Abstract
Rationale: The clinical course of chronic lung allograft dysfunction (CLAD) is heterogeneous. Forced vital capacity (FVC) loss at onset, which may suggest a restrictive phenotype, was associated with worse survival for bilateral lung transplant recipients in one previously published single-center study.
Objectives: We sought to replicate the significance of FVC loss in an independent, retrospectively identified cohort of bilateral lung transplant recipients and to investigate extended application of this approach to single lung recipients.
Methods: FVC loss and other potential predictors of survival after the onset of CLAD were assessed using Kaplan-Meier and Cox proportional hazards models.
Measurements and Main Results: FVC loss at the onset of CLAD was associated with higher mortality in an independent cohort of bilateral lung transplant recipients (hazard ratio [HR], 2.75; 95% confidence interval [CI], 2.02–3.73; P < 0.0001) and in a multicenter cohort of single lung recipients (HR, 1.80; 95% CI, 1.09–2.98; P = 0.02). Including all subjects, the deleterious impact of FVC loss on survival persisted after adjustment for other relevant clinical variables (HR, 2.36; 95% CI, 1.77–3.15; P < 0.0001). In patients who develop CLAD without FVC loss, chest computed tomography features suggestive of pleural or parenchymal fibrosis also predicted worse survival in both bilateral (HR, 2.01; 95% CI, 1.16–5.20; P = 0.02) and single recipients (HR, 2.47; 95% CI, 1.24–10.57; P = 0.02).
Conclusions: We independently validated the prognostic significance of FVC loss for bilateral lung recipients and demonstrated that this approach to CLAD classification also confers prognostic information for single lung transplant recipients. Improved understanding of these discrete phenotypes is critical to the development of effective therapies.
Keywords: lung transplantation, spirometry, graft rejection, bronchiolitis obliterans
Lung transplantation is a therapeutic option for end-stage pulmonary diseases. However, the median survival after lung transplant is less than 6 years (1). Although survival has improved over the past decade, chronic allograft dysfunction remains the primary obstacle to better long-term survival. The physiologic hallmark of chronic allograft dysfunction is a persistent decline in the FEV1. Early on, this physiologic event was correlated with the histologic finding of obliterative bronchiolitis (2), and thus the clinical condition was termed bronchiolitis obliterans syndrome. The syndrome has subsequently been defined by the International Society of Heart and Lung Transplantation (ISHLT) as an irreversible decline in the FEV1 of 20% or more relative to the highest post-transplant baseline (3).
Bronchiolitis obliterans syndrome has proven to be a useful descriptor of allograft dysfunction. However, it is increasingly recognized that lung allograft pathologies other than obliterative bronchiolitis may lead to persistent decline in FEV1 and be accompanied by clinical features inconsistent with our understanding of bronchiolitis obliterans syndrome (4–6). Therefore, the term chronic lung allograft dysfunction (CLAD) was introduced in 2010 as an overarching descriptor that encompasses all forms of chronic lung dysfunction after transplantation, including bronchiolitis obliterans syndrome (7).
Recently, CLAD has been more precisely defined as a persistent (at least 3 wk), often unexplained decline in pulmonary function (FEV1 with/without FVC) compared with the best postoperative baseline (8). Distinguishing clinically meaningful phenotypes within CLAD is critical to inform patients as to prognostic implications, support investigations of the precise mechanisms that lead to the pathogenesis of these conditions, and ensure homogenous patient populations for studies in therapeutic trials. However, the optimal methods for classification of CLAD phenotypes have not yet been established.
The best-described form of CLAD other than bronchiolitis obliterans syndrome is characterized by restrictive physiology, initially defined by longitudinal total lung capacity (TLC) measurements (9). Restrictive allograft syndrome (RAS) was defined as CLAD (decline in FEV1 ≥ 20%, analogous to bronchiolitis obliterans syndrome) accompanied by a persistent decline in TLC of at least 10%. Distinct from bronchiolitis obliterans syndrome, the characteristic radiologic finding of RAS was interstitial changes, including upper lobe–dominant fibrosis (9). The histopathologic characteristics of RAS include diffuse alveolar damage and fibrosis of the alveolar interstitium, visceral pleura, and interlobular septa, with or without obliterative bronchiolitis lesions (9). In addition, pleuroparenchymal fibroelastosis can be seen as a histopathologic feature of RAS (4). Importantly, RAS was associated with worse post-CLAD survival than bronchiolitis obliterans syndrome (9). Unfortunately, TLC measurement is not routinely performed as a part of follow-up monitoring at most transplant centers, making it difficult to widely apply the RAS definition.
Todd and colleagues proposed an alternative approach to identify this restrictive phenotype at the time of CLAD onset using FVC (10). They submitted that FVC loss, which may suggest a restrictive ventilatory defect, is a generalizable and clinically applicable method to distinguish a restrictive phenotype of CLAD, analogous to RAS. FVC loss was defined as an FVC/FVCBest < 0.8 at the onset of CLAD (10). Subjects not meeting criteria for FVC loss were classified as having bronchiolitis obliterans syndrome. In this single-center study of bilateral lung recipients, patients with FVC loss at CLAD onset experienced worse survival after CLAD than those in whom FVC was preserved (bronchiolitis obliterans syndrome), similar to what has been described for RAS as determined by the TLC method (10).
The FVC loss approach has not been subjected to external validation, nor has it been applied to single lung recipients. In addition, it remains uncertain whether consideration of radiographic findings may confer additional prognostic information. The primary objectives of this study were to (1) validate the significance of FVC loss in an independent cohort of bilateral lung transplant recipients, (2) extend the FVC loss criteria to a cohort of single lung transplant recipients from two large transplant centers, and (3) consider risk factors for death after CLAD in all subjects with CLAD from both centers. A secondary objective was to explore whether incorporation of radiographic characteristics provides additional prognostic information over and above that provided by physiological CLAD phenotype. Preliminary results were presented as an oral abstract presentation at the 2014 ISHLT Annual Conference (11).
Methods
Study Cohorts
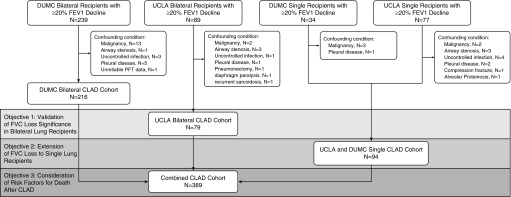
We conducted a multicenter retrospective cohort study with all relevant clinical data obtained through medical record review at each center. Figure 1 outlines the patient cohorts used to achieve each study objective.
Figure 1.
Flow chart for derivation of each chronic lung allograft dysfunction (CLAD) cohort and analysis. DUMC = Duke University Medical Center; UCLA = University of California at Los Angeles.
CLAD was defined as a sustained, greater than or equal to 20% decline in FEV1 as compared with the average of the two best post-transplant FEV1 measured at least 3 weeks apart in the absence of other clinical confounders (2). Between January 1, 2000 and June 30, 2012, 277 adults (≥18 yr old) received a first bilateral and 244 received a first single lung transplant at the University of California at Los Angeles (UCLA). UCLA patients were followed to death, retransplantation, or the time of last pulmonary function test (PFT) before July 31, 2013. After exclusion of patients with confounding conditions, 79 bilateral lung recipients and 64 single lung recipients with CLAD were included in this study.
Similarly, between January 1, 1998 and December 31, 2010, 682 adults received a first bilateral and 91 received a first single lung transplant at Duke University Medical Center (DUMC). DUMC patients were followed to death, retransplantation, or the time of last PFT before May 31, 2012.
After exclusion of patients with confounding conditions (Figure 1), 216 bilateral recipients and 30 single recipients with CLAD were included in the study. Among the excluded patients were eight subjects with uncontrolled infection, defined by concurrent infection at the time of FEV1 decline, and failing to demonstrate clinical and radiographic recovery to allow for the clear diagnosis of CLAD as judged by the treating pulmonologist. Median follow-up time for the study cohort was 4.21 years, with an interquartile range of 2.56 to 6.85.
All patients received standardized immunosuppression, PFT follow up, surveillance bronchoscopies, and other clinical management as previously described (12) and summarized briefly for each center in the online supplement. Institutional review boards at each center approved the study, with protocol numbers 10-001492 at UCLA and Pro00029129 at DUMC.
CLAD Phenotype Classification
Patients were considered to have FVC loss if at CLAD onset the FVC/FVCBest was less than 0.8. The FVCBest was defined as the average of the two FVC measurements that paired with the two best post-transplant FEV1 measurements used in the CLAD calculation. Stable FVC at CLAD onset was defined as the FVC/FVCBest greater than or equal to 0.8.
Radiology Review
Chest computed tomography (CT) scans performed 30 days before and up to 90 days after CLAD onset were eligible for inclusion. For subjects with multiple eligible CT scans, the scan performed nearest to the time of CLAD onset was selected. In the event of equal temporal distribution, the post-CLAD CT scan was selected. Clinical radiology reports were reviewed by a single pulmonologist at each center, blinded to CLAD phenotype. The following findings were systematically noted: small pleural effusion (moderate or large effusion was considered an alternative etiology for FEV1 decline, and these patients were excluded from study), pleural thickening, ground-glass opacities, nodular or tree-in-bud infiltrates, consolidative opacities, bronchiectasis or bronchial wall thickening, septal thickening or reticular opacities, and air trapping. Air trapping was considered assessable only if the CT scan was performed with both inspiratory and expiratory imaging.
Allograft Assessments
Acute cellular rejection and lymphocytic bronchiolitis were determined and graded in all transbronchial biopsies obtained before CLAD onset according to 1996 ISHLT criteria (13). Any pre-CLAD acute rejection or lymphocytic bronchiolitis of grade 1 or greater was considered positive in subsequent statistical models. Primary graft dysfunction grade 3 at 72 hours post-reperfusion was evaluated according to ISHLT guidelines (14). Early-onset CLAD (EO-CLAD) was defined according to prior literature as the onset of CLAD within 2 years of transplantation, and severe-onset CLAD was defined as a decline in FEV1 to less than or equal to 65% of the post-transplant baseline at CLAD onset (15).
Statistical Analysis
Demographic and baseline characteristics were summarized using descriptive statistics. Continuous variables were summarized using mean and SD or median and interquartile range. Categorical variables were summarized using counts and percentages. Nonparametric Wilcoxon t tests (two-tailed P value) were used to assess differences for demographic and time-independent baseline characteristics across groups; chi-square or Fisher exact tests, as appropriate, were used to evaluate frequency differences. Survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test. Univariable Cox proportional hazard models were used to estimate the impact of potentially relevant clinical characteristics, including FVC loss on survival after CLAD (time to death or retransplantation). A final multivariable model was constructed by entering all covariates in the final model.
Results
Validation of FVC Loss as Risk Factor for Death in Bilateral Lung Recipients with CLAD
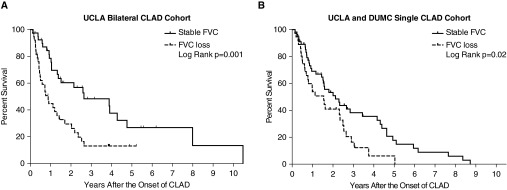
The UCLA bilateral CLAD cohort (N = 79, Figure 1) was used to independently replicate the previously described association between FVC loss at CLAD onset and worse post-CLAD survival. The characteristics of this bilateral lung validation cohort are described in Table 1. There were 38 (48%) cases meeting criteria for FVC loss. We found no significant differences in spirometric sampling frequency per transplant year between subjects with and without FVC loss, and sampling rates for the first 2 years post-transplant are included in Table 1. Notably, FVC loss at CLAD onset was associated with a significantly worse post-CLAD survival (P < 0.0001; unadjusted hazard ratio [HR], 2.75; 95% confidence interval [CI], 2.02–3.73), with 1- and 3-year Kaplan-Meier post-CLAD survival estimates of 44 and 11% for the FVC loss group, compared with 79 and 48% for the stable FVC group (P = 0.001, Figure 2A), similar to what was previously described (10).
Table 1.
Clinical characteristics of the chronic lung allograft dysfunction cohorts
| UCLA Bilateral CLAD Cohort |
UCLA and DUMC Single CLAD Cohort |
DUMC Bilateral CLAD Cohort |
Combined CLAD Cohort |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Stable FVC (n = 41) | FVC loss (n = 38) | Stable FVC (n = 56) | FVC loss (n = 38) | Stable FVC (n = 151) | FVC loss (n = 65) | Stable FVC (n = 248) | FVC loss (n = 141) | P Value* | |
| Median age at transplant (IQR), yr | 56 (48–61) | 55 (46–59) | 63 (59–66) | 64 (61–66) | 55 (39–61) | 55 (40–62) | 57 (46–63) | 58 (48–63) | 0.42 |
| Recipient sex | 0.01 | ||||||||
| Male | 24 (59) | 26 (68) | 41 (73) | 16 (42) | 96 (64) | 29 (45) | 161 (69) | 87 (55) | |
| Female | 17 (41) | 12 (32) | 15 (27) | 22 (58) | 55 (36) | 36 (55) | 71 (31) | 70 (45) | |
| Race | 0.09 | ||||||||
| White | 32 (78) | 24 (63) | 49 (88) | 30 (79) | 130 (86) | 60 (92) | 211 (85) | 114 (81) | |
| African American | 2 (5) | 3 (8) | 3 (5) | 3 (8) | 19 (13) | 5 (8) | 24 (10) | 11 (8) | |
| Other | 7 (17) | 11 (29) | 4 (7) | 5 (13) | 2 (1) | 0 (0) | 13 (5) | 16 (11) | |
| Native disease category | 0.20 | ||||||||
| Obstructive | 13 (32) | 9 (24) | 34 (61) | 19 (50) | 59 (39) | 22 (34) | 106 (43) | 50 (35) | |
| Restrictive | 16 (39) | 21 (55) | 22 (39) | 19 (50) | 56 (37) | 29 (44) | 94 (38) | 69 (49) | |
| Cystic | 5 (12) | 2 (5) | 0 (0) | 0 (0) | 30 (20) | 12 (20) | 35 (14) | 15 (11) | |
| Other | 7 (17) | 6 (16) | 0 (0) | 0 (0) | 6 (4) | 1 (2) | 13 (5) | 7 (5) | |
| CMV high risk† | 12 (29) | 4 (11) | 13 (23) | 4 (11) | 41 (27) | 14 (22) | 66 (27) | 22 (16) | 0.01 |
| PGD grade 3 at 72 h | 3 (7) | 3 (8) | 8 (14) | 5 (13) | 12 (8) | 0 (0) | 23 (9) | 8 (6) | 0.20 |
| Median No. of PFTs (IQR) | |||||||||
| First post-transplant year | 9 (8–14) | 9 (8–11) | 11 (8–13) | 11 (9–14) | 13 (11–15) | 14 (12–16) | 12 (9–15) | 12 (9–15) | 0.97 |
| Second post-transplant year | 5 (3–8) | 4 (3–6) | 5 (4–8) | 6 (3–8) | 5 (4–7) | 5 (3–6) | 5 (4–7) | 5 (3–6) | 0.07 |
| Median days to CLAD (IQR) | 739 (507–1,476) | 671 (379–1,101) | 821 (455–1,202) | 874 (553–1,413) | 1191 (559–1,978) | 1030 (442–1,552) | 989 (532–1,644) | 848 (462–1,383) | 0.12 |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction; CMV = cytomegalovirus; DUMC = Duke University Medical Center; IQR = interquartile range; PFT = pulmonary function tests; PGD = primary graft dysfunction; UCLA = University of California at Los Angeles.
Data presented as n (%) unless otherwise noted.
Chi-squared or Wilcoxon test comparing stable FVC vs. FVC loss in combined cohort.
Defined as recipient CMV seronegative with donor CMV seropositive.
Figure 2.
A physiologic pattern of FVC loss at chronic lung allograft dysfunction (CLAD) onset was associated with worse post-CLAD survival among bilateral recipients at University of California at Los Angeles (UCLA) (P = 0.001) (A), similar to what has been previously described. Among single lung recipients, FVC loss was also associated with worse post-CLAD survival (P = 0.02) (B). DUMC = Duke University Medical Center.
Application of the FVC Loss Criteria to Single Lung Recipients
We next analyzed the UCLA and DUMC single lung CLAD cohort, which included 94 single lung recipients with CLAD (UCLA, n = 64 and Duke, n = 30; Figure 1) to explore the extended application of the FVC loss criteria to single lung transplant recipients. The clinical characteristics of this UCLA and DUMC single lung CLAD cohort are described in Table 1. Among single lung recipients at two transplant centers, 38 (40%) met criteria for FVC loss. Again, there was a significant association between FVC loss at CLAD onset and worse post-CLAD survival (P = 0.02; unadjusted HR, 1.80; 95% CI, 1.09–2.98), with 1- and 3-year Kaplan-Meier post-CLAD survival estimates for the FVC loss group of 53 and 16%, in contrast to 69 and 38% in the stable FVC group (P = 0.02, Figure 2B).
Risk Factors for Death after CLAD in Bilateral and Single Lung Recipients
To optimize statistical power, risk factors for death after CLAD were then considered in a combined CLAD cohort that included all bilateral and single lung recipients with CLAD from both UCLA and Duke (N = 389, Figure 1). The characteristics of this combined CLAD cohort are described in Table 1.
In this large multicenter cohort of single and bilateral lung recipients, a physiologic pattern of FVC loss significantly predicted mortality after the onset of CLAD (Table 2). Other characteristics significantly associated with mortality after CLAD onset in univariate analyses included EO-CLAD, restrictive native lung disease, female sex, prior history of acute rejection, and prior organizing pneumonia histology in the allograft. The impact of FVC loss on post-CLAD survival remained significant in the multivariable model (Table 2).
Table 2.
Univariable and multivariable Cox models for death after chronic lung allograft dysfunction in the combined multicenter chronic lung allograft dysfunction cohort
| Univariable |
Multivariable Model |
|||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| FVC loss | 2.47 (1.90–3.20) | <0.0001 | 2.36 (1.77–3.15) | <0.0001 |
| Early-onset CLAD* | 1.45 (1.13–1.85) | 0.004 | 1.61 (1.23–2.10) | 0.0005 |
| Prior acute rejection | 1.40 (1.05–1.88) | 0.02 | 1.47 (1.08–2.02) | 0.01 |
| Female recipient | 1.40 (1.13–2.03) | 0.009 | 1.37 (1.05–1.77) | 0.02 |
| Primary graft dysfunction grade 3 at 72 h | 1.34 (0.87–1.97) | 0.18 | 1.64 (1.04–2.46) | 0.03 |
| Prior organizing pneumonia | 1.50 (1.13–1.97) | 0.006 | 1.37 (1.02–1.82) | 0.04 |
| Center (DUMC) | 0.89 (0.69–1.16) | 0.39 | 1.23 (0.90–1.69) | 0.20 |
| Restrictive native lung disease | 1.37 (1.06–1.77) | 0.02 | 1.20 (0.90–1.58) | 0.21 |
| Single lung | 1.16 (0.88–1.52) | 0.29 | 1.12 (0.81–1.53) | 0.47 |
| Prior lymphocytic bronchiolitis | 1.24 (0.97–1.61) | 0.09 | 1.07 (0.81–1.43) | 0.63 |
| Age at transplant > 65 yr | 1.31 (0.94–1.79) | 0.10 | 1.12 (0.78–1.59) | 0.52 |
| Severe-onset CLAD* | 1.27 (0.97–1.66) | 0.09 | 1.02 (0.76–1.38) | 0.87 |
Definition of abbreviations: CI = confidence interval; CLAD = chronic lung allograft dysfunction; DUMC = Duke University Medical Center; HR = hazard ratio.
Early onset CLAD and severe-onset CLAD were defined according to prior literature as the onset of CLAD within 2 years of transplantation (early onset) or as a decline in FEV1 to <65% of the post-transplant baseline at CLAD onset (severe onset).
Receiver operator characteristic analyses for FVC loss as a predictor of 1-year mortality after CLAD onset are presented in Figure E1 in the online supplement. When stratified by stable FVC and FVC loss groups, EO-CLAD was associated with worse post-CLAD mortality for both physiological CLAD phenotypes (Figure E2). Tests for interactions were negative and not included in the final model. Specifically, there was no significant interaction between FVC loss and native lung disease, even among single lung recipients. Survival curves stratified by native lung disease among single lung recipients are provided in the online supplement (Figure E3).
Exploratory Incorporation of Radiographic Findings to Refine CLAD Phenotypes
A subset of the multicenter combined CLAD cohort, including 170 (58%) bilateral lung recipients and 55 (58%) single lung recipients, had an eligible chest CT near the time of CLAD onset for review. Characteristics of subsets of subjects with and without an eligible CT are described in Table E2. Table 3 and Table E3 describe the radiographic findings as stratified by CLAD phenotype. Air trapping was more common with stable FVC than with FVC loss but was relatively common in both groups. Pleural abnormalities, ground-glass opacities, consolidative opacities, and interstitial changes were all significantly more common with FVC loss than with stable FVC (Table 3). Examples of CT images with these characteristic findings are provided in the online supplement (Figure E4).
Table 3.
Radiologic features on computed tomography concurrent with chronic lung allograft dysfunction onset in subjects with an eligible computed tomography scan in the combined multicenter chronic lung allograft dysfunction cohort
| FVC Loss (n = 109) | Stable FVC (n = 116) | P Value | |
|---|---|---|---|
| Air trapping* | 20 (49) | 37 (71) | 0.03 |
| Bronchiectasis/thickening | 30 (28) | 43 (37) | 0.13 |
| Nodular or tree-in-bud infiltrate | 25 (23) | 33 (28) | 0.34 |
| Pleural abnormality | 64 (59) | 32 (28) | <0.0001 |
| Consolidative opacities | 38 (35) | 21 (18) | 0.004 |
| Ground-glass opacities | 55 (50) | 37 (32) | 0.005 |
| Reticulation or septal thickening | 41 (38) | 16 (14) | <0.0001 |
Data presented as n (%).
Denominator is based on number of computed tomography scans assessed for air trapping: FVC loss, n = 41; stable FVC, n = 52.
On the basis of the previously described radiologic (9, 10) and histopathologic features of the restrictive phenotype of CLAD or RAS (4, 9), we further explored whether consideration of CT features that may suggest parenchymal or pleural fibrosis, specifically septal thickening/reticulation or the combination of either ground-glass or consolidative opacities with pleural abnormality (thickening or small effusion), would improve on the prognostic information conferred by physiological CLAD phenotype. Among the subset of patients with FVC loss and an eligible CLAD CT, these CT features were observed in 50 (60%) bilateral and 16 (62%) single lung transplant recipients. In contrast, only 18 (21%) bilateral and 9 (31%) single lung transplant recipients with stable FVC had CT features suggestive of underlying pleural or parenchymal fibrosis.
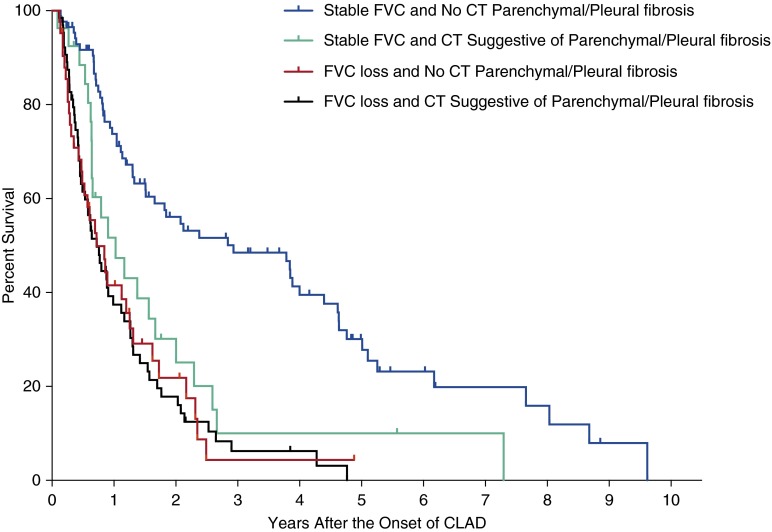
Among patients with FVC loss, consideration of these specific CT features did not add prognostic information (P = 0.93; unadjusted HR, 1.02; 95% CI, 0.67–1.58) (Figure 3 and Figure E5). However, among patients with stable FVC, the presence of CT features that may suggest underlying pleural or parenchymal fibrosis was associated with significantly worse post-CLAD survival (P = 0.003; unadjusted HR, 2.23; 95% CI, 1.32–3.63), similar to the prognosis observed with FVC loss (Figure 3 and Figure E5).
Figure 3.
Exploration of computed tomography (CT) findings for the refinement of chronic lung allograft dysfunction (CLAD) phenotypes. In patients with a stable FVC at CLAD onset, findings suggestive of parenchymal or pleural fibrosis on CT scan were associated with worse post-CLAD survival, similar to patients with FVC loss.
Among subjects with stable FVC at CLAD diagnosis who had available post-CLAD spirometry (N = 109), CT features of pleural-parenchymal fibrosis were significantly more likely to precede the subsequent development of FVC loss within 6 months of CLAD onset. The 6-month incidences of FVC loss were 75% (n = 18 of 24) and 39% (n = 33 of 85) for subjects with and without imaging features of pleural-parenchymal fibrosis, respectively (P = 0.002).
Discussion
In an external cohort of bilateral lung transplant recipients, we have validated the clinical significance of physiologic CLAD phenotype classification using routinely available spirometric data. Specifically, we have validated independently that among bilateral lung recipients, patients with FVC loss at CLAD onset have significantly worse survival after CLAD than those with stable FVC. Importantly, we have demonstrated for the first time that FVC loss carries clinical and prognostic importance in single lung allograft recipients as well, although the association may not be as strong as that observed in bilateral recipients. Finally, using all patients with CLAD across two centers inclusive of single and bilateral recipients, we demonstrate that FVC loss is the most important determinant of survival after CLAD onset, independent of other factors in a multivariable analysis.
We observed radiographic differences between patients with CLAD with and without FVC loss, similar to those previously described (10). Patients with stable FVC were more likely to have radiographic air trapping, but air trapping was frequently noted with FVC loss as well. Patients with CLAD with FVC loss were more likely to have radiographic findings of septal thickening/reticulation and ground-glass or consolidative opacities. In addition, we show that pleural abnormalities (thickening or small effusions) were noted more frequently on chest CT scan in patients with FVC loss than in those with stable FVC. Pleural involvement, specifically visceral pleural fibrosis, was recognized in the first histopathologic description of RAS (9). Recently, the same group confirmed that varying degrees of pleural fibrosis are present in all cases of RAS (4).
A novelty of our study explored the application of chest CT scan to add prognostic value to physiologic classification of CLAD. We demonstrated radiographic features that may suggest pleural or parenchymal fibrosis were associated with worse post-CLAD mortality in patients with stable FVC. Furthermore, among patients with stable FVC at CLAD onset, those with pleural-parenchymal fibrotic findings on CT scan were more likely to develop FVC loss over the following 6 months than those without such findings. These results suggest that among subjects who develop CLAD with stable FVC, chest CT scan findings may help identify a subset of patients who progress to a more deleterious phenotype, imparting increased risk of mortality. In light of these findings, additional studies involving the application of prospective chest CT scan at CLAD onset, particularly in the subset without FVC loss, could confer useful prognostic information.
In this study, we observed that recipient female sex is associated with FVC loss and with a significantly worse prognosis after CLAD onset, even after multivariable adjustment. We speculate that a greater incidence of human leukocyte antigen (HLA) sensitization in women could explain this relationship (16). One limitation of the present study was the absence of uniform standardized HLA sensitization data among recipients that would allow for exploration of this hypothesis. Alternatively, female recipient–male donor sex mismatch has been described as a risk factor for mortality in both hematologic and solid organ transplantation (17, 18). Cytotoxic T lymphocytes directed against male-specific minor histocompatibility antigens have been described with acute graft-versus-host disease and graft rejection (19). If confirmed, future studies could investigate the mechanisms responsible for the worse prognosis after CLAD onset in women.
This study also confirms that the timing of CLAD onset is an independent risk factor for post-CLAD mortality. In the previous study within the Duke bilateral recipient cohort, there was an interaction between EO-CLAD and spirometric phenotype, with the significance of EO-CLAD limited to only the bronchiolitis obliterans syndrome phenotype (10). However, in this larger study, EO-CLAD was associated with a worse prognosis for both stable FVC and FVC loss phenotypes. We also confirm that the severity of FEV1 decline at CLAD onset was not significantly related to post-CLAD survival in the multivariable analysis.
Limitations
Although this is the first multicenter study validating a method for CLAD classification and demonstrates consistent findings across two large transplant cohorts of bilateral and single lung allograft recipients with CLAD, inherent limitations exist. We cannot exclude residual confounding in our multivariable analyses due to potentially relevant covariables that were unavailable in our records, including HLA sensitization data as previously noted. Prior studies suggest that in addition to the presence of lymphocytic bronchiolitis, its severity may have additional negative prognostic value (20). We adjusted for the presence of absence of lymphocytic bronchiolitis, but uniform data to account for lymphocytic bronchiolitis severity were unavailable over the study period.
Antibody-mediated rejection has also been implicated as a potentially important risk factor for CLAD (21). Over the study period, antibody-mediated rejection was poorly characterized, and we were unable to evaluate for this relevant confounder. Although the PFT measures used to determine CLAD were collected prospectively, the retrospective nature of our study limits the ability to exclude alternative causes of PFT decline with absolute confidence. Other spirometric findings (such as declining FEV1/FVC ratio) may have some diagnostic or prognostic utility in the phenotypic characterization of CLAD, although in analyses of this cohort and our prior publication, the FEV1/FVC ratio at the time of CLAD onset did not add any prognostic information over and above FVC loss (10).
Native lung hyperinflation in single lung recipients could cause spirometric changes mimicking CLAD. Complex lung function testing or CT may help assess for native lung changes, but these studies were not routinely available in all subjects. Thus, it is possible that native lung hyperinflation or progressive native lung disease could have been misclassified as CLAD in this study and may represent a potential explanation for the weaker association of FVC loss with poor outcomes in single lung recipients.
We acknowledge the limitations of using FVC loss alone in assessing restrictive physiology. Lung volume measurements were not routinely performed during the time frame of this study, and no direct comparison to RAS can be made. Regardless, it is clear that FVC loss at CLAD onset predicts a worse prognosis, analogous to outcomes reported for RAS. Radiologic features associated with FVC loss were also similar to those previously described for RAS. However, FVC loss at CLAD onset is associated with poor survival even in the absence of fibrotic changes on chest CT.
Of note, the subset of patients with CLAD with an eligible CT scan had worse survival than patients with CLAD who did not have a chest CT scan, suggesting that clinical circumstances leading to the CT scan portend a worse outcome. Our study did not include central radiology review of included CT scans. This lack of a standardized radiology review likely explains some of the differences in the frequency of radiographic findings between centers. However, our methods correspond to clinical practice where the treating physician must rely on clinical interpretations to guide prognosis and decision making.
Conclusions
In conclusion, we have validated the prognostic implications of distinct physiologic phenotypes determined at CLAD onset in bilateral lung allograft recipients using commonly obtained spirometric indices. Furthermore, we have extended the application of the FVC loss criteria to single lung allograft recipients and demonstrated a similar clinical significance.
Our approach incorporating FVC loss at CLAD onset can be immediately and widely adopted across transplant centers to aid in prognosis discussions with patients. However, because there is currently no effective treatment for CLAD, future studies investigating mechanisms that contribute to CLAD should incorporate CLAD phenotypes.
Although studies suggest that diffuse alveolar damage precedes a restrictive CLAD phenotype and lymphocytic bronchiolitis is probably a precursor to bronchiolitis obliterans syndrome (22, 23), additional work is required to better elucidate phenotype-specific risk factors. Further refinement of phenotypic homogeneity, possibly incorporating chest CT as suggested by this study, is also critical to improving our understanding of CLAD pathogenesis and for discovering novel therapeutic strategies.
Acknowledgments
Acknowledgment
The authors thank the study participants and the research staff at the University of California at Los Angeles and Duke University Medical Center.
Footnotes
Supported by National Institutes of Health grants R01HL112990 (J.B.) and U01AI113315 (S.M.P.).
Author Contributions: All authors were involved in the conception of design of the present study, interpretation of results, revision and drafting of the manuscript, and final approval of the version presented for publication. All authors agree to be accountable for all aspects of the present work. A.D., J.L.T., S.M.P., and S.S.W. were responsible for the initial drafting of the submitted manuscript, acquisition and analysis of the relevant data, and approval of the research at their respective institutional review boards.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, et al. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, Mauer J, Paradis I, Patterson GA, Smith C, et al. International Society for Heart and Lung Transplantation. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 4.Ofek E, Sato M, Saito T, Wagnetz U, Roberts HC, Chaparro C, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26:350–356. doi: 10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 5.Pakhale SS, Hadjiliadis D, Howell DN, Palmer SM, Gutierrez C, Waddell TK, Chaparro C, Davis RD, Keshavjee S, Hutcheon MA, et al. Upper lobe fibrosis: a novel manifestation of chronic allograft dysfunction in lung transplantation. J Heart Lung Transplant. 2005;24:1260–1268. doi: 10.1016/j.healun.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Paraskeva M, McLean C, Ellis S, Bailey M, Williams T, Levvey B, Snell GI, Westall GP. Acute fibrinoid organizing pneumonia after lung transplantation. Am J Respir Crit Care Med. 2013;187:1360–1368. doi: 10.1164/rccm.201210-1831OC. [DOI] [PubMed] [Google Scholar]
- 7.Woodrow JP, Shlobin OA, Barnett SD, Burton N, Nathan SD. Comparison of bronchiolitis obliterans syndrome to other forms of chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1159–1164. doi: 10.1016/j.healun.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, Haroon A, Wagnetz D, Chaparro C, Singer LG, Hutcheon MA, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 10.Todd JL, Jain R, Pavlisko EN, Finlen Copeland CA, Reynolds JM, Snyder LD, Palmer SM. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189:159–166. doi: 10.1164/rccm.201306-1155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DerHovanessian A, Li N, Shino M, Sayah DM, Gregson AL, Lynch Iii JP, Saggar R, Ardehali A, Ross DJ, Elashoff RM, et al. Defining clinically relevant CLAD phenotypes in bilateral and single lung transplant recipients [abstract] J Heart Lung Transplant. 2014;33:S42. [Google Scholar]
- 12.Weigt SS, Copeland CA, Derhovanessian A, Shino MY, Davis WA, Snyder LD, Gregson AL, Saggar R, Lynch JP, III, Ross DJ, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant. 2013;13:919–927. doi: 10.1111/ajt.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 14.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010;182:784–789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder LD, Wang Z, Chen DF, Reinsmoen NL, Finlen-Copeland CA, Davis WA, Zaas DW, Palmer SM. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest. 2013;144:226–233. doi: 10.1378/chest.12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willner DL, Hugenholtz P, Yerkovich ST, Tan ME, Daly JN, Lachner N, Hopkins PM, Chambers DC. Reestablishment of recipient-associated microbiota in the lung allograft is linked to reduced risk of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2013;187:640–647. doi: 10.1164/rccm.201209-1680OC. [DOI] [PubMed] [Google Scholar]
- 18.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, Urbano-Ispizua A, Pavletic SZ, Haagenson MD, Zhang MJ, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popli R, Sahaf B, Nakasone H, Lee JY, Miklos DB. Clinical impact of H-Y alloimmunity. Immunol Res. 2014;58:249–258. doi: 10.1007/s12026-014-8514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033–1040. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 21.Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, Bennett Bain K, Alexander Patterson G, Mohanakumar T, Trulock EP, Hachem RR. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. 2013;32:1034–1040. doi: 10.1016/j.healun.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M, Hwang DM, Ohmori-Matsuda K, Chaparro C, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S. Revisiting the pathologic finding of diffuse alveolar damage after lung transplantation. J Heart Lung Transplant. 2012;31:354–363. doi: 10.1016/j.healun.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Shino MY, Weigt SS, Li N, Palchevskiy V, Derhovanessian A, Saggar R, Sayah DM, Gregson AL, Fishbein MC, Ardehali A, et al. CXCR3 ligands are associated with the continuum of diffuse alveolar damage to chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2013;188:1117–1125. doi: 10.1164/rccm.201305-0861OC. [DOI] [PMC free article] [PubMed] [Google Scholar]