Abstract
Transplantation of embryonic cortical tissue is considered as a promising therapy for brain injury. Grafted neurons can reestablish neuronal network and improve cortical function of the host brain. Microglia is a key player in regulating neuronal survival and plasticity, but its activation and dynamics in grafted cortical tissue remain unknown. Using two-photon intravital imaging and parabiotic model, here we investigated the proliferation and source of microglia in the donor region by transplanting embryonic cortical tissue into adult cortex. Live imaging showed that the endogenous microglia of the grafted tissue were rapidly lost after transplantation. Instead, host-derived microglia infiltrated and colonized the graft. Parabiotic model suggested that the main source of infiltrating cells is the parenchyma of the host brain. Colonized microglia proliferated and experienced an extensive morphological transition and eventually differentiated into resting ramified morphology. Collectively, these results demonstrated that donor tissue has little contribution to the activated microglia and host brain controls the microglial population in the graft.
The adult brain has a limited capacity of self-repairing after neuronal loss caused by trauma or disease. During last three decades, many experimental studies have explored the possibility to restore the lost functions by replacing damaged brain area with embryonic neural tissues. These studies have achieved remarkable results in showing that grafted neurons can successfully survive and synaptic connections can be established between the host and donor cells1,2,3. Importantly, grafted neurons are electrophysiologically active in responding to adjacent host cortex4,5,6, and the lost functions can be partially recovered after transplantation7,8,9.
For a successful transplantation, the entire cell populations of cortical tissue including neurons and different subsets of glial cells have to be reestablished. However, previous studies mostly focused on the survival and development of grafted neurons, and little information was available regarding the other essential cell subsets in cortical tissue. One of the key cell subsets that may affect the survival of graft tissue and reestablishment of synaptic connections is microglia. As the immunocompetent cells in the central nervous system (CNS)10,11,12, microglia show remarkable functional diversity under different conditions in brain. During early development, yolk sac-derived microglia enter the developing CNS and distribute widely in the brain13,14,15. Colonized microglia actively participate in regulating the number of neural precursor cells and wiring the axonal input16,17. In the adult brain, the number of microglia remains relatively stable but microglial processes are highly motile18. Resting microglia are involved in the surveillance and support of the CNS microenvironment19,20,21. In addition, they also contribute to synapse elimination and learning-dependent synapse formation22,23. Under pathological conditions, microglia are rapidly activated and play vital roles in immunoreactions and CNS pathologies20,24,25. Activated microglia can derive from different sources in different neuropathological conditions in adult brain26,27,28,29. The dynamics and sources of microglia are well described in healthy and diseased brain, but it is still undefined about the microglial activation and population dynamics in grafted embryonic tissue.
In this study, we took advantage of transgenic mice that express green fluorescent protein (GFP) in microglia and in vivo two-photon imaging to investigate the survival of endogenous microglia and the infiltration, repopulation and differentiation of host-derived microglia in an embryonic cortical tissue transplantation model. A parabiosis model was used to determine the fate and origin of both endogenous and host-derived microglia after successful transplantation. Our data demonstrated that vast majority of endogenous microglia in embryonic cortical tissue disappeared shortly after transplantation. Host-derived microglia infiltrated into the graft and restored the microglial population in the transplanted tissue, and local resident microglia in host brain parenchyma was the main source of colonized microglia.
Results
Endogenous microglia of the grafted tissue were lost rapidly after transplantation
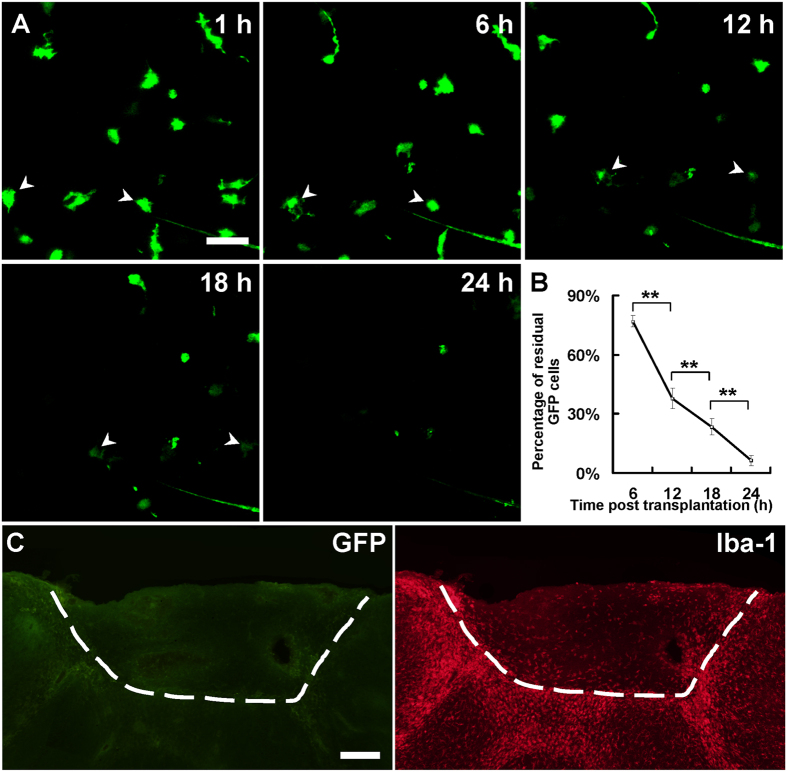
We first established a transplantation model by transplanting embryonic cortical tissue from E14~E15 YFP H-line fetuses into wild-type adult mouse brains (Supplementary Fig. 1A,C). Consistent to the results of a previous study7, we found that grafted neurons can survive and differentiate in the host brains (Supplementary Fig. 2). Importantly, we found the somata of the grafted neurons located only in grafted tissue, but their axons and dendrites projected into the host tissue (Supplementary Fig. 2D–F). In addition, we also performed experiments to transplant embryonic cortical tissue from wild type pregnant mice to adult YFP H-line mice, and we did not find any soma of the YFP-marked host neurons in grafted tissue (Supplementary Fig. 2C), suggesting neurons in grafted tissue are not from the host brains. To investigate whether the endogenous microglia of the grafted embryonic tissue could survive and repopulate in host brain, we transplanted embryonic cortical tissue from E14~E15 CX3CR1GFP/+ fetus into the lesion cavity of the cortex of an adult wild-type mouse. Two-photon imaging through the open-skull windows was used to examine microglial dynamics in the transplants (Supplementary Fig. 1A,B). We observed drastic atrophy of the endogenous microglia within hours after transplantation and the number of GFP+ cells decreased gradually in grafted tissue (Fig. 1A). There were only 37.7 ± 5.2% and 6.3 ± 2.7% of GFP+ cells left at 12 h and 24 h after transplantation, respectively (Fig. 1B), and repeated imaging showed that no GFP+ cells was found in the transplants at 36 h after transplantation (Supplementary Fig. 3A). To reduce the possible impact of repeated imaging on microglial survival, we imaged the transplanted tissues only once at 36 hours after grafting, and still no GFP+ cells was found in the graft (Supplementary Fig. 3B). TUNEL staining of the grafted tissue at 6 h after transplantation showed that some of the grafted microglia were TUNEL-positive (Supplementary Fig. 4), suggesting the grafted microglia can undergo apoptotic cell death.
Figure 1. Loss of endogenous microglia in grafted tissue.
(A) In vivo two-photon time-lapse imaging of GFP+ microglia in grafted tissue at 1, 6, 12, 18 and 24 h after transplantation. Most GFP+ cells were lost within 24 h after transplantation. (B) Percentage of residual GFP+ cells in grafted area at 6, 12, 18 and 24 h after transplantation; compared with the number of cells at 1 h. N = 2–3 sections/mouse, 3–6 mice/group, **P < 0.01. (C) An example showing GFP+ endogenous microglia disappeared in grafted tissue at 7 d after transplantation in most cases. Note the host animal was wild-type C57BL/6 mouse and the transplanted tissue was from a CX3CR1GFP/+ mouse. The white dash line shows the boundary between donor and recipient tissue. Scale bar, 25 μm (A); 200 μm (C).
To further confirm the loss of endogenous microglia and determine if any microglia existed in the transplants, brain sections of the grafted tissue from 7 days to 60 days after transplantation were harvested and stained with IBA-1 antibody. We found that abundant of IBA-1 positive microglia were distributed in grafted tissue at 7 d and 60 d (Fig. 1C, Supplementary Fig. 5A). However, we failed to find any GFP+ cells in the grafted tissues of most of the animals (13 out of 15 mice, Fig. 1C). Exceptionally, very few sparsely distributed GFP+ cells were found survived in the transplants in 2 animals (2 out of 15 mice, Supplementary Fig. 5A). These GFP+ cells only constitute about 0.1% of total IBA-1 positive cells, but they exhibited a similar morphology to neighboring ramified microglia (Supplementary Fig. 5B). Together, these results demonstrated that vast majority of the donor-derived microglia in the grafted tissue failed to survive in host brain after transplantation.
Microglia survived in the grafted tissue are colonizers from the host
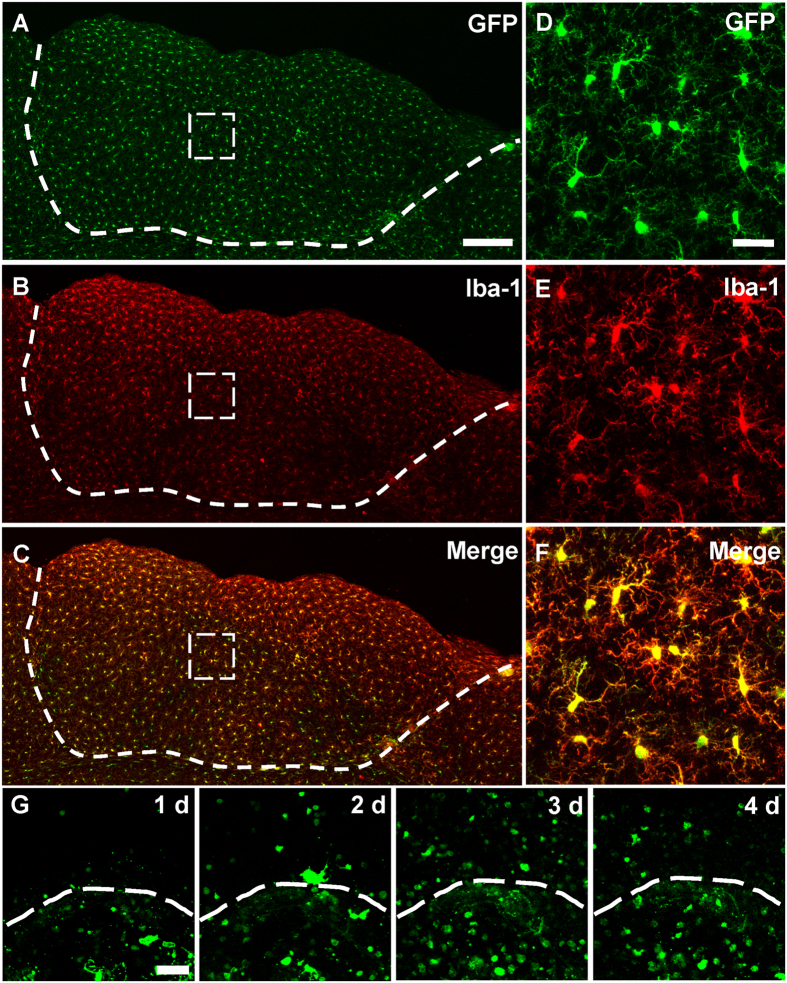
The distribution of IBA-1 positive but GFP-negative microglia in the transplants suggested that host microglia can infiltrate the donor tissue. To confirm this, embryonic cortical tissue from wild-type fetus was grafted into the lesion cavity of adult CX3CR1GFP/+ mouse (Supplementary Fig. 1A,C). At 60 days after transplantation, we found that GFP+ cells were ubiquitously distributed in the graft (Fig. 2A). IBA-1 staining indicated that 98.9 ± 0.3% of the GFP+ cells in the graft were IBA-1-positive and 99.6 ± 0.3% of the IBA-1 positive cells were GFP+ (Fig. 2A–F). Theses data suggested that host brain controlled the great majority of microglial population in grafted tissue.
Figure 2. Colonization of host-derived microglia in grafted tissue.
(A) GFP+ host-derived microglia (green) existed homogeneously in grafted tissue at 60 d after transplantation. (B) IBA-1 antibody staining (red) showing microglia in transplanted cortical tissue. (C) Merge of channel (A,B) showing that the majority of GFP+ microglia were also IBA-1 positive. N = 4–5 sections/mouse, 3 mice. (D–F) Representative higher magnification image of boxed-region in (A–C). (G) Images taken through an open-skull window showing the migration of microglia from CX3CR1GFP/+ mouse cortex to wild-type embryonic cortical graft at 1, 2, 3, and 4 days after transplantation. Only sporadic GFP+ microglia crossed the border line at 1 day after transplantation, and the cell density of GFP-positive cells increased in the grafted tissue from 1–3 d, then decreased at 4 d. Note the host animal was CX3CR1GFP/+ mouse and the transplanted tissue was from a wild-type C57BL/6 mouse. The white dash line shows the boundary between donor and recipient tissue. Scale bar, 200 μm (A–C); 25 μm (D–F); 50 μm (G).
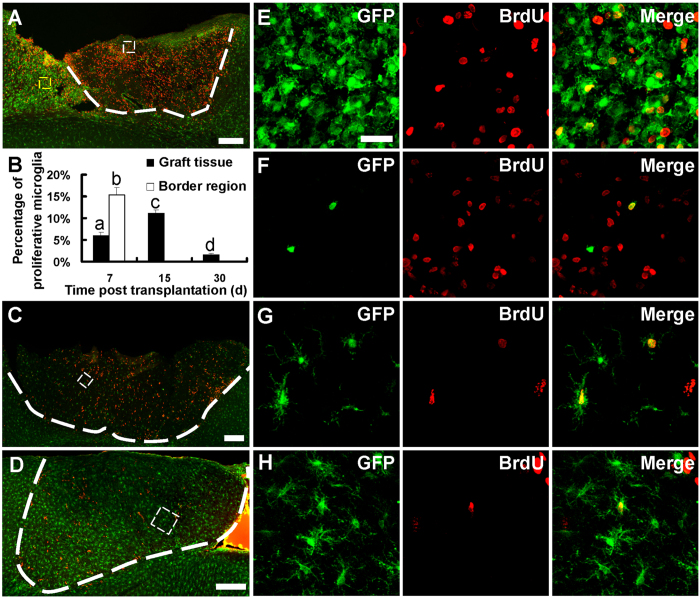
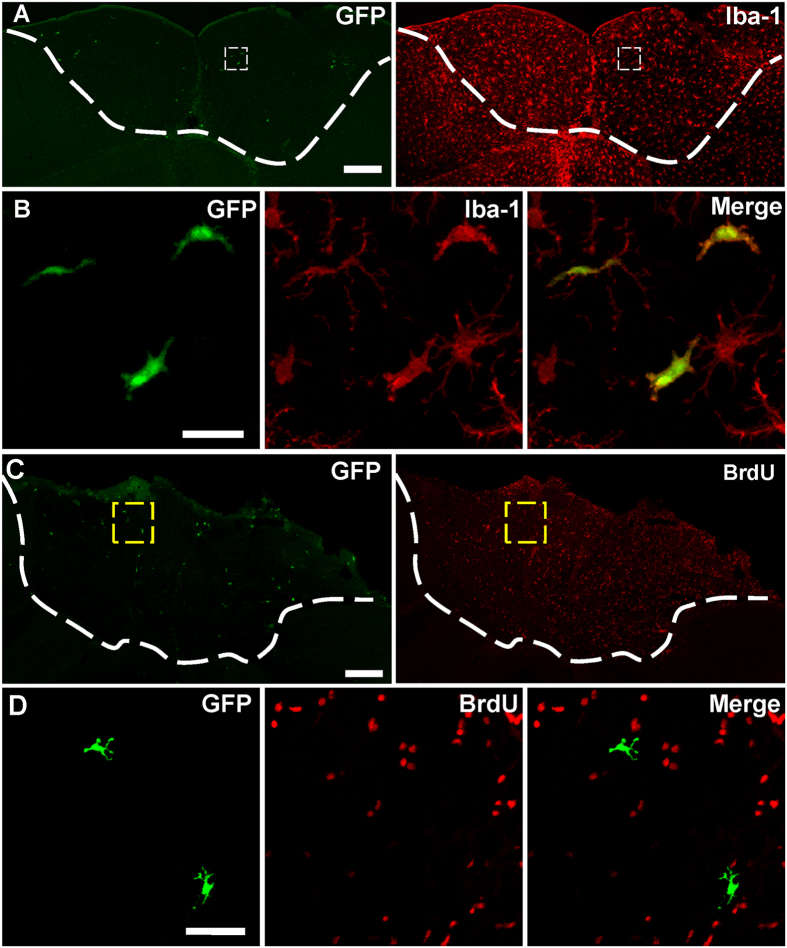
We next examined the dynamics of microglial infiltration. Daily imaging through an open-skull window showed that GFP-marked cells from the host brain invaded into grafted tissue within 1 d after transplantation. The number of GFP-positive cells increased in the grafted tissue from 1–3 d, and then decreased at 4 d (Fig. 2G; cell densities were 128.0 ± 34.6/mm2, 296.7 ± 42.6/mm2, 770.5 ± 110.0/mm2 and 446.6 ± 41.0/mm2+ for 1 d, 2 d, 3 d and 4 d, respectively). Immunostaining of the fixed tissue indicated that microglia were activated in the host tissue near the border region during the first week after transplantation (Fig. 3A). We found that 15.3 ± 1.7% of the activated microglia were proliferative (both GFP-positive and BrdU-positive) in the host tissue near the border region (Fig. 3B,E) at 7 d after transplantation. However, no proliferative microglia was found in the host tissue along the border region (tissue surrounding the graft) at 15 d and later time points (Supplementary Fig. 6A,B). These results further suggested that microglia from host brain infiltrated into the donor tissue at early stage after transplantation.
Figure 3. Proliferation of host-derived microglia at different time points after transplantation.
(A,C,D) Confocal images showing proliferative microglia (BrdU-positive and GFP-positive) at 7 d, 15 d and 30 d after transplantation. Note the graft was from a wild-type mouse and host animal was a CX3CR1GFP/+ mouse. White dash line shows the boundary of grafted tissue. (B) Percentage of proliferative microglia in grafted tissue and host part of border region at different time points. N = 3 sections/mouse, 3–4 mice/group. a, b, c, d indicating significant differences between the two groups. P < 0.01. (E) Higher magnification image of the yellow box region in (A) showing BrdU-positive microglia in host part of the border region next to grafted tissue at 7 d. (F–H) Higher magnification image of the white box region in (A,C,D) showing BrdU-positive microglia in grafted tissue at 7 d, 15 d and 30 d after transplantation. Scale bar, 200 μm (A,C,D); 25 μm (E–H).
Infiltrated CX3CR1GFP/+ cells restored the microglial population in grafted tissue
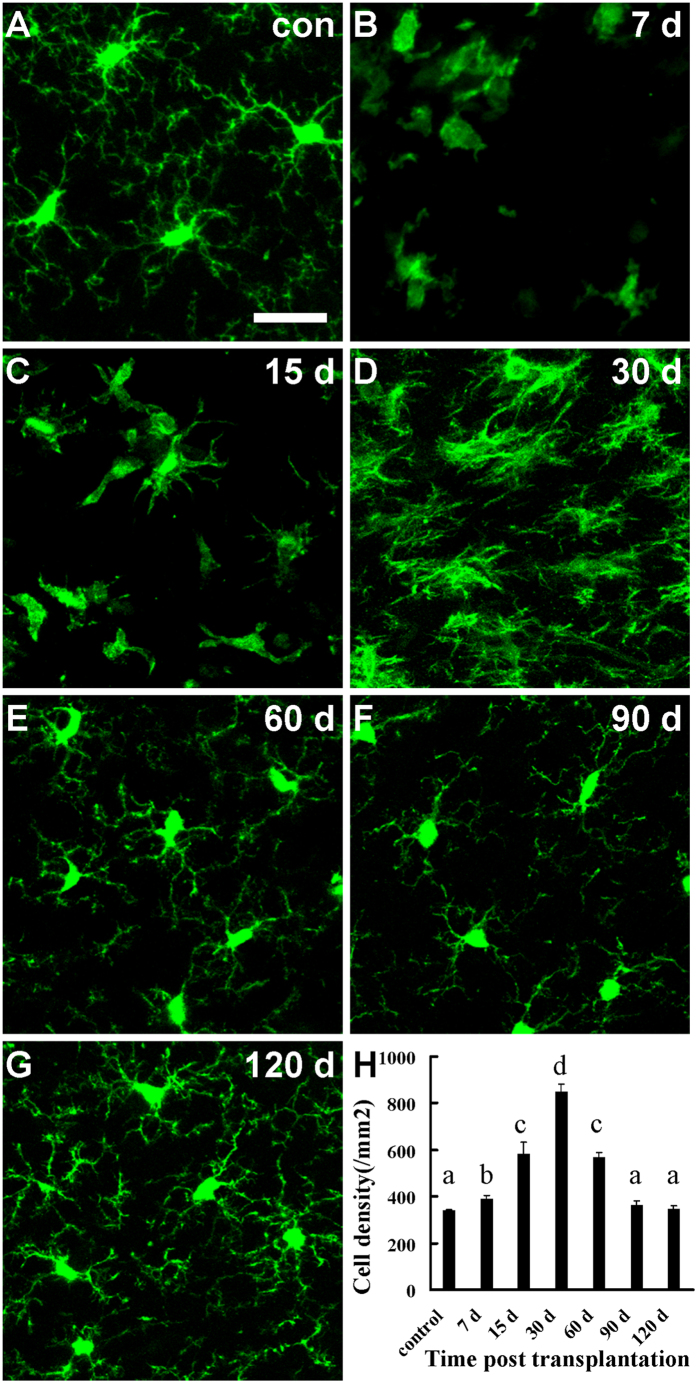
To better understand the migration and transition of host microglia, we evaluated the density and morphological transformation of microglia in transplanted tissue using fixed brain slices harvested from 7 d to 120 d after transplantation. Microglia in the graft had already reached a density higher than that in control animals at 7 d after transplantation (Fig. 4A,B and H; 338.6 ± 6.4/mm2 for control, 389.8 ± 15.5/mm2 for 7 d; n = 5 mice). Among these cells, 6.0 ± 0.7% of them were BrdU positive (Fig. 3F). The number of microglia increased gradually and reached a peak density at 30 d after transplantation (Fig. 4D,H; 848.0 ± 35.7/mm2, n = 5 mice), and then decreased to control level at 90 d and 120 d (Fig. 4F–H; 364.8 ± 16.5/mm2 for 90 d, 346.9 ± 12.8/mm2 for 120 d; n = 3 mice). Consistent to the density increase in microglia, we found that 11.1 ± 0.7% and 1.6 ± 0.3% of microglia were proliferative at 15 d and 30 d after transplantation respectively (Fig. 3G,H), but there was no BrdU positive microglia present in the graft at the subsequent time points after microglia reached a peak density (Supplementary Fig. 6C,D). Colonized microglia in the graft underwent a drastic morphological transition from amoeboid phenotype to ramified phenotype after transplantation (Fig. 4B–G). During the first two weeks after transplantation, amoeboid microglia with thick twigs was the predominant phenotype (Fig. 4C). Microglia transformed into “bushy microglia” with dense dendrites at 30 d30,31 (Fig. 4D) and restored to resting ramified morphology from 60 d to 120 d after transplantation (Fig. 4E–G).
Figure 4. Changes in density and transition in morphology of host-derived microglia in grafted tissue following transplantation.
(A) Resting microglia in healthy CX3CR1GFP/+ mouse exhibited ramified structures. (B–G) Morphological changes of intruding microglia in grafted tissue at 7 d (B) 15 d (C) 30 d (D) 60 d (E) 90 d (F) and 120 d (G) after transplantation. Amoeboid microglia was the major phenotype at 7 d (B), microglia extended thick branches at 15 d (C), developed dense dendrites at 30 d (D) and restored gradually to ramified phenotype after 60 d following transplantation. (H) Quantification of the density of microglia in the graft cortical tissue at different time points following transplantation. N = 5–6 sections/mouse, 3–5 mice/group. a, b, c, and d indicating significant differences between the two groups. P < 0.01. Scale bar, 25 μm (A–G).
Parenchymal microglia in host brain is the main source of the colonized microglia
Above data revealed that microglia in the grafted tissue were from host brain. However, it remains unclear whether infiltrating microglia are from peripheral circulating cells or resident microglial pool in brain parenchyma. To investigate the origin of colonized microglia, parabiotic surgery was first performed between a wild-type mouse and a CX3CR1GFP/+ mouse, and embryonic cortical tissue from C57BL/6 fetus was grafted to the wild-type mouse of the parabiotic pairs (Supplementary Fig. 1D). We observed only a small number of CX3CR1GFP/+ cells distributed in grafted tissue (Fig. 5A). All of these infiltrating cells were IBA-1 positive (Fig. 5B), but these infiltrating cells (GFP-positive and IBA-1 positive) accounted for only 3.8 ± 1.2% of the total IBA-1 positive cells in the graft. BrdU assay showed that none of these infiltrating cells were BrdU-positive (Fig. 5C,D), suggesting infiltrating cells from bloodstream do not proliferate. Thus, circulating cells from bloodstream do not contribute to proliferative microglia and parenchymal microglia of the host brain is the main source of the microglial population in the graft tissue.
Figure 5. Infiltration of CX3CR1GFP/+ circulating cells into grafted tissue.
(A) Distribution of CX3CR1GFP/+ infiltrating cells (green) and IBA-1 positive cells (red) in grafted tissue at 15 d after transplantation. (B) Higher magnification image of the boxed region in (A). Note that all of the GFP+ cells were IBA-1 positive but most of IBA-1 positive cells were not GFP+. N = 3–4 sections/mouse, 3 mice. (C) Distribution of CX3CR1GFP/+ infiltrating cells (green) and BrdU-positive cells (red) in grafted tissue at 15 d after transplantation. (D) Higher magnification image of the boxed region in (C), showing the CX3CR1GFP/+ infiltrating cells (green) were not BrdU-positive (red) in grafted tissue. N = 3 sections/mouse, 3 mice. Parabiotic surgery was performed between a wild-type mouse and a heterozygous CX3CR1GFP/+ mouse. Embryonic cortical tissue from a wild-type fetus was transplanted into the wild-type animal of the parabiotic pairs. The white dash line shows the boundary between donor and recipient tissue. Scale bar, 200 μm (A,C); 25 μm (B,D).
Discussion
Our data suggest that parenchymal microglia from the host brain is the main source of microglial population in grafted cortical tissue. These results revealed an interactive relationship between the grafted tissue and the host brain after transplantation. The host brain benefited from grafted neurons in promoting cortical circuit reconstruction and function recovery. Meanwhile, infiltrating cells from host brain restored the microglial population of grafted tissue and may play an important role in monitoring and supporting the microenvironment of grafted tissue.
Repairing the injured neural network by transplantation of embryonic cortical tissue has provided a potential therapy in the treatment of brain injuries and diseases7,9,32. Grafted neurons can differentiate and project efferent myelinated fibers to appropriate cortical and subcortical targets2,4,7. Consistently, we found that donor-derived neurons can survive and integrate successfully into the host brain. Unlike the graft neurons, our results revealed that vast majority of the microglia from graft tissue disappeared after the transplantation. The survival of neurons but not microglia in grafted tissue indicates that microglia are more vulnerable than neurons in host environment. An in vitro model suggest that hypoxia/reperfusion can induce apoptosis of microglia, but not neurons under certain conndition33. Microglia exhibit various degree of viability in different experimental models. Microglia can maintain their population in cell cultures34,35 and slice cultures36,37,38, but are vulnerable to ischemia and nutrition deprivation39,40,41,42. Some studies have shown that grafted microglia can survive in host region in cell suspension transplantations43,44, but another cell suspension transplantation study indicate that grafted microglia are negligible to the overall microglial population within the graft area45. We observed endogenous microglia of the graft underwent a rapid atrophy and only in two rare cases that few endogenous CX3CR1GFP/+ cells survived in the transplants, suggesting that the traumatic environment used in our study doesn’t support the survival of embryonic microglia. The few CX3CR1GFP/+ cells observed in two animals in our study suggested that microglia from embryonic transplants can survive in the host brains if properly treated. Nevertheless, the rapid loss of endogenous microglia suggested that microglia from the embryonic tissue may not play an essential role in graft survival and differentiation.
When other different types of cells, such as mesenchymal stem cells, were transplanted into CNS, the transplants can cause strong immune response within the host46,47,48. During this process, massive activated microglia from host were observed to surround and invade into the grafted area46,47. Similarly, increasing number of activated CX3CR1GFP/+ cells from host were found to invade the grafted tissue in a few days after transplantation in the present study. In addition, these infiltrating cells resulted in reestablishing the normal microglial density of grafted tissue, and accounted for overwhelming majority of the population of the IBA-1 positive cells in the grafted tissue. These results indicated that invaded microglia from host dominated and restored the microglial population of grafted tissue, which had lost this important cell subset rapidly after transplantation.
Combination of transplantation surgery and parabiosis model demonstrated that the main source of colonized microglia in the transplant were parenchymal microglia of the host brain. Previous studies suggest that local expansion of reactive microglia, infiltration of circulating cells, or proliferation of latent progenitors in the CNS can contribute to activated microglia under various pathological conditions26,27,49,50. Consistent with our previous study in a stroke model26, here we observed that circulating cells infiltrated into the transplant, but the number of infiltrating cells constitutes only a small portion of IBA1-labeled cells. Importantly, these infiltrating cells do not proliferate and may not contribute to the sustained microglial population.
Despite the continuous increase in microglia density in the transplant during the first month after transplantation, we found proliferative microglia existed only within the first weeks in the host tissue near the border region, suggesting microglial infiltration occurred only during the early stage after transplantation. Infiltrated microglia were proliferative within the graft before they reached peak density. We observed that colonized microglia underwent a progressive transformation before they developed into highly ramified structures. These morphological and density changes largely recapitulate the developmental process of microglia during embryonic and postnatal periods51,52,53. Microglia are inflammatory cells in the CNS11,20,54 and one of the key players for circuit rewiring and synapse plasticity16,17,22,53. Their roles in the survival and differentiation of grafted embryonic tissue remain to be determined.
Methods
Animals
CX3CR1GFP/+, YFP H-line transgenic mice and C57BL/6 wild-type mice were used in this study. The animals were purchased from Jackson Laboratory and bred on a 12 h/12 h light/dark cycle with food and water ad libitum in the Laboratory Center for Medical Sciences, Lanzhou University. Mice aged 3–4 months of both sexes were used in this study. All experimental procedures and protocols in the study were in accordance with the ‘Guidance of the Care and Use of Laboratory Animals’ approved by Ethic Committee of Experimental Animals of Lanzhou University, China.
Transplantation surgery
The transplanting procedure used in this study was modified from a method described previously7. Briefly, adult mice (as recipients) were anesthetized with an intraperitoneal injection of 20 mg/ml ketamine and 2 mg/ml xylazine. A piece of cranial bone of approximately 2 mm in diameter, in the skull of the recipient animal was removed by using dental drill at the position about 2.5 mm posterior to the Bregma and 2.5 mm lateral to the midline. Embryonic cortical tissue was dissected out from E14-15 d fetus cortex and cut into small fragments of about 1 mm3. When the embryonic cortical block was ready for use, a unilateral traumatic lesion, about 1 mm in diameter and depth, was made in the cortex of the recipient animal by using a dental drill. Care was taken to avoid making a through hole in cortex and keeping away from large vessels when drilling the hole. Immediately after the lesion, embryonic cortical graft was deposited into the lesion cavity. For brain harvest after transplantation, the removed cranial bone was put back to cover the exposed brain. Then, the separated skull was glued with the cranial bone by adhesives. After transplantation, the scalp was sutured and disinfected carefully, and animals were put back to the cages and housed for different time periods. For in vivo imaging, exposed brain was covered by a circular cover glass and the edge of the cover glass were sealed with dental acrylic as the methods previously reported55.
Parabiosis
The parabiotic surgery was carried out following the procedure as previously reported56,57. A C57BL/6 (wild-type) mouse and a weight-matched heterozygous CX3CR1GFP/+ mouse were selected for the parabiosis surgery. Mice were anaesthetized by intraperitoneal injection with xylazine and ketamine, and the opposite flank of each mouse were shaved and sterilized with 75% ethanol followed by betadine solution. Then, a straight incision from the shoulder to the hip was made on the cleaned skins of each animal. Corresponding scapulas of each mouse were fixed together with non-absorbable 4-0 silk and the posterior muscles were sutured together by absorbable 5-0 chromic gut. Finally, the corresponding dorsal and ventral skin edges of each mouse were sutured with 4-0 silk. Pairs of mice were kept warm before completely recovered from anesthesia, and then they were put back to the cages and fed with food and water at 20 ± 2 °C room temperature. It took 14–15 days to establish sufficient anastomotic blood circulation and complete blood sharing between the pair of mice. Then, embryonic cortical tissue from C57BL/6 fetus was grafted to the wild-type mouse of the parabiotic pair.
Immunohistochemistry
Mice were anesthetized intraperitoneally with a lethal dose of ethyl urethane and then transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde (PFA). The mouse brains were dissected out and soaked in 4% PFA for at least 36 hours at 4 °C. The fixed mouse brains were serially sectioned into 30 μm coronal slices on a vibrating microtome (TV1000S, Leica). For antibody staining, brain slices were pre-incubated with PBS-T (0.3% Twen-20 in 0.1 M PBS) for 0.5 h at room temperature (22 ± 2 °C). The slices were washed three times with PBS and then stained overnight in primary antibodies at 4 °C on a shaker. For primary antibodies, we used a rabbit antibody to IBA-1 as a microglia marker (1:500, Wako), mouse antibody to NeuN as a neuron marker (1:250; Millipre). Second antibodies were TRITC-conjugated goat anti-rabbit (1:100; ZSGB-BIO), FITC-conjugated goat anti-rabbit (1:100; ZSGB-BIO), and FITC-conjugated goat anti-mouse (1:100; ZSGB-BIO).
To mark proliferating cells, bromodeoxyuridine (BrdU, 50 mg/kg; Sigma) was injected intraperitoneally per 2 hours for four times one day before brain harvest. For BrdU staining, brain slices were rinsed in double distilled water, and treated in 2 M HCl at 37 °C for 30 min, then neutralized with 0.1 M borate buffer (PH 8.5) for 3 × 15 min and incubated overnight in rat anti-BrdU (1:500; AbD Serotec). Second antibodies were TRITC-conjugated goat anti-rat (1:100; ZSGB-BIO) and the sections were stained for 1.5 h at room temperature in the dark.
In vivo two-photon and confocal microscopy imaging
Brain sections after fluorescent staining were imaged with UPlan SApo ×40/0.85 objective on a confocal laser scanning microscope (Olympus FV1000). Stacks (1024 × 1024 pixel arrays) were captured at 1 μm intervals in the Z dimension. In vivo two-photon imaging was performed as described previously58. Briefly, mice were deeply anesthetized by intraperitoneal injection of xylazine and ketamine. For stable imaging, the optical window was fixed to a custom-made steel plate. To excite GFP fluorescence, the mouse was fitted into a two-photon microscope and imaged with ×25 water-immersion objective (×25/1.05; Olympus) at laser of 890 nm.
Data analysis
To calculate the disappearance rate of endogenous microglia, numbers of GFP+ cells at 6 h, 12 h, 18 h, 24 h after transplantation were compared with the number of GFP+ cells at 1 h after transplantation under the same imaging area. To compare the density of the microglia in grafted tissue at different time point, a random area in grafted tissue was selected to count the microglia number. To quantitate the percentage of proliferating microglia, three slices from each mouse brain were randomly chosen, and all of the microglia and BrdU-positive microglia in grafted tissue and host part of border region (accumulation area contain large number of both activated microglia and BrdU-positive cells near the grafted tissue in host brain) were counted. All areas are measured by using ImageJ software. Statistical analyses of all dates were done by using IBM SPSS Statistics 19 software (One way ANOVA for three or more groups, and unpaired t-test for two groups). All date are presented as mean ± SEM.
Additional Information
How to cite this article: Wang, C. et al. Infiltrating cells from host brain restore the microglial population in grafted cortical tissue. Sci. Rep. 6, 33080; doi: 10.1038/srep33080 (2016).
Supplementary Material
Acknowledgments
This study was supported by National Natural Science Foundation of China (Nos 81171174 and 31471045) and Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP: 20130211110002). The authors also wish to thank Liping Guan and Qingxiang Gao at Core Facility of School of Life Sciences, Lanzhou University for technical support, and Lei Wang and Wenguang Xie for assistance with preliminary experiments.
Footnotes
Author Contributions C.W., S.T., Y.F., J.G. and L.Z. performed experiments; C.W. and S.T. analyzed the experiments data; C.W. and S.Z. participated in experiments designing and manuscript writing.
References
- Gaillard A. & Roger M. Early commitment of embryonic neocortical cells to develop area-specific thalamic connections. Cereb Cortex 10, 443–453 (2000). [DOI] [PubMed] [Google Scholar]
- Gaillard F., Domballe L. & Gaillard A. Fetal cortical allografts project massively through the adult cortex. Neuroscience 126, 631–637, doi: 10.1016/j.neuroscience.2004.04.011 S0306452204002751 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- Gaillard F. Heterotopic, not homotopic, fetal occipital allografts in adult hosts project to visual-related extracortical targets. Restor Neurol Neurosci 25, 161–175 (2007). [PubMed] [Google Scholar]
- Santos-Torres J. et al. Electrophysiological and synaptic characterization of transplanted neurons in adult rat motor cortex. J Neurotrauma 26, 1593–1607, doi: 10.1089/neu.2008.0702 (2009). [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L. et al. Embryonic amygdalar transplants in adult rats with motor cortex lesions: a molecular and electrophysiological analysis. Front Neurol 2, 59, doi: 10.3389/fneur.2011.00059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard F. & Domballe L. Fetal tissue allografts in the damaged adult visual cortex: physiology and connectivity. Restor Neurol Neurosci 26, 267–277 (2008). [PubMed] [Google Scholar]
- Gaillard A. et al. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci 10, 1294–1299, doi: nn1970 [pii]10.1038/nn1970 (2007). [DOI] [PubMed] [Google Scholar]
- Plumet J., Ebrahimi A., Guitet J. & Roger M. Partial recovery of skilled forelimb reaching after transplantation of fetal cortical tissue in adult rats with motor cortex lesion - anatomical and functional aspects. Restor Neurol Neurosci 6, 9–27, doi: 10.3233/RNN-1993-6102L7V1W25552852K41 [pii] (1993). [DOI] [PubMed] [Google Scholar]
- Riolobos A. S. et al. Functional recovery of skilled forelimb use in rats obliged to use the impaired limb after grafting of the frontal cortex lesion with homotopic fetal cortex. Neurobiol Learn Mem 75, 274–292, doi: 10.1006/nlme.2000.3979 (2001). [DOI] [PubMed] [Google Scholar]
- Bergwerf I. et al. Recognition of cellular implants by the brain’s innate immune system. Immunol Cell Biol 89, 511–516, doi: icb2010141 [pii]10.1038/icb.2010.141 (2011). [DOI] [PubMed] [Google Scholar]
- Kofler J. & Wiley C. A. Microglia: key innate immune cells of the brain. Toxicol Pathol 39, 103–114, doi: 0192623310387619 [pii]10.1177/0192623310387619 (2011). [DOI] [PubMed] [Google Scholar]
- Tambuyzer B. R., Ponsaerts P. & Nouwen E. J. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol 85, 352–370, doi: jlb.0608385 [pii]10.1189/jlb.0608385 (2009). [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. & Cardona A. E. The myeloid cells of the central nervous system parenchyma. Nature 468, 253–262, doi: nature09615[pii]10.1038/nature09615 (2010). [DOI] [PubMed] [Google Scholar]
- Prinz M. & Mildner A. Microglia in the CNS: immigrants from another world. Glia 59, 177–187, doi: 10.1002/glia.21104 (2011). [DOI] [PubMed] [Google Scholar]
- Alliot F., Godin I. & Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117, 145–152, doi: S0165380699001133 [pii] (1999). [DOI] [PubMed] [Google Scholar]
- Cunningham C. L., Martinez-Cerdeno V. & Noctor S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33, 4216–4233, doi: 33/10/4216 [pii]10.1523/JNEUROSCI.3441-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P. et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep 8, 1271–1279, doi: S2211-1247(14)00626-3 [pii]10.1016/j.celrep.2014.07.042 (2014). [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F. & Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318, doi: 1110647 [pii]10.1126/science.1110647 (2005). [DOI] [PubMed] [Google Scholar]
- Davalos D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–758, doi: nn1472 [pii]10.1038/nn1472 (2005). [DOI] [PubMed] [Google Scholar]
- Saijo K. & Glass C. K. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11, 775–787, doi: nri3086 [pii]10.1038/nri3086 (2011). [DOI] [PubMed] [Google Scholar]
- Michell-Robinson M. A. et al. Roles of microglia in brain development, tissue maintenance and repair. Brain 138, 1138–1159, doi: awv066[pii]10.1093/brain/awv066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609, doi: S0092-8674(13)01481-5 [pii]10.1016/j.cell.2013.11.030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H., Kirchhoff F. & Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron 77, 10–18, doi: S0896-6273(12)01162-2 [pii]10.1016/j.neuron.2012.12.023 (2013). [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D. & Perry V. H. Microglial dynamics and role in the healthy and diseased brain: a paradigm of functional plasticity. Neuroscientist 21, 169–184, doi: 1073858414530512 [pii]10.1177/1073858414530512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A., Barres B. A. & Bennett M. L. Microglia: scapegoat, saboteur, or something else? Science 339, 156–161, doi: 339/6116/156 [pii]10.1126/science.1227901 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. et al. Proliferation of parenchymal microglia is the main source of microgliosis after ischaemic stroke. Brain 136, 3578–3588, doi: awt287 [pii]10.1093/brain/awt287 (2013). [DOI] [PubMed] [Google Scholar]
- Elmore M. R. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397, doi: S0896-6273(14)00171-8[pii]10.1016/j.neuron.2014.02.040 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. K. et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785, doi: S0092-8674(10)00374-0[pii]10.1016/j.cell.2010.03.055 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y. et al. Dynamics of spinal microglia repopulation following an acute depletion. Sci Rep 6, 22839, doi: srep22839 [pii]10.1038/srep22839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes K. R., Loane D. J., Stoica B. A., Zhang J. & Faden A. I. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J Neuroinflammation 9, 43, doi: 1742-2094-9-43 [pii]10.1186/1742-2094-9-43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys Z., Ziaja M., Pawlinski R., Setkowicz Z. & Janeczko K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res 63, 90–97, doi: [pii] (2001). [DOI] [PubMed] [Google Scholar]
- Tuszynski M. H. Rebuilding the brain: resurgence of fetal grafting. Nat Neurosci 10, 1229–1230, doi: 10.1038/nn1007-1229 (2007). [DOI] [PubMed] [Google Scholar]
- Chen C. M. et al. Green Tea Catechin Prevents Hypoxia/Reperfusion-Evoked Oxidative Stress-Regulated Autophagy-Activated Apoptosis and Cell Death in Microglial Cells. J Agric Food Chem 64, 4078–4085, doi: 10.1021/acs.jafc.6b01513 (2016). [DOI] [PubMed] [Google Scholar]
- Saura J., Tusell J. M. & Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia 44, 183–189, doi: 10.1002/glia.10274 (2003). [DOI] [PubMed] [Google Scholar]
- Lai A. Y., Dibal C. D., Armitage G. A., Winship I. R. & Todd K. G. Distinct activation profiles in microglia of different ages: a systematic study in isolated embryonic to aged microglial cultures. Neuroscience 254, 185–195, doi: S0306-4522(13)00781-1 [pii]10.1016/j.neuroscience.2013.09.010 (2013). [DOI] [PubMed] [Google Scholar]
- Swinnen N. et al. Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 61, 150–163, doi: 10.1002/glia.22421 (2013). [DOI] [PubMed] [Google Scholar]
- Dailey M. E., Eyo U., Fuller L., Hass J. & Kurpius D. Imaging microglia in brain slices and slice cultures. Cold Spring Harb Proto 2013, 1142–1148, doi:2013/12/pdb.prot079483 [pii]10.1101/pdb.prot079483 (2013). [DOI] [PubMed] [Google Scholar]
- Noctor S. C. Time-lapse imaging of fluorescently labeled live cells in the embryonic mammalian forebrain. Cold Spring Harb Protoc 2011, 1350–1361, doi: 2011/11/pdb.prot066605 [pii]10.1101/pdb.prot066605 (2011). [DOI] [PubMed] [Google Scholar]
- Yenari M. A. & Giffard R. G. Ischemic vulnerability of primary murine microglial cultures. Neurosci Lett 298, 5–8, doi: S0304394000017249 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- Lyons S. A. & Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab 18, 521–530, doi: 10.1097/00004647-199805000-00007 (1998). [DOI] [PubMed] [Google Scholar]
- Eyo U. B., Miner S. A., Ahlers K. E., Wu L. J. & Dailey M. E. P2X7 receptor activation regulates microglial cell death during oxygen-glucose deprivation. Neuropharmacology 73, 311–319, doi: S0028-3908(13)00246-3 [pii]10.1016/j.neuropharm.2013.05.032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo U. & Dailey M. E. Effects of oxygen-glucose deprivation on microglial mobility and viability in developing mouse hippocampal tissues. Glia 60, 1747–1760, doi: 10.1002/glia.22394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y. et al. Intracerebroventricular injection of microglia protects against focal brain ischemia. J Pharmacol Sci 94, 203–206 (2004). [DOI] [PubMed] [Google Scholar]
- Ma W., Zhao L., Fontainhas A. M., Fariss R. N. & Wong W. T. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One 4, e7945, doi: 10.1371/journal.pone.0007945 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell N. A. & Streit W. J. Colonization of neural allografts by host microglial cells: relationship to graft neovascularization. Cell Transplant 6, 221–230, doi: S0963689797000304 [pii] (1997). [DOI] [PubMed] [Google Scholar]
- De Vocht N. et al. Quantitative and phenotypic analysis of mesenchymal stromal cell graft survival and recognition by microglia and astrocytes in mouse brain. Immunobiology 218, 696–705, doi: S0171-2985(12)00463-9 [pii]10.1016/j.imbio.2012.08.266 (2013). [DOI] [PubMed] [Google Scholar]
- Le Blon D. et al. Distinct spatial distribution of microglia and macrophages following mesenchymal stem cell implantation in mouse brain. Immunol Cell Biol 92, 650–658, doi: icb201449 [pii]10.1038/icb.2014.49 (2014). [DOI] [PubMed] [Google Scholar]
- Praet J. et al. Cell type-associated differences in migration, survival, and immunogenicity following grafting in CNS tissue. Cell Transplant 21, 1867–1881, doi: ct0547praet [pii]10.3727/096368912×636920 (2012). [DOI] [PubMed] [Google Scholar]
- Djukic M. et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain 129, 2394–2403, doi: awl206 [pii]10.1093/brain/awl206 (2006). [DOI] [PubMed] [Google Scholar]
- Li T. & Zhang S. Microgliosis in the Injured Brain: Infiltrating Cells and Reactive Microglia Both Play a Role. Neuroscientist 22, 165–170, doi: 1073858415572079 [pii]10.1177/1073858415572079 (2016). [DOI] [PubMed] [Google Scholar]
- Nayak D., Roth T. L. & McGavern D. B. Microglia Development and Function. Annu Rev Immunol. doi: 10.1146/annurev-immunol-032713-120240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski D., Soltys Z. & Janeczko K. Morphological development of microglia in the postnatal rat brain. A quantitative study. Int J Dev Neurosci 21, 445–450, doi: S0736574803001114 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y., Hoshikawa K., Goldman J. E., Sekino Y. & Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34, 2231–2243, doi: 34/6/2231[pii]10.1523/JNEUROSCI.1619-13.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber M. B. Changing face of microglia. Science 330, 783–788, doi: 330/6005/783 [pii]10.1126/science.1190929 (2010). [DOI] [PubMed] [Google Scholar]
- Chen B. E., Trachtenberg J. T., Holtmaat A. J. & Svoboda K. Long-term, high-resolution imaging in the neocortex in vivo. CSH Protoc 2008, pdb prot4902 (2008). [DOI] [PubMed] [Google Scholar]
- Ajami B., Bennett J. L., Krieger C., McNagny K. M. & Rossi F. M. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14, 1142–1149, doi: nn.2887 [pii]10.1038/nn.2887 (2011). [DOI] [PubMed] [Google Scholar]
- Rossi F. M. et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol 6, 626–634, doi: ni1203 [pii]10.1038/ni1203 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang S., Boyd J., Delaney K. & Murphy T. H. Rapid reversible changes in dendritic spine structure in vivo gated by the degree of ischemia. J Neurosci 25, 5333–5338, doi:25/22/5333 [pii]10.1523/JNEUROSCI.1085-05.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.