Abstract
The last two decades have seen significant advancement in our understanding of colorectal tumors with DNA mismatch repair (MMR) deficiency. The ever-emerging revelations of new molecular and genetic alterations in various clinical conditions have necessitated constant refinement of disease terminology and classification. Thus, a case with the clinical condition of hereditary non-polyposis colorectal cancer as defined by the Amsterdam criteria may be one of Lynch syndrome characterized by a germline defect in one of the several MMR genes, one of the yet-to-be-defined “Lynch-like syndrome” if there is evidence of MMR deficiency in the tumor but no detectable germline MMR defect or tumor MLH1 promoter methylation, or “familial colorectal cancer type X” if there is no evidence of MMR deficiency. The detection of these conditions carries significant clinical implications. The detection tools and strategies are constantly evolving. The Bethesda guidelines symbolize a selective approach that uses clinical information and tumor histology as the basis to select high-risk individuals. Such a selective approach has subsequently been found to have limited sensitivity, and is thus gradually giving way to the alternative universal approach that tests all newly diagnosed colorectal cancers. Notably, the universal approach also has its own limitations; its cost-effectiveness in real practice, in particular, remains to be determined. Meanwhile, technological advances such as the next-generation sequencing are offering the promise of direct genetic testing for MMR deficiency at an affordable cost probably in the near future. This article reviews the up-to-date molecular definitions of the various conditions related to MMR deficiency, and discusses the tools and strategies that have been used in detecting these conditions. Special emphasis will be placed on the evolving nature and the clinical importance of the disease definitions and the detection strategies.
Keywords: Lynch syndrome, Lynch-like syndrome, CMMR-D, Sporadic MSI cancer, Microsatellite instability, MMR IHC
Introduction
Knowledge about colorectal cancers with microsatellite instability (MSI) has grown significantly since the initial discovery of MSI in colorectal cancer in 1993.1–3 The genetic basis for MSI has been found to be a defective DNA mismatch repair (MMR) system and two major mechanisms responsible for causing such deficiencies have been uncovered. One is germline mutation in one of the major MMR genes, namely, MLH1 (mutL homolog 1), MSH2 (mutS homolog 2), MSH6 (mutS homolog 6), and PMS2 (postmeiotic segregation increased 2), or deletions in the EPCAM gene that cause allele-specific MSH2 inactivation. Germline mutation in an MMR gene or EPCAM defines Lynch syndrome (LS). The second major mechanism relates to a somatic occurrence of promoter methylation of MLH1, often in the context of CpG island methylator phenotype (CIMP). This mechanism leads to sporadic MSI colorectal cancers.
MSI colorectal cancers are found to have distinctive clinicopathological features. They arise primarily in the right colon and tend to be locally bulky, but are less likely to have nodal or distant metastasis. While most MSI colorectal cancers are gland forming similar to their non-MSI counterparts, they tend to have conspicuous tumor-infiltrating lymphocytes and often contain a second or third histologic pattern, which results in histologic heterogeneity. Other histological features over-represented in MSI cancers include mucinous and signet-ring-cell components, and the so-called “medullary” pattern. Significantly, the MSI phenotype has been shown to impact on prognosis and treatment response. In general, MSI colorectal cancers have a better prognosis than their non-MSI counterparts, and patients with MSI colorectal cancers may not benefit from 5-fluorouracil therapy.4,5 Furthermore, ongoing efforts on immunotherapeutic approaches targeting the MSI phenotype and its associated tumor-infiltrating lymphocytes may offer further treatment options for MSI colorectal cancers.6
Perhaps, the most significant development in the field of MSI in recent years is the growing awareness of LS and the widespread efforts on its detection and management.1 There have been data indicating that the cancer incidence and mortality in LS patients and their family members can be significantly reduced by stringent surveillance protocols3 and preventive measures.7 Consequently, there has been a steady increase in the attention and effort devoted to the detection of this syndrome.
Today, as knowledge about LS and MSI cancers translates to better clinical practice, it is particularly worth directing our attention to the most updated definitions of these conditions, and also to the specific utilities of the various testing tools and strategies that are being used to detect these conditions. Incorporation of the most up-to-date knowledge and technology is the key to ensure the most precise diagnosis and consequently the most precise management of these diseases.
A brief historical overview
MSI in colorectal cancer was first discovered in 1993. This discovery was preceded by decades of clinical investigation that dated back to 1913 when a pathologist, Aldred Warthin, first documented the phenomenon of cancer heredity. Significant developments in the decades of clinical investigation following Warthin’s initial observation include the description of hereditary non-polyposis colorectal cancer (HNPCC) by Henry Lynch, the formation of the international collaborative group on HNPCC (ICG-HNPCC)8, and the creation of the Amsterdam criteria (AC, Table 1)8,9 by the ICG-HNPCC. In 1993, accompanying the discovery of MSI, studies on HNPCC families detected a cancer susceptibility locus on chromosome 2p. Built on knowledge that a defective MMR system could cause MSI in bacteria and yeast, the gene involved on chromosome 2p was soon found to be MSH2, and the mutations in MSH2 and the MSI phenotype were found to be causally related. Shortly afterward, MLH1 was discovered in another HNPCC family. These breakthroughs thus ushered in a molecular era wherein Warthin’s family G and similar families are defined by their molecular traits and genetic status, and the Amsterdam criteria used to detect these families are complemented and, in many cases, supplanted by molecular genetic testing.
Table 1.
The Amsterdam criteria.
| Amsterdam criteria | Amsterdam II criteria |
|---|---|
| Three or more family members, one of whom is a first-degree relative of the other two, with a confirmed diagnosis of colorectal cancer |
Three or more family members (one of whom is a first-degree relative of the other two) with HNPCC-related cancers (including colorectal, endometrial, stomach, small intestinal, hepatobiliary, renal pelvic, or ureteral cancers) |
| Two successive affected generations | Two successive affected generations |
| One or more colon cancers diagnosed before the age of 50 years | One or more of the HNPCC-related cancers diagnosed before the age of 50 years |
| Exclusion of familial adenomatous polyposis (FAP) | Exclusion of familial adenomatous polyposis (FAP) |
HNPCC, hereditary non-polyposis colorectal cancer.
Updated molecular definitions for Lynch syndrome and its related conditions
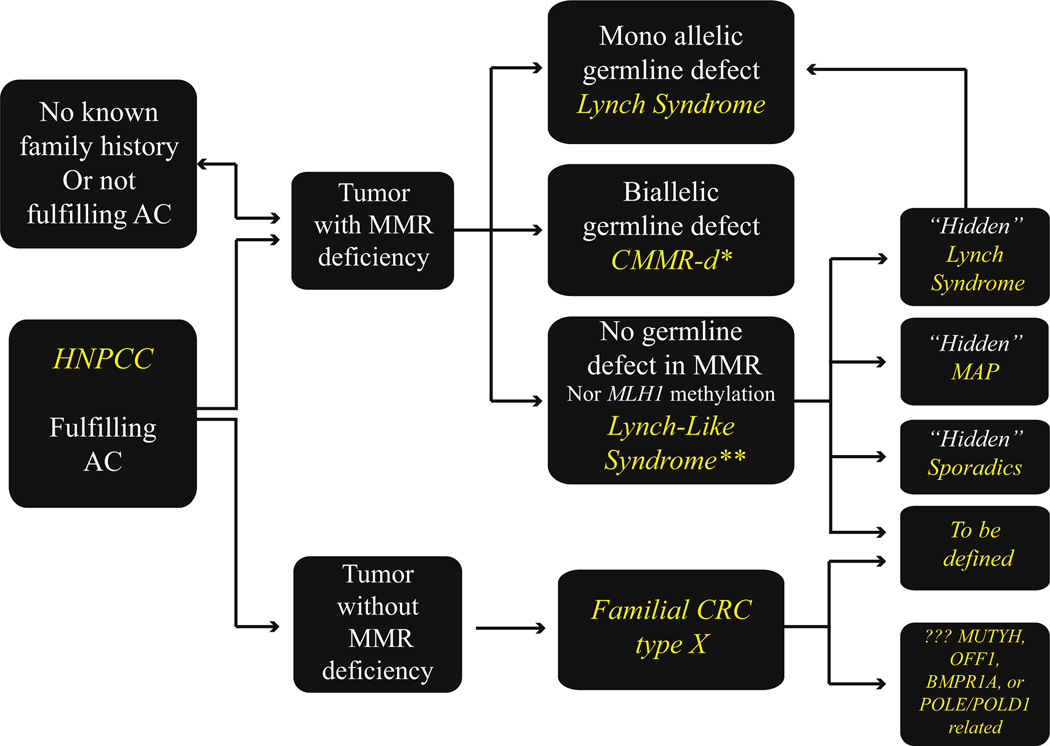
The knowledge gained since the discovery of MSI in colorectal carcinoma has allowed a new molecular approach to the classification of MMR deficiency-associated familial and sporadic colorectal cancers. Over the last two decades, as new data continually emerged and concepts evolved, the naming of the syndrome and its related conditions changed. Fig. 1 summarizes this dynamic process. The current definitions for the various conditions are described below.
Fig. 1.
Subclassification of colorectal cancer cases associated with “hereditary nonpolyposis colorectal cancer” and microsatellite instability. AC, Amsterdam criteria; HNPCC, hereditary nonpolyposis colorectal cancer; MMR, mismatch repair; CMMR-D, constitutional MMR deficiency; CRC, colorectal cancer; MAP, MUTYH associated polyposis. *May present with LS type cancers in early adulthood, **WHO term “probably Lynch syndrome.”
Lynch syndrome (LS) (MIM no. 120435-6) is currently defined as an autosomal dominant hereditary predisposition to colorectal cancer and certain other malignancies (including cancers in the endometrium, ovary, stomach, hepatobiliary tract, upper urinary tract, small bowel, pancreas, and brain) as a result of a deleterious germline mutation in one of the four DNA mismatch repair genes MLH1, MSH2, MSH6, and PMS2 or deletions in EPCAM. Thus, LS is now defined by germline mutation. The diagnosis applies to both individuals with an existing cancer and those who have not yet developed cancer. This definition is endorsed by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group in 200910 and by the 2010 WHO Classification of Tumours of the Digestive System.11 It is also supported by many major publications.
The definition of LS also applies to its two named variants. One is the Muir-Torre syndrome (MTS) (MIM no. 158320), which shows an association of sebaceous gland tumors with LS-type internal malignancies. The other is the Turcot syndrome (TS) (MIM no. 158320), which entails primary brain tumors coexisting with multiple colorectal adenomas. Notably, TS may be related to different genetic defects. Cases typically presenting with glioblastomas and colorectal polyps may represent either LS as defined above or constitutional MMR deficiency (CMMRD, see discussion below), whereas cases with medulloblastomas and colorectal adenomas may represent familial adenomatous polyposis caused by APC mutation.
The germline defect in LS and its named variants MTS and TS is monoallelic. In non-neoplastic cells, the wild-type allele maintains the DNA mismatch repair function and protects the genome from DNA damage. Tumors develop when there is loss of the wild-type allele, commonly through somatic point mutations or loss of heterozygosity. Thus, the tumors have biallelic loss of the affected gene, consequently, the tumors lose MMR function, exhibit MSI and show absence of the affected MMR protein(s) by immunohistochemistry. It is believed that LS colorectal cancers evolve from adenomas, via a significantly faster progression rate than sporadic cases (estimated adenoma-carcinoma transformation time of 1–3 years vs. 8–17 years in sporadic cases).12–14
Notably, only about one third of LS cases meet the Amsterdam criteria defined by the ICG-HNPCC.15 This, therefore, highlights the fact that family history, when not ascertained with care and thoroughness, is of relatively limited value in LS detection.
Lynch-like syndrome (LLS) is a recently proposed designation for cases with clinicopathologic features similar to LS but lacking a detectable germline mutation or promoter methylation in the MMR genes.16,17 Specifically, these individuals develop colorectal cancers that manifest MSI and show abnormal DNA MMR protein immunohistochemistry not only for MLH1 as with sporadic MSI cancers but also for the other MMR proteins, i.e., MSH2, MSH6, and PMS2, as for true LS cancers. Clinically, these patients show a mean age of onset similar to LS patients (53.7 ± 16.8 years in LLS vs. 48.5 ± 14.1 years in LS16). The main difference detected thus far between LS and LLS seems to be lower standardized incidence ratios for colorectal cancer (2.12 vs. 6.04) and for non-colonic LS-associated cancers (1.69 vs. 2.81) in LLS.16 Such intermediate ratios could imply that LLS encompasses heterogeneous conditions. In general, these Lynch-like individuals and their family members are being managed similar to those with bona fide LS.
It should be emphasized that LLS is an evolving term; the conditions under this name may diverge into different entities as new knowledge emerges. This is best exemplified by the case of EPCAM deletions.18 Patients with germline EPCAM deletions may present as a classic MSH2-type LS, only there is no detectable germline mutation in the MSH2 gene. These patients, therefore, would have fallen into the category of LLS before the definition of LS included the EPCAM gene. The discovery of EPCAM deletion causing LS was made in 2009. The EPCAM gene is located immediately upstream of MSH2; deletions of the terminal portion of EPCAM can result in continued transcription into MSH2, causing MSH2 promoter methylation and gene inactivation, but not affecting the gene’s sequence. This discovery thus explained these cases, necessitated the modification of the definition of LS to include EPCAM mutation, and called for the re-classification of these cases into the true LS category. This is significant as studies suggest that EPCAM deletions account for up to 30% of the patients with MSH2-negative tumors or about 20% of all LLS patients.19
Similarly, a small subset of LS cases (about 0.6%) has been found to be causally related to constitutional inactivation of the MLH1 by methylation (or MLH1 epimutation).19,20 For these cases, the genetic defect would not be detectable by the commonly used diagnostic techniques (i.e., sequence analysis, and deletion/duplication analysis of MLH1); consequently, the cases could be miscategorized.
More recently, studies have teased apart further subsets of LLS cases and placed them into either the true LS family,21 true sporadic MSI cancers,22 or a different hereditary syndrome.23,24
Rhees et al.,21 by using allelic dropout in long PCR to look for potential regions of rearrangement in the MSH2 gene, found inversion of exons 1–7 in this gene to be the underlying etiology for 6 of their 12 LLS cases that had absent MSH2 protein expression and positive MSI in the tumor tissues. This defect was not detectable by commercial genetic reference laboratories; thus, these patients would have been erroneously categorized as LLS had they not been tested for the MSH2 inversion.
Biallelic somatic mutations in the MMR genes—a mechanism indicating a sporadic event—have recently been found in some LLS cases. The phenomenon was first reported by Sourrouille et al.25 in 2013. Subsequently, a study by Mensenkamp et al.22 that used Sanger and ion semiconductor sequencing techniques found biallelic somatic mutations to be the explanation for 13 of 25 LLS tumors, eight in MLH1 and five in MSH2. Two more recent studies26,27 have further corroborated such findings and showed that a significant proportion (up to about 70%) of the MSI tumors of LLS patients had biallelic somatic MMR gene alterations. Thus, these cases are most likely members of the family of “sporadic MSI cancers”. However, one caveat in the interpretation of these findings relates to the fact that the germline studies were targeting the MMR genes. It is unclear whether and to what extent the possibility has been explored that such biallelic somatic events are secondary to germline defects in other genes.
To this point, it is particularly interesting to note that biallelic somatic mutations of the MMR genes have been shown to occur in patients with germline mutations in the human Mut Y homolog gene (MUTYH; MIM no. 604933), i.e., MUTYH-associated polyposis (MAP; MIM no. 608456). As referenced by Morak et al.,23 six MAP patients with MSI-H tumors were on record. Morak et al.23 described a seventh case in which the MMR deficiency in a sebaceous carcinoma was attributable to two somatic MAP-specific G > T trans-version mutations in the MSH2 gene. This patient had an LLS phenotype. Most recently, Castillejo et al.24 reported yet another seven LLS patients harboring MUTYH biallelic mutations. These seven patients were detected among a series of 225 LLS cases, resulting in a prevalence of 3.1%.
These findings are clinically significant as they allow the patients and their family members to receive the most appropriate management. For example, LLS cases that are found to be truly sporadic would no longer need to follow the stringent surveillance protocol that they and their family members would otherwise be recommended to follow, and the surveillance regimen can instead be based on their family history, which most often reduces the frequency and starting age of colonoscopies and eliminates the need for surveillance for extra-colonic LS-associated tumors.22
Constitutional MMR deficiency (CMMRD) (MIM no. 276300) refers to biallelic germline mutations in one of the four MMR genes, a rare condition first described in 1999. It also encompasses a subset of Turcot syndrome. A recently established European consortium “Care for CMMRD” (C4CMMRD)28 reviewed 146 such patients known to date and identified that 58% of the patients/families carried biallelic PMS2 mutations, while the remainder were more or less equally distributed among biallelic MSH6, MLH1, and MSH2 mutants. CMMRD individuals have a high risk of developing malignancies in childhood and adolescence, and the tumor spectrum is often wide encompassing hematological malignancies, brain/central nervous system tumors, and colorectal and other cancers that are typically seen in LS patients at a later age. These patients also tend to show features reminiscent of neurofibromatosis 1 (NF1), particularly multiple café au lait spots.
Diagnosis of CMMRD has been challenging due to the lack of awareness of this rare condition and the varied clinical manifestations. The C4CMMRD has proposed a set of criteria28 that hold the promise of improving recognition of this clinically significant condition. Those CMMRD cases that present in adulthood with tumors typically seen in LS may undergo LS workup. In such a context, it is important to bear in mind that MMR defects may not only be seen in tumor tissues but also in normal tissues, i.e., germline MMR protein loss or MSI can occur. Thus, by immunohistochemistry, absence of staining in the non-neoplastic cells should not be interpreted as a failure of proper staining. The interpretation of MSI in CMMRD is further complicated by the fact that altered microsatellites are present only in a small proportion of the cells from normal tissues, which is technically more challenging to detect. In non-typical LS-type tumors, the shifts of microsatellite alleles may be more subtle than what can be detected by the commonly used methods.
Sporadic MSI colorectal cancers accounts for the majority of MSI colorectal cancers. Overall, about 12% of all colorectal carcinomas are sporadic MSI cancers and only about 3% are LS associated. The majority of sporadic MSI colorectal carcinomas have MLH1 promoter hypermethylation, often, but not always, as a manifestation of CIMP. These cancers may evolve from serrated polyps. About 60% of them harbor the BRAF V600E mutation. Clinically, the tumors tend to occur in females, in the proximal colon, and have a better prognosis when compared to their non-MSI counterparts. However, it has recently been recognized that the better prognosis in MSI cancers may not apply to all histologic subtypes (see discussion below).
Notably, mechanisms other than MLH1 promoter methylation may underlie sporadic MSI cancers. As discussed above,22,27 some sporadic MSI cancers are likely to be caused by somatic biallelic hits. Additionally, deficiency in an epi-genetic histone mark, the H3K36 trimethyltransferase SETD2, has been suggested to cause MSI by preventing the binding of MutS-alpha (MSH2-MSH6 complex) to chromatin,29 and as such, constitutes another potential mechanism. Alterations of genes regulating MSH2 degradation [i.e., FRAP1 (also known as MTOR), HERC1, PRKCZ, and PIK3C2B] may represent further etiologies for somatic MSI.30
Hereditary non-polyposis colorectal cancer (HNPCC) is now used as a clinical term for colorectal cancer patients who fulfill the Amsterdam criteria.8,9 Thus, in contrast to LS which is now defined by germline mutation, HNPCC is defined by family history.
Roughly 2–5% of colorectal cancer patients fulfill the AC, i.e., have HNPCC. About 60% of the HNPCC patients have tumors that are MMR-deficient, the majority of which are Lynch syndrome as defined above, with some as-yet-undefined minor subsets being either CMMRD or LLS. The remaining 40% of the HNPCC patients develop tumors that do not show MMR deficiency. These patients, the “other half of HNPCC,” are currently categorized under the term familial colorectal cancer type X.31
Familial colorectal cancer type X (FCCTX), a designation suggested by Lindor et al.,31 refers to families who fulfill AC I but in whose tumors no DNA MMR gene defect is evident. The “X” is used to acknowledge the lack of understanding of the etiology. Although this was initially intended for families fulfilling AC I, more recent studies have included those fulfilling AC II with microsatellite stable tumors as well. Interestingly, as a group, the phenotype of FCCTX differs from LS in that the FCCTX colorectal cancers are more often distal in the colon, extracolonic cancers are less frequent, and the age of onset is later. A recent epidemiologic study32 showed that FCCTX patients are less likely to be current tobacco users when compared to LS or non-AC I colorectal cancer cases. Histopathologically, FCCTX tumors do not typically exhibit the MSI-H histology but frequently exhibit venous invasion.32 For the majority of these families, the underlying genetic alteration is not known. However, defects in non-MMR genes, such as MUTYH, OGG1, and BMPR1A, are occasionally detected.33–35 Efforts to molecularly demystify the “X” are ongoing.
The tools and strategies for detecting Lynch syndrome and related conditions
The task of detecting LS probands among colorectal cancer patients is at the current time carried out via a screening strategy. Various tools that bear informative value in predicting MMR deficiency are being pursued in an algorithmic fashion.
Detection tools
Family history and other clinical data
Clinical information was what enabled Warthin to detect “cancer heredity” more than a century ago. When ascertained with accuracy, it is a powerful tool. It forms the basis for the stringent Amsterdam criteria (Table 1). It also constitutes an important element in the less stringent Bethesda guidelines (Table 2)36 and the various other risk assessment models such as the PREMM1,2,6 Model37 and MMRpro.38 Limitations of clinical data and particularly of family history arise, however, when the families are small, widely distributed, or complicated by false paternity. The utility of family history in detecting LS can be further lessened by factors such as incomplete penetrance of the pathogenic mutation, cancer risk reduction as a result of screening or prophylactic surgery, and early death.
Table 2.
The Revised Bethesda Guidelines for testing colorectal tumors for microsatellite instability (MSI)
Tumors from individuals should be tested for MSI in the following situations
|
Hereditary non-polyposis colorectal cancer (HNPCC)-related tumors include colorectal, endometrial, stomach, ovarian, pancreas, ureter and renal pelvis, biliary tract, and brain (usually glioblastoma as seen in Turcot syndrome) tumors, sebaceous gland adenomas and keratoacanthomas in Muir–Torre syndrome, and carcinoma of the small bowel.
MSI-H, microsatellite instability–high in tumors. (Refers to changes in two or more of the five National Cancer Institute-recommended panels of microsatellite markers.)
Presence of tumor-infiltrating lymphocytes, Crohn’s-like lymphocytic reaction, mucinous/signet-ring differentiation, or medullary growth pattern.
There was no consensus among the workshop participants on whether to include the age criteria in guideline three above; participants voted to keep less than 60 years of age in the guidelines.
Tumor morphology
A number of morphological patterns have been shown to bear informative value in predicting MSI.2,39 They include increased tumor-infiltrating lymphocytes, tumor heterogeneity, the presence of minor or major components of mucinous, medullary, or signet-ring-cell elements, and Crohn’s-type lymphoid reaction. Of these, tumor-infiltrating lymphocytes have the highest sensitivity. Although morphology can provide an excellent clue in typical cases, as a screening tool, morphology alone cannot reliably identify some 40% of MSI-H cancers, and some 6% of MSI-H cancers may not have any discriminating features at all.40 Such limitations are well recognized and constitute part of the reason for the limited utility of the revised Bethesda guidelines which partly relied on tumor morphology.
MMR protein IHC
In contrast to tumor morphology, MMR protein IHC has proven to be a valuable tool in the screening of colorectal carcinoma for Lynch syndrome.41,42 It has a high sensitivity (about 93%) and nearly perfect specificity in predicting MSI. It has the advantage of identifying which gene is most likely to be affected. However, both MSI testing and MMR IHC will miss cases. For MMR IHC, some MLH1 mutation positive cases or even cases with MLH1 promoter methylation may show false-positive nuclear staining for MLH1. While the mechanism for false-positive staining in cases with MLH1 promoter methylation remains to be determined, some pathogenic missense mutations in MLH1 are believed to result in mutant peptides that are antigenically active, and thus react with the antibody used for IHC. MMR mutations that function as a dominant negative43 represent yet another etiology for false normal MMR IHC, as they impair MMR function but do not destroy the protein. A more detailed discussion on MMR IHC is available in a recent review article.2
MSI testing
PCR-based MSI testing compares the sizes of microsatellite marker sets in tumor DNA with corresponding DNA isolated from a normal tissue sample of the same patient via electrophoresis. A 1997 NCI consensus meeting44 recommended testing a core panel of five microsatellite markers consisting of two mononucleotide markers and three dinucleotide markers. This panel remains the most commonly used, although many laboratories are now using a variety of panels. MSI-high (MSI-H) is defined as two or more of the core panel of five markers, or more than 30% of the markers for other panels, showing instability; MSI-low (MSI-L) is defined as one of the five markers or fewer than 30% of the markers showing instability; and microsatellite stable (MSS) is defined as 0% of the markers showing instability.
It is generally agreed upon that MSI testing and MMR IHC are almost equally valuable in the detection of LS, and the decision of which to use as the first screening test hinges on local resources and expertise.
It is noteworthy that as newer methods stemming from the widespread use of genome sequencing become available, new technology for MSI detection is emerging as well. The “MSI-sensor” described by Niu et al.45 represents such an example. This is a software tool that quantifies MSI in paired tumor-normal genome sequencing data and reports the somatic status of corresponding microsatellite sites in the human genome.45 Salipante et al.46 also described a next-generation sequencing-based approach to detect MSI and showed it to be advantageous over existing PCR-based methods. Such advanced methodology holds the promise of becoming a routine part of diagnosis and treatment procedures.
MLH1 methylation testing
Detection of promoter methylation in MLH1 can serve as a screening tool in the detection of LS as MLH1 methylation is the underlying defect in the vast majority of sporadic MSI cancers and is usually not seen in LS. It has been shown that MLH1 promoter methylation of the “C region” in particular can serve as a predictor of MMR mutation-negative status in MSI-H colorectal cancer cases, whereas methylation of the “A region” was not predictive.47 However, caveats exist. As mentioned above, constitutional methylation may occur in LS, albeit infrequently. In such cases, MLH1 methylation will not be informative. Some20 have recommended that the seemingly sporadic colorectal and endometrial cancer cases whose tumors exhibit MLH1 methylation be considered for follow-up testing for a germline mutation and/or epimutation of MLH1 if they present with cancer under the age of 60 years and/or the tumor is BRAF wild-type.
BRAF V600E mutation analysis
This analysis detects the oncogenic V600E hotspot mutation within BRAF, a kinase-encoding gene from the RAS/RAF/ MAPK pathway. This mutation occurs in about 15% of colorectal cancers. It is found to be associated with sporadic MSI-H CRC as a result of MLH1 promoter methylation, and only rarely in LS.47 Consequently, detection of the V600E BRAF mutation in an MSI-H CRC is evidence against the presence of a germline mutation in either MLH1 or MSH2. Hence, screening for MMR genes will not be necessary in those patients who are positive for the V600E mutation, when there is no other significant evidence suggesting an MMR-associated Lynch syndrome mutation. Notably, BRAF pathogenic variants are not common in endometrial cancers; thus, BRAF testing is not helpful in distinguishing endometrial cancers that are sporadic from those that are LS-related.48
Detection strategy
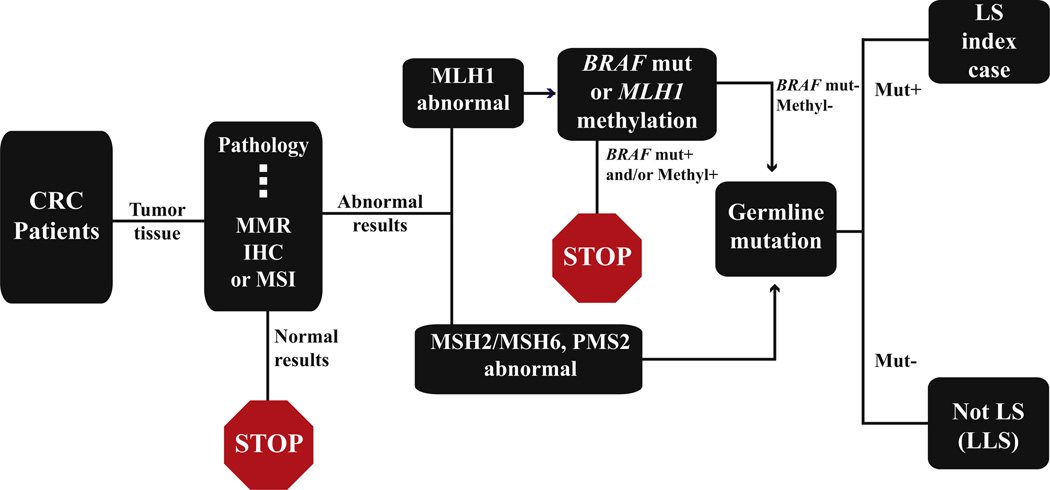
Most LS screening protocols pursue the various tests described above in an algorithmic fashion. Fig. 2 illustrates a commonly utilized algorithm. According to this schema, MMR IHC and/or MSI are used as first screening tools. Tumors showing normal results with either test are regarded as at low or negligible risk for LS, and no further tests are to be pursued. For cases with abnormal results, the triaging to subsequent tests will depend on which MMR protein is abnormal by MMR IHC. If there is abnormality in MLH1/ PMS2, MLH1 methylation and BRAF mutation may be tested. Positive MLH1 methylation and positive BRAF mutation would indicate sporadic MSI and no further testing is necessary. In cases where there is MLH1 methylation but no BRAF mutation, or there is no MLH1 methylation but positive BRAF mutation, the decision on whether there should be germline mutation testing will depend on the clinical suspicion for Lynch syndrome, and germline mutation testing is recommended when there is a suspicion. If MMR IHC shows abnormality in MSH2 and/or MSH6, or PMS2 alone, MMR gene germline mutation testing should be performed. Not surprisingly, issues exist in the application of such screening protocols.
Fig. 2.
An algorithmic detection schema utilizing screening tests to select at-risk patients for germline mutation testing for diagnosing Lynch syndrome. CRC, colorectal cancer; MMR, mismatch repair; IHC, immunohistochemistry; MSI, microsatellite instability testing; Methyl, methylation; Mut, mutation; LS, Lynch syndrome; LLS, Lynch-like syndrome.
Selective vs. universal approach
The question of who should be screened has been a contentious point ever since the screening tools became available. The Bethesda guidelines first developed more than a decade ago represented recommendations at the time and were based on personal/family history and tumor morphology. These guidelines are more sensitive than older criteria, particularly the Amsterdam criteria. However, they still fail to identify approximately 28% of LS mutation carriers.49 Thus, the alternative universal approach, i.e., testing all newly diagnosed colorectal cancers, was proposed and has gained increasing popularity. Indeed, cost-effective analysis performed by the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group10 and other investigators50 has found evidence favoring the universal approach. Another approach that is more relaxed than the Bethesda guidelines but less so than the universal approach is to test all colorectal cancers diagnosed in patients under the age of 70 years.51 Accordingly, the current NCCN guideline on this issue is to perform screening tests either on all colorectal patients (the universal approach) or on patients under the age of 70 years and those aged 70 years and older who meet the clinical criteria.52
However, the true effectiveness of the relaxed or universal approach in clinical practice (outside the research setting) remains to be confirmed. Some authors have reported findings that call into question the incremental benefit of universal screening as compared with the selective approach in real clinical practice. A 6-year experience of 392 colorectal cancer patients from the University of California, San Francisco (UCSF) medical center as reported by a recent study53 found that although the selective criteria failed to identify one of eight LS cases from the universal group, the universal strategy screened 166 additional tumors to find this additional patient.
Proper follow-up of test results and the associated medico-legal ramifications
An important point about the screening strategies is that their effectiveness hinges on proper follow-up and proper patient uptake of abnormal results. In real clinical practice, many hurdles exist in the chain of action required to effect a positive test result, and the rate of patient and family member uptake has been disturbingly low.46,47,53 The study from the UCSF53 that compared the outcomes of the selective approach vs. the universal approach in detecting LS reported that the proportion of patients with MMR-IHC abnormal tumors who underwent genetic counseling was 87% in the selective group and only 58% in the universal group. Overall, only 59% of those screened positive actually underwent germline testing. However, of those who received genetic counseling, 85% underwent germline testing. Thus, the barrier appears to be in convincing patients to undergo genetic counseling, and this barrier appears even more difficult to overcome in cases detected via a universal approach.
Obviously, continued efforts are needed in this area. As we strive to detect all LS individuals, attention and efforts are particularly needed in achieving a multidisciplinary approach involving surgeons, oncologists, pathologists, and genetic counselors. Proper communication among all involved health care providers and patient education is not only the key to ensure effective screening, it also helps avoid unnecessary medico-legal issues. The ethics of LS screening with reflex testing initiated by the pathologist without patient consent has been discussed in the literature.54 Surveys show that informed consent for screening is rarely obtained, and most investigators are of the opinion that it is not needed as these tests detect phenotypic changes only, even though the genotype may be inferred in some scenarios.54
Testing for purposes other than Lynch syndrome detection
Although the detection of MMR deficiency is primarily centered on the diagnosis of LS individuals, the MSI status of a colorectal carcinoma also bears prognostic and therapeutic implications. This may be factored into the decision-making process when implementing test protocols.
A better prognosis in patients with MSI colorectal cancers when compared to patients whose tumors are microsatellite stable has been well demonstrated in the literature. An interesting conundrum that is gaining increasing attention has been the conflicting prognostic implications between the MSI phenotype and the histologic grade. Specifically, a significant proportion of MSI cancers are of medullary or signet-ring-cell type, or poorly differentiated type with or without mucin production, all of which have been shown to be poor prognostic factors. Does the better prognosis conferred by MSI apply to tumors with these high grade histologic types? The WHO has recommended that all MSI-H colorectal tumors be regarded as low grade tumors, irrespective of their histologic appearance or grade.55 However, there are already data indicating that the WHO approach may not be valid for signet-ring-cell carcinomas as the MSI status did not affect the survival in two independent patient cohorts whose tumors were of signet-ring-cell type.56,57 Questions have also been raised with regard to poorly differentiated carcinomas, although the data in this group are less straightforward.58 Further studies are necessary to address the prognostic value of MSI in tumors with high histologic grade.
The therapeutic implications of the tumor’s MSI status in a patient with colorectal carcinoma are particularly pertinent when the tumor falls into the clinical stage II category with high-risk features requiring adjuvant chemotherapy. In this scenario, the standard chemotherapy regimen is 5-FU, and yet existing data have suggested that patients with MSI cancers do not respond to this regimen. In fact, this treatment may actually be harmful to these patients.4,5 Thus, it becomes important to know the tumor’s MSI status, and to steer treatment regimens away from the otherwise standard 5-FU-based therapy if the tumor shows MSI. However, it should be pointed out that there have been data59 indicating that patients with stage III MSI cancers may benefit from 5-FU-based adjuvant therapy compared with observation or no 5-FU treatment, and interestingly, the treatment benefit seemed to be restricted to patients with suspected germline deficiency (i.e., LS) vs. sporadic MSI tumors.
Challenges in performing and interpreting screening tests
The various screening tests, particularly MMR IHC and MSI testing, may be associated with technical and interpretational issues (see Ref. 2). One particularly noteworthy and underrecognized scenario where pitfalls exist relates to the detection of MMR in extra-colonic tissues.
MMR IHC in extra-colonic tissues tends to be less robust than in colonic tissues; the internal control can appear weak. There are two major related reasons. First, the extent of MMR protein expression is proportional to the rate of cell proliferation, and many extra-colonic tissues are less proliferative than colonic tissues.2,60 Second, the MMR IHC optimization in most laboratories is achieved by using colorectal tissue samples. In dealing with such cases, it is helpful to look for proliferative cells, such as germinal center cells and activated lymphocytes, for internal control. In equivocal cases, communication with the caring physician/geneticist and correlation with clinical findings are important to determine if further testing may be necessary.
Notably, not all extra-colonic tumors in LS will show microsatellite instability on PCR testing even when there is evidence of MMR protein loss.60 Thus, a negative MSI test on an extra-colonic tumor sample does not necessarily rule out the possibility of MMR deficiency. Various tumor types (including adrenal cortical tumors and mesotheliomas) have been shown to be MSS but with MMR protein loss in LS cases.60,61 It is believed that the accumulation of detectable unstable microsatellites requires a sufficient amount of cell cycling,62 and in some low proliferative tumors, there may not have been sufficient cell cycling for the tumor to acquire MSI, even though the MMR protein is lost.
The evolving nature of mismatch repair testing in colorectal carcinoma
The field of MSI and LS detection is one that has been continuously evolving as technology has advanced and new knowledge has emerged. The current universal approach is being achieved after we have gone through phases of relying solely on family history (the era of Amsterdam criteria) to incorporate MSI testing into family history and other clinical data (the era of Bethesda guidelines). As we are now immersed in an era of massively parallel sequencing and individualized medicine, the detection tools and strategies for LS may undergo further changes of a scale as yet unparalleled.
The effects of the advanced sequencing techniques in the field of LS detection are being reflected by the emergence of new MMR gene sequencing assays. These assays take advantage of the next-generation sequencing (NGS) platforms. Recently, a highly processive gDNA assay (ColoSeq, University of Washington, Seattle, WA)63 based on targeted capture followed by NGS has been rolled out, which simultaneously analyzes MMR-related and MMR-unrelated cancer predisposing genes. Such assays offer a higher sensitivity in detecting genetic alterations than the traditional tests. The application of these high-throughput techniques may then impact on the pre-genetic screening tests. It may be anticipated that, in the future, these high-throughput sequencing techniques may find more widespread clinical application, and offer the status of the MMR genes as well as other cancer predisposing genes at a reasonably low cost and with a reasonably fast turnaround time, and as such, eliminating the need to perform any screening tests. A recent cost-effectiveness analysis estimated that if charges for germline testing dropped to $633–1518, universal germline testing of all newly diagnosed colorectal cancer cases would be more cost-effective than the various commonly used screening strategies.64
However, NGS-based techniques are not without limitations. For example, NGS does not necessarily provide insights into the pathogenic role of variants of unknown significance (VUS) that are more likely to be detected by these highly processive methods. The genomic-based approaches are also not designed to define the pathogenic potential of cis- and trans-acting variants that affect gene expression. More recently, assays65 that integrate germline allele-specific expression (ASE) analysis with gDNA-based assays are being introduced and offer the promise of overcoming the limitations of NGS, although only to a certain degree. In this context, it is important to bear in mind that, as we prepare to embrace truly individualized medicine, the various tests that have been used as screening tests up to the current time, including MMR protein IHC, will still offer utility in functional analyses in the determination of the pathogenicity of VUS, and therefore, will remain valuable tools even when screening strategies are no longer necessary for LS diagnosis, and the pathologists will remain significant players in the field.
Acknowledgments
This work is supported by NIH R01 grant CA164944-01A1.
REFERENCES
- 1.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087. doi: 10.1053/j.gastro.2009.12.064. e2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shia J, Holck S, Depetris G, Greenson JK, Klimstra DS. Lynch syndrome-associated neoplasms: a discussion on histopathology and immunohistochemistry. Fam Cancer. 2013;12(2):241–260. doi: 10.1007/s10689-013-9612-4. [DOI] [PubMed] [Google Scholar]
- 3.Lynch HT, Drescher K, Knezetic J, Lanspa S. Genetics, bio-markers, hereditary cancer syndrome diagnosis, heterogeneity and treatment: a review. Curr Treat Options Oncol. 2014 doi: 10.1007/s11864-014-0293-5. [DOI] [PubMed] [Google Scholar]
- 4.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinopoulos PA, Matulonis UA. POLE mutations as an alternative pathway for microsatellite instability in endometrial cancer: implications for Lynch syndrome testing. Cancer. 2014 doi: 10.1002/cncr.29057. [DOI] [PubMed] [Google Scholar]
- 7.Burn J, Gerdes AM, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 9.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 10.EGAPP Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. doi: 10.1097/GIM.0b013e31818fa2ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltomaki P, Offerhaus GJA, Vasen HFA. Lynch syndrome. In: Bosman FTCF, Carneiro F, Hruban RH, Theise ND, et al., editors. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. pp. 152–155. [Google Scholar]
- 12.De Jong AE, Morreau H, Van Puijenbroek M, et al. The role of mismatch repair gene defects in the development of adenomas in patients with HNPCC. Gastroenterology. 2004;126(1):42–48. doi: 10.1053/j.gastro.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal R, Sheahan K, O’Connell PR, Hanly AM, Martin ST, Winter DC. Lynch syndrome: an updated review. Genes. 2014;5(3):497–507. doi: 10.3390/genes5030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Dis Colon Rectum. 2014;57(8):1025–1048. doi: 10.1097/DCR.000000000000000. [DOI] [PubMed] [Google Scholar]
- 15.Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44(6):353–362. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Soler M, Perez-Carbonell L, Guarinos C, et al. Risk of cancer in cases of suspected Lynch syndrome without germline mutation. Gastroenterology. 2013;144(5):926–932. doi: 10.1053/j.gastro.2013.01.044. [e921; quiz e913–e924] [DOI] [PubMed] [Google Scholar]
- 17.Boland CR. The mystery of mismatch repair deficiency: Lynch or Lynch-like? Gastroenterology. 2013;144(5):868–870. doi: 10.1053/j.gastro.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tutlewska K, Lubinski J, Kurzawski G. Germline deletions in the EPCAM gene as a cause of Lynch syndrome-literature review. Hered Cancer Clin Pract. 2013;11(1):9. doi: 10.1186/1897-4287-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niessen RC, Hofstra RM, Westers H, et al. Germline hyper-methylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosomes Cancer. 2009;48(8):737–744. doi: 10.1002/gcc.20678. [DOI] [PubMed] [Google Scholar]
- 20.Hitchins MP, Lynch HT. Dawning of the epigenetic era in hereditary cancer. Clin Genet. 2014;85(5):413–416. doi: 10.1111/cge.12369. [DOI] [PubMed] [Google Scholar]
- 21.Rhees J, Arnold M, Boland CR. Inversion of exons 1–7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Fam Cancer. 2014;13(2):219–225. doi: 10.1007/s10689-013-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mensenkamp AR, Vogelaar IP, van Zelst-Stams WA, et al. Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gastroenterology. 2014;146(3):643–646. doi: 10.1053/j.gastro.2013.12.002. [e648] [DOI] [PubMed] [Google Scholar]
- 23.Morak M, Heidenreich B, Keller G, et al. Biallelic MUTYH mutations can mimic Lynch syndrome. Eur J Hum Genet. 2014 doi: 10.1038/ejhg.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillejo A, Vargas G, Castillejo MI, et al. Prevalence of germline MUTYH mutations among Lynch-like syndrome patients. Eur J Cancer. 2014 doi: 10.1016/j.ejca.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Sourrouille I, Coulet F, Lefevre JH, et al. Somatic mosaicism and double somatic hits can lead to MSI colorectal tumors. Fam Cancer. 2013;12(1):27–33. doi: 10.1007/s10689-012-9568-9. [DOI] [PubMed] [Google Scholar]
- 26.Haraldsdottir S, Hampel H, Tomsic J, et al. Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geurts-Giele WR, Leenen CH, Dubbink HJ, et al. Somatic aberrations of mismatch repair genes as a cause of microsatellite-instable cancers. J Pathol. 2014 doi: 10.1002/path.4419. [DOI] [PubMed] [Google Scholar]
- 28.Wimmer K, Kratz CP, Vasen HF, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘Care for CMMRD’ (C4CMMRD) J Med Genet. 2014;51(6):355–365. doi: 10.1136/jmedgenet-2014-102284. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Mao G, Tong D, et al. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell. 2013;153(3):590–600. doi: 10.1016/j.cell.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diouf B, Cheng Q, Krynetskaia NF, et al. Somatic deletions of genes regulating MSH2 protein stability cause DNA mismatch repair deficiency and drug resistance in human leukemia cells. Nat Med. 2011;17(10):1298–1303. doi: 10.1038/nm.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindor NM. Familial colorectal cancer type X: the other half of hereditary nonpolyposis colon cancer syndrome. Surg Oncol Clin N Am. 2009;18(4):637–645. doi: 10.1016/j.soc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiovitz S, Copeland WK, Passarelli MN, et al. Characterisation of Familial Colorectal Cancer Type X, Lynch syndrome, and non-familial colorectal cancer. Br J Cancer. 2014 doi: 10.1038/bjc.2014.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterlongo P, Mitra N, Sanchez de Abajo A, et al. Increased frequency of disease-causing MYH mutations in colon cancer families. Carcinogenesis. 2006;27(11):2243–2249. doi: 10.1093/carcin/bgl093. [DOI] [PubMed] [Google Scholar]
- 34.Nieminen TT, Abdel-Rahman WM, Ristimaki A, et al. BMPR1A mutations in hereditary nonpolyposis colorectal cancer without mismatch repair deficiency. Gastroenterology. 2011;141(1):e23–e26. doi: 10.1053/j.gastro.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 35.Morak M, Massdorf T, Sykora H, Kerscher M, Holinski-Feder E. First evidence for digenic inheritance in hereditary colorectal cancer by mutations in the base excision repair genes. Eur J Cancer. 2011;47(7):1046–1055. doi: 10.1016/j.ejca.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastrinos F, Steyerberg EW, Mercado R, et al. The PREMM (1,2,6) model predicts risk of MLH1, MSH2, and MSH6 germline mutations based on cancer history. Gastroenterology. 2011;140(1):73–81. doi: 10.1053/j.gastro.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Wang W, Lee S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. J Am Med Assoc. 2006;296(12):1479–1487. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shia J, Ellis NA, Paty PB, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003;27(11):1407–1417. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with micro-satellite instability. Am J Pathol. 2001;158(2):527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shia J, Klimstra DS, Nafa K, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29(1):96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- 42.Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008;10(4):293–300. doi: 10.2353/jmoldx.2008.080031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Lopez JV, Barrios Y, Medina-Arana V, et al. The hMSH2 (M688R) Lynch syndrome mutation may function as a dominant negative. Carcinogenesis. 2012;33(9):1647–1654. doi: 10.1093/carcin/bgs199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 45.Niu B, Ye K, Zhang Q, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2013 doi: 10.1093/bioinformatics/btt755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salipante SJ, Scroggins SM, Hampel HL, Turner EH, Pritchard CC. Microsatellite instability detection by next generation sequencing. Clin Chem. 2014;60(9):1192–1199. doi: 10.1373/clinchem.2014.223677. [DOI] [PubMed] [Google Scholar]
- 47.Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012;49(3):151–157. doi: 10.1136/jmedgenet-2011-100714. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi M, Yanokura M, Banno K, et al. Analysis of a correlation between the BRAF V600E mutation and abnormal DNA mismatch repair in patients with sporadic endometrial cancer. Int J Oncol. 2009;34(6):1541–1547. doi: 10.3892/ijo_00000283. [DOI] [PubMed] [Google Scholar]
- 49.Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155(2):69–79. doi: 10.7326/0003-4819-155-2-201107190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boland CR, Shike M. Report from the Jerusalem workshop on Lynch syndrome-hereditary nonpolyposis colorectal cancer. Gastroenterology. 2010;138(7):2197.e1–2197.e7. doi: 10.1053/j.gastro.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hampel H. NCCN increases the emphasis on genetic/familial high-risk assessment in colorectal cancer. J Natl Compr Canc Netw. 2014;12(Suppl 5):829–831. doi: 10.6004/jnccn.2014.0200. [DOI] [PubMed] [Google Scholar]
- 53.Kidambi TD, Blanco A, Myers M, Conrad P, Loranger K, Terdiman JP. Selective versus universal screening for Lynch syndrome: a six-year clinical experience. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3234-z. [DOI] [PubMed] [Google Scholar]
- 54.Beamer LC, Grant ML, Espenschied CR, et al. Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30(10):1058–1063. doi: 10.1200/JCO.2011.38.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman FTCF, Carneiro F, Hruban RH, Theise ND, et al., editors. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. pp. 134–146. [Google Scholar]
- 56.Kakar S, Smyrk TC. Signet ring cell carcinoma of the colorectum: correlations between microsatellite instability, clinicopathologic features and survival. Mod Pathol. 2005;18(2):244–249. doi: 10.1038/modpathol.3800298. [DOI] [PubMed] [Google Scholar]
- 57.Hartman DJ, Nikiforova MN, Chang DT, et al. Signet ring cell colorectal carcinoma: a distinct subset of mucin-poor micro-satellite-stable signet ring cell carcinoma associated with dismal prognosis. Am J Surg Pathol. 2013;37(7):969–977. doi: 10.1097/PAS.0b013e3182851e2b. [DOI] [PubMed] [Google Scholar]
- 58.Xiao H, Yoon YS, Hong SM, et al. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140(3):341–347. doi: 10.1309/AJCP8P2DYNKGRBVI. [DOI] [PubMed] [Google Scholar]
- 59.Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol. 2012;43(10):1677–1687. doi: 10.1016/j.humpath.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Medina-Arana V, Delgado L, Gonzalez L, et al. Adrenocortical carcinoma, an unusual extracolonic tumor associated with Lynch II syndrome. Fam Cancer. 2011;10(2):265–271. doi: 10.1007/s10689-010-9416-8. [DOI] [PubMed] [Google Scholar]
- 62.Blake C, Tsao JL, Wu A, et al. Stepwise deletions of polyA sequences in mismatch repair-deficient colorectal cancers. Am J Pathol. 2001;158(5):1867–1870. doi: 10.1016/S0002-9440(10)64143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pritchard CC, Smith C, Salipante SJ, et al. ColoSeq provides comprehensive Lynch and polyposis syndrome mutational analysis using massively parallel sequencing. J Mol Diagn. 2012;14(4):357–366. doi: 10.1016/j.jmoldx.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gould-Suarez M, El-Serag HB, Musher B, Franco LM, Chen GJ. Cost-effectiveness and diagnostic effectiveness analyses of multiple algorithms for the diagnosis of Lynch syndrome. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Lellis L, Aceto GM, Curia MC, et al. Integrative analysis of hereditary nonpolyposis colorectal cancer: the contribution of allele-specific expression and other assays to diagnostic algorithms. PLoS One. 2013;8(11):e81194. doi: 10.1371/journal.pone.0081194. [DOI] [PMC free article] [PubMed] [Google Scholar]