Abstract
Purpose
Nocturnal cardiac conduction abnormalities are commonly observed in patients with sleep-disordered breathing (SDB). However, few population-based studies have examined the association between SDB and daytime cardiac conduction abnormalities.
Methods
We examined a random sample of 471 community-dwelling men, aged ≥67 years, enrolled in the multi-center Outcomes of Sleep Disorders in Older Men (MrOS Sleep) study. SDB severity was categorized using percent of total sleep time with oxygen saturation <90 % (%TST < 90) and apnea hypopnea index (AHI). Cardiac conduction parameters were assessed by resting 12-lead electrocardiography (ECG). All analyses were adjusted for age, site, β-blocker use, coronary heart disease, calcium channel blocker use, and use of antiarrhythmic medications.
Results
Mean age was 77 ± 6 years, median %TST < 90 was 0.7 (IQR 0.00–3.40), and median AHI was 7.06 (IQR 2.55–15.32). Men with greater nocturnal hypoxemia (%TST < 90 ≥ 3.5 %) compared with those without hypoxemia (%TST < 90 < 1.0 %) had a lower odds of bradycardia (OR 0.55 [0.32–0.94]) and right bundle branch block (RBBB) (OR 0.24 [0.08–0.75]) but a higher odds of ventricular paced rhythm (OR 4.42 [1.29–15.19]). Heart rate (HR) increased in a graded manner with increasing %TST < 90 (p-trend 0.01) and increasing AHI (p-trend 0.006), but these gradients were small in absolute magnitude. There were no associations of SDB measures with other ECG conduction parameters.
Conclusions
Greater nocturnal hypoxemia in older men was associated with a lower prevalence of daytime sinus bradycardia and RBBB, a higher prevalence of ventricular paced rhythm, and higher resting HR.
Keywords: Electrocardiography, Pacemaker, Sleep-disordered breathing
Introduction
Sleep-disordered breathing (SDB) is a highly prevalent condition affecting 10–20 % of adults in the USA [1]. SDB is associated with cardiovascular disease (CVD) risk factors including hypertension [2] and metabolic syndrome [3]. Some, but not all, prospective studies conducted in primarily middle-aged adults have reported associations of SDB with adverse CVD outcomes including coronary heart disease (CHD), heart failure, stroke, and cardiovascular mortality [4–6]. In addition, a number of studies in selected populations have reported an association between SDB and nocturnal cardiac conduction abnormalities [7, 8]. However, few studies have examined the relationship of SDB with daytime conduction abnormalities. The resting 12-lead electrocardiography (ECG) is a widely available, minimally burdensome, and noninvasive method to evaluate sinoatrial, atrioventricular, or intraventricular conduction in the myocardium. Furthermore, the 12-lead ECG allows better differentiation of the conduction abnormalities and more precise interval measurement compared to ambulatory ECG monitoring. While conduction abnormalities identified on 12-lead ECG are in large part subclinical in nature, prospective studies have reported associations of selected subclinical ECG abnormalities with a higher risk of clinical cardiac arrhythmias, sudden cardiac death, and all-cause mortality [9–13].
We used data from the baseline examination of the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) study to test the hypothesis that increasing severity of SDB is associated with higher prevalence of daytime ECG conduction abnormalities in community-dwelling older men, not selected on the basis of SDB or cardiac conduction disease.
Methods
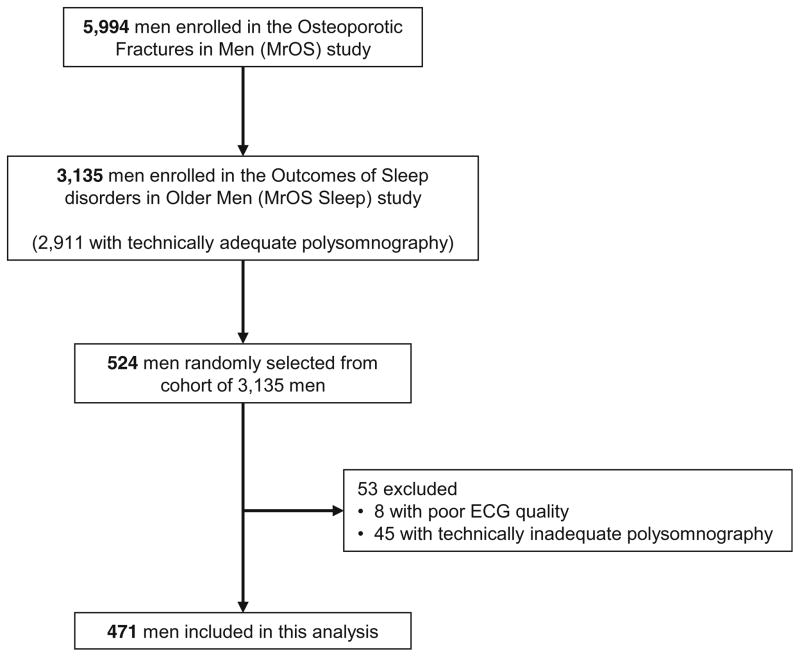
The MrOS sleep study (n = 3135) is an ancillary study of the parent cohort Osteoporotic Fractures in Men (MrOS, n = 5994) study that enrolled community-dwelling older men from six geographic regions in the USA: Birmingham, AL; Minneapolis, MN; Palo Alto, CA; the Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA [14, 15]. Enrollment in the MrOS Sleep ancillary study exceeded the study recruitment goal of 3000 men. For this study, we randomly selected 524 men enrolled in the baseline exam of the MrOS Sleep Study that included an in-home overnight polysomnography study and resting 12-lead ECG. After excluding men with poor ECG quality (n = 8) and those without technically adequate polysomnography (n = 45), the final cohort for this analysis was comprised of 471 men (Fig. 1). The research protocols were approved by the institutional review boards at each participating institution and all participants gave written informed consent.
Fig. 1.
Participant flow
An overnight unattended in-home polysomnography (PSG; Safiro, Compumedics, Inc., Melbourne, Australia) was performed. A standard recording montage included electroencephalograms (C3/A2 and C4/A1), bilateral electrooculograms, and a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (by nasal-oral thermocouple and nasal pressure cannula), finger pulse oximetry, lead I ECG, body position (mercury switch sensor), and bilateral tibialis leg movements (piezoelectric sensors). Data were scored by certified research sleep technologists at the central Sleep Reading Center using standardized criteria [16–18].
Severity of nocturnal hypoxemia was assessed by the percent of total sleep time with pulse oximetry arterial oxygen saturation below 90 % (%TST < 90) and considered normal if %TST < 90 was less than 1 % (first and second quartiles), mild if %TST < 90 was 1.0 % to less than 3.5 % (third quartile), and at least moderate if %TST < 90 was 3.5 % or greater (fourth quartile). Apneas were defined as absence or near absence of airflow for ≥ 10 s. Hypopneas were determined when there was ≥30 % reduction of breathing amplitude lasting ≥10 s linked to ≥4 % oxygen desaturation. Apnea hypopnea index (AHI) was defined by total number of apneas and hypopneas per hour of sleep. Severity of sleep apnea was defined by clinical criteria, normal if AHI was <5, mild if AHI was 5 to <15, and moderate to severe if AHI was ≥15 [19]. Central sleep apnea was defined by central apnea index (CAI) ≥5.
A resting 12-lead ECG was obtained during the MrOS clinic visit (median time interval to overnight sleep study 1 day). ECGs were recorded for 10 s with the participant lying down in a comfortable position. Printed ECGs were read by two physicians (YK and KP) blinded to SDB status and confirmed by a cardiac electrophysiologist (SA), also blinded to SDB status. Heart rate (HR), PR interval, QRS duration, and QT interval were automatically derived from the ECG, the latter three measures representing average values from 12 leads. Corrected QT (QTc) was calculated using Bazett’s Formula (QT Interval/√ [RR interval]) [20]. The Minnesota Code measurement rules and classification system was used in identifying the following measures [21]: atrial rhythm, sinoatrial block, atrioventricular (AV) block, right bundle branch block (RBBB), left bundle branch block (LBBB), left anterior fascicular block (LAFB), left posterior fascicular block (LPFB), intraventricular conduction delay, and ventricular paced rhythm. The following definitions were used: “sinus bradycardia” for HR <60 beats per minute, “first-degree AV block” for PR interval >200 msec, “QRS prolongation” for QRS interval >120 msec, “QT and prolongation” for QTc >440 msec.
Demographic data and medical history were assessed by administration of a questionnaire. CHD was defined by a self-reported history of a physician diagnosis of angina, myocardial infarction, angioplasty, or coronary artery bypass. Participants attended an in-clinic visit for measurements including blood pressure, body weight, and height. Participants were asked to bring all medication containers used within the preceding 30 days to the clinic visit. Medications were identified, recorded by the clinic staff, and stored in an electronic medication inventory database [22].
Participant characteristics were compared across the three categories of %TST < 90 using ANOVA (or non-parametric equivalent, i.e., Kruskal-Wallis test) for continuous variables, chi-square, or Fisher’s exact test for categorical variables. Logistic regression models were used to examine the associations of SDB measures with dichotomous measures of sinus bradycardia, AV conduction abnormalities, QRS prolongation (QRS duration >120 msec), and QT prolongation (QTc >440 msec). Analyses examining the association of SDB measures with dichotomous ECG outcomes were limited to those ECG outcomes that were identified in at least 20 men. In addition, we determined the association of SDB measures with continuous ECG parameters including HR, PR interval, QRS duration, and QTc using multiple linear regression. Twenty-two men with a 12-lead ECG showing ventricular paced rhythm were excluded from all analyses except for the specific analysis examining the association of SDB exposures with ventricular paced rhythm. In addition, 23 men with atrial fibrillation or flutter were excluded from analyses examining PR interval as an outcome. All analyses were adjusted for age and site and participant characteristics (i.e., β-blocker use, prevalent CHD, calcium channel blocker use, and use of antiarrhythmic medications) that both varied across category of %TST < 90 in the cohort and for which evidence from previous studies suggested associations with cardiac conduction abnormalities. To ensure that results in the overall cohort were not driven by findings among men with central sleep apnea, we performed sensitivity analyses excluding participants with central apnea index ≥5 (n = 41).
Given the sample size of 471 men, the minimal detectable regression coefficient per 1 standard deviation (SD) change in the SDB predictor variables was 0.15 SD (in ECG parameter) at 90 % power and 0.13 SD (in ECG parameter) at 80 % power. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA.).
Results
Mean age of the cohort was 76.9 ± 5.8 years, 92.6 % were Caucasian, and 32.5 % reported a history of CHD. A total of 238 men (50.5 %) had no evidence of nocturnal hypoxemia (%TST < 90 < 1.0 %), 116 (24.6 %) had mild hypoxemia (%TST < 90 1.0 to <3.5 %), and 117 (24.8 %) had at least moderate hypoxemia (%TST < 90 ≥ 3.5 %). SDB as manifested by higher AHI was prevalent in this cohort of older men with 34 % of the participants having an AHI 5.0 to <15.0 and 26 % having an AHI ≥15.0. Baseline characteristics of the study participants overall and by category of nocturnal hypoxemia are shown in Table 1. Participants with greater nocturnal hypoxemia tended to be more obese; had a higher prevalence of hypertension, chronic obstructive pulmonary disease, and CHD; and were more likely to be taking β-blockers, calcium channel blockers, and antiarrhythmic medications.
Table 1.
Baseline characteristics of 471 participants
| Characteristic | Entire cohort (n = 471) | Percent of total sleep time with oxygen saturation <90 %
|
P value | ||
|---|---|---|---|---|---|
| Normal (<1.0) (n = 238) | Mild (1.0 to <3.5) (n = 116) | At least moderate (≥3.5) (n = 117) | |||
| Age, years, mean (SD) | 76.9 (5.8) | 76.5 (6.0) | 76.4 (5.7) | 78.0 (5.4) | 0.04 |
| Caucasian, n (%) | 436 (92.6) | 216 (90.8) | 107 (92.2) | 113 (96.6) | 0.13 |
| Diabetes, n (%) | 58 (12.3) | 33 (13.9) | 13 (11.2) | 12 (10.3) | 0.57 |
| Hypertension, n (%) | 338 (71.8) | 163 (68.5) | 79 (68.1) | 96 (82.1) | 0.02 |
| Body mass index, kg/m2, mean (SD) | 27.0 (3.6) | 26.0 (3.2) | 27.3 (3.6) | 28.7 (3.7) | <0.001 |
| Coronary heart disease, n (%) | 153 (32.5) | 72 (30.3) | 29 (25.0) | 52 (44.4) | 0.004 |
| Stroke, n (%) | 19 (4.0) | 9 (3.8) | 5 (4.3) | 5 (4.3) | 0.91 |
| Congestive heart failure, n (%) | 24 (5.1) | 11 (4.6) | 7 (6.0) | 6 (5.1) | 0.79 |
| COPD or emphysema, n (%) | 23 (4.9) | 7 (2.9) | 4 (3.4) | 12 (10.3) | 0.01 |
| Smoking, n (%) | 0.36 | ||||
| Never | 193 (41.0) | 102 (42.9) | 48 (41.4) | 43 (36.8) | |
| Former | 267 (56.7) | 131 (55.0) | 63 (54.3) | 73 (62.4) | |
| Current | 11 (2.3) | 5 (2.1) | 5 (4.3) | 1 (0.9) | |
| Use of antidepressants, n (%) | 36 (7.6) | 19 (8.0) | 9 (7.8) | 8 (6.8) | 0.95 |
| Use of β-blockers, n (%) | 140 (29.7) | 61 (25.6) | 35 (30.2) | 44 (37.6) | 0.07 |
| Use of calcium channel blockers, n (%) | 62 (13.2) | 23 (9.7) | 18 (15.5) | 21 (17.9) | 0.07 |
| Use of digoxin, n (%) | 26 (5.5) | 13 (5.5) | 5 (4.3) | 8 (6.8) | 0.70 |
| Use of antiarrhythmic medications, n (%) | 13 (2.8) | 4 (1.7) | 1 (0.9) | 8 (6.8) | 0.02 |
| AHI (median, IQR) | 7.1 (2.6–15.3) | 3.2 (1.0–6.6) | 10.7 (6.7–15.9) | 21.0 (10.4–31.8) | <0.001 |
| AHI categories, n (%) | <0.001 | ||||
| Normal (<5.0) | 189 (40.1) | 160 (67.2) | 14 (12.1) | 15 (12.8) | |
| Mild (5.0 to <15.0) | 162 (34.3) | 65 (27.3) | 68 (58.6) | 29 (24.8) | |
| At least moderate (≥15.0) | 120 (25.5) | 13 (5.5) | 34 (29.3) | 73 (62.4) | |
| Overall average O2 saturation, mean (SD) | 94.0 (1.6) | 95.0 (1.2) | 93.8 (1.2) | 92.3 (1.3) | <0.001 |
AHI apnea hypopnea index, COPD chronic obstructive pulmonary disease
Sinus bradycardia was the most common ECG abnormality (40 %) followed by first-degree AV block (25 %) (Table 2). RBBB and LAFB were found in 9.1 and 15.4 % of the cohort, respectively. LBBB was rare (2 %). None of the participants had ECG evidence of sinoatrial block, high-grade (second/third degree) AV block or LPFB.
Table 2.
Associations of measures of sleep-disordered breathing with daytime ECG conduction abnormalities
| Measure of SDBa | Odds ratio (95 % confidence interval)
|
||||||
|---|---|---|---|---|---|---|---|
| Sinus bradycardia (n = 197) | First-degree AV block (n = 110) | RBBB (n = 43) | LAFB (n = 73) | Ventricular paced rhythm (n = 22) | QRS prolongation (n = 61) | QTc prolongation (n = 90) | |
| %TST < 90 | |||||||
| <1.0 (n = 238) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 1.0 to <3.5 (n = 116) | 0.93 (0.58–1.51) | 1.12 (0.64–1.97) | 0.76 (0.36–1.62) | 0.99 (0.53–1.88) | 1.78 (0.40–8.03) | 0.84 (0.43–1.64) | 0.79 (0.42–1.48) |
| ≥3.5 (n = 117) | 0.55 (0.32–0.94) | 1.29 (0.72–2.32) | 0.24 (0.08–0.75) | 0.97 (0.49–1.90) | 4.42 (1.29–15.19) | 0.50 (0.23–1.08) | 1.70 (0.94–3.08) |
| P value for trend | 0.04 | 0.27 | 0.01 | 0.95 | 0.01 | 0.09 | 0.14 |
| AHI | |||||||
| <5.0 (n = 189) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 5.0 to <15.0 (n = 162) | 0.63 (0.39–0.99) | 0.88 (0.51–1.49) | 1.05 (0.51–2.12) | 1.07 (0.59–1.93) | 1.25 (0.32–4.94) | 1.03 (0.55–1.93) | 0.77 (0.44–1.36) |
| ≥15.0 (n = 120) | 0.62 (0.37–1.06) | 0.93 (0.51–1.69) | 0.60 (0.24–1.48) | 0.83 (0.41–1.68) | 2.31 (0.65–8.19) | 0.59 (0.27–1.27) | 0.89 (0.48–1.65) |
| P value for trend | 0.05 | 0.77 | 0.32 | 0.66 | 0.19 | 0.22 | 0.64 |
Twenty-two men with permanent pacemaker excluded from all analyses except those examining association between SDB and presence of permanent pacemaker; 23 men with atrial fibrillation were also excluded from analyses examining association between SDB and first-degree AV block. Sinus bradycardia—resting heart rate <60 bpm, first-degree AV block—PR interval >200 ms, QRS prolongation—QRS >120 ms, QTc prolongation—QTc >440 ms
%TST < 90 percent of total sleep time with oxygen saturation <90 %, AHI apnea hypopnea index, LAFB left anterior fascicular block, RBBB right bundle branch block
Models adjusted for age, site, β-blocker use, coronary heart disease, calcium channel blocker use, and use of antiarrhythmic medications
After adjustment for age, site, β-blocker use, CHD, calcium channel blocker use, and use of antiarrhythmic medications, there were no significant associations of either SDB measure and the ECG outcomes of first-degree AV block, LAFB, QRS prolongation, or QT prolongation (Table 2). Men with at least moderate nocturnal hypoxemia (%TST < 90 ≥ 3.5 %) compared to those without hypoxemia (%TST < 90 < 1.0 %) had a lower odds of having sinus bradycardia (OR 0.55 [0.32–0.94]) and RBBB (OR 0.24 [0.08–0.75]) but had a fourfold higher odds of having a ventricular paced rhythm (OR 4.42 [1.29–15.19]). Men with at least moderate SDB as manifested by an AHI ≥15 compared to those with an AHI <5 appeared to have a lower likelihood of sinus bradycardia (OR 0.62 [0.37–1.06]), but the association did not quite reach the level of statistical significance. No significant associations were observed between AHI category and either RBBB or ventricular paced rhythm.
In terms of associations of SDB measures with continuous ECG parameters, there was evidence of a trend of increasing resting HR with greater degree of nocturnal hypoxemia (p-trend 0.01) and higher AHI (p-trend 0.03), but gradients were small in absolute magnitude (Table 3). There was no evidence of associations of either measure of SDB with PR interval, QRS duration, or QTc interval.
Table 3.
Associations of measures of sleep-disordered breathing with continuous ECG parameters
| Measure of SDBa | N | Mean (95 % confidence interval)
|
|||
|---|---|---|---|---|---|
| Resting HR | PR interval | QRS duration | QTc interval | ||
| %TST < 90 | |||||
| <1.0 (referent) | 238 | 61.7 (60.3–63.0) | 186.6 (182.0–192.0) | 102.2 (99.7–104.8) | 422.3 (419.0–425.7) |
| 1.0 to <3.5 | 116 | 62.4 (60.5–64.3) | 184.9 (177.7–192.1) | 101.9 (98.3–105.5) | 419.0 (414.3–423.9) |
| ≥3.5 | 117 | 64.9 (62.9–67.0) | 187.7 (180.0–195.4) | 99.2 (95.4–103.1) | 427.6 (422.5–432.7) |
| P for linear trend | 0.01 | 0.99 | 0.24 | 0.25 | |
| AHI | |||||
| <5.0 (referent) | 189 | 61.5 (60.0–63.0) | 188.6 (183.0–194.3) | 102.2 (99.4–105.1) | 423.3 (419.6–427.1) |
| 5.0 to <15.0 | 162 | 62.7 (61.1–64.3) | 187.1 (181.1–193.1) | 101.6 (98.6–104.7) | 421.8 (417.7–425.8) |
| ≥15.0 | 120 | 64.4 (62.4–66.3) | 182.5 (175.0–190.0) | 100.0 (96.3–103.6) | 423.1 (418.2–428.0) |
| P for linear trend | 0.03 | 0.22 | 0.37 | 0.87 | |
Twenty-two men with permanent pacemaker excluded from all analyses except those examining association between SDB and presence of permanent pacemaker; 23 men with atrial fibrillation were also excluded from analyses examining association between SDB and first-degree AV block
%TST < 90 percent of total sleep time with oxygen saturation <90 %, AHI apnea hypopnea index, HR heart rate, SDB sleep-disordered breathing
Models adjusted for age, site, β-blocker use, coronary heart disease, calcium channel blocker use, and use of antiarrhythmic medications
Findings were not altered in sensitivity analyses excluding 41 men with central apnea index ≥5.
Discussion
In this large cohort of community-dwelling older men, SDB as manifested by greater nocturnal hypoxemia was associated with a lower likelihood of sinus bradycardia and RBBB, but a higher likelihood of ventricular paced rhythm identified on daytime resting 12-lead ECG. When severity of SDB was defined by AHI, no significant associations of SDB with these outcomes were observed. HR increased with greater burden of hypoxemia and with higher AHI, but the magnitude of absolute changes in HR was small.
This investigation comprehensively evaluated the association between SDB and conduction abnormalities on daytime 12-lead ECG in older men not selected on the basis of SDB or cardiac conduction abnormalities. Associations of measures of SDB with daytime manifestation of cardiac conduction abnormalities (i.e., independent of sleep state or sleep-related respiratory events) have been understudied. One previous population-based study in middle-aged adults reported no association of SDB as defined by greater AHI and the composite outcome of conduction abnormalities identified on resting ECG [23]. However, this study did not specify the different types of conduction abnormalities and did not investigate nocturnal hypoxemia as a predictor. Transient bradyarrhythmia due to autonomic modulation in different sleep states [24, 25] and nocturnal bradyarrhythmia triggered by obstructive respiratory events have previously been described in younger, selected populations [8]. Our study of an unselected population of community-dwelling older men found that greater nocturnal hypoxemia was associated with a lower odds of daytime sinus bradycardia.
In this study, older men with a greater nocturnal hypoxemia burden had a fourfold increase in the odds of having a ventricular paced rhythm on ECG compared to those without nocturnal hypoxemia despite adjustment for several potential confounders. This finding is consistent with disproportionately high prevalence of SDB observed among patients with a permanent pacemaker [26]. However, the biological mechanism underlying the association between SDB and ventricular paced rhythm (a potential indication marker for symptomatic cardiac conduction abnormalities) is unclear. Given that symptomatic bradyarrhythmia is a major indication for pacemaker implantation, our finding of a lower odds of sinus bradycardia in older men with SDB is intriguing. It is possible that older men with greater nocturnal hypoxemia simply have higher burden of unmeasured CVD and are more subject to cardiac conduction disease leading to implantation of a permanent pacemaker. Additional studies with a prospective design are warranted to determine whether SDB is associated with a greater risk of future permanent pacemaker implantation.
Greater nocturnal hypoxemia burden and higher AHI were each associated with a higher resting HR in this study. Although the increase in HR was modest, this finding is consistent with the results from both clinical and animal studies in which SDB was associated with higher sympathetic [27] and diminished parasympathetic activity during the daytime [28]. This association of SDB with higher daytime resting HR, albeit of modest magnitude, may have public health implications given the well-known association of higher resting HR with cardiovascular mortality and sudden cardiac death [29]. It is possible that increased sympathetic activity, manifested by a higher HR, may be one of the mediating mechanisms between SDB and adverse cardiovascular outcomes. This hypothesis warrants testing in future prospective studies.
This study also observed a lower prevalence of RBBB in men with greater nocturnal hypoxemia. While RBBB can be seen in individuals without structural heart disease, recent studies have reported associations of RBBB with increased cardiovascular risk [30] and higher incidence of PPM placement [31]. Further, one might have postulated a positive association between the severity of SDB and RBBB given the SDB’s association with right ventricular hypertrophy [32]. In this regard, the association of nocturnal hypoxemia with lower likelihood of RBBB was unanticipated. Given the multiple statistical comparisons made in our study, this finding may be due to chance alone. Future prospective studies are needed to further clarify the association of SDB with RBBB in the aged population. In the Wisconsin Sleep Cohort study, the prevalence of major conduction abnormalities comprising composite endpoints of intraventricular conduction delay, and RBBB/LBBB did not differ by presence of SDB [23]. In addition, the prevalence of ventricular paced rhythm was also not higher among younger and middle-aged adults with SDB in that study.
In this cohort of older men, we found no significant association between SDB and AV conduction delay or QRS duration. The lack of association between SDB and QRS duration is in line with the finding of Gupta et al. in which severity of sleep apnea was associated with prolonged QRS in women, but not in men [33]. Since prolonged QTc is a well-known ECG marker for cardiovascular risk including sudden cardiac death [34, 35], an association of SDB with QTc prolongation might be a mechanism underlying the reported associations of SDB with adverse cardiovascular outcome and sudden cardiac death [36, 37]. However, we did not find any association of SDB with daytime QTc in this study. In contrast, a recent case-control study [38] in younger subjects (30–50 years old) reported longer daytime QTc in patients with SDB compared to those without. However, no associations were reported between OSA severity defined by AHI and daytime QTc.
Our study has several strengths including the community-based population of older men not selected on the basis of SDB or cardiac conduction abnormalities, objective measurements of SDB using PSG, and comprehensive examination of resting 12-lead daytime ECGs. However, because our design was cross-sectional, the temporal relationship between SDB and the ECG cardiac conduction abnormalities cannot be established. Associations of SDB measures with severe conduction abnormalities including sinus node dysfunction or high-degree AV block could not be examined because these disorders were uncommon in our cohort. Finally, our study had an observational design and the possibility of residual confounding cannot be eliminated.
In conclusion, in older men, SDB as manifested by the degree of nocturnal hypoxemia was associated with a lower prevalence of daytime sinus bradycardia and RBBB, but a higher prevalence of ventricular paced rhythm. SDB severity as expressed by greater nocturnal hypoxemia and higher AHI was also associated with higher daytime resting HR. No associations were found between these measures of SDB and commonly derived ECG intervals (or PR, QRS, and QTc interval). Future prospective studies are needed to further clarify the association of SDB with cardiac conduction abnormalities in the aged population.
Acknowledgments
Funding The Osteoporotic Fractures in Men (MrOS) Study is supported by the National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System.
The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs or the United States government.
Footnotes
Conflict of interest All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers’ bureaus, membership, employment, consultancies, stock ownership, or other equity interest, and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, Cruz Moron I, Duran-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186:909–916. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cintra FD, Leite RP, Storti LJ, Bittencourt LA, Poyares D, Castro LD, Tufik S, Paola AD. Sleep apnea and nocturnal cardiac arrhythmia: a populational study. Arq Bras Cardiol. 2014;103:368–374. doi: 10.5935/abc.20140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–494. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 9.Adabag AS, Grandits GA, Prineas RJ, Crow RS, Bloomfield HE, Neaton JD, Group MR. Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am J Cardiol. 2008;101:1437–1443. doi: 10.1016/j.amjcard.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, Benjamin EJ, Vasan RS, Wang TJ. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. Jama. 2009;301:2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coumbe AG, Naksuk N, Newell MC, Somasundaram PE, Benditt DG, Adabag S. Long-term follow-up of older patients with Mobitz type I second degree atrioventricular block. Heart. 2013;99:334–338. doi: 10.1136/heartjnl-2012-302770. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RJ, Bengtson J, Chen ZY, Anderson KM, Locati E, Levy D. Duration of the QT interval and total and cardiovascular mortality in healthy persons (the Framingham Heart Study experience) Am J Cardiol. 1991;67:55–58. doi: 10.1016/0002-9149(91)90099-7. [DOI] [PubMed] [Google Scholar]
- 13.Kurl S, Makikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation. 2012;125:2588–2594. doi: 10.1161/CIRCULATIONAHA.111.025577. [DOI] [PubMed] [Google Scholar]
- 14.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 16.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized techniques and scoring system for sleep stages of human subjects. National Institutes of Health; Washington, D.C: 1968. NIH Publication No. 204. [Google Scholar]
- 18.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1. American Academy of Sleep Medicine; Westchester: 2007. [Google Scholar]
- 20.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–e250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn H, Keys A, Simonson E, Rautaharju P, Punsar S. The electrocardiogram in population studies. A classification system. Circulation. 1960;21:1160–1175. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- 22.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL Osteoporotic Fractures in Men Study G. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59:2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hla KM, Young T, Finn LA, Peppard PE, Kinsey TJ, Ende D. Electrocardiographically indicated cardiovascular disease in sleep-disordered breathing. Sleep Breath. 2008;12:251–258. doi: 10.1007/s11325-007-0168-0. [DOI] [PubMed] [Google Scholar]
- 24.Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol. 1977;39:390–395. doi: 10.1016/s0002-9149(77)80094-5. [DOI] [PubMed] [Google Scholar]
- 25.Zwillich C, Devlin T, White D, Douglas N, Weil J, Martin R. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest. 1982;69:1286–1292. doi: 10.1172/JCI110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrigue S, Pepin JL, Defaye P, Murgatroyd F, Poezevara Y, Clementy J, Levy P. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European Multicenter Polysomnographic Study. Circulation. 2007;115:1703–1709. doi: 10.1161/CIRCULATIONAHA.106.659706. [DOI] [PubMed] [Google Scholar]
- 27.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyavanapalli J, Jameson H, Dergacheva O, Jain V, Alhusayyen M, Mendelowitz D. Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons. J Physiol. 2014;592:2799–2811. doi: 10.1113/jphysiol.2014.273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 30.Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J. 2013;34:138–146. doi: 10.1093/eurheartj/ehs291. [DOI] [PubMed] [Google Scholar]
- 31.Kusumoto S, Kawano H, Makita N, Ichimaru S, Kaku T, Haruta D, Hida A, Sera N, Imaizumi M, Nakashima E, Maemura K, Akahoshi M. Right bundle branch block without overt heart disease predicts higher risk of pacemaker implantation: the study of atomic-bomb survivors. Int J Cardiol. 2014;174:77–82. doi: 10.1016/j.ijcard.2014.03.152. [DOI] [PubMed] [Google Scholar]
- 32.Guidry UC, Mendes LA, Evans JC, Levy D, O’Connor GT, Larson MG, Gottlieb DJ, Benjamin EJ. Echocardiographic features of the right heart in sleep-disordered breathing: the Framingham Heart Study. Am J Respir Crit Care Med. 2001;164:933–938. doi: 10.1164/ajrccm.164.6.2001092. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Cepeda-Valery B, Romero-Corral A, Shamsuzzaman A, Somers VK, Pressman GS. Association between QRS duration and obstructive sleep apnea. J Clin Sleep Med. 2012;8:649–654. doi: 10.5664/jcsm.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 35.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 36.Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, Herges RM, Howard DE, Somers VK. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62:610–616. doi: 10.1016/j.jacc.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 38.Shamsuzzaman A, Amin RS, van der Walt C, Davison DE, Okcay A, Pressman GS, Somers VK. Daytime cardiac repolarization in patients with obstructive sleep apnea. Sleep Breath. 2015 doi: 10.1007/s11325-015-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]