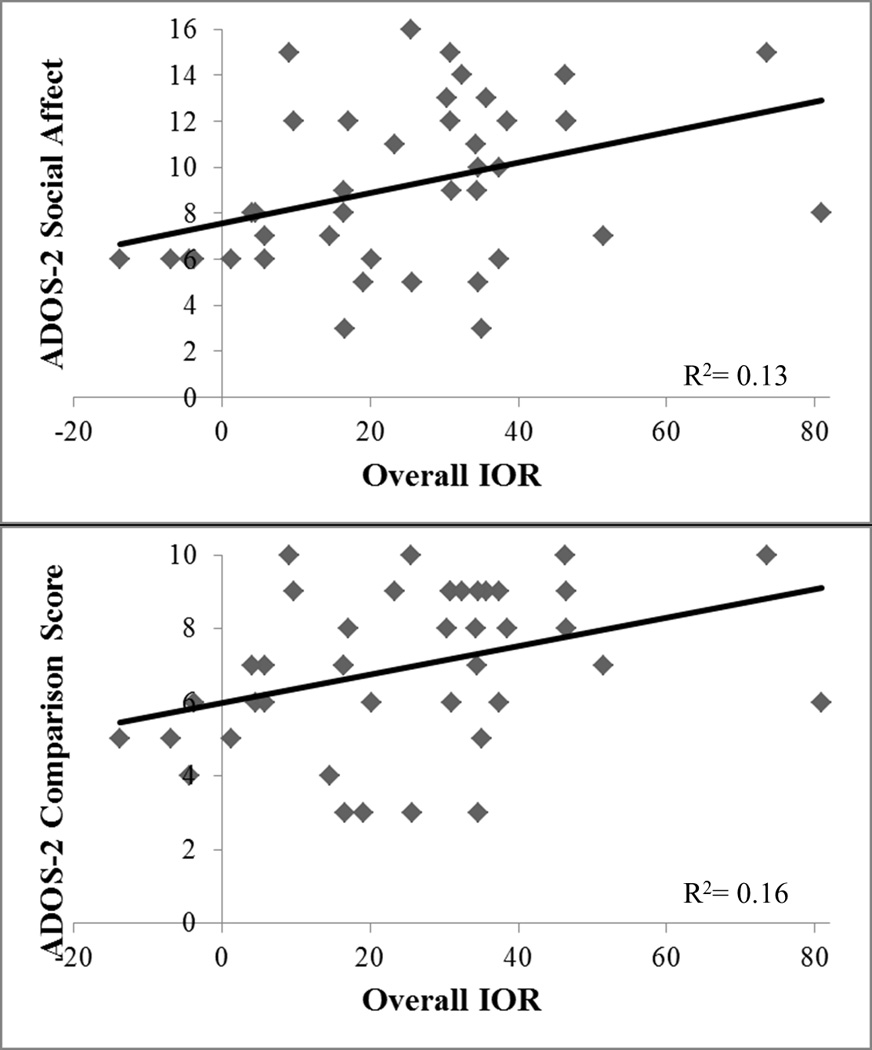
Correlation between Overall IOR effect and ADOS-2 scores. Top graph displays ADOS-2 Social Affect raw score.
Follow-Up Analysis
We observed a significant difference in Catch trial performance, documenting the large response bias in the ASD group (d=0.74). Because the IOR effect may be influenced by a response bias, we ran additional analyses to evaluate whether performance difference on Catch trials played a role in the significant difference between groups in Overall IOR. Groups were significantly different in number of participants with catch errors, X2(1, N = 66) = 9.48, p<0.01, therefore we dropped all participants who made these errors (TDC=2; ASD=18), leaving a sample of 23 TDC and 23 ASD participants.
In this sample we found no main effects or interactions for accuracy (all Fs<0.80, all ps>0.37, all η2<0.01). Consistent with the original analysis, we found a similar pattern of results in RT with no main effect of group, F(1, 44)=0.02, p=0.88, η2<0.001, a significant main effect of cue, F(1, 44)=57.37, p<0.001, η2=0.02, and a significant group-by-cue interaction, F(1, 44)=8.49, p<0.01, η2<0.01. There were no other significant effects or interactions (all Fs<0.52, all ps>0.47, all η2<0.001). Overall IOR continued to be significantly different between groups, t(43.59)=−2.91, p<0.01, d=0.87, and correlated with Social Affect symptoms (rho=0.43, p=0.04), and calibrated severity (rho=0.46, p=0.03).
Discussion
The present study demonstrated that the orienting component of visual attention is disrupted in ASD. Both groups responded faster to the Invalid trials than the Valid trials, reflecting the IOR effect; however, the ASD group was less accurate and had a stronger IOR effect than the TDC group. The IOR effect in the ASD group was positively correlated with social affect and total ASD symptom severity, but was not significantly correlated with ADHD or anxiety symptoms. Thus, this study identified an early mechanism of attention that contributes to atypical attention in ASD, and is associated with core social deficits but not common co-occurring symptoms.
Although the TDC group was predicted to demonstrate a reduced IOR effect for angry facial expressions relative to neutral facial expressions, this effect was not observed. This lack of difference between emotional expressions fails to replicate a prior study in young adults using a similar emotional spatial cueing task (Fox et al., 2002). These discrepant findings may be accounted for by differences in the methodology and samples, which are outlined below.
First, Fox and colleagues (2002) used schematic drawings of faces while the present study used photographs of social-emotional faces. The use of real photographs regardless of facial expression may have captured visual attention to a greater degree across all trial types than schematic faces, diminishing the valence effect (i.e. angry > neutral). Our observation that the TDC group had a weaker IOR effect than what has been observed previously (Fillmore, Milich, & Lorch, 2009; MacPherson, Klein, & Moore, 2003; Marotta et al., 2013; Rinehart et al., 2008), is consistent with this argument.
Secondly, the present study’s paradigm blocked the emotion conditions rather than interspersing them. As noted above, this choice limited potential ‘bleeding’ effect of the anger expression condition into the neutral expression condition, but it may have also led to the TDC participants to habituate to the emotional stimuli.
Third, there are differences between samples in the present and prior study. The present study’s TDC group included youth, whereas the prior investigation included college age students. There may be developmental differences in how emotional stimuli influence the IOR effect. Also, the present study’s TDC group inclusion criteria required no significant psychopathology, and this may have limited variability in their anxiety (See Table 1), which would have contributed to null findings in the present study’s Emotion conditions. This point is supported by a recent study showing that young adults with both high-trait anxiety and worry demonstrated a weakened IOR effect with emotional stimuli (Verkuil et al., 2009).
The present study extends the field’s knowledge by identifying an early stage of attention (orienting) as altered in children with ASD, and by linking impaired orienting with social impairments in ASD. This study’s key findings provide empirical support for Keehn et al.’s (2013) hypothesis that early attention processes contribute to core ASD symptoms. While studies have demonstrated altered development of attention orienting in children with ASD and infants at high-risk to develop ASD (Elison et al., 2013; Elsabbagh et al., 2009; Keehn, Lincoln, Müller, & Townsend, 2010), none have demonstrated a linear relationship between orienting of attention and social impairments. The present study’s large sample size may have led to the observation of this relationship. Moreover, other social motivation hypotheses of ASD have argued that visual attention prioritizes social signals and these hypotheses would also predict that altered attention orienting would be one potential process to have a cascading effect on social development (Chevallier et al., 2012; Geraldine Dawson, Webb, & McPartland, 2005; Schultz, 2005). While the findings from the present study cannot distinguish between a domain general impairment in attention orienting and a specific impairment for orienting attention with social information, the present study lays the groundwork for future investigations in this area.
Prior studies of the IOR effect in children with ASD using flashing boxes as peripheral cues found similar effects across ASD and control groups (Marotta et al., 2013; Rinehart et al., 2008). The use of non-social cue stimuli, as well as the small sample size (n’s<15 per group) may have limited their ability to detect group differences. Despite the small sample sizes, one study reported trends of a stronger IOR effect in an Asperger’s syndrome subset (Rinehart et al., 2008). The present study revealed significant group differences in the IOR effect, likely because the present study’s sample size was nearly three times larger than prior studies, and because the present study used pictorial social-emotional cues. While speculative, the pictorial stimuli may have captured attention in both groups to a greater degree and led to larger suppression/inhibition in the ASD group, which in turn led to the larger IOR effect. This speculation is consistent with the hypothesis that children with ASD do not prioritize social stimuli to the same degree as TDC children (Chevallier et al., 2012; Dawson et al., 2005; Dichter et al., 2010; Kohls, Chevallier, Troiani, & Schultz, 2012).
Interestingly, previous work has demonstrated that children with Asperger’s syndrome display a weaker IOR effect when the spatial cueing task placed faces in the center of the screen and used eye gaze to direct attention to the periphery (Marotta et al., 2013). Comparing this finding to the present study is difficult, because the present study’s paradigm did not require children to make explicit use of social information. Furthermore, cues at the center of the screen that direct attention to another location (endogenous cues) are known to influence visual attention differently than peripheral cues that are drawing visual attention to a specific location (exogenous cues; Lupiáñez et al., 2004). In future studies, it may be useful to independently manipulate implicit and explicit processing of social-emotional information, as well as endogenous and exogenous cueing to examine how these factors influence orienting of visual attention in ASD.
The positive correlation between the stronger IOR effect and ASD symptoms, but not anxiety or ADHD symptoms, demonstrates that the orienting process of visual attention may be specific to ASD deficits. Indeed, if ADHD or anxiety symptoms were influencing the task performance, we would also have observed an overall diminished IOR effect or an interaction with Emotion condition, respectively. These effects were not observed in the overall ANOVA. Therefore, it is unlikely that the results can be explained by these co-occurring symptoms.
Future research can expand on the present findings in several ways. First, it is important to tease apart whether the stronger IOR effect in the ASD group and its relationship to ASD symptoms are specific to social-emotional peripheral cues. If the IOR effect correlates with ASD symptoms when non-social emotional stimuli are used as peripheral cues, then this would suggest that the orienting component of visual attention is generally disturbed in ASD. Interventions targeting improved orienting of visual attention could support individuals with ASD. Such interventions have already been piloted in healthy toddlers (Wass, Porayska-Pomsta, & Johnson, 2011). If the effect is specific to social-emotional stimuli, then this is compatible with a burgeoning field of research to improve attention to social-emotional information in ASD (Clark-Elford et al., 2015; Tanaka et al., 2010).
Second, normative studies have demonstrated that the IOR effect has strong connections to visual search ability. Larger IOR effects are thought to support enhanced visual search capabilities. Thus, this enhanced IOR effect may be one mechanism to support the putative enhanced visual search capabilities in ASD (Jolliffe & Baron-Cohen, 1997; Kaldy, Kraper, Carter, & Blaser, 2011; O’Riordan, Plaisted, Driver, & Baron-Cohen, 2001; Rutherford, Richards, Moldes, & Sekuler, 2007). Future studies should confirm this finding in ASD by pairing spatial cueing tasks with visual search paradigms.
Third, future studies should examine the time course of both IOR and facilitation effects using manual and saccadic responses in individuals with ASD. These two effects unfold over different time courses (Briand, Larrison, & Sereno, 2000), and initial evidence suggests that the IOR effects may be similar in magnitude, but have an earlier time course in adults with ASD versus controls (Pieron, Seassau, Leboyer, & Zalla, 2014). The present study only examined one cue-target onset asynchrony that could elicit the IOR effect, similar to paradigms used in prior studies (Fox et al., 2002; Marotta et al., 2013; Rinehart et al., 2008). Thus, future investigations may examine whether the present finding of a stronger IOR effect in ASD is influenced by the cue-target onset asynchrony, as well as the potential role of response format. Furthermore, it will be important to dissociate the effects of exogenous vs. endogenous attention at the mechanistic level, which support distinct underlying attentional processes. For example, exogenous attentional orienting has been linked to programming of unexecuted eye movements whereas endogenous attention is thought to reflect top-down preparation in order to maintain goal-directed behavior. Because these two systems rely on partially distinct, yet interacting neural substrates (Chica, Bartolomeo, & Lupiáñez, 2013; Pinto, van der Leij, Sligte, Lamme, & Scholte, 2013), future work examining these differences may lead to the more precise neural link underlying visual attention deficits in ASD.
Finally, the lack of a correlation between the IOR effect and co-occurring anxiety and ADHD symptoms suggests this alteration in visual attention may be specific to ASD. The lack of a relationship between anxiety and Overall IOR effect converges with recent findings that co-occurring anxiety symptoms has little influence on attention vis à vis attentional biases in ASD (Hollocks, Ozsivadjian, Matthews, Howlin, & Simonoff, 2013; May, Cornish, & Rinehart, 2015). Furthermore, the lack of a correlation between the Overall IOR effect with ADHD symptoms in the present study aligns with a prior study showing no differences among ASD and ADHD groups’ orienting during the complex attentional network task (Samyn, Roeyers, Bijttebier, & Wiersema, 2013). Thus, the potential specificity of this finding to ASD should be followed up in future investigations. Reliability of the stronger IOR effect with social-emotional cues in ASD, and relationship to ASD symptoms require replication. If these findings are replicated and reliability is established, then future research may explore measuring the IOR effect in children at high-risk for an ASD to predict diagnosis.
It is important to note that the present study only included children with ASD without intellectual disability. Therefore, it is imperative to replicate this study in individuals with intellectual disabilities to confirm whether the pronounced IOR effect and its relationship to social impairment are present across the cognitive spectrum.
Conclusion
In summary, when using social-emotional cues, the ASD group had a significantly stronger IOR effect compared to the TDC group. This pronounced IOR effect did not correlate with ADHD or anxiety symptoms, but did correlate with measures of social affect and severity in the ASD group. These findings suggest that this attention impairment may be specific to ASD. The pronounced IOR effect in the ASD group indicates greater deficits in orienting visual attention in the context of social-emotional stimuli. This finding is in line with hypotheses regarding deficits in early visual attention mechanisms having a cascading effect on atypical social development in ASD.
Acknowledgments
We thank the many children and families for their participation in the study. The study was sponsored by grants from the National Institute of Mental Health (K23MH086111; PI: B.E. Yerys, R21MH092615; PI: B.E. Yerys, RC1MH088791; R.T. Schultz), and a New Program Development Award to B.E. Yerys through the Intellectual and Developmental Disabilities Research Center funded by the National Institute of Child and Human Development (P30HD026979; PI: M. Yudkoff), a grant from the Philadelphia Foundation, a grant from the Pennsylvania Department of Health (SAP #4100042728) to R.T. Schultz, a grant from the Pennsylvania Department of Health (SAP # 4100047863) to R.T. Schultz, a grant from Pfizer to R.T. Schultz, and a grant from the Robert Wood Johnson Foundation, #6672 to R.T. Schultz.
Footnotes
Author contributions: B.E.Y. conceived the study. B.E.Y. and V.T. programmed the experiment and generated the stimuli. M.G.M. and L.A. recruited, enrolled, and completed the experimental measures. B.E.Y. conducted or supervised almost all clinical evaluations. L.A. completed all data analyses. L.A., M.G.M., V.T., and B.E.Y contributed to manuscript preparation.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition. 4th. Arlington, VA: American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Briand KA, Larrison AL, Sereno AB. Inhibition of return in manual and saccadic response systems. Perception and Psychophysics. 2000;62(8):1512–1524. doi: 10.3758/bf03212152. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012 doi: 10.1016/j.tics.2012.02.007. http://doi.org/10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P, Lupiáñez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behavioural Brain Research. 2013;237:107–123. doi: 10.1016/j.bbr.2012.09.027. http://doi.org/10.1016/j.bbr.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Clark-Elford R, Nathan PJ, Auyeung B, Mogg K, Bradley BP, Sule A, Baron-Cohen S. Effects of Oxytocin on Attention to Emotional Faces in Healthy Volunteers and Highly Socially Anxious Males. International Journal of Neuropsychopharmacology. 2015;18(2):pyu012–pyu012. doi: 10.1093/ijnp/pyu012. http://doi.org/10.1093/ijnp/pyu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Heller W. Paying attention to emotion: an fMRI investigation of cognitive and emotional stroop tasks. Cognitive, Affective & Behavioral Neuroscience. 2003;3(2):81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research. 2009;166(2–3):210–222. doi: 10.1016/j.psychres.2008.02.005. http://doi.org/10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T. Performance on the emotional Stroop task in groups of anxious, expert, and control subjects: A comparison of computer and card presentation formats. Cognition & Emotion. 1995;9:341–362. [Google Scholar]
- Dawson G, Lewy A. Arousal, attention, and the socioemotional impairments of individuals with autism. In: Dawson G, editor. Autism: Nature, diagnosis, and treatment. New York: Guilford Press; 1989. pp. 49–74. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. http://doi.org/10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2010;7(2):160–172. doi: 10.1093/scan/nsq095. http://doi.org/10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-5 for Children and Adolescents: Checklists, norms, and clinical interpretation. New York: Guilford Press; 2016. [Google Scholar]
- Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Piven J. White Matter Microstructure and Atypical Visual Orienting in 7-Month-Olds at Risk for Autism. The American Journal of Psychiatry. 2013 doi: 10.1176/appi.ajp.2012.12091150. http://doi.org/10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales-II (DAS-II) San Antonio, TX: Pearson Assessments; 2007. [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, Johnson MH. Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(5):637–642. doi: 10.1111/j.1469-7610.2008.02051.x. http://doi.org/10.1111/j.1469-7610.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Milich R, Lorch EP. Inhibitory deficits in children with attention-deficit/hyperactivity disorder: intentional versus automatic mechanisms of attention. Development and Psychopathology. 2009;21(2):539–554. doi: 10.1017/S0954579409000297. http://doi.org/10.1017/S0954579409000297. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional Bias for Threat: Evidence for Delayed Disengagement from Emotional Faces. Cognition & Emotion. 2002;16(3):355–379. doi: 10.1080/02699930143000527. http://doi.org/10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Childhood Symptom Inventory - Fourth Edition. Stony Brook, NY: Checkmate Plus; 2000. [Google Scholar]
- Gadow KD, Sprafkin J. Child & Adolescent Symptom Inventory - Fourth Edition Revised. Stony Brook, NY: Checkmate Plus; 2010. [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. http://doi.org/10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FG. Wechsler IQ profile and theory of mind in autism: a research note. Journal of Child Psychology and Psychiatry. 1994;35(8):1461–1471. doi: 10.1111/j.1469-7610.1994.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Miller J, Pandey J, Schultz RT. Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. 2015 doi: 10.1093/scan/nsw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, Heller W. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion (Washington, D.C.) 2005;5(2):200–207. doi: 10.1037/1528-3542.5.2.200. http://doi.org/10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Holle C, Neely JH, Heimberg RG. The effects of blocked versus random presentation and semantic relatedness of stimulus words on response to a modified Stroop task among social phobics. Cognitive Therapy & Research. 1997;21:681–697. [Google Scholar]
- Hollocks M, Jones C, Pickles A, Baird G, Happe F, Simonoff E. The association between social cognition and executive functioning and symptoms of anxiety and depression in adolescents with autism spectrum disorders. Autism Research. 2014;7(2):216–228. doi: 10.1002/aur.1361. http://doi.org/10.1002/aur.1361. [DOI] [PubMed] [Google Scholar]
- Hollocks M, Ozsivadjian A, Matthews C, Howlin P, Simonoff E. The relationship between attentional bias and anxiety in children and adolescents with autism spectrum disorders. Autism Research. 2013;6(4):237–247. doi: 10.1002/aur.1285. http://doi.org/10.1002/aur.1285. [DOI] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS Domain Scores: Separating Severity of Social Affect and Restricted and Repetitive Behaviors. Journal of Autism and Developmental Disorders. 2012;44(10):2400–2412. doi: 10.1007/s10803-012-1719-1. http://doi.org/10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Lord C. The Autism Diagnostic Observation Schedule, Module 4: Revised Algorithm and Standardized Severity Scores. Journal of Autism and Developmental Disorders. 2014;44(8):1996–2012. doi: 10.1007/s10803-014-2080-3. http://doi.org/10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational Modelling Of Visual Attention. Nature Reviews Neuroscience. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. http://doi.org/10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. Are People with Autism and Asperger Syndrome Faster than Normal on The Embedded Figures Test? Journal of Child Psychology and Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. http://doi.org/10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Kaldy Z, Kraper C, Carter AS, Blaser E. Toddlers with Autism Spectrum Disorder are more successful at visual search than typically developing toddlers. Developmental Science. 2011;14(5):980–988. doi: 10.1111/j.1467-7687.2011.01053.x. http://doi.org/10.1111/j.1467-7687.2011.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Lincoln AJ, Müller R-A, Townsend J. Attentional networks in children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51(11):1251–1259. doi: 10.1111/j.1469-7610.2010.02257.x. http://doi.org/10.1111/j.1469-7610.2010.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Müller R-A, Townsend J. Atypical Attentional Networks and the Emergence of Autism. Neuroscience and Biobehavioral Reviews. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. http://doi.org/10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Senju A, Akechi H, Tojo Y, Osanai H, Hasegawa T. Atypical disengagement from faces and its modulation by the control of eye fixation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41(5):629–645. doi: 10.1007/s10803-010-1082-z. http://doi.org/10.1007/s10803-010-1082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. http://doi.org/10.1016/S1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kohls G, Chevallier C, Troiani V, Schultz RT. Social “wanting” dysfunction in autism: neurobiological underpinnings and treatment implications. Journal of Neurodevelopmental Disorders. 2012;4(1):10. doi: 10.1186/1866-1955-4-10. http://doi.org/10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koven NS, Heller W, Banich M, Miller GA. Relationships of Distinct Affective Dimensions to Performance on an Emotional Stroop Task. Cognitive Therapy & Research. 2003;27:671–680. [Google Scholar]
- Landry R, Bryson SE. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. http://doi.org/10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Law AS, Burton AM, Schweinberger SR. Attention capture by faces. Cognition. 2008;107(1):330–342. doi: 10.1016/j.cognition.2007.07.012. http://doi.org/10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Li C-SR, Chang H-L, Lin S-C. Inhibition of return in children with attention deficit hyperactivity disorder. Experimental Brain Research. 2003;149(1):125–130. doi: 10.1007/s00221-002-1362-8. http://doi.org/10.1007/s00221-002-1362-8. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, second edition (ADOS-2) manual (Part I): Modules 1–4. Torrance, CA: Westerm Psychological Services; 2012. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. http://doi.org/7814313. [DOI] [PubMed] [Google Scholar]
- Lupiáñez J, Decaix C, Siéroff E, Chokron S, Milliken B, Bartolomeo P. Independent effects of endogenous and exogenous spatial cueing: inhibition of return at endogenously attended target locations. Experimental Brain Research. 2004;159:447–457. doi: 10.1007/s00221-004-1963-5. http://doi.org/10.1007/s00221-004-1963-5. [DOI] [PubMed] [Google Scholar]
- MacPherson AC, Klein RM, Moore C. Inhibition of return in children and adolescents. Journal of Experimental Child Psychology. 2003;85(4):337–351. doi: 10.1016/s0022-0965(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Marotta A, Pasini A, Ruggiero S, Maccari L, Rosa C, Lupiáñez J, Casagrande M. Inhibition of Return in Response to Eye Gaze and Peripheral Cues in Young People with Asperger’s Syndrome. Journal of Autism and Developmental Disorders. 2013;43(4):917–923. doi: 10.1007/s10803-012-1636-3. http://doi.org/10.1007/s10803-012-1636-3. [DOI] [PubMed] [Google Scholar]
- May T, Cornish K, Rinehart NJ. Mechanisms of Anxiety Related Attentional Biases in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2015 doi: 10.1007/s10803-015-2500-z. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. Journal of Child Psychology and Psychiatry. 2003;44(6):904–913. doi: 10.1111/1469-7610.00174. [DOI] [PubMed] [Google Scholar]
- Nakano T, Tanaka K, Endo Y, Yamane Y, Yamamoto T, Nakano Y, Kitazawa S. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proceedings. Biological Sciences / The Royal Society. 2010;277(1696):2935–2943. doi: 10.1098/rspb.2010.0587. http://doi.org/10.1098/rspb.2010.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior Visual Search in Autism. Journal of Experimental Psychology. Human Perception and Performance. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. http://doi.org/10.1037/0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Ortega R, López V, Carrasco X, Anllo-Vento L, Aboitiz F. Exogenous orienting of visual-spatial attention in ADHD children. Brain Research. 2013;1493:68–79. doi: 10.1016/j.brainres.2012.11.036. http://doi.org/10.1016/j.brainres.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Pieron M, Seassau M, Leboyer M, Zalla T. Accelerated time course of saccadic inhibition of return in individuals with autism spectrum disorders. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-4152-1. http://doi.org/10.1007/s00221-014-4152-1. [DOI] [PubMed] [Google Scholar]
- Pinto Y, van der Leij AR, Sligte IG, Lamme VA, Scholte HS. Bottom-up and top-down attention are independent. Journal of Vision. 2013;13(3):16. doi: 10.1167/13.3.16. http://doi.org/10.1167/13.3.16. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The Attention System of the Human Brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. http://doi.org/10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4(7):1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese CE, Anthony LG, Haake C, Martucci JL, Granader Y, Kenworthy L. Relations Between Anxiety and Executive Function in Youth with ASD; Oral presented at the annual International Meeting for Autism Research; Salt Lake City, UT. 2015. May, Retrieved from https://imfar.confex.com/imfar/2015/webprogram/Paper19927.html. [Google Scholar]
- Riby DM, Brown PH, Jones N, Hanley M. Brief report: faces cause less distraction in autism. Journal of Autism and Developmental Disorders. 2012;42(4):634–639. doi: 10.1007/s10803-011-1266-1. http://doi.org/10.1007/s10803-011-1266-1. [DOI] [PubMed] [Google Scholar]
- Rice K, Moriuchi JM, Jones W, Klin A. Parsing Heterogeneity in Autism Spectrum Disorders: Visual Scanning of Dynamic Social Scenes in School-Aged Children. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(3):238–248. doi: 10.1016/j.jaac.2011.12.017. http://doi.org/10.1016/j.jaac.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Bradshaw JL, Moss SA, Brereton AV, Tonge BJ. Brief report: Inhibition of return in young people with autism and Asperger’s disorder. Autism. 2008;12(3):249–260. doi: 10.1177/1362361307088754. http://doi.org/10.1177/1362361307088754. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Richards ED, Moldes V, Sekuler AB. Evidence of a divided-attention advantage in autism. Cognitive Neuropsychology. 2007;24(5):505–515. doi: 10.1080/02643290701508224. http://doi.org/10.1080/02643290701508224. [DOI] [PubMed] [Google Scholar]
- Sacrey L-AR, Bryson SE, Zwaigenbaum L. Prospective Examination of Visual Attention during Play in Infants at High-Risk for Autism Spectrum Disorder: A Longitudinal Study from 6 to 36 Months of Age. Behavioural Brain Research. 2013 doi: 10.1016/j.bbr.2013.08.028. http://doi.org/10.1016/j.bbr.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Samyn V, Roeyers H, Bijttebier P, Wiersema JR. Attentional Networks in Boys With ADHD or Autism Spectrum Disorder and the Relationship With Effortful Control. Journal of Attention Disorders. 2013 doi: 10.1177/1087054712473183. http://doi.org/10.1177/1087054712473183. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. http://doi.org/10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1999;23(4):613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion Dysregulation in Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2014;171(3):276–293. doi: 10.1176/appi.ajp.2013.13070966. http://doi.org/10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzig J, Bruning N, Morsch D, Lehmkuhl G. Attention profiles in autistic children with and without comorbid hyperactivity and attention problems. Acta Neuropsychiatrica. 2008;20(4):207–215. doi: 10.1111/j.1601-5215.2008.00292.x. http://doi.org/10.1111/j.1601-5215.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, Vitiello B. Parent-Rated Anxiety Symptoms in Children with Pervasive Developmental Disorders: Frequency and Association with Core Autism Symptoms and Cognitive Functioning. Journal of Abnormal Child Psychology. 2008;36(1):117–128. doi: 10.1007/s10802-007-9165-9. http://doi.org/10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, Wheelwright S. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry. 1998;39(5):747–753. http://doi.org/9690937. [PubMed] [Google Scholar]
- Tanaka JW, Wolf JM, Klaiman C, Koenig K, Cockburn J, Herlihy L, Schultz RT. Using computerized games to teach face recognition skills to children with autism spectrum disorder: the Let’s Face It! program. Journal of Child Psychology and Psychiatry. 2010;51(8):944–952. doi: 10.1111/j.1469-7610.2010.02258.x. http://doi.org/10.1111/j.1469-7610.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Weaver B, Watson FL. Inhibition of return to successively cued spatial locations: Commentary on Pratt and Abrams (1995) Journal of Experimental Psychology: Human Perception and Performance. 1996;22(5):1289–1293. doi: 10.1037//0096-1523.22.5.1289. http://doi.org/http://dx.doi.org/10.1037/0096-1523.22.5.1289. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. http://doi.org/10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, Putman P, Thayer BF. Interacting effects of worry and anxiety on attentional disengagement from threat. Behaviour Research & Therapy. 2009;47(2):146–152. doi: 10.1016/j.brat.2008.11.003. http://doi.org/10.1016/j.brat.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klein RM. Searching for inhibition of return in visual search: A review. Vision Research. 2010;50(2):220–228. doi: 10.1016/j.visres.2009.11.013. http://doi.org/10.1016/j.visres.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Wass S, Porayska-Pomsta K, Johnson MH. Training attentional control in infancy. Current Biology: CB. 2011;21(18):1543–1547. doi: 10.1016/j.cub.2011.08.004. http://doi.org/10.1016/j.cub.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Nitz AB, Craske MG, Johnson C. The effects of anxiety upon attention allocation to affective stimuli. Behaviour Research and Therapy. 2007;45(4):763–774. doi: 10.1016/j.brat.2006.07.002. http://doi.org/10.1016/j.brat.2006.07.002. [DOI] [PubMed] [Google Scholar]
- White HA. Inhibitory control of proactive interference in adults with ADHD. Journal of Attention Disorders. 2007;11(2):141–149. doi: 10.1177/1087054706295604. http://doi.org/10.1177/1087054706295604. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Kenworthy L, Jankowski KF, Strang J, Wallace GL. Separate Components of Emotional Go/No-Go Performance Relate to Autism Versus Attention Symptoms in Children With Autism. Neuropsychology. 2013;27(5):537–545. doi: 10.1037/a0033615. http://doi.org/10.1037/a0033615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Ruiz E, Strang J, Sokoloff J, Kenworthy L, Vaidya CJ. Modulation of attentional blink with emotional faces in typical development and in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2012;54(6):636–643. doi: 10.1111/jcpp.12013. http://doi.org/10.1111/jcpp.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. http://doi.org/10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]


