Abstract
Reversal learning paradigms are among the most widely used tests of cognitive flexibility and have been used as assays, across species, for altered cognitive processes in a host of neuropsychiatric conditions. Based on recent studies in humans, non-human primates, and rodents, the notion that reversal learning tasks primarily measure response inhibition, has been revised. In this review, we describe how cognitive flexibility is measured by reversal learning and discuss new definitions of the construct validity of the task that are serving as an heuristic to guide future research in this field. We also provide an update on the available evidence implicating certain cortical and subcortical brain regions in the mediation of reversal learning, and an overview of the principle neurotransmitter systems involved.
Keywords: frontal cortex, striatum, amygdala, dopamine, serotonin, glutamate
Introduction
Cognitive flexibility, the ability to rapidly change behavior in the face of changing circumstances, is disrupted in many psychiatric and neurological disorders. Determining the neural basis of cognitive flexibility is therefore important for understanding the pathophysiology of these disorders and potentially developing treatments. To study the neural substrates of cognitive flexibility in rodents, nonhuman primates, and humans, researchers have often used a set of paradigms collectively referred to as reversal learning. Across species, these paradigms are subtly different, but importantly they all assess cognitive flexibility by evaluating adaptive responding in the face of changing stimulus-outcome (S-O) or response-outcome (R-O) contingencies.
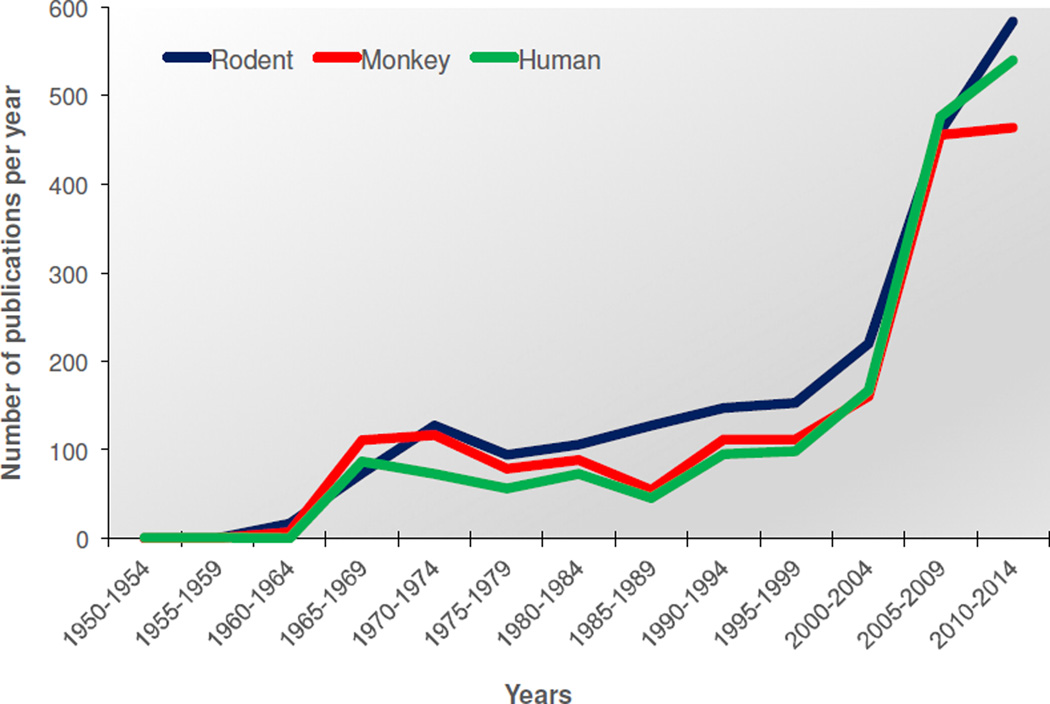
Over the years, reversal learning has become a pre-eminent test of cognitive flexibility and has been used to characterize altered cognitive processes in a host of neuropsychiatric disorders, including substance abuse, obsessive compulsive disorder, psychopathy, Parkinson’s disease, schizophrenia, and to assess cognition at certain developmental time periods such as adolescence (Swainson et al., 2000, Remijnse et al., 2006, Finger et al., 2008, Brigman et al., 2009, Leeson et al., 2009, van der Schaaf et al., 2011, Izquierdo and Jentsch, 2012). Despite its long history of use, reversal learning continues to be an essential experimental paradigm for assessing cognitive function. Indeed, recent years have seen a precipitous rise in the number of published studies using reversal learning, with almost equal focus on rodent, monkey and human subjects (Figure 1).
Figure 1. Publications of reports on reversal learning in rodent, monkey, and human subjects.
Pubmed search terms “reversal learning” from 1950–2014. The early-to-mid 2000’s witnessed the steepest rise in the number of publications on reversal learning. Reversal learning continues to be a widely-used paradigm for assessing cognitive function, with an almost equal focus on rodent, monkey and human subjects.
While the literature on the neural basis of reversal and the interpretation of findings using this task have been reviewed elsewhere (Clark et al., 2004, Izquierdo and Jentsch, 2012, Costa et al., 2015, Hamilton and Brigman, 2015, Wassum and Izquierdo, 2015), our aim here is to: 1) consider how reversal can be measured and compare different versions of the paradigm across species; 2) provide an updated perspective on the construct validity of reversal learning paradigms; 3) discuss current thinking on the major neural circuits mediating the ability to flexibly change behavior; and 4) review the neurochemical modulation of the cognitive processes engaged during reversal learning.
Reversal learning paradigms across species
In the classic reversal learning paradigm used in humans (Fellows and Farah, 2003a), monkeys (Butter, 1969) and rodents (Schoenbaum et al., 2000), subjects are trained to discriminate between two visual stimuli or spatial locations, one of which is rewarded every time it is chosen and the other which is not. After successful discrimination learning has been demonstrated by reaching a criterion level of performance, the outcomes associated with the two stimuli are reversed and subjects are again trained until they meet a performance criterion. Note, that while in this review we focus on instrumental, appetitive forms of reversal learning, Pavlovian associations can also be reversed and outcomes can also be aversive (Morris and Dolan, 2004, Burke et al., 2009).
An advantage of reversal learning paradigms is that they can be employed in multiple species and, as such, can have significant translational value for understanding the neural bases of cognitive flexibility. Reflecting this, many of the key findings concerning the neural mechanisms of reversal learning have been replicated across rodent, non-human primate and human subjects, as discussed later in this review. We briefly review some of the common procedures employed to test reversal learning in different species noting the differences in paradigms, but also their similarities.
For reversal learning tasks in rodents, behavioral apparatus are often outfitted with either two levers, nosepoke portals or a touch-sensitive screen. Mazes are also commonly used to test spatial discriminations and reversals (Jentsch and Taylor, 2001, Bannerman et al., 2003, Palencia and Ragozzino, 2004). With mazes, levers and portals, reversal may be performed solely using information about spatial location or incorporate the use of visual or auditory cues (Neill et al., 2001, Widholm et al., 2003, Boulougouris et al., 2007, Castañé et al., 2010). When a touchscreen is used, a wider variety of visual stimuli become available and spatial and egocentric strategies better controlled for (Izquierdo et al., 2006, Mar et al., 2013, Graybeal et al., 2014). In nonhuman primates, modified versions of the Wisconsin General Testing Apparatus (WGTA) have been used to test reversal learning (Jones and Mishkin, 1972, Stern and Passingham, 1995, Izquierdo et al., 2004a). In one paradigm, an opaque screen is lowered while one of two food wells is baited with a reward. When the screen is raised the monkey is tasked with displacing one of the objects to reveal the reward. Alternatively, animals can be presented with visual stimuli on cards or a touchscreen (Crofts et al., 1999, Clarke et al., 2005, Walker et al., 2009). With either method, selection of the correct stimulus results in delivery or access to a reward. Thus, the paradigms used in both rodents and monkeys are very similar. However, where reversal learning tasks differ between species is in the number of reversals typically completed by the animals. In the standard WGTA version of the task for macaque monkeys, subjects often complete more than seven serial reversals (Izquierdo et al., 2004b), but more recent versions of the paradigm deliver multiple reversals in a single session. In marmoset monkeys, subjects usually complete approximately four reversals across multiple sessions, whereas in rodents, only a single reversal is often completed (c.f. Schoenbaum et al., 2002).
There are also a range of approaches to assaying reversal learning in human volunteers and patient populations, including via the presentation of visual stimuli on a screen that can be responded to with a screen touch or keyboard stroke (Lawrence et al., 1999, Swainson et al., 2000, Cools et al., 2002, Hvoslef-Eide et al., 2015). Reward in these experiments involves a simple notification that a response was correct or the accumulation of simulated or actual monetary compensation (Cools et al., 2002, Fellows and Farah, 2003b). Thus, a difference with most reversal tasks in non-human animals is that rewards are almost always secondary, conditioned reinforcers rather than primary reinforcers such as food or drugs, and can often entail delayed access and a mental representation of reward accrual.
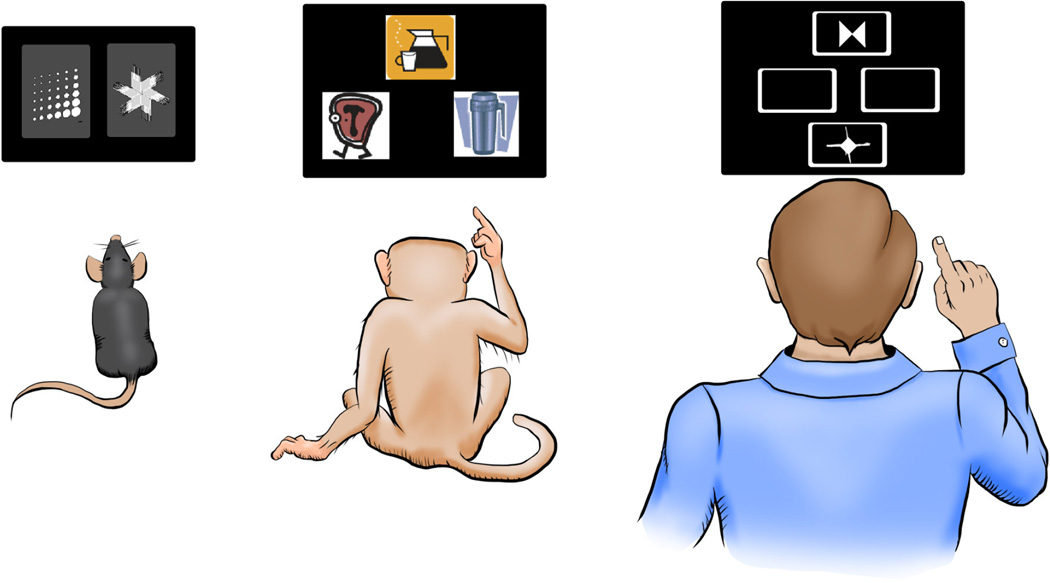
Across species and platforms, the relationship between stimuli and outcomes can in principle be either fully (deterministic) or partially predictive (probabilistic) (Bari et al., 2010a, Ineichen et al., 2012, Dalton et al., 2014, Rygula et al., 2014). Indeed, S-O outcomes can be reversed at the mid-point of sessions with differing probabilistic reinforcement schedules (e.g., 80% rewarded or 60% rewarded) as a way to modulate the subject’s (in this case, a monkey’s) anticipation of upcoming reversals (Walton et al., 2010, Costa et al., 2015). Reversal paradigms in humans are almost always probabilistic, and probabilistic associations can occur numerous times during a single session, in some cases in fewer than five trials (Rolls et al., 1994a, Hampton et al., 2007). Probabilistic tasks slow the rate of learning (which can be fast in human subjects) and helps to reduce the use of simple strategies (as reviewed later) and allows for contrasting of ‘true errors’ and ‘probabilistic errors’ (Cools et al., 2002). Other methodological factors challenge the notion that there are preserved features of reversal learning across species. One such factor is sensory modality of learning. Learning in some modalities (e.g., involving odor or digging-medium discriminanda) is easily acquired in rodents, whereas visual discrimination paradigms are learned more readily in primates. The differences in length of training that result from these species- specific propensities could potentially engage different neural circuitry, thereby complicating translation. Nonethless, despite some differences in the paradigms, there is more convergence and comparability of methods for testing reversal learning across species than disagreement (Figure 2).
Figure 2. Reversal learning assessments using touchscreens in rodent, monkey, and human subjects.
Despite some differences in the paradigms, there is more convergence and comparability of methods for testing reversal learning across species than disagreement. Shown is an example of the touchscreen platform across species. In rats and monkeys the relationship between stimuli and outcomes are either fully (deterministic) or partially predictive (probabilistic), whereas reversal paradigms in humans are almost always probabilistic.
What does reversal learning measure?
Reversal learning requires a subject to flexibly adjust their behavior when the reward-related contingencies that they have previously learned are reversed. For some time, a widely-voiced idea was that reversal leaning paradigms primarily measured inhibitory control of responding (Jones and Mishkin, 1972). Based on experiments in humans, monkeys, and rodents (Fellows and Farah, 2005, Chudasama et al., 2007, Schoenbaum et al., 2009a), this view has been revisited and other tasks have been designed to directly test more specific aspects of cognition related to attention and motor inhibition (for example, stop signal task, 5-choice serial reaction time task). It appears that the brain regions underlying performance on these various tasks are overlapping with those implicated in reversal learning, though there are also functional dissociations (Jentsch et al., 2014).
In the paragraphs below, we review current thinking that reversal learning paradigms require dynamic reward representations including an abstraction of accrued reward and an expectation (belief) of the possibility for change. We discuss how, to perform optimally, the subject’s most adaptive strategy is rule use (similar to a “learning set,” described below) combined with reward learning, and not simply inhibitory (motor response) control. These conclusions have arisen in large measure from important refinements and variants of reversal learning tasks that we will also detail. For instance, in addition to general measures such as the number of correct responses and errors the subject makes after each switch in reward contingencies, experimenters can use consecutive (‘correction’) errors to assess a failure to disengage from ongoing behavior (perseverative responses), monitor performance throughout different stages of learning (e.g., below vs. above chance performance, early, middle and/or late stages) (Jones and Mishkin, 1972, Chudasama and Robbins, 2003, Izquierdo et al., 2006, Brigman et al., 2008, Graybeal et al., 2011, DePoy et al., 2013), and study the microstructure of learning derived from trial-by-trial responses to positive and negative feedback (Rudebeck and Murray, 2008, Brigman et al., 2013, Izquierdo et al., 2013, Stolyarova et al., 2014, Klanker et al., 2015). These measures assess how subjects are learning from the feedback that they received on each trial, and therefore assume that reversal learning is solved by a single reward learning process that integrates reward and non-reward experiences across trials to drive performance. As an example, increased omissions during reversal learning can index a failure to overcome non-reward experiences (Tait and Brown, 2007).
Variations in task-design which allow for different ways of assessing the reward learning process including administering serial reversals, in which the subject learns to perform multiple reversals, for example via repeated stimulus-outcome (S-O) switching (Boulougouris et al., 2007, Castañé et al., 2010, Kosaki and Watanabe, 2012), concurrent reversals - where subjects learn multiple pairs of stimuli - some of which are then reversed and some not (Wilson and Gaffan, 2008), and introducing an additional option for choice. In the latter variant, when a three- or four-choice reversal procedure is used, it becomes easier to distinguish between regressive errors, perseverative errors, and new learning errors (Ragozzino et al., 2003, Kim and Ragozzino, 2005, Lee et al., 2007, Ragozzino and Rozman, 2007, Rudebeck et al., 2008, Seu et al., 2009, Kosaki and Watanabe, 2012, Riceberg and Shapiro, 2012). Increasing the option space beyond two possibilities also has the potential for decoding exploitative versus explorative strategies, consistent with optimal foraging, in the service of maximizing reward procurement.
Evidence from both human and monkey studies shows that strategies or rules are also adopted during reversal learning and in some cases can come to dominate responding (Murray and Gaffan, 2006). Indeed this is the main reason why probabilistic learning tasks are predominantly used with human subjects (Rolls et al., 1994a, Hampton et al., 2007) and increasingly in monkeys (Walton et al., 2010, Costa et al., 2015, Rygula et al., 2015) where, as noted above, learning can proceed very (too) quickly. Probabilistic learning paradigms, especially those with more than two options, reduce the ability of subjects to apply a simple strategy, such as win-stay/lose-shift, as they have to use an integrated history of choices and outcomes to determine the best stimulus to choose.
Probabilistic (or bandit) tasks require the subject to choose from a set of options with unknown reward rates that do not depend on choice history. Probabilistic reversal paradigms also have another advantage: they are more amenable to the application of reinforcement learning (RL) models that can estimate parameters for reward decisions, providing another window into cognitive flexibility (Sutton and Barto, 1998). This is especially true for probabilistic tasks because RL models estimate choice behavior based on an integrated history of previous reward and non-reward experience - a critical feature when “correct” responses are not always rewarded. Within the basic RL model equation, different free parameters such as the learning rate, how quickly values are updated after each trial, and the inverse temperature parameter, also known as the exploration parameter (Katahira, 2015) influence how choice outcomes are integrated into option values (i.e., learning rate) and then used to drive reward-guided behavior. These parameters can be fit to the data and compared across groups, helping to further dissociate the processes involved in reversal learning (Costa et al., 2015). Ultimately, the application of these RL models allows an even more fine-grained understanding of what drives subjects’ choices during reversal learning.
Within the past decade, empirical work on reinforcement learning has been greatly influenced by computational theories (Daw and Doya, 2006), and this is likely to continue. One key feature of the theoretical work related to reversal learning is model-free vs. model-based learning (Doya, 1999, Daw et al., 2005, Dayan and Berridge, 2014). Model-free learning does not require a representation, or map, of how to respond or even an understanding of the identity of the end state, and is most associated with acquiring information about the world on a trial-by-trial basis, experiencing stimuli and their outcomes (as described above). There is certainly one aspect of reversal learning that incorporates this, and as we review in the Neural Substrates section, subcortical regions such as striatum and amygdala may help to generate a reward history from these experiences. By contrast, model-based learning incorporates choices guided by a map or abstract cognitive-type structure that the subject can refer to in guiding their choices. This type of learning may also be engaged in reversal learning, and is associated with cortical function. Both model-free and model-based systems may be online during behavior but one could dominate depending on the task variant. For example, simple deterministic tasks involve model-free learning since the task can be solved by implementing a win-stay/lose shift strategy. However, in a task variant with both options rewarded but with different outcomes (Keiflin et al., 2013), this problem can only be solved by model-based learning. Therefore, reversal learning tasks can incorporate both model-free and model-based aspects, depending on the method employed.
Recently, Costa et al. (2015) proposed a novel Bayesian framework for studying both reward learning and strategy use, in reversal learning (Costa et al., 2015). Within this framework, one model is closely associated with a reward learning process that tracks whether the subject did or did not receive a reward for a particular choice. This is similar to an accumulator model in which a decision reflects evidence accrued from information based on discrete time versus continuous state space, that is not unlike initial pairwise discrimination learning (Vickers, 1970). A second model, which likely interacts with the first, represents the subject’s belief that reversals or changes in reward contingency can occur. It does so by accumulating a different kind of evidence over multiple reversals to generate a likelihood estimate (known as a prior probability) that a reversal can occur. Using this framework, Costa and colleagues were able to model the subject’s “belief” that a reversal could occur, even before the first reversal, in both standard deterministic (Jang et al., 2015) and probabilistic reversal learning tasks (Costa et al., 2015, Jang et al., 2015). The utilization of such beliefs is consistent with model-based learning, described above.
The notion that reward learning and belief that reversals occur likely both contribute to efficient performance of reversal learning and a prospective coding of the anticipated trials is consistent with concepts such as learning set (Murray and Gaffan, 2006), and potentially also fits with formulations of ‘task space’ (Wilson et al., 2014). The idea of task space suggests that subjects generate an abstraction of the current state of performance on-task that is not bound to the stimulus or cue, but instead to a more ‘cognitive’ representation of some hidden variable that is relevant to the task, such as the possibility of changing S-O or task context (Wilson et al., 2014, Saez et al., 2015). Like the formulation of a belief that reversals can occur, the map or task space model is particularly informative when explaining serial reversal learning or switching S-O probe trials - where it can account for improvements in performance resulting from experience. How notions of task space differ from the belief that a reversal can occur is, however, yet to be determined.
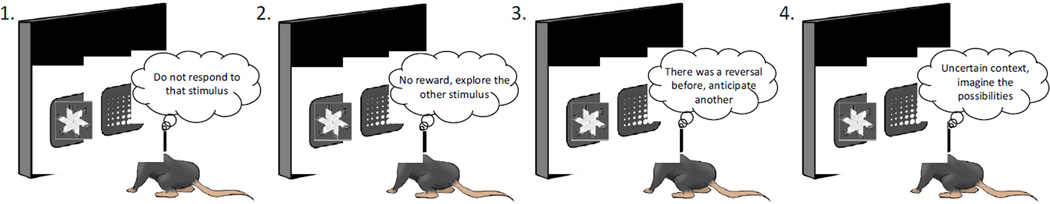
Taken together, these studies suggest that we should consider a revised definition of the construct validity of reversal learning. Irrespective of their exact design, reversal learning paradigms all likely test the ability to: 1) learn from the rewards received (as well as absence of reward, or reward omission) upon choosing different stimuli (Stalnaker et al., 2015); 2) estimate the likelihood or prior probability that reversals can occur (Costa et al., 2015, Jang et al., 2015); and/or 3) generate an understanding of task or option space (Wilson et al., 2014, Saez et al., 2015) (Figure 3). Defining these different sub-processes in future work will be important, as it also allows more straightforward comparisons between species and paradigms, and the parsing of specific neural systems that contribute to these different aspects of cognitive flexibility. Another challenge will be to determine the concordance of reversal learning sub-processes with other tasks of cognitive flexibility, such as discounting tasks, set shifting tasks, and responses during reinforcer devaluation or in contingency degradation paradigms.
Figure 3. Sub-processes in reversal learning that contribute to a revised definition for the construct validity of the task.
The widely-accepted idea that reversal leaning paradigms primarily measure inhibitory control (1) of responding, has fallen out of favor. Instead, reversal learning paradigms all likely test the ability to learn from rewards and non-rewards upon choosing different stimuli (2), estimate the likelihood or prior probability that reversals can occur (3), and/or generate an understanding of task or option space (4).
Neural substrates of reversal
Cortical regions
Neuroimaging studies report increased activity in orbitofrontal cortex (OFC) and medial prefrontal cortex (mPFC) in human subjects performing reversals (Nagahama et al., 2001, Cools et al., 2002, Kringelbach and Rolls, 2003, Remijnse et al., 2005, Ghahremani et al., 2010), and patients with lesions of these regions exhibit reversal learning deficits (Rolls et al., 1994b, Fellows and Farah, 2003b, Hornak et al., 2004). Furthermore, a consistent finding from over fifty years of work in nonhuman primates, has been that frontal lobectomies and ablations (Battig et al., 1962, Butter et al., 1963), or more localized aspiration lesions of the OFC, impair various measures of flexible responding for reward (Jones and Mishkin, 1972, Izquierdo et al., 2004a, Machado and Bachevalier, 2007) (for recent reviews, see (Hamilton and Brigman, 2014, Rudebeck and Murray, 2014). In addition, excitotoxic lesions of the OFC in marmosets, that were presumed to spare fibers of passage, were found to selectively impair reversal learning, while leaving other forms of behavioral flexibility intact (Dias et al., 1996b, a, 1997). Similarly, recordings of single neuron activity in the OFC during reversal learning also show that neurons track changes in reward contingency (Thorpe et al., 1983, Schoenbaum et al., 1999, Morrison and Salzman, 2009).
Extending these studies in nonhuman primates, research in rodents has shown that excitotoxic lesions and glutamatergic manipulation of the OFC impairs reversals based on visual stimuli (Bohn et al., 2003, Graybeal et al., 2011, Brigman et al., 2013, Izquierdo et al., 2013), as well as reversal of auditory cues (Burke et al., 2009), spatial responses (Ghods-Sharifi et al., 2008, Young and Shapiro, 2009) and spatially-cued operant responses (Boulougouris et al., 2007, Mar et al., 2011). OFC inactivations or lesions also impair reversal of olfactory and tactile cues in rats and mice (Ferry et al., 2000, Schoenbaum et al., 2002, McAlonan and Brown, 2003, Schoenbaum et al., 2003, Ragozzino, 2007, Bissonette et al., 2008) (Ragozzino, 2007, Churchwell et al., 2009). The importance of the OFC to reversal learning may depend upon the amount of discrimination training the subject receives before reversal. Setting a stringent performance criterion for discrimination produces improvements in reversal learning performance when the OFC is inactive, whereas loss of OFC function causes reversal impairment when the discrimination criterion was easier to attain (Riceberg and Shapiro, 2012). This is possibly due to the role of the OFC varying as a function of the relative ‘stability’ in reward experience.
The role of the mPFC in reversal is distinct from that of the OFC. A majority of studies in rodents report that reversal learning is insensitive to mPFC damage (Birrell and Brown, 2000, Bissonette et al., 2008, Floresco et al., 2008, Churchwell et al., 2009, Cordova et al., 2014) and can even be enhanced by lesions or stress-associated dysfunction in this region (Salazar et al., 2004, Graybeal et al., 2011, Bryce and Howland, 2015). Interestingly, several studies have suggested the mPFC may be recruited in reversal when attentional processes are taxed with difficult discriminanda (Bussey et al., 1997, Brigman and Rothblat, 2008), when multiple contingency changes are tracked continuously (Kosaki and Watanabe, 2012) or when discrete cues are coupled with a high visual or visuospatial component (Meunier et al., 1991, Li and Shao, 1998, Ragozzino et al., 1999, Chudasama and Robbins, 2003, Schwabe et al., 2004, Pickens et al., 2005, Young and Shapiro, 2009, Shaw et al., 2013). Similarly, lesions of the mPFC do not produce gross effects on reversal learning in monkeys (Meunier et al., 1997, Rudebeck et al., 2006). Instead, macaques with mPFC lesions exhibit a slight deficit in the ability to maintain the correct response following a reversal (Chudasama et al., 2013). This also fits with a recent neurophysiology study showing the neurons in the anterior cingulate cortex track rewarded and unrewarded choices over multiple trials during reversal learning (Kawai et al., 2015). Thus, the mPFC may have a more circumscribed role in reversal than the OFC, one that primarily manifests in tasks with a high demand on attention and performance monitoring; consistent with ideas about the involvement of the mPFC in stimulus detection, timing, and error detection (Laubach et al., 2015). However, the precise contribution of the mPFC in reversal learning remains enigmatic and a question for future work.
Understanding the place of the OFC in reversal learning is also evolving. Some authors frame the OFC’s role in reversal as one representing expected outcomes (Schoenbaum et al., 2009b, Rhodes and Murray, 2013, Stalnaker et al., 2015) and task space (Wilson et al., 2014). This outcome representation could utilize value information stored in the OFC (Padoa-Schioppa, 2007, Cai and Padoa-Schioppa, 2014), and/or derive outcome information from subcortical networks tracking critical parameters of the reward environment (e.g., the history and uncertainty of reward availability, its cost and incentive value) (Wassum and Izquierdo, 2015). These conceptualizations are consistent with the finding that OFC lesions do not decrease responding to reward-related cues learned and presented after devaluation (Pickens et al., 2003, Pickens et al., 2005), the observation that OFC is required to store response-outcome associations during reversal learning (Keiflin et al., 2013), and the observation from in vivo electrophysiological recordings that OFC neurons respond to the expected outcome and track reward value across reversal learning (Schoenbaum et al., 2000, Bissonette et al., 2008, Moorman and Aston-Jones, 2014). Moreover, problems updating the value of reward-related cues after OFC lesions could also account for the finding that reversal deficits are typically seen on initial, but not subsequent, reversals (Schoenbaum et al., 2002, Boulougouris et al., 2007, Klanker et al., 2013), given the subject will have accrued more updated information by a second reversal that could serve to mitigate the impact of a suboptimal outcome-valuation system.
Contrasting with earlier data from aspiration lesions in macaque monkeys and excitotoxic lesions in marmosets, fiber-sparing excitotoxic targeting of OFC subregions limited to Walker’s areas 11, 13 and 14 in macaque monkeys have recently been shown to leave reversal intact (Rudebeck and Murray, 2011, Rudebeck et al., 2013). Moreover, aspirating a narrow ‘strip’ of the posterior OFC reinstated reversal deficits. This raises the possibility that damage to white matter tracts near the OFC could at least partially account for impairments attributed to OFC damage (Rudebeck et al., 2013, Rudebeck and Murray, 2014). It is important to note that these findings do not rule out the possibility that areas near to the OFC in macaque monkeys may be important for performance on reversal learning. Indeed given that even small amounts of damage to white matter near OFC cause deficits in reversal learning it seems likely that areas laterally adjacent to the OFC in macaques are engaged during the task, a possibility that has recently received some support (Chau et al., 2015). The importance of the OFC was further demonstrated by the finding that serotonergic depletion in this region impaired reversal learning in marmosets (Clarke et al., 2004, Clarke et al., 2005, Clarke et al., 2007, Walker et al., 2009). As we review in more detail later, levels of serotonin in the OFC, together with DA in putamen, have been estimated to account for a significant proportion of the variance in reversal learning performance in vervet monkeys (Groman et al., 2013). Determining the precise brain regions and systems involved in reversal learning in macaques and how these relate to areas in rodents, other species of monkey, and humans are important avenues for future research.
Striatal and amygdalar regions
In humans, neuroimaging studies demonstrate recruitment of the dorsal (Rogers et al., 2000) and ventral regions of the striatum (Cools et al., 2002) during reversal, while lesions of the basal ganglia are associated with impairments in reversal learning (Rogers et al. 2009). In the rodent, anterograde tracing shows that the OFC projects to the nucleus accumbens (NAc) and dorsomedial striatum (DMS) (Haber et al., 1995, Schilman et al., 2008) and receives reciprocal input from the striatum through the mediodorsal nucleus of the thalamus (Middleton and Strick, 1996). The DMS is implicated in reversal learning by studies demonstrating that neurotoxic lesions of this region impair various forms of reversal learning in marmosets (Clarke et al., 2008, Robbins et al., 2008) and rats (Ragozzino, 2007, Castañé et al., 2010) (Braun and Hauber, 2011). Likewise, neurotoxic lesion of the NAc disrupts spatial, but not visual, reversal learning in monkeys (Stern and Passingham, 1995), as well as probabilistic reversal learning in rats (Dalton et al., 2014), although there is as much, if not more, evidence of unaffected reversal learning following lesions to NAc (Burk and Mair, 2001, Schoenbaum and Setlow, 2003, Castañé et al., 2010).
In addition to receiving strong cortical inputs, the amygdala projects to the NAc and DMS, forming a functional circuit that may support reversal learning. Recent models propose projections from the BLA to the NAc and DMS influence motivated behavior and action selection involving both rewards and punishments, while BLA-OFC interactions generate a high-resolution representation of expected outcome value (Wassum and Izquierdo, 2015). However, various lines of evidence have provided only equivocal support for a contribution of the BLA to reversal learning (Schoenbaum et al., 2003, Stalnaker et al., 2007, Churchwell et al., 2009, Izquierdo et al., 2013) – likely due to variation across studies in the specific methods employed. For example, the BLA may be less engaged in tasks that can be solved without the need to form representations of different outcomes, as in two-choice and/or deterministic reversal tasks where outcome-specific representations do not aid performance. The outcomes encoded by BLA are restricted to the cues that predict them; distinct from the ‘expected outcomes’ that are linked to OFC function. The specific outcomes represented in BLA may be used by OFC to generate expectations, or conversely, OFC may set the learning rate parameter in the RL model by which specific representations are formed in BLA. In two-choice tasks, lesions of rat BLA or whole amygdala in monkey facilitate reversal learning, presumably by enabling a simpler stimulus-response strategy that is not based on outcome representations (Rudebeck and Murray, 2008, Izquierdo et al., 2013). It also may matter when the amygdala goes ‘offline.’ Inactivation or lesions made before specific outcome representations are formed may alter the way the association is learned and, therefore, the way the animal adapts to later changes in contingency, when assessed in the reversal phase. In tasks that do necessitate outcome-specific representations, for example where animals are required to compare outcomes (sucrose versus quinine), or when BLA inactivations occur after initial training but prior to the reversal phase, BLA manipulations disrupt performance (Schoenbaum et al., 2003, Churchwell et al., 2009).
Given these considerations, the BLA can be conceptualized as supporting reversal learning by tracking prior outcomes and comparing them to current outcomes. This idea is supported by computational modeling (Jang et al., 2015) and in vivo recordings showing that monkey amygdala neurons respond according to the deviation between cached and expected rewards (Belova et al., 2007, Bermudez and Schultz, 2010). This is also consistent with the notion that amygdala encodes a dynamic representation of outcome that incorporates both the stimuli that predict those outcomes, and a history of reward (Paton et al., 2006, Morrison and Salzman, 2010).
Neurochemical modulation of reversal
The molecular and neurochemical factors influencing reversal learning and the associated cognitive domains affected are yet to be fully understood (Izquierdo et al., 2012). Here we consider what have been the most intensively studied neurotransmitter systems in reversal learning - serotonin, dopamine and glutamate.
Serotonin
Systemic depletions of serotonin produce generalized effects on reward learning that are not specific to reversal learning (Lapiz-Bluhm et al., 2009). For example, rats systemically treated with para-chloroamphetamine (PCA) or parachlorophenylalanine (PCPA), both of which produce significant depletion of brain serotonin, showed impaired stimulus-reward, discrimination and reversal learning (Masaki et al., 2006, Izquierdo et al., 2012). However, serotonin and serotonin transporter levels in the rodent OFC predict individual variation in reversal learning performance, suggesting a more circumscribed role for the transmitter (Stolyarova et al., 2014, Barlow et al., 2015). A study in vervet monkeys by Groman and colleagues also reported that serotonin levels in ventrolateral PFC/Area 47 correlated with reversal learning performance (Groman et al., 2013). In relation to the previous discussion on the role of OFC in reversal learning, note that Area 47 is outside and laterally adjacent to the areas considered to constitute the OFC in rhesus monkeys. Nonetheless, this result does fit with recent studies suggesting that areas outside of the OFC may be necessary for reversal learning in macaques (Rudebeck et al., 2013).
Specific serotonergic manipulations have ranged from 1) region-selective neuron destruction and depletions (e.g., in the OFC or amygdala) (Park et al., 1994, Rogers et al., 1999, Clark et al., 2004, Clarke et al., 2005, Clarke et al., 2007, Finger et al., 2007, West et al., 2013, Ochoa et al., 2015, Rygula et al., 2015), 2) serotonin receptor blockade (Boulougouris and Robbins, 2010), 3) treatment with serotonin reuptake inhibitors (Brigman et al., 2010b, Brown et al., 2012, Furr et al., 2012a, Wallace et al., 2014), to 4) phenotyping gene deletions and polymorphisms (Homberg et al., 2007, Izquierdo et al., 2007, Vallender et al., 2009, Brigman et al., 2010b, Jedema et al., 2010). A consistent finding to emerge from this work has been that reduced serotonin signaling (in the cortex, rather than striatum) increases perseveration and impairs reversal learning, whereas increasing serotonin generally facilitates reversal learning (Clarke et al., 2004).
There are, however, problems with this blanket conclusion, most notably because specific serotonin receptor subtypes have differential effects. For instance, in rats antagonism of 5-HT2A (via MDL 100–907) impairs reversal performance, while 5-HT2C antagonism (with SB 242084) facilitates performance (Boulougouris et al., 2008, Boulougouris and Robbins, 2010, Furr et al., 2012b, Nilsson et al., 2012), though there is also a report of an overall impairment after this same treatment (Alsio et al., 2015). Performance effects of serotonergic manipulations are likely also influenced by sensory modality, whether ligands are administered centrally or peripherally, and the amount of pre-reversal training allowed. As an example of the latter, post-training probabilistic spatial reversal learning is impaired after 5,7-DHT lesions in rats (Bari et al., 2010a) yet rats failed to learn to touch stimuli to bring about reward after PCPA administration, before any training with contingencies (Izquierdo et al., 2012).
There is then the question of the cognitive processes by which serotonin modulates reversal learning. Some authors have proposed that serotonin signaling affects punishment or absence-of-reward processing by subcortical structures, but also regulates processes ascribed to cortical regions, such as outcome-tracking (Dayan and Huys, 2009, van der Schaaf et al., 2011, den Ouden et al., 2013). The relative balance between these effects may vary with the duration of serotonin alterations, with acute manipulations increasing the gain on negative feedback, whereas chronic treatments affect reward sensitivity (Bari et al., 2010b, Rygula et al., 2014). In rats, BLA serotonin depletions failed to affect deterministic reversal performance, possibly due to the timing of the manipulation relative to training and testing (Ochoa et al., 2015). However, when Rygula and colleagues compared the effects of localized serotonin depletions on a probabilistic visual reversal task in marmosets they found impairments after amygdala depletions were related to increased sensitivity to misleading reward and punishment, while OFC depletion impairments were associated with poor response suppression (Rygula et al., 2014). In summary, serotonergic tone modulates reversal learning across species and appears to do so, at least in part, through affecting processing in OFC.
Dopamine
Dopamine mediates synaptic plasticity in brain regions subserving optimal reversal learning performance, notably the cortex and striatum (Reynolds and Wickens, 2002, Cagniard et al., 2006, Calabresi et al., 2007). In addition, dopamine neurons encode so-called reward prediction errors that coincide with violations in expected outcomes (Schultz, 2013), and can be manipulated to generate and disrupt various forms of learning (Tsai et al., 2009, Adamantidis et al., 2011, Steinberg and Janak, 2013). Indeed, optogenetic excitation of dopamine neurons in the ventral tegmental area (VTA) or substantia nigra pars compacta (SNc) improves spatial reversal learning performance in rats (Adamantidis et al., 2011, Rossi et al., 2013). It is perhaps surprising then that, in contrast to serotonin depletions, depleting dopamine content in the OFC did not disrupt touchscreen reversal learning in marmosets (Clarke et al., 2007). Conversely, depleting dopamine, but not serotonin, in the marmoset striatum led to non-perseverative reversal impairments (Clarke et al., 2011).
This dissociation suggests a prominent role of striatal dopamine in cognitive flexibility – a contention supported by recent empirical data. In human subjects, methylphenidate has been shown to improve reversal learning specifically when it results in an increase in striatal dopamine levels (Clatworthy et al., 2009). Using fast-scan cyclic voltammetry to measure phasic dopamine transients in the rat nucleus accumbens, Klanker and colleagues found phasic dopamine increases in response to unexpected rewards (positive prediction error) during a cued lever-based reversal learning paradigm (Klanker et al., 2015). This fits with the observation that striatal dopamine transporter binding correlates with individual differences in the use of positive feedback during reversal learning (Stolyarova et al., 2014). Together, these findings suggest dopamine might serve as a neurochemical substrate for the use of Bayesian prior beliefs in reversal learning whereby, as discussed earlier, performance is guided by evidence of reversal occurring from prior experience (Jang et al., 2015).
An important corollary question is that of the relative roles of specific dopamine receptor subtypes in reversal learning. A study in mice demonstrated early touchscreen visual-cue reversal deficits following systemic treatment with a D1 receptor agonist (SKF81297) (Izquierdo et al., 2006). The locus of this systemic drug effect remains to be determined, but the nucleus accumbens and BLA are prime candidates. One salient finding here is that BLA-NAc projections invigorate reward-related behaviors in a D1 receptor-dependent manner (Stuber et al., 2011). Whether a similar subcortical D1 receptor mechanism contributes to the early stages of reversal learning performance when new cue-reward associations are being formed is yet to be determined. There is also the outstanding question of cortical D1 receptors given their singular contribution to other forms of cognition (Floresco, 2013, Wass et al., 2013).
Regarding other dopamine receptor subtypes, low dopamine D2 receptor availability correlates with poor reversal learning in mice (Laughlin et al., 2011), monkeys (Groman et al., 2011) and humans (Jocham et al., 2009). Furthermore, D2 receptor gene deletion impairs reversal learning performance in mice (Kruzich and Grandy, 2004, Kruzich et al., 2006, De Steno and Schmauss, 2009), while either D2/D3 receptor agonists (quinpirole, 7-OH-DPAT) or antagonists (raclopride), impair spatial and olfactory reversals in rats (Boulougouris et al., 2009), monkeys (Smith et al., 1999, Lee et al., 2007) or healthy human volunteers (Mehta et al., 2001). These findings suggest that reversal learning depends upon an optimal balance of dopamine D2 receptor function (Izquierdo et al., 2012).
Experience with reward events may calibrate the impact of subsequent events on D2 receptor signaling; events that may be negative or suboptimal by comparison, as in the case of acute drug-withdrawal (Thompson et al., 2015a). More generally, this role is consistent with the idea that dopamine tone scales the performance of previously learned behavior and that poor reward conditions, as experienced in early reversal learning, are characterized by weaker dopamine signaling, co-occurring with D2 receptor activation (Cagniard et al., 2006, Calabresi et al., 2007). In support of this, in monkeys with extensive experience on a two-arm bandit reversal task, neither systemic administration of L-Dopa or haloperidol affected learning, but haloperidol led to increased reliance on prior beliefs about when a reversal would occur and monkeys displayed anticipatory switching (Costa et al., 2015).
Glutamate: N-methyl-D-aspartate receptors (NMDAR)
Glutamate is key substrate for synaptic plasticity and many forms of learning. Consequently, a number of investigators have examined the role of the NMDAR, as well as other glutamate receptors and transporters (Karlsson et al., 2009, Barkus et al., 2012), in reversal learning and other forms of cognitive flexibility (for review, see (Jentsch and Roth, 1999). This work has led to reports that acute, systemic NMDAR blockade (with MK-801) can cause impairments in spatial reversal learning (Lobellova et al., 2013), but also fail to affect reversal performance and instead disrupt set-shifting (Svoboda et al., 2015). The literature on the effects of subchronic NMDAR antagonism with phencyclidine (PCP) (considered a potential model of schizophrenia (Brigman et al., 2010a) is also equivocal. While early studies found reversal learning impairments with acute or subchronic PCP treatment in rodents (Abdul-Monim et al., 2007), more recent reports find no change (Brigman et al., 2009, Janhunen et al., 2015) or an improvement in late-stage reversal learning (Dix et al., 2010, Fellini et al., 2014, McAllister et al., 2015). As with other apparently discrepant data in the reversal literature, the potential for methodological variation to explain some of these differences should not be discounted.
Using gene manipulations and pharmacology, some of these studies have attempted to parse the role of the specific subunits that constitute the heteromeric NMDAR complex and influence its function. Brain-wide deletion of the GluN2A subunit was found to impair touchscreen-based reversal learning in mice; a phenotype that likely reflected a general learning deficit given it was accompanied by impaired discrimination and occurred late in reversal when learning, rather than flexibility, is presumed to be taxed (Brigman et al., 2008, Marquardt et al., 2014). As with any gene knockout approach, the extent to which constitutive GluN2A deletion can be attributed solely to loss of the subunit, and not to more generalized alterations in NMDAR function, is unclear. However, a pharmacological approach to targeting NMDAR subunits, particularly GluN2A, also has issues relating to specificity (Neyton and Paoletti, 2006). Notwithstanding, a very recent study failed to find a deficit in reversal in a touchscreen location-based task following acute systemic treatment with a GluN2A preferring antagonist (NVP-AAM077), whereas treatment with either a subunit non-specific blocker (MK-801) or a GluN2B antagonist (CP 101–606) impaired the same behavior (Kumar et al., 2015).
The ability of GluN2B antagonism or gene deletion to impair reversal learning performance has now been reported across a number of behavioral paradigms. Dalton and colleagues demonstrated that systemic administration of a GluN2B blocker (Ro 25–6891) increased perseveration in a spatial location reversal task (Dalton et al., 2011), without affecting initial discrimination learning. Brigman and colleagues also found selective, perseveration-driven, (touchscreen visual-cue) reversal deficits following cortex-wide GluN2B deletion (Radke et al., 2015) or local blockade of GluN2B (via Ro 25–6981) in the lateral OFC (Brigman et al., 2013, Thompson et al., 2015b). With more widespread forebrain GluN2B deletion or localized antagonism in the dorsolateral striatum, the same authors found that deficits extended to discrimination learning – suggesting GluN2B expressed in different brain regions has dissociable roles in discrimination and reversal phases of a stimulus reversal learning task.
These studies of GluN2B raise the question of whether NMDARs, more generally, exert effects on reversal in a region-specific manner. Antagonizing NMDARs (with MK-801 or AP-5) in the DMS of adult or weanling rats impairs spatial reversal learning (Palencia and Ragozzino, 2004, Watson and Stanton, 2009). Ragozzino and colleagues found that these deficits were accompanied by decreases in acetylcholine efflux (Palencia and Ragozzino, 2006) and could be mimicked by blocking muscarinic M1 (via pirenzepine) or stimulating M2 (via oxotremorine sesquifumurate), but not by antagonizing nicotinic (via mecamylamine), acetylcholine receptors in the DMS (Tzavos et al., 2004, McCool et al., 2008, Ragozzino et al., 2009). Somewhat surprisingly, given this evidence of a key role for DMS NMDARs in reversal, a recent study found no effect of DMS NMDAR antagonism (via AP5) on a visual-cue reversal learning task that was impaired by NMDAR blockade in the NAc (Ding et al., 2014). It may be that differences in the way reversal was tested across these studies accounts for this discrepancy, but further investigation would be needed to draw any firm conclusions.
Concluding remarks
In this review we summarized evidence that reversal learning paradigms generally test the ability to learn specific stimulus-outcome associations, to estimate the likelihood that reversals can occur given accumulated evidence, and/or to generate a representation of option or task space. More investigation is needed to design behavioral tasks to extract and further describe the neural mechanisms of these individual processes, which should then allow even better comparisons between species and across paradigms. With the availability of higher resolution neuroscience techniques, it should be possible to assign cell type- specific roles to each process and therefore, to distinct aspects of cognitive flexibility.
Highlights.
-
-
Reversal learning is a widely used test of cognitive flexibility across species
-
-
The idea that this learning primarily measures response inhibition has been revised
-
-
We describe how it is measured and present new definitions for its construct validity
-
-
We also present an update of the brain regions and neurotransmitters that support it
Acknowledgments
We are very grateful to Dr. Russel Morton for constructing some of the figures and helpful comments from the Izquierdo lab on a previous version of this manuscript. AI is supported by the UCLA Division of Life Sciences Recruitment and Retention fund. JLB is supported by NIAAA grant 1P50AA022534-01. PHR is supported by generous seed funds from the Icahn School of Medicine at Mount Sinai and a Brain and Behavior Research Foundation Young Investigator NARSAD award. AKR and AH are supported by the NIAAA intramural research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors report no conflicts of interest.
References
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsio J, Nilsson SR, Gastambide F, Wang RA, Dam SA, Mar AC, Tricklebank M, Robbins TW. The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: a cross-site study. Psychopharmacology (Berl) 2015;232:4017–4031. doi: 10.1007/s00213-015-3963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Seeburg PH, Rawlins JNP. GluR-A-Deficient mice display normal acquisition of a hippocampus-dependent spatial reference memory task but are impaired during spatial reversal. Behavioral neuroscience. 2003;117:866. doi: 10.1037/0735-7044.117.4.866. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010a;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010b;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, Kiselycznyk C, Schmitt W, Sanderson DJ, Rawlins JN, Saksida LM, Bussey TJ, Sprengel R, Bannerman D, Holmes A. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–1272. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RL, Alsio J, Jupp B, Rabinovich R, Shrestha S, Roberts AC, Robbins TW, Dalley JW. Markers of serotonergic function in the orbitofrontal cortex and dorsal raphe nucleus predict individual variation in spatial-discrimination serial reversal learning. Neuropsychopharmacology. 2015;40:1619–1630. doi: 10.1038/npp.2014.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battig K, Rosvold HE, Mishkin M. Comparison of the effects of frontal and caudate lesions on discrimination learning in monkeys. J Comp Physiol Psychol. 1962;55:458–463. doi: 10.1037/h0047328. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez MA, Schultz W. Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity) J Neurophysiol. 2010;103:1158–1170. doi: 10.1152/jn.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double Dissociation of the Effects of Medial and Orbital Prefrontal Cortical Lesions on Attentional and Affective Shifts in Mice. Journal of Neuroscience. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. Orbital prefrontal cortex and guidance of instrumental behaviour in rats under reversal conditions. Behav Brain Res. 2003;143:49–56. doi: 10.1016/s0166-4328(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2009;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural brain research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hauber W. The dorsomedial striatum mediates flexible choice behavior in spatial tasks. Behav Brain Res. 2011;220:288–293. doi: 10.1016/j.bbr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nature Neuroscience. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Graybeal C, Holmes A. Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front Neurosci. 2010a;4 doi: 10.3389/neuro.01.013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A. Effects of Subchronic Phencyclidine (PCP) Treatment on Social Behaviors, and Operant Discrimination and Reversal Learning in C57BL/6J Mice. Front Behav Neurosci. 2009;3:2. doi: 10.3389/neuro.08.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010b;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Rothblat LA. Stimulus specific deficit on visual reversal learning after lesions of medial prefrontal cortex in the mouse. Behav Brain Res. 2008;187:405–410. doi: 10.1016/j.bbr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Brown HD, Amodeo DA, Sweeney JA, Ragozzino ME. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. J Psychopharmacol. 2012;26:1443–1455. doi: 10.1177/0269881111430749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce CA, Howland JG. Stress facilitates late reversal learning using a touchscreen-based visual discrimination procedure in male Long Evans rats. Behav Brain Res. 2015;278:21–28. doi: 10.1016/j.bbr.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk JA, Mair RG. Effects of dorsal and ventral striatal lesions on delayed matching trained with retractable levers. Behav Brain Res. 2001;122:67–78. doi: 10.1016/s0166-4328(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Burke KA, Takahashi YK, Correll J, Leon Brown P, Schoenbaum G. Orbitofrontal inactivation impairs reversal of Pavlovian learning by interfering with ‘disinhibition’of responding for previously unrewarded cues. European Journal of Neuroscience. 2009;30:1941–1946. doi: 10.1111/j.1460-9568.2009.06992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablations of frontal cortex in rhesus monkeys. Exp Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Cai X, Padoa-Schioppa C. Contributions of orbitofrontal and lateral prefrontal cortices to economic choice and the good-to-action transformation. Neuron. 2014;81:1140–1151. doi: 10.1016/j.neuron.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Castañé A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behavioural brain research. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BK, Sallet J, Papageorgiou GK, Noonan MP, Bell AH, Walton ME, Rushworth MF. Contrasting Roles for Orbitofrontal Cortex and Amygdala in Credit Assignment and Learning in Macaques. Neuron. 2015;87:1106–1118. doi: 10.1016/j.neuron.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH, Murray EA. The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cerebral cortex. 2013;23:2884–2898. doi: 10.1093/cercor/bhs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Kralik JD, Murray EA. Rhesus monkeys with orbital prefrontal cortex lesions can learn to inhibit prepotent responses in the reversed reward contingency task. Cereb Cortex. 2007;17:1154–1159. doi: 10.1093/cercor/bhl025. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. The Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins T. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain and cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Clarke H, Walker S, Crofts H, Dalley J, Robbins T, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. The Journal of Neuroscience. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron J-C, Fryer TD. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. The Journal of Neuroscience. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. The Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova CA, Jackson D, Langdon KD, Hewlett KA, Corbett D. Impaired executive function following ischemic stroke in the rat medial prefrontal cortex. Behavioural Brain Research. 2014;258:106–111. doi: 10.1016/j.bbr.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Costa VD, Tran VL, Turchi J, Averbeck BB. Reversal learning and dopamine: a bayesian perspective. J Neurosci. 2015;35:2407–2416. doi: 10.1523/JNEUROSCI.1989-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts H, Muggleton N, Bowditch A, Pearce P, Nutt D, Scott E. Home cage presentation of complex discrimination tasks to marmosets and rhesus monkeys. Laboratory animals. 1999;33:207–214. doi: 10.1258/002367799780578174. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Ma LM, Phillips AG, Floresco SB. Blockade of NMDA GluN2B receptors selectively impairs behavioral flexibility but not initial discrimination learning. Psychopharmacology. 2011;216:525–535. doi: 10.1007/s00213-011-2246-z. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG, Floresco SB. Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. The Journal of Neuroscience. 2014;34:4618–4626. doi: 10.1523/JNEUROSCI.5058-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Doya K. The computational neurobiology of learning and reward. Curr Opin Neurobiol. 2006;16:199–204. doi: 10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Dayan P, Berridge KC. Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cogn Affect Behav Neurosci. 2014;14:473–492. doi: 10.3758/s13415-014-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Huys QJ. Serotonin in affective control. Annual review of neuroscience. 2009;32:95–126. doi: 10.1146/annurev.neuro.051508.135607. [DOI] [PubMed] [Google Scholar]
- De Steno DA, Schmauss C. role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden HE, Daw ND, Fernandez G, Elshout JA, Rijpkema M, Hoogman M, Franke B, Cools R. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80:1090–1100. doi: 10.1016/j.neuron.2013.08.030. [DOI] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci U S A. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Qiao Y, Piao C, Zheng X, Liu Z, Liang J. N-methyl-D-aspartate receptor-mediated glutamate transmission in nucleus accumbens plays a more important role than that in dorsal striatum in cognitive flexibility. Front Behav Neurosci. 2014;8:304. doi: 10.3389/fnbeh.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology (Berl) 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999;12:961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Fellini L, Kumar G, Gibbs S, Steckler T, Talpos J. Re-evaluating the PCP challenge as a pre-clinical model of impaired cognitive flexibility in schizophrenia. Eur Neuropsychopharmacol. 2014;24:1836–1849. doi: 10.1016/j.euroneuro.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain : a journal of neurology. 2003a;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003b;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal--thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Experimental brain research. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Buzas B, Kamel N, Rhodes R, Vythilingham M, Pine DS, Goldman D, Blair JR. The impact of tryptophan depletion and 5-HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks. Neuropsychopharmacology. 2007;32:206–215. doi: 10.1038/sj.npp.1301182. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of general psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Frontiers in neuroscience. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012a;15:1295–1305. doi: 10.1017/S1461145711001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2012b;15:1295–1305. doi: 10.1017/S1461145711001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cerebral Cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiology of learning and memory. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Bachu M, Mozhui K, Saksida LM, Bussey TJ, Sagalyn E, Williams RW, Holmes A. Strains and stressors: an analysis of touchscreen learning in genetically diverse mouse strains. PLoS One. 2014;9:e87745. doi: 10.1371/journal.pone.0087745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Seu E, Crawford MA, Harpster SN, Jentsch JD. Monoamine levels within the orbitofrontal cortex and putamen interact to predict reversal learning performance. Biological psychiatry. 2013;73:756–762. doi: 10.1016/j.biopsych.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JDJ. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. 2011 doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D, Brigman JL. Behavioral flexibility in rats and mice: Contributions of distinct frontocortical regions. Genes Brain Behav. 2014 doi: 10.1111/gbb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Brigman JL. Behavioral flexibility in rats and mice: Contributions of distinct frontocortical regions. Genes, Brain and Behavior. 2015 doi: 10.1111/gbb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O’Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Pattij T, Janssen MCW, Ronken E, De Boer SF, Schoffelmeer ANM, Cuppen E. Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. The European journal of neuroscience. 2007;26:2066–2073. doi: 10.1111/j.1460-9568.2007.05839.x. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty JE, Bramham J, Rolls ET, Morris RG, Bullock P, Polkey C. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Cognitive Neuroscience, Journal of. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hvoslef-Eide M, Mar A, Nilsson S, Alsiö J, Heath C, Saksida L, Robbins T, Bussey T. The NEWMEDS rodent touchscreen test battery for cognition relevant to schizophrenia. Psychopharmacology. 2015;232:3853–3872. doi: 10.1007/s00213-015-4007-x. [DOI] [PubMed] [Google Scholar]
- Ineichen C, Sigrist H, Spinelli S, Lesch K-P, Sautter E, Seifritz E, Pryce CR. Establishing a probabilistic reversal learning test in mice: evidence for the processes mediating reward-stay and punishment-shift behaviour and for their modulation by serotonin. Neuropharmacology. 2012;63:1012–1021. doi: 10.1016/j.neuropharm.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Carlos K, Ostrander S, Rodriguez D, McCall-Craddolph A, Yagnik G, Zhou F. Impaired reward learning and intact motivation after serotonin depletion in rats. Behavioural brain research. 2012;233:494–499. doi: 10.1016/j.bbr.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Darling C, Manos N, Pozos H, Kim C, Ostrander S, Cazares V, Stepp H, Rudebeck PH. Basolateral amygdala lesions facilitate reward choices after negative feedback in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4105–4109. doi: 10.1523/JNEUROSCI.4942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Newman TK, Higley JD, Murray EA. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. The Journal of Neuroscience. 2004a;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004b;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Jang AI, Costa VD, Rudebeck PH, Chudasama Y, Murray EA, Averbeck BB. The Role of Frontal Cortical and Medial-Temporal Lobe Brain Areas in Learning a Bayesian Prior Belief on Reversals. J Neurosci. 2015;35:11751–11760. doi: 10.1523/JNEUROSCI.1594-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen SK, Svard H, Talpos J, Kumar G, Steckler T, Plath N, Lerdrup L, Ruby T, Haman M, Wyler R, Ballard TM. The subchronic phencyclidine rat model: relevance for the assessment of novel therapeutics for cognitive impairment associated with schizophrenia. Psychopharmacology (Berl) 2015;232:4059–4083. doi: 10.1007/s00213-015-3954-6. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Molecular psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. The Journal of Neuroscience. 2009;29:3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus--reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira K. The relation between reinforcement learning parameters and the influence of reinforcement history on choice behavior. J Math Psychol. 2015;66:59–69. [Google Scholar]
- Kawai T, Yamada H, Sato N, Takada M, Matsumoto M. Roles of the Lateral Habenula and Anterior Cingulate Cortex in Negative Outcome Monitoring and Behavioral Adjustment in Nonhuman Primates. Neuron. 2015;88:792–804. doi: 10.1016/j.neuron.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Reese RM, Woods CA, Janak PH. The orbitofrontal cortex as part of a hierarchical neural system mediating choice between two good options. J Neurosci. 2013;33:15989–15998. doi: 10.1523/JNEUROSCI.0026-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of learning and memory. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanker M, Post G, Joosten R, Feenstra M, Denys D. Deep brain stimulation in the lateral orbitofrontal cortex impairs spatial reversal learning. Behavioural Brain Research. 2013;245:7–12. doi: 10.1016/j.bbr.2013.01.043. [DOI] [PubMed] [Google Scholar]
- Klanker M, Sandberg T, Joosten R, Willuhn I, Feenstra M, Denys D. Phasic dopamine release induced by positive feedback predicts individual differences in reversal learning. Neurobiol Learn Mem. 2015;125:135–145. doi: 10.1016/j.nlm.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki Y, Watanabe S. Dissociable roles of the medial prefrontal cortex, the anterior cingulate cortex, and the hippocampus in behavioural flexibility revealed by serial reversal of three-choice discrimination in rats. Behavioural Brain Research. 2012;227:81–90. doi: 10.1016/j.bbr.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grandy DK. Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC neuroscience. 2004;5:12. doi: 10.1186/1471-2202-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Mitchell SH, Younkin A, Grandy DK. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cognitive, affective & behavioral neuroscience. 2006;6:86–90. doi: 10.3758/cabn.6.1.86. [DOI] [PubMed] [Google Scholar]
- Kumar G, Talpos J, Steckler T. Strain-dependent effects on acquisition and reversal of visual and spatial tasks in a rat touchscreen battery of cognition. Physiol Behav. 2015;144:26–36. doi: 10.1016/j.physbeh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach M, Caetano MS, Narayanan NS. Mistakes were made: neural mechanisms for the adaptive control of action initiation by the medial prefrontal cortex. J Physiol Paris. 2015;109:104–117. doi: 10.1016/j.jphysparis.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biological psychiatry. 2011;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian B, Rogers R, Hodges J, Robbins T. Discrimination, reversal, and shift earning in Huntington’s disease: mechanisms of impaired response selection. Neuropsychologia. 1999;37:1359–1374. doi: 10.1016/s0028-3932(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biological psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shao J. Restricted lesions to ventral prefrontal subareas block reversal learning but not visual discrimination learning in rats. Physiology & Behavior. 1998;65:371–379. doi: 10.1016/s0031-9384(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Lobellova V, Entlerova M, Svojanovska B, Hatalova H, Prokopova I, Petrasek T, Vales K, Kubik S, Fajnerova I, Stuchlik A. Two learning tasks provide evidence for disrupted behavioural flexibility in an animal model of schizophrenia-like behaviour induced by acute MK-801: a dose-response study. Behav Brain Res. 2013;246:55–62. doi: 10.1016/j.bbr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Mar AC, Horner AE, Nilsson SR, Alsiö J, Kent BA, Kim CH, Holmes A, Saksida LM, Bussey TJ. The touchscreen operant platform for assessing executive function in rats and mice. Nature protocols. 2013;8:1985–2005. doi: 10.1038/nprot.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable Effects of Lesions to Orbitofrontal Cortex Subregions on Impulsive Choice in the Rat. Journal of Neuroscience. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt K, Saha M, Mishina M, Young JW, Brigman JL. Loss of GluN2A–containing NMDA receptors impairs extra-dimensional set-shifting. Genes, brain, and behavior. 2014;13:611–617. doi: 10.1111/gbb.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki D, Yokoyama C, Kinoshita S, Tsuchida H, Nakatomi Y, Yoshimoto K, Fukui K. Relationship between limbic and cortical 5-HT neurotransmission and acquisition and reversal learning in a go/no-go task in rats. Psychopharmacology. 2006;189:249–258. doi: 10.1007/s00213-006-0559-0. [DOI] [PubMed] [Google Scholar]
- McAllister KA, Mar AC, Theobald DE, Saksida LM, Bussey TJ. Comparing the effects of subchronic phencyclidine and medial prefrontal cortex dysfunction on cognitive tests relevant to schizophrenia. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4018-7. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian B, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D2 agonist bromocriptine in human volunteers. Psychopharmacology. 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Meunier M, Jaffard R, Destrade C. Differential involvement of anterior and posterior cingulate cortices in spatial discriminative learning in a T-maze in mice. Behavioural brain research. 1991;44:133–143. doi: 10.1016/s0166-4328(05)80018-x. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar output influences non-motor function. Mol Psychiatry. 1996;1:429–433. [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orbitofrontal Cortical Neurons Encode Expectation-Driven Initiation of Reward-Seeking. Journal of Neuroscience. 2014;34:10234–10246. doi: 10.1523/JNEUROSCI.3216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Dolan R. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22:372–380. doi: 10.1016/j.neuroimage.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. Re-valuing the amygdala. Curr Opin Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Gaffan D. Prospective memory in the formation of learning sets by rhesus monkeys (Macaca mulatta) Journal of experimental psychology Animal behavior processes. 2006;32:87–90. doi: 10.1037/0097-7403.32.1.87. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cerebral Cortex. 2001;11:85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- Neill JC, Sarkisian MR, Wang Y, Liu Z, Yu L, Tandon P, Zhang G-r, Holmes GL, Geller AI. Enhanced auditory reversal learning by genetic activation of protein kinase C in small groups of rat hippocampal neurons. Molecular brain research. 2001;93:127–136. doi: 10.1016/s0165-3806(01)00204-8. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SRO, Ripley TL, Somerville EM, Clifton PG. Reduced activity at the 5-HT(2C) receptor enhances reversal learning by decreasing the influence of previously non-rewarded associations. Psychopharmacology. 2012;224:241–254. doi: 10.1007/s00213-012-2746-5. [DOI] [PubMed] [Google Scholar]
- Ochoa JG, Stolyarova A, Kaur A, Hart EE, Bugarin A, Izquierdo A. Post-training depletions of basolateral amygdala serotonin fail to disrupt discrimination, retention, or reversal learning. Frontiers in neuroscience. 2015;9:155. doi: 10.3389/fnins.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C. Orbitofrontal cortex and the computation of economic value. Ann N Y Acad Sci. 2007;1121:232–253. doi: 10.1196/annals.1401.011. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiology of learning and memory. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The effect of N-methyl-D-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neuroscience. 2006;143:671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Coull J, McShane R, Young A, Sahakian B, Robbins T, Cowen P. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–588. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Nakazawa K, Holmes A. Cortical GluN2B deletion attenuates punished suppression of food reward-seeking. Psychopharmacology. 2015;232:3753–3761. doi: 10.1007/s00213-015-4033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behavioral neuroscience. 2003;117:1054. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behavioral neuroscience. 2007;121:698. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26:609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Archives of general psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Reynolds JNJ, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural networks : the official journal of the International Neural Network Society. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rhodes SE, Murray EA. Differential effects of amygdala, orbital prefrontal cortex, and prelimbic cortex lesions on goal-directed behavior in rhesus macaques. J Neurosci. 2013;33:3380–3389. doi: 10.1523/JNEUROSCI.4374-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riceberg JS, Shapiro ML. Reward stability determines the contribution of orbitofrontal cortex to adaptive behavior. J Neurosci. 2012;32:16402–16409. doi: 10.1523/JNEUROSCI.0776-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MJ, Critchlow HM, Lloyd A, Cilia J, Clarke JD, Bond B, Jones DN, Maycox PR. Differential expression of IEG mRNA in rat brain following acute treatment with clozapine or haloperidol: a semi-quantitative RT-PCR study. J Psychopharmacol. 2008;22:536–542. doi: 10.1177/0269881107081521. [DOI] [PubMed] [Google Scholar]
- Rogers R, Blackshaw A, Middleton H, Matthews K, Hawtin K, Crowley C, Hopwood A, Wallace C, Deakin J, Sahakian B. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology. 1999;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Andrews TK, Grasby P, Brooks D, Robbins T. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Cognitive Neuroscience, Journal of. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of neurology, neurosurgery, and psychiatry. 1994a;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery & Psychiatry. 1994b;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH. Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One. 2013;8:e65799.. doi: 10.1371/journal.pone.0065799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014;84:1143–1156. doi: 10.1016/j.neuron.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Clarke HF, Cardinal RN, Cockcroft GJ, Xia J, Dalley JW, Robbins TW, Roberts AC. Role of Central Serotonin in Anticipation of Rewarding and Punishing Outcomes: Effects of Selective Amygdala or Orbitofrontal 5-HT Depletion. Cereb Cortex. 2015;25:3064–3076. doi: 10.1093/cercor/bhu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Clarke HF, Cardinal RN, Cockcroft GJ, Xia J, Dalley JW, Robbins TW, Roberts ACTH. Role of Central Serotonin in Anticipation of Rewarding and Punishing Outcomes: Effects of Selective Amygdala or Orbitofrontal 5- Cereb Cortex pii: bhu102. 2014 doi: 10.1093/cercor/bhu102. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Rigotti M, Ostojic S, Fusi S, Salzman CD. Abstract Context Representations in Primate Amygdala and Prefrontal Cortex. Neuron. 2015;87:869–881. doi: 10.1016/j.neuron.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar RF, White W, Lacroix L, Feldon J, White IA. NMDA lesions in the medial prefrontal cortex impair the ability to inhibit responses during reversal of a simple spatial discrimination. Behavioural Brain Research. 2004;152:413–424. doi: 10.1016/j.bbr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci. 2009a;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews Neuroscience. 2009b;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci. 2003;23:9833–9841. doi: 10.1523/JNEUROSCI.23-30-09833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe K, Enkel T, Klein S, Schutte M, Koch M. Effects of neonatal lesions of the medial prefrontal cortex on adult rat behaviour. Behavioural Brain Research. 2004;153:21–34. doi: 10.1016/j.bbr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology. 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CL, Watson GDR, Hallock HL, Cline KM, Griffin AL. The role of the medial prefrontal cortex in the acquisition, retention, and reversal of a tactile visuospatial conditional discrimination task. Behavioural Brain Research. 2013;236:94–101. doi: 10.1016/j.bbr.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Neill JC, Costall B. The dopamine D3/D2 receptor agonist 7-OH-DPAT induces cognitive impairment in the marmoset. Pharmacology, biochemistry, and behavior. 1999;63:201–211. doi: 10.1016/s0091-3057(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nature neuroscience. 2015;18:620–627. doi: 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Janak PH. Establishing causality for dopamine in neural function and behavior with optogenetics. Brain research. 2013;1511:46–64. doi: 10.1016/j.brainres.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Passingham RE. The nucleus accumbens in monkeys (Macaca fascicularis) Experimental brain research. 1995;106:239–247. doi: 10.1007/BF00241119. [DOI] [PubMed] [Google Scholar]
- Stolyarova A, O’Dell SJ, Marshall JF, Izquierdo A. Positive and negative feedback learning and associated dopamine and serotonin transporter binding after methamphetamine. Behavioural brain research. 2014;271:195–202. doi: 10.1016/j.bbr.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Barto A. Reinforcement Learning. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Svoboda J, Stankova A, Entlerova M, Stuchlik A. Acute administration of MK-801 in an animal model of psychosis in rats interferes with cognitively demanding forms of behavioral flexibility on a rotating arena. Front Behav Neurosci. 2015;9:75. doi: 10.3389/fnbeh.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson R, Rogers R, Sahakian B, Summers B, Polkey C, Robbins T. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- Thompson AB, Stolyarova A, Ying Z, Zhuang Y, Gomez-Pinilla F, Izquierdo A. Methamphetamine blocks exercise effects on Bdnf and Drd2 gene expression in frontal cortex and striatum. Neuropharmacology. 2015a;99:658–664. doi: 10.1016/j.neuropharm.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Josey M, Holmes A, Brigman JL. Conditional loss of GluN2B in cortex and hippocampus impairs attentional set formation. Behav Neurosci. 2015b;129:105–112. doi: 10.1037/bne0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Experimental brain research. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavos A, Jih J, Ragozzino ME. Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsomedial striatum on response reversal learning. Behav Brain Res. 2004;154:245–253. doi: 10.1016/j.bbr.2004.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallender EJ, Lynch L, Novak MA, Miller GM. Polymorphisms in the 3’ UTR of the serotonin transporter are associated with cognitive flexibility in rhesus macaques. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2009;150B:467–475. doi: 10.1002/ajmg.b.30835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaaf ME, Warmerdam E, Crone EA, Cools R. Distinct linear and non-linear trajectories of reward and punishment reversal learning during development: Relevance for dopamine’s role in adolescent decision making. Developmental cognitive neuroscience. 2011;1:578–590. doi: 10.1016/j.dcn.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers D. Evidence for an accumulator model of psychophysical discrimination. Ergonomics. 1970;13:37–58. doi: 10.1080/00140137008931117. [DOI] [PubMed] [Google Scholar]
- Walker S, Robbins T, Roberts A. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cerebral Cortex. 2009;19:889–898. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A, Pehrson AL, Sánchez C, Morilak DA. Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2014;17:1695–1706. doi: 10.1017/S1461145714000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Buckley MJ, Rudebeck PH, Rushworth MF. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–939. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass C, Pizzo A, Sauce B, Kawasumi Y, Sturzoiu T, Ree F, Otto T, Matzel LD. Learning & memory. Vol. 20. NY: Cold Spring Harbor; 2013. Dopamine D1 sensitivity in the prefrontal cortex predicts general cognitive abilities and is modulated by working memory training; pp. 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A. The basolateral amygdala in reward learning and addiction. Neuroscience and biobehavioral reviews. 2015 doi: 10.1016/j.neubiorev.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Stanton ME. Spatial discrimination reversal learning in weanling rats is impaired by striatal administration of an NMDA-receptor antagonist. Learn Mem. 2009;16:564–572. doi: 10.1101/lm.1448009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Forcelli PA, McCue DL, Malkova L. Differential effects of serotonin-specific and excitotoxic lesions of OFC on conditioned reinforcer devaluation and extinction in rats. Behavioural brain research. 2013;246:10–14. doi: 10.1016/j.bbr.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Seo B-W, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicology and teratology. 2003;25:459–471. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Wilson CR, Gaffan D. Prefrontal-inferotemporal interaction is not always necessary for reversal learning. J Neurosci. 2008;28:5529–5538. doi: 10.1523/JNEUROSCI.0952-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Shapiro ML. Double Dissociation and Hierarchical Organization of Strategy Switches and Reversals in the Rat PFC. Behavioral Neuroscience. 2009;123:1028–1035. doi: 10.1037/a0016822. [DOI] [PMC free article] [PubMed] [Google Scholar]