Abstract
In the past decade drug research community has started to appreciate the indispensable role of ligand–receptor binding kinetics (BK) in drug discovery. Next to the classical equilibrium-based drug evaluation process with affinity and potency values as outcomes, kinetic investigation of the ligand–receptor interaction can aid compound triage in the hit-to-lead campaign and provide additional information to understand the molecular mechanism of drug action. Translational models incorporating BK are emerging as well, which represent powerful tools for the prediction of in vivo effects. In this viewpoint we will summarize some recent findings and discuss and emphasize the added value of ligand–receptor binding kinetics in drug research.
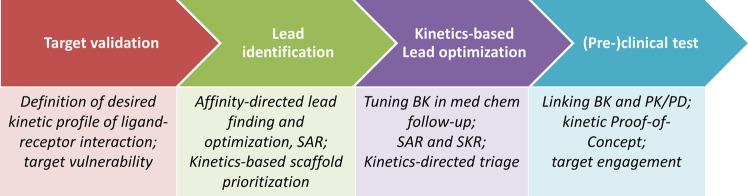
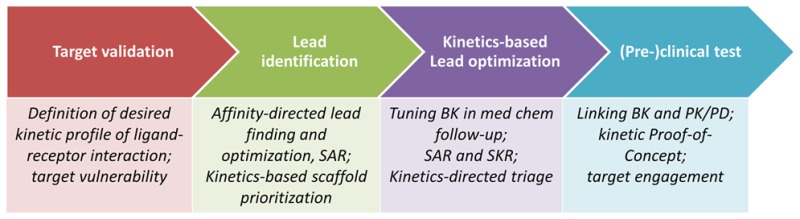
Ligand–receptor (in the context of this viewpoint, “receptor” stands for any macromolecular drug target) binding kinetics (BK) has been and is receiving increasing attention in drug discovery.1,1b In particular, drug-target residence time (RT), the time a target is occupied by a ligand (RT = 1/koff), has been suggested to predict in vivo pharmacological activity better than binding affinity per se.2 This is because binding affinity is often determined under equilibrium conditions in a closed system (i.e., a drug and a target are present at constant concentrations, for instance, in a test tube), which, however, are seldom met in an open system (such as the human body). Moreover, the BK concept takes into account the conformational dynamics of target macromolecules that affect drug binding (exemplified by the association rate constant kon) and unbinding (exemplified by the dissociation rate constant koff).3 The concept, and in particular its relevance, was first emphasized by Copeland and colleagues in 2006.2 In the meantime BK has evolved from a theoretical concept of drug–receptor interaction to an emerging paradigm that appears to have an increasing impact on drug discovery.3 Recently, a drug discovery pipeline that integrates ligand–receptor BK has been proposed as a prospective strategy for drug discovery and development.1 As shown in Figure 1, it might be beneficial to take BK into account immediately from the target validation stage and onward. One should consider whether a long or short RT is desired for improved efficacy, optimal duration of action, decreased mechanism-based toxicity, or desired on-set of drug action, respectively. Factors that influence target vulnerability (e.g., target desensitization, internalization, or recycling) should be taken into consideration as well in this step. Next, after an affinity-directed hit-to-lead campaign, medium- to high-throughput kinetic screening (see the review1b and references therein for detailed information) can be initiated to prioritize scaffolds with desired BK for further lead optimization. This can be performed by extensive structure–kinetics relationship (SKR) studies in combination with classical equilibrium-based structure–activity relationship (SAR) studies. In this step BK can be a powerful tool to triage candidate compounds to a further (pre)clinical stage. The selected leads, preferably with divergent BK profile, will be examined for kinetic proof-of-concept in the (pre)clinical stages of drug discovery in which pharmacokinetic/pharmacodynamics (PK/PD) modeling plays an important role. Of note, if the PK elimination of two drugs is slower than their BK, the latter will not be the cause of differences in duration of action and/or efficacy.4 At this stage, special attention should be paid to evaluating target engagement in the target tissue and linking preclinical to clinical readouts. One may wish to consider, for instance, the blood–brain barrier or the abundance of efflux transporter proteins to ensure an accurate evaluation of receptor occupancy. Similarly, factors such as lipid partitioning in the receptor-embedded membrane or target rebinding should be taken into account as well since they can further extend the duration of target occupancy.5 Taken together, such information may facilitate in vitro/in vivo translation and perhaps direct future design and discovery of kinetically favorable drug candidates.
Figure 1.
Proposed pipeline for kinetics-directed drug discovery. The figure has been adapted with permission from ref (1). Copyright 2015 Wiley.
1. BK Investigation Can Facilitate Compound Triage in Hit-to-Lead Campaigns
Understanding ligand–receptor BK can be beneficial at all stages of the drug discovery process, but its highest impact is probably in the lead identification and optimization phase.1 At this stage, a series of candidate compounds can be systematically examined for their SKR, which is complementary to the classical SAR. Such inclusive application of kinetic metrics in the triage and advancement of best leads can be very informative, especially in the context of a series of compounds that are otherwise chemically and/or biologically similar.1,1b
The integrated use of kinetics-based evaluations also facilitates a more complete selectivity profiling of a lead compound on a number of targets. Approaches that utilize prolonged occupancy of the drug on the designated target while minimizing binding to off-target proteins may improve a drug candidate’s therapeutic index and thus yield compounds with desired kinetic selectivity.6 One recent example toward this purpose comes from our own lab, where both equilibrium- and kinetic-based measurements were undertaken to examine the selectivity of a number of compounds for three adenosine receptor (A1AR, A2AAR, and A3AR) subtypes. As a result, XAC and LUF5964, two AR antagonists, were kinetically more selective for the adenosine A1AR or A3AR, respectively, although they were nonselective in terms of their affinity. In comparison, another AR antagonist, LUF5967, displayed a strong equilibrium-based selectivity for the A1AR over the A2AAR, yet its kinetic selectivity thereon was less pronounced.7 Similarly, Ayaz et al. performed kinetic profiling of Roniciclib (BAY 1000394), a potent type I pan-CDK (cyclin-dependent kinase) inhibitor in xenograft cancer models. It was found that the compound was kinetically more selective on CDK2 and CDK9 over other CDKs with prolonged target RTs. These results were in accordance with a sustained inhibitory effect on retinoblastoma protein phosphorylation, indicating that the target RT on CDK2 may contribute to sustained target engagement and antitumor efficacy.8 Importantly, kinetic selectivity can only occur when a drug’s RT outlasts its PK profile.
2. BK Investigation Can Improve the Understanding of the Molecular Mechanism of Drug Action
Compelling evidence shows that the spatiotemporal pattern of drug action may be quite different depending on various ligand–receptor binding kinetics (see the review3 and references therein for detailed information). One recent study is exemplified on the dopamine D2 receptor in which Lane and colleagues investigated the role of kinetic context in apparent biased agonism at GPCRs.9 For example, bifeprunox, a D2R agonist, displayed substantial bias at earlier time points toward the activation of Gαo G proteins versus inhibition of cAMP production (Gαi). In contrast, at later time points the agonist displayed a reversed bias toward inhibition of cAMP production. Such alternation in observed bias was manifested both in the increase in potency over time due to the compound’s slower dissociation kinetics and different patterns of changing potency of the reference ligand ropinirole showing faster kinetics. Evidently, kinetic context must be taken into account in the design and interpretation of studies of biased agonism.
Another topic catching increasing attention is the relationship between the effect and the duration of action of an agonist. There is accumulating evidence indicating that a desensitized/internalized receptor may not always lead to the dissociation of the ligand or the termination of downstream signalizing cascades. A number of clues come from recent studies examining the subcellular localization of fluorescently tagged agonists and receptors. As observed, an internalized ligand–receptor binary complex with continued ligand binding, i.e., long receptor residence time, can still trigger sustained signaling from cytosolic compartments (see the review10 and references therein for detailed information). Thus, studying the kinetics of ligand–receptor interaction adds new facets to the understanding of the molecular basis of drug action.
3. Translation of in Vitro Binding Kinetics into Cellular and in Vivo Effects
Although translation of in vitro binding kinetics into cellular and in vivo effects remains elusive, notable progress has been made to improve our understanding of their relationships. One of the emerging models is reported by colleagues from Janssen Pharmaceuticals, who investigated the application of an integrated model consisting of pharmacokinetics (PK), BK, and systems biology to predict the anticoagulant effects of FXa inhibitors in healthy subjects.11 The authors showed that the integrated model predicted the clotting time and anti-FXa effects reasonably well, and the role of BK was highlighted by sensitivity analysis and simulations. The model has the potential to serve as a tool for new compound and/or target selection in the prevention of thrombosis. In another recent study, Walkup et al. described a mechanistic pharmacodynamics (PD) model that includes drug-target kinetic parameters.6 This model predicted time-dependent antibacterial activity for inhibitors of LpxC, a zinc metalloenzyme involved in the biosynthesis of cell wall lipopolysaccharides from Pseudomonas aeruginosa in intact cells and in an animal model of infection. This study represents a prospective example that integrates ligand–receptor BK to predict drug efficacy.
To facilitate the translation between the in vitro BK and in vivo activities of a given drug, De Witte et al. discussed and summarized the most relevant experimental conditions, such as in vitro temperature, in vivo displacement method, and the presence of endogenous ligand (i.e., with its own BK), which may bridge the apparent gap between in vitro and in vivo drug action.12 Furthermore, the authors provide evidence that all kinetic processes, which influence the in vivo kinetics of a drug’s effect, need to be considered (as discussed already above).
Concluding Remarks
Ligand–receptor BK is receiving increasing attention in the drug research community. There is mounting evidence suggesting that BK investigations offer added value in the drug design and discovery process. This has been reflected in recent progress in understanding ligand–receptor interactions and the molecular mechanism of drug action. The translation of in vitro binding kinetics into cellular and in vivo effects remains elusive, yet integrated mechanistic models are emerging as powerful tools to predict drug efficacy in vivo. We envision that a rapid expansion of the field of ligand–receptor binding kinetics can be expected in the near future, which hopefully will further facilitate the drug design and discovery process.
Acknowledgments
This study was partly undertaken within the framework of the “Kinetics for Drug Discovery (K4DD) consortium”. The K4DD project is supported by the Innovative Medicines Initiative Joint Undertaking (IMI JU) under grant agreement no. 115366, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. This study was supported by National Natural Science Foundation of China (no. 81603170 to D.G.) and Natural Science Foundation of Jiangsu Province (no. BK20160234 to D.G.).
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- Guo D.; Heitman L. H.; IJzerman A. P. The Role of Target Binding Kinetics in Drug Discovery. ChemMedChem 2015, 10 (11), 1793–1796. 10.1002/cmdc.201500310. [DOI] [PubMed] [Google Scholar]
- Guo D.; Hillger J. M.; IJzerman A. P.; Heitman L. H. Drug-target residence time-a case for G protein-coupled receptors. Med. Res. Rev. 2014, 34 (4), 856–892. 10.1002/med.21307. [DOI] [PubMed] [Google Scholar]
- Copeland R. A.; Pompliano D. L.; Meek T. D. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discovery 2006, 5 (9), 730–9. 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- Copeland R. A. The drug-target residence time model: a 10-year retrospective. Nat. Rev. Drug Discovery 2016, 15 (2), 87–95. 10.1038/nrd.2015.18. [DOI] [PubMed] [Google Scholar]
- Dahl G.; Akerud T. Pharmacokinetics and the drug-target residence time concept. Drug Discovery Today 2013, 18 (15–16), 697–707. 10.1016/j.drudis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Vauquelin G.; Charlton S. J. Long-lasting target binding and rebinding as mechanisms to prolong in vivo drug action. Br. J. Pharmacol. 2010, 161 (3), 488–508. 10.1111/j.1476-5381.2010.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup G. K.; You Z.; Ross P. L.; Allen E. K.; Daryaee F.; Hale M. R.; O’Donnell J.; Ehmann D. E.; Schuck V. J.; Buurman E. T.; Choy A. L.; Hajec L.; Murphy-Benenato K.; Marone V.; Patey S. A.; Grosser L. A.; Johnstone M.; Walker S. G.; Tonge P. J.; Fisher S. L. Translating slow-binding inhibition kinetics into cellular and in vivo effects. Nat. Chem. Biol. 2015, 11 (6), 416–23. 10.1038/nchembio.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D.; Dijksteel G. S.; van Duijl T.; Heezen M.; Heitman L. H.; IJzerman A. P. Equilibrium and kinetic selectivity profiling on the human adenosine receptors. Biochem. Pharmacol. 2016, 105, 34–41. 10.1016/j.bcp.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Ayaz P.; Andres D.; Kwiatkowski D. A.; Kolbe C. C.; Lienau P.; Siemeister G.; Lucking U.; Stegmann C. M. Conformational Adaption May Explain the Slow Dissociation Kinetics of Roniciclib (BAY 1000394), a Type I CDK Inhibitor with Kinetic Selectivity for CDK2 and CDK9. ACS Chem. Biol. 2016, 11 (6), 1710–9. 10.1021/acschembio.6b00074. [DOI] [PubMed] [Google Scholar]
- Klein Herenbrink C.; Sykes D. A.; Donthamsetti P.; Canals M.; Coudrat T.; Shonberg J.; Scammells P. J.; Capuano B.; Sexton P. M.; Charlton S. J.; Javitch J. A.; Christopoulos A.; Lane J. R. The role of kinetic context in apparent biased agonism at GPCRs. Nat. Commun. 2016, 7, 10842. 10.1038/ncomms10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothersall J. D.; Brown A. J.; Dale I.; Rawlins P. Can. residence time offer a useful strategy to target agonist drugs for sustained GPCR responses?. Drug Discovery Today 2016, 21 (1), 90–6. 10.1016/j.drudis.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Huntjens D.; Gilissen R. A Systems Pharmacology Model for Predicting Effects of Factor Xa Inhibitors in Healthy Subjects: Assessment of Pharmacokinetics and Binding Kinetics. CPT: Pharmacometrics Syst. Pharmacol. 2015, 4 (11), 650–9. 10.1002/psp4.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte W. E.; Wong Y. C.; Nederpelt I.; Heitman L. H.; Danhof M.; van der Graaf P. H.; Gilissen R. A.; de Lange E. C. Mechanistic models enable the rational use of in vitro drug-target binding kinetics for better drug effects in patients. Expert Opin. Drug Discovery 2016, 11 (1), 45–63. 10.1517/17460441.2016.1100163. [DOI] [PubMed] [Google Scholar]