Abstract
Objective
The performance of an ossicular replacement prosthesis (ORP) is influenced by its alignment and appropriate tension between the tympanic membrane and the stapes footplate. A novel ORP with a flexible element that potentially allows for length adjustment in situ is presented and tested for acoustic performance.
Study Design
Laser Doppler vibrometry in fresh human cadaveric temporal bones was used to test the acoustic performance of the adjustable ORP relative to standard prostheses used for ossiculoplasty.
Methods
The 3D velocity of the stapes posterior crus was measured in the 0.2–20kHz range using a Polytec CLV-3D laser Doppler vibrometer. The middle ear cavity was accessed through a facial recess approach. After measuring the normal response, the incus was removed and stapes velocity was measured in the disarticulated case, then after insertion of the new prosthesis, a conventional prosthesis (Kurz BELL Dusseldorf type), and a sculpted autologous incus prosthesis in each temporal bone. The 3D stapes velocity transfer function (SVTF) was calculated for each case and compared.
Results
The novel ORP design restored stapes velocity to within 6 dB (on average) of the intact response. No significant differences in 3D-SVTF were found between the new, conventional, or autologous ORPs.
Conclusion
The inclusion of an in situ adjustable element into the ORP design did not adversely affect its acoustic performance. The adjustable element may increase the ease of achieving optimal ORP placement, especially through a facial recess approach.
Keywords: Ossicular Reconstruction, Adjustable Prosthesis, Temporal Bones, 3D Vibrometry
Introduction
Ossicular reconstruction surgery is a commonly performed procedure in the case of a discontinuous or fixed ossicular chain, and there exist a large variety of ossicular replacement prostheses (ORPs) that have been designed for this purpose. Previous studies have examined the relative efficacy of different prosthesis designs, but the results of these studies vary widely.1, 2 A number of studies have shown that the performance of an implanted ORP depends highly on its position, alignment, and tension between the tympanic membrane and the stapes head or footplate.3–7 Because of this, there has been interest in the design of adjustable-length prostheses that can be fit to the exact anatomy of an individual ear in order to optimize placement.8–11
The traditional method to access the middle ear for ossicular reconstruction is through a transcanal approach. In this, the surgeon elevates the skin of the medial external auditory canal in continuity with the posterior tympanic membrane to gain a lateral view of the ossicles. The ossicular chain can be assessed, and the appropriate prosthesis style and size selected and placed. The tympanic membrane can then be reconstructed as needed and repositioned over the prosthesis. In these cases, there can be uncertainty of where the tympanic membrane will ultimately rest when fully healed. The variability associated with this can adversely affect both the alignment and tension of the prosthesis, leading to suboptimal hearing results.
In some cases, access for ossicular reconstruction can instead be gained through a facial recess approach.12 The facial recess is a space between the posterior tympanic membrane laterally and facial nerve medially. When opened through a mastoidectomy, this affords a posterior view of the middle ear and ossicular chain without the need to detach the tympanic membrane from its bony attachments. If the surgeon is able to place an optimally sized prosthesis through this constrained exposure, less variability is expected with healing. The majority of cases in which this is an option include second look procedures for cholesteatoma. This has motivated the development of a prosthesis designed specifically to be placed through the facial recess, which also allows the surgeon the possibility of adjusting its length in situ to optimally span the distance from the TM to the stapes.
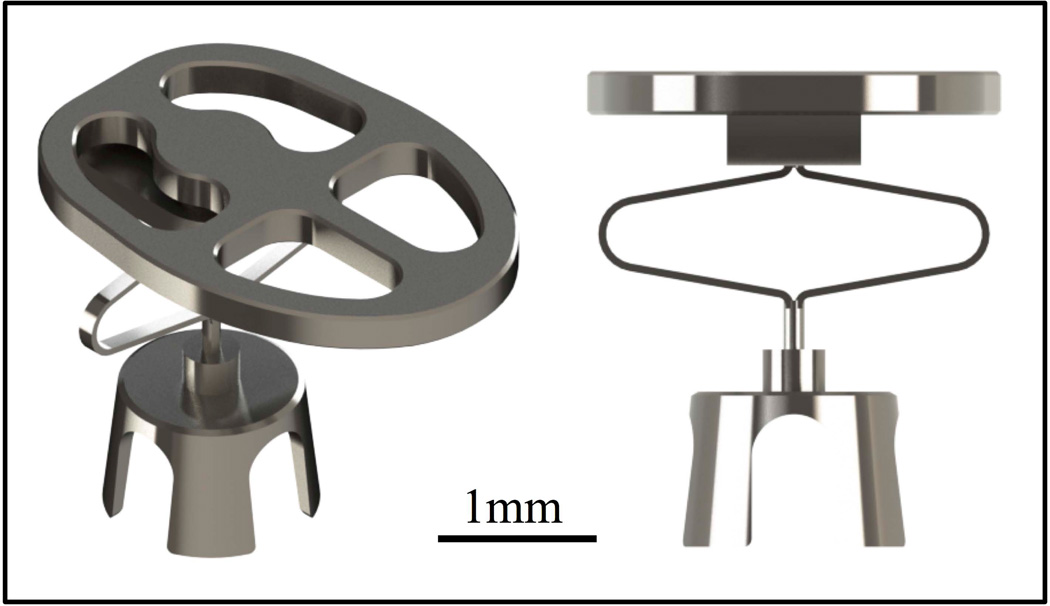
In this study, a new adjustable ORP (A-ORP) with a flexible element that potentially allows for in situ length adjustment is evaluated. It has an initial compact length that makes it easy to insert and align through the facial recess before the flexible element is expanded to achieve optimal tension (figure 1). The design is proposed in both partial (A-PORP) and total (A-TORP) models.
Figure 1.
Rendering of A-PORP (mass 7.9 mg) showing adjustable element in its compressed state. The design includes a ball joint between the adjustable shaft and the prosthesis head to maximize surface contact between the ORP and the TM.
The goal of this work is to establish a basis for future work on the A-ORP design by testing if the addition of this flexible element does not degrade the acoustic performance of the prosthesis relative to standard prostheses commonly used for ossiculoplasty, such as a non-adjustable titanium ORP or a sculpted autologous incus prosthesis (I-PORP).
Due to variations in anatomy and pathology between patients, it is difficult to compare the efficacy of different prosthesis designs clinically, especially with a limited sample size.13 Cadaveric temporal bone models have been used to characterize ORP efficacy by measuring stapes velocity or cochlear pressure in response to acoustic pressure in the ear canal.3, 14 This allows multiple designs to be tested in a single specimen and directly compared, which helps mitigate potentially confounding factors such as anatomical variations.6, 7 This study specifically does not evaluate the ease of use of this prosthesis of its ability to be adjusted in situ. These factors will be addressed in the future.
Laser Doppler vibrometry (LDV) measurements have traditionally been used to measure sound transmission through the middle ear.15, 16 Typically, a single-axis LDV system is used to measure the velocity of a single point on the stapes. This velocity is then used as a proxy for cochlear input. The stapes, however, is known to have complex, frequency-dependent vibration modes that drive the cochlea.17–19 Single-point, single-axis LDV measurements may not capture the full complex motions of the stapes because they are only able to record motions along the measurement axis of the laser. Therefore, these measurements may provide a false estimate of cochlear input and the acoustic performance of the reconstructed middle ear.
In this study, a 3D LDV system was used to simultaneously measure vibration velocity along three orthogonal axes and the resultant 3D motion of a single point on the stapes from 0.2 to 20 kHz is reported for the first time. These measurements were performed on both normal ossicular chains and those reconstructed using the new A-ORP, a standard ORP, and an autologous incus prosthesis (I-PORP) placed between the stapes head and either the tympanic membrane or the manubrium.
Materials and Methods
Materials
Twelve fresh adult temporal bones were obtained from Anatomy Gifts Registry (Hanover, MD). All were from donors with no history of otologic disease. Measurements were completed in six bones from two male and four female donors. The average age at death was 63.8 years (range 51–72). The temporal bones were kept refrigerated until the time of use.
Preparation
Following removal of soft tissues and the cartilaginous portion of the external ear canal, the facial recess was exposed through a cortical mastoidectomy. In order to provide a wide enough window to accommodate the three lasers of the 3D LDV system, the horizontal and vertical portions of the facial nerve were removed along with the stapedius tendon and a portion of the pyramidal process. Care was taken to ensure that the ossicular chain and supporting ligaments remained intact during the procedure. Retroreflective microspheres were placed on the posterior crus of the stapes and the cochlear promontory to act as targets for the LDV. The temporal bone was visualized through the facial recess to check for signs of drying and measurements were completed within five hours.
Measurement system
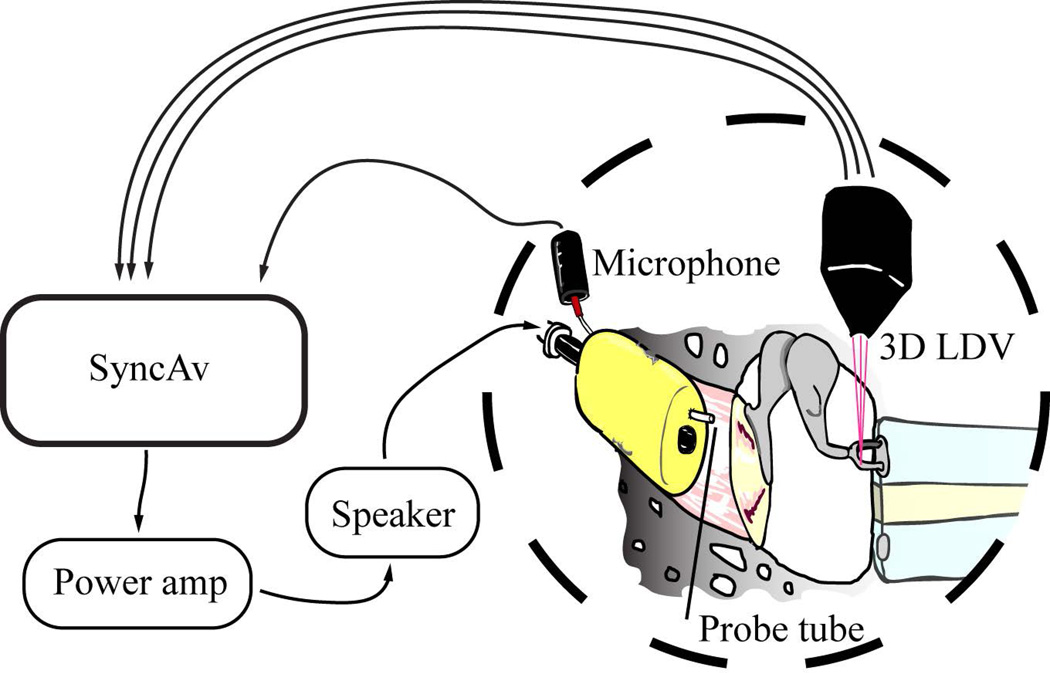
The measurement software, SyncAv (v0.26), is a custom Labview (National Instruments, Austin, TX)-based data acquisition program that performs synchronous averaging between up to two output and six input channels. For the present experiment, a National Instruments PXI data acquisition computer with a maximum sampling rate of 204.8kHz was used. The sampling rate, FFT length, and number of averages were 48kHz, 4096, and 10 respectively. Figure 2 shows the experimental setup.
Figure 2.
Experimental setup. SyncAv was used to generate an acoustic signal, which was amplified by a QSC power amplifier. Sound was produced by an ultrasonic tweeter (Tymphany, Sausalito, CA, USA). A foam earplug with a sound delivery tube adapter and microphone probe tube hole was inserted into the ear canal to create a closed acoustic space. The tweeter was coupled through a sound delivery tube to the foam earplug. Ear canal pressure was measured using an ER-7C probe tube microphone inserted through the foam earplug.
A CLV-3D LDV system (Polytec GmbH, Waldbronn, Germany) was mounted on three motorized linear translation stages and the LDV lasers were reflected into the specimen by a dichroic mirror so that the specimen could be imaged through a Zeiss OPMI-1 operating microscope during measurements. SyncAv is also used to operate the translation stages and focus the LDV system on the measurement targets.
Measurement protocol
Sound stimuli consisting of 80 pure tones logarithmically spaced from 200Hz to 20kHz and equalized to a constant pressure using the SyncAv StimEq function were presented. The ear canal pressure and the three LDV output channels were simultaneously recorded. Stapes velocity was compared to the ASTM standard F2504 for temporal bone models.20 Two temporal bones were discarded because their responses deviated significantly from this standard.
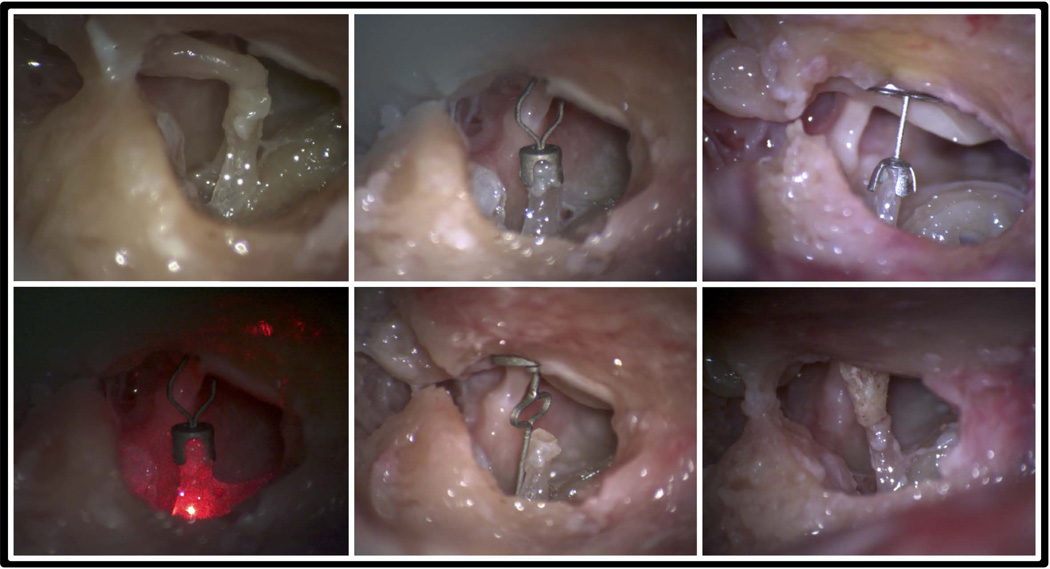
Following baseline measurements on the intact specimen, the incudo-stapedial and incudo-malleolar joints were disarticulated using a Rosen needle and the incus was removed. Measurements were repeated on the disarticulated specimen. The ossicular chain was then reconstructed in the following order and measured: (1) A-PORP, (2) conventional PORP (Kurz BELL Dusseldorf type titanium), (3) I-PORP placed between the stapes head and the tympanic membrane, (4) I-PORP placed between the stapes head and manubrium, and (5) A-TORP (figure 3). Additional cartilage was not used between the prostheses and the tympanic membrane. Coupling was evaluated qualitatively by gently touching the malleus head to observe stapes motion. Optimal length prostheses were selected or created by an experienced otologist (Dr. Li) as would be used clinically.
Figure 3.
Photos of specimens implanted with different prostheses. Clockwise from upper left: intact, A-PORP, PORP, I-PORP, A-TORP, A-PORP under LDV.
For the A-ORP designs, the length was adjusted before insertion by using forceps to squeeze the adjustable element. Coupling was evaluated by Dr. Li by gently palpating the malleus and observing stapes motion. If satisfactory coupling, according to his clinical experience, was not achieved, the A-ORP was removed and the length adjusted again. For the conventional PORP, a 2.5mm model was first inserted. If the coupling was not optimal, a 2.75mm or 2.25mm model was used instead. Care was taken not to damage the stapes superstructure or footplate or the tympanic membrane. If at any point during the experiment these structures were damaged, the experiment was halted (N=4). A specialized tool for expanding the prosthesis in-situ is under development.
Data analysis
Data analysis was performed in MATLAB (Mathworks, Natick, MA, USA). The SyncAv Toolbox, a custom set of scripts designed to interface with SyncAv output files, was used to organize, analyze, and plot the data.
The stapes velocity transfer function (SVTF), defined as the stapes velocity divided by ear canal pressure, was calculated for all cases. Since three orthogonal velocity components were recorded during each measurement, the SVTF was first calculated for each individual velocity component before calculating the resultant 3D-SVTF. The 3D resultant velocity is defined as the maximum magnitude of the 3D velocity vector over one time period. A sinusoidally varying 3D vector traces an ellipse in space, so the maximum magnitude of the 3D velocity vector is half the length of the major axis of that ellipse. From this point forward, SVTF refers to the 3D resultant velocity transfer function.
ORP performance was evaluated by dividing the A-PORP and disarticulated SVTF by the baseline (intact) SVTF. Ratios were then converted to dB and means and standard errors were calculated. A Savitsky-Golay smoothing filter was applied to the mean. Finally, in order to remove the possibility of variations between specimens confounding the analysis, transfer function ratios between ORP designs were calculated within each specimen before calculating the average ratio across the specimens. The ratios calculated were A-PORP to PORP, A-TORP, I-TORP (TM), and I-TORP (manubrium), respectively.
Results
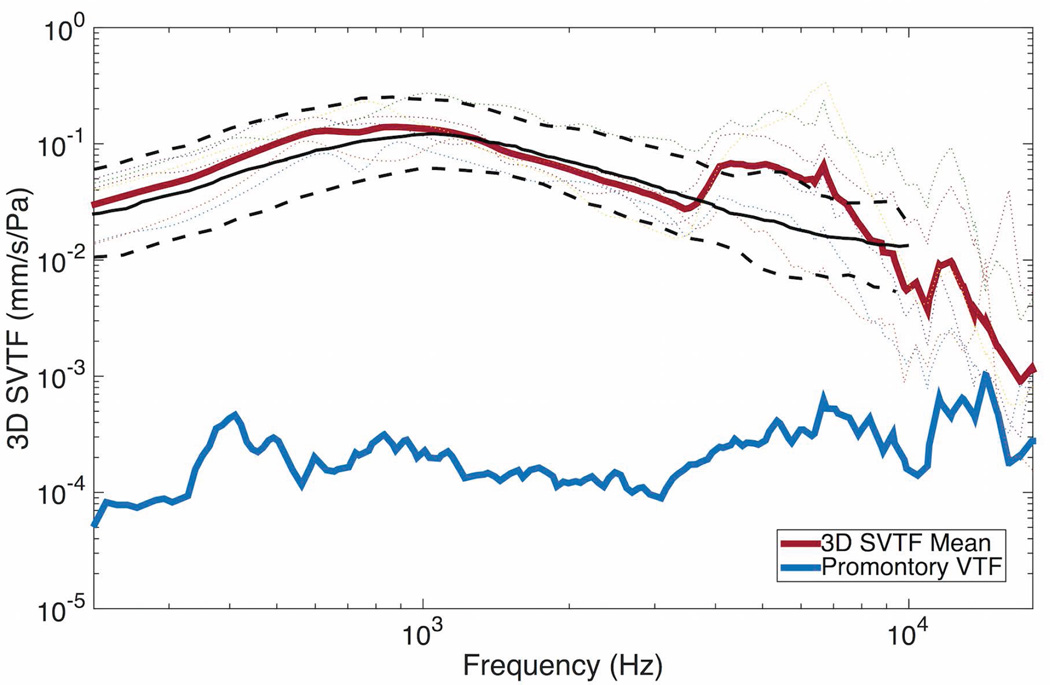
The mean baseline response fell within the measurement standards previously established21 below 5kHz (figure 4). Above 5kHz, the mean SVTF was higher than the standard by up to 6 dB. Cochlear promontory sound-driven transfer function is shown as a measure of artifact levels within the measurement system. The noise floor of the LDV was at least 20 dB below the promontory velocity at all frequencies.
Figure 4.
Intact (baseline) 3D-SVTF. The black lines show the mean (solid) and 95% confidence interval (dashed) SVTF from the ASTM F2504 standard. The thin dotted lines show individual specimens. The cochlear promontory velocity transfer function (VTF) is shown in blue as a measure of artifact levels.
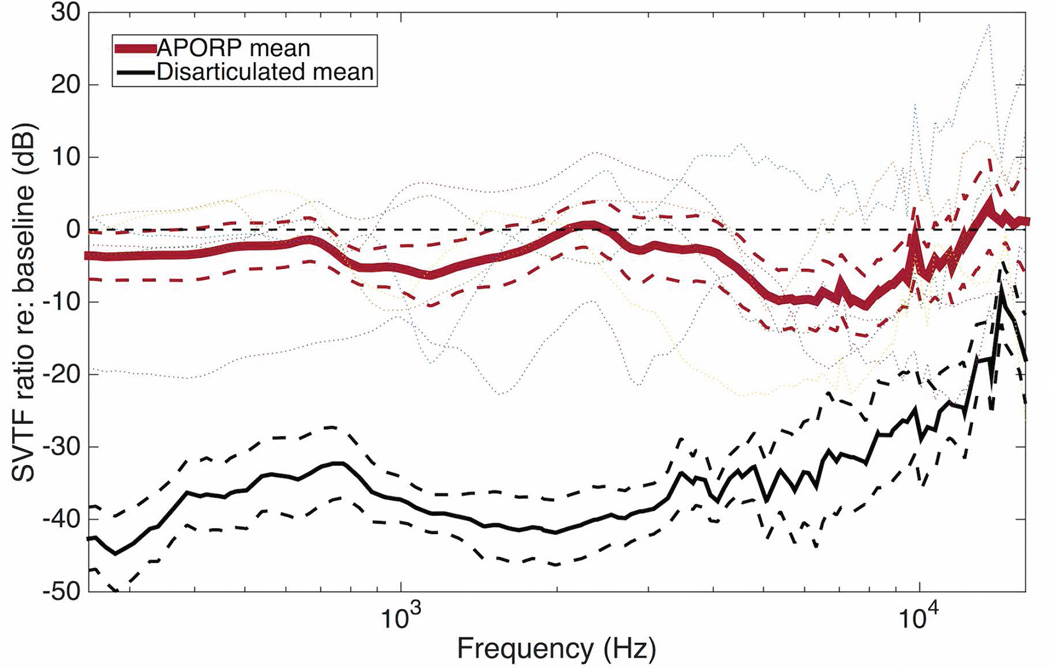
Figure 5 shows the ratio of the A-PORP and disarticulated SVTFs relative to the intact case. The mean A-PORP ratio was more than 20 dB greater than the mean disarticulated ratio below 8 kHz, and more than 10 dB greater above even as the disarticulated SVTF ratio increased. Significant variability was found between specimens, but even in the worst case, the A-PORP restored the SVTF to within 20 dB of the intact case.
Figure 5.
A-PORP and disarticulated SVTF divided by baseline case for each specimen. Means (solid) and standard errors (dashed) are shown. The thin dotted lines show individual specimens.
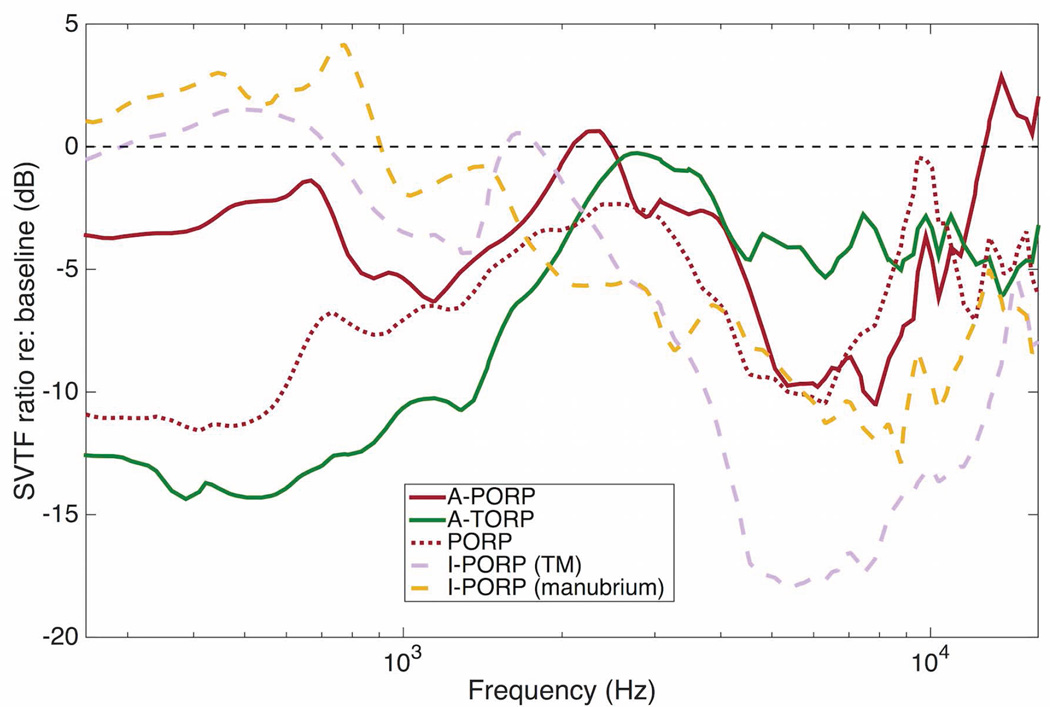
Figure 6 shows the mean SVTF ratios for each prosthesis design. The A-PORP and A-TORP designs restored the mean SVTF to within 4 and 6 dB (on average) of the intact response, respectively. The difference between their performances is largely due to the inferior performance of the A-TORP at frequencies below 1 kHz, where the mean SVTF was more than 10 dB lower than the intact case. The mean A-PORP SVTF ratio was higher than the mean PORP ratio at all frequencies except for a narrow band between 7 and 10 kHz. Both I-PORP cases performed well at low frequencies and poorly at high frequencies. Overall, no significant differences were found between any of the ORP designs over the whole frequency range.
Figure 6.
Mean of prosthesis SVTFs for each specimen divided by baseline case. The standard error for each of these cases was comparable to the A-PORP case shown in figure 5, so error bars are not shown.
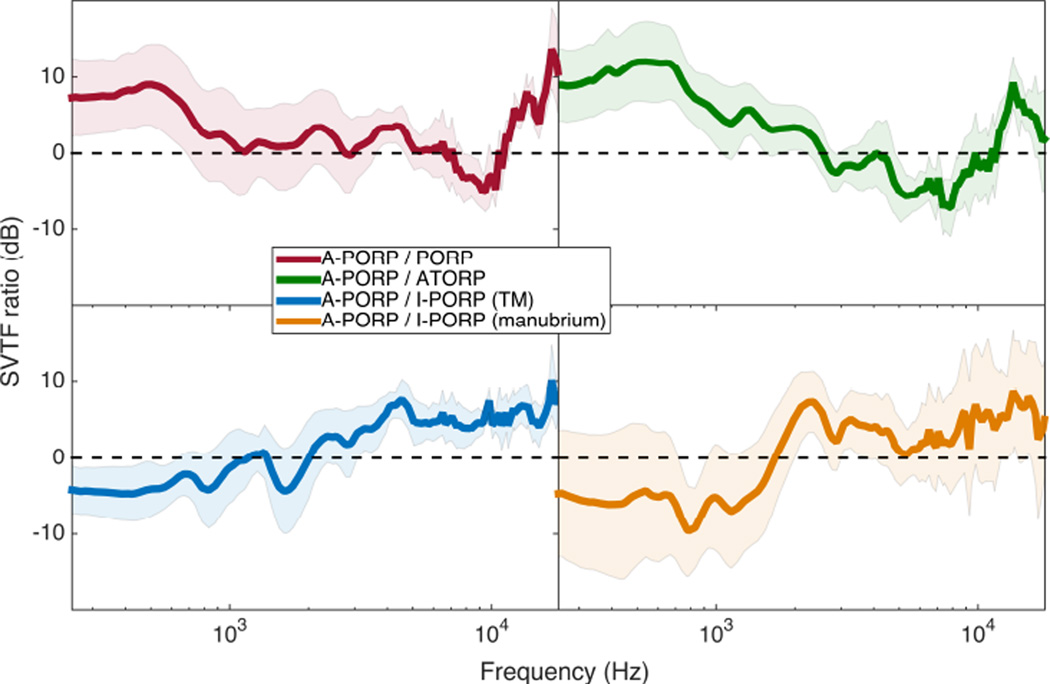
Figure 7 shows direct comparisons of the ORP-to-intact SVTF ratios for four cases. The A-PORP design performed slightly better than the PORP and A-TORP designs at most frequencies. Both I-PORP designs (manubrium and TM contact) performed slightly better than the A-PORP design at frequencies below about 2 kHz, but the A-PORP mean was greater at higher frequencies. Again, overall, no significant differences were found between the ORP designs.
Figure 7.
Mean and standard error for A-PORP divided by PORP, A-TORP, and both I-PORP cases. Means were taken after calculating ratios for each specimen.
Discussion
Baseline transfer function and 3D measurements
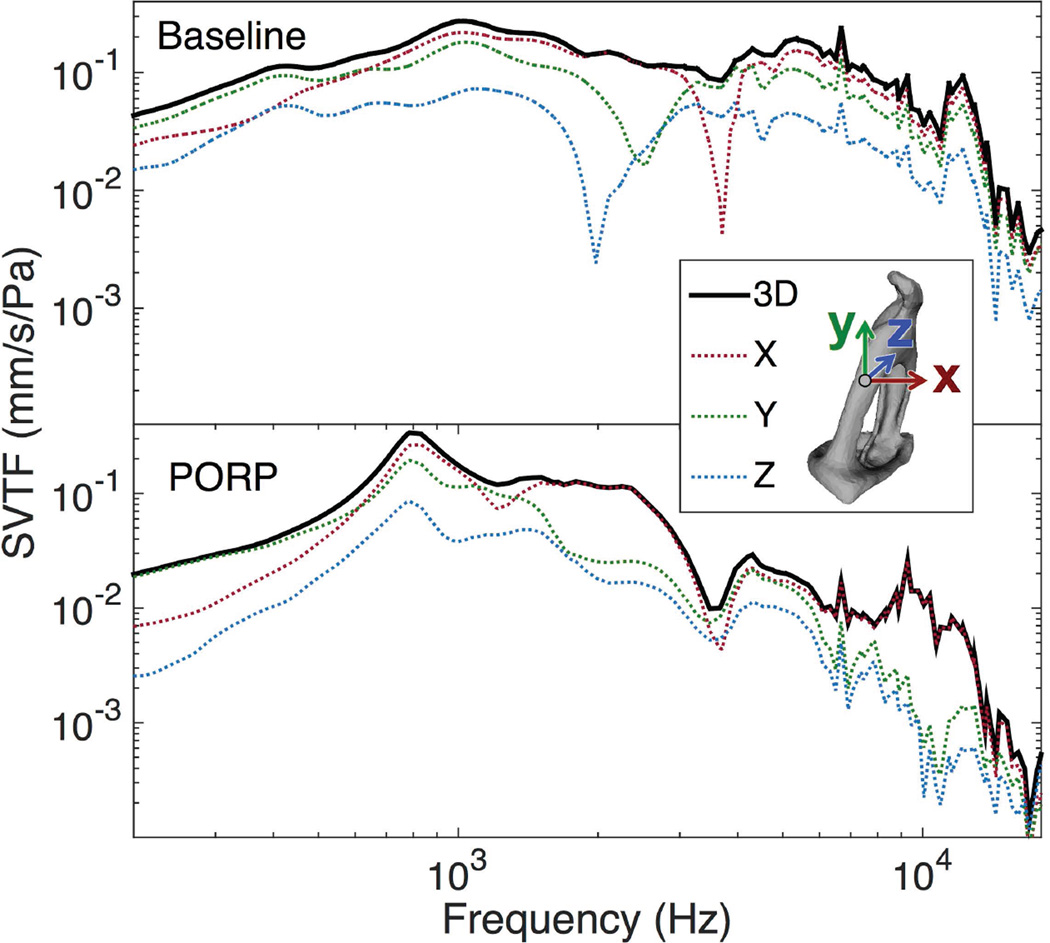
In this experiment, the 3D-SVTF was calculated and reported rather than the more common single-axis SVTF. Figure 8 shows a comparison of the 3D-SVTF and its three orthogonal components for an intact and PORP case. Two features are evident. First, while the component SVTFs of the intact case have prominent localized resonances, the 3D-SVTF is smoother because those resonances occur at different frequencies. This also suggests that the stapes has several different vibration modes over the measured frequency range. Second, the relationship between the component SVTFs differs greatly between the intact and PORP cases, which indicates that the PORP not only affects the stapes vibration magnitude but also the vibration mode.
Figure 8.
Comparison of the single-component and 3D-SVTFs for the baseline and PORP cases in a representative specimen. The coordinate axes are shown relative to the measurement location on the stapes crus.
The use of the 3D-STVF may account for the increase in the SVTF seen at higher frequencies in this study relative to previously reported single-axis SVTFs15, 16, 21 since, depending on the choice of axis, a single-axis measurement would likely underestimate the true vibration magnitude of the stapes. A further source of differences between these results may arise from the variation in measurement locations, since in the current study the velocity of a point on the stapes posterior crus was reported rather than that of the stapes footplate.
3D velocity measurements are therefore an important tool, since a single axis measurement might incorrectly 1) indicate that the SVTF has a notable resonance, or 2) underestimate the true SVTF in the normal and reconstructed ears.
ORP performance
In an intact middle ear, the lever ratio between the malleus and the incus is about 2.1, which causes a 6 dB decrease in velocity between the umbo and the stapes.22 Therefore, a simple model predicts that bypassing the incus with a prosthesis would increase stapes velocity by this amount. That the prostheses tested here generally reduced stapes velocity relative to the intact case (figure 6) points to an importance of the complex geometry of the ossicles beyond this lever ratio.
The PORP design used in this experiment (Kurz BELL) was previously shown to be clinically effective as measured by a post-operative air-bone gap of less than 20 dB in more than 80% of evaluated cases,23 so the comparable performance of the A-ORP designs to the PORP design demonstrate the clinical promise of the A-ORP designs.
Although no significant differences were found, a qualitative analysis suggests that some designs seemed to perform better in certain frequency ranges. For example, the I-PORPs responded well at low frequencies but poorly at high frequencies, which may be explained by the relatively higher mass of these prostheses. The lack of any significant difference between the titanium ORPs and the I-PORP has also been seen clinically,24 and makes a case for the use of I-PORPs since they are autologous tissue and are therefore inherently biocompatible. No significant difference was found between the performance of an I-PORP contacting the manubrium or the TM, suggesting that this choice should be made based on the specific anatomy of the patient.
Implications and future directions
Previous studies have shown that ORP alignment and tension are important factors in the ultimate performance of the prosthesis.3, 4, 8 The results of this study reinforce that conclusion, and furthermore it appears that these factors are far more important than the material or geometrical differences between the prostheses tested in this study. Rather, the design of the ORP is only important insofar as it facilitates optimal placement.
Adjustable prostheses (e.g. Grace Medical ALTO) are currently available for clinical use, but none to our knowledge are adjustable in situ. There is one commercially available self-adjusting prosthesis (Magnan-Babighian telescopic total and partial prostheses, Audio Technologies, Milan, Italy), but even this only maintains a constant tension and does not facilitate insertion and alignment. Furthermore, a previous study found that it performed significantly worse than a fixed-length prosthesis, especially at low frequencies.9 Since the A-PORP and A-TORP designs perform as well as non-adjustable ORPs, and they potentially allow for in situ optimization of length and alignment, they seem to be promising designs, especially when inserting the ORP through the constrained facial recess approach.
This study was designed to demonstrate feasibility of the A-ORP designs on a small sample. Now that the design has been shown to be comparable in acoustic performance to a conventional ORP, future work will continue to improve and test the A-ORP designs. The A-ORP designs additionally provide a better way of testing the effects of ORP tension on acoustic performance, since tension can be adjusted without removing and replacing the ORP. The next studies will directly test whether adjusting the A-ORPs in situ allows for better alignment and tension by using a specially designed tool for adjusting the ORP length, which is under development. The ease of inserting the prosthesis through the facial recess and adjusting the length in situ without damaging surrounding structures, such as the tympanic membrane and stapes footplate, will also need to be investigated before this device can be clinically evaluated.
Conclusion
This study introduced a new prosthesis design with an adjustable element. It was tested in a temporal bone model using 3D vibrometry for the first time, which may provide a more comprehensive estimate of cochlear input. No significant difference was found between the acoustic performance of the new A-ORP designs and a conventional titanium PORP or an autologous incus PORP. Since the new designs potentially allow for in situ length adjustment and easier alignment and tension optimization, they represent a promising new direction for ORPs.
Acknowledgments
This work was supported in part by the National Institute on Deafness and Other Communication Disorders (DC005960 and FDC013943A).
Footnotes
Conflicts of Interest: Drs. Blevins and Monfared have licensed the adjustable prosthesis design used in these experiments to Grace Medical.
References
- 1.Goldenberg RA, Emmet JR. Current use of implants in middle ear surgery. Otology & Neurotology. 2001;22:145–152. doi: 10.1097/00129492-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Merchant S, Rosowski J. Surgical Reconstruction and Passive Prostheses. In: Puria S, Fay RR, Popper A, editors. The Middle Ear. New York, NY: Springer; 2013. pp. 253–272. [Google Scholar]
- 3.Bance M, Morris DP, VanWijhe RG, Kiefte M. Comparison of the mechanical performance of ossiculoplasty using a prosthetic malleus-to-stapes head with a tympanic membrane-to-stapes head assembly in a human cadaveric middle ear model. Otology & Neurotology. 2004;25:903–909. doi: 10.1097/00129492-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Morris DP, Bance M, van Wijhe RG, Kiefte M, Smith R. Optimum tension for partial ossicular replacement prosthesis reconstruction in the human middle ear. The Laryngoscope. 2004;114:305–308. doi: 10.1097/00005537-200402000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Merchant SN, McKenna MJ, Rosowski JJ. Current status and future challenges of tympanoplasty. European Archives of Oto-Rhino-Laryngology; 1998;255:221–228. doi: 10.1007/s004050050047. [DOI] [PubMed] [Google Scholar]
- 6.Murugasu E, Puria S, Roberson JB., Jr Malleus-to-footplate versus malleus-to-stapes-head ossicular reconstruction prostheses: temporal bone pressure gain measurements and clinical audiological data. Otology & Neurotology. 2005;26:572–582. doi: 10.1097/01.mao.0000178151.44505.1b. [DOI] [PubMed] [Google Scholar]
- 7.Puria S, Kunda LD, Roberson JB, Jr, Perkins RC. Malleus-to-footplate ossicular reconstruction prosthesis positioning: cochleovestibular pressure optimization. Otology & Neurotology. 2005;26:368–379. doi: 10.1097/01.mao.0000169788.07460.4a. [DOI] [PubMed] [Google Scholar]
- 8.Zhao S, Hato N, Goode RL. Experimental study of an adjustable-length titanium ossicular prosthesis in a temporal bone model. Acta oto-laryngologica. 2005;125:33–37. doi: 10.1080/00016480410018250. [DOI] [PubMed] [Google Scholar]
- 9.Goode RL, Yamada H, Hearing Researchl 2010 A constant tension middle ear replacement prosthesis: Why don’t we have one? [Abstract] Hearing Research. 2010;263:233–252. [Google Scholar]
- 10.Praetorius M, Kirchenbauer SS, Buss S, Klingmann C, Plinkert P, Baumann I. First experiences with a new adjustable length titanium ossicular prosthesis (ALTO) Acta Oto-Laryngologica. 2010;130:1237–1241. doi: 10.3109/00016489.2010.490238. [DOI] [PubMed] [Google Scholar]
- 11.Yamada H, Goode RL. A self-adjusting ossicular prosthesis containing polyurethane sponge. Otology & Neurotology. 2010;31:1404–1408. [PubMed] [Google Scholar]
- 12.Blevins NH. Transfacial recess ossicular reconstruction: technique and early results. Otology & Neurotology. 2004;25:236–241. doi: 10.1097/00129492-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Neudert M, Zahnert T, Lasurashvili N, Bornitz M, Lavcheva Z, Offergeld C. Partial ossicular reconstruction: comparison of three different prostheses in clinical and experimental studies. Otology & Neurotology. 2009;30:332–338. doi: 10.1097/MAO.0b013e31819679dd. [DOI] [PubMed] [Google Scholar]
- 14.Nishihara S, Goode RL. Experimental study of the acoustic properties of incus replacement prostheses in a human temporal bone model. Otology & Neurotology. 1994;15(4):485–494. [PubMed] [Google Scholar]
- 15.Voss SE, Rosowski JJ, Merchant SN, Peake WT. Acoustic responses of the human middle ear. Hearing Research. 2000;150:43–69. doi: 10.1016/s0378-5955(00)00177-5. [DOI] [PubMed] [Google Scholar]
- 16.Aibara R, Welsh JT, Puria S, Goode RL. Human middle-ear sound transfer function and cochlear input impedance. Hearing Research. 2001;152:100–109. doi: 10.1016/s0378-5955(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 17.Heiland KE, Goode RL, Asai M, Huber AM. A human temporal bone study of stapes footplate movement. Otology & Neurotology. 1999;20:81–86. [PubMed] [Google Scholar]
- 18.Hato N, Stenfelt S, Goode RL. Three-dimensional stapes footplate motion in human temporal bones. Audiology and Neurotology. 2003;8:140–152. doi: 10.1159/000069475. [DOI] [PubMed] [Google Scholar]
- 19.Sim JH, Chatzimichalis M, Lauxmann M, Roosli C, Eiber A, Huber A. Complex stapes motions in human ears. Journal of the Association for Research in Otolaryngology. 2010;11:329–341. doi: 10.1007/s10162-010-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ASTM Standard 2504, 2005 (2014) Standard Practice for Describing System Output of Implantable Middle Ear Hearing Devices. West Conshohocken, PA: ASTM International; 2014. [Google Scholar]
- 21.Rosowski J, Huber A, Ravicz M, Goode R. Are temporal bones useful models of human middle ear mechanics? Abtracts of the Twenty-Seventh Meeting of the Association for Research in Otolaryngology. 2004:275. [Google Scholar]
- 22.Gyo K, Aritomo H, Goode RL. Measurement of the ossicular vibration ratio in human temporal bones by use of a video measuring system. Acta Oto-Laryngologica. 1987;103:87–95. doi: 10.3109/00016488709134702. [DOI] [PubMed] [Google Scholar]
- 23.Krueger W, Feghali JG, Shelton C, et al. Preliminary ossiculoplasty results using the Kurz titanium prostheses. Otology & Neurotology. 2002;23:836–839. doi: 10.1097/00129492-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kawatra R, Maheshwari P. A comparative study of surgical outcomes of ossiculoplasty using biomaterials and autologous implants. Bangladesh Journal of Otorhinolaryngology. 2013;19:29–35. [Google Scholar]