Abstract
The endoplasmic reticulum (ER) is a prime mediator of cellular signaling due to its functions as an internal cellular store for calcium, as well as a site for synthesis of proteins and lipids. Its peripheral network of sheets and tubules facilitate calcium and lipid signaling, especially in areas of the cell that are more distant to the main cytoplasmic network. Specific membrane proteins shape the peripheral ER architecture and influence the network stability in order to project into restricted spaces. The signaling microdomains are anatomically separate from the cytoplasm as a whole and exhibit localized protein, ion channel and cytoskeletal element expression. Signaling can also occur between the ER and other organelles, such as the Golgi or mitochondria. Lipids made in the ER membrane can be sent to the Golgi via specialized transfer proteins and specific phospholipid synthases are enriched at ER-mitochondria junctions to more efficiently expedite phospholipid transfer. As a hub for protein and lipid synthesis, a store for intracellular calcium [Ca2+]i, and a mediator of cellular stress, the ER is an important cellular organelle. Its ability to organize into tubules and project into restricted spaces allows for discrete and temporal signaling, which is important for cellular physiology and organism homeostasis.
Keywords: endoplasmic reticulum, intracellular signaling, lipid transfer, calcium microdomains
Introduction
The endoplasmic reticulum (ER) is the largest organelle in most cells and is present in all cells but mature erythrocytes. For over five decades, it has been recognized as a key source for internal sequestration of calcium and protein translation. Although the lumen and calcium store within the ER are essentially continuous (Park et al., 2000; Wu and Bers, 2006), the ER is divided into distinct cellular regions: rough ER (rER, characterized by ribosomes on the surface), smooth ER (sER), and a third region, comprised in part by the nuclear membrane.
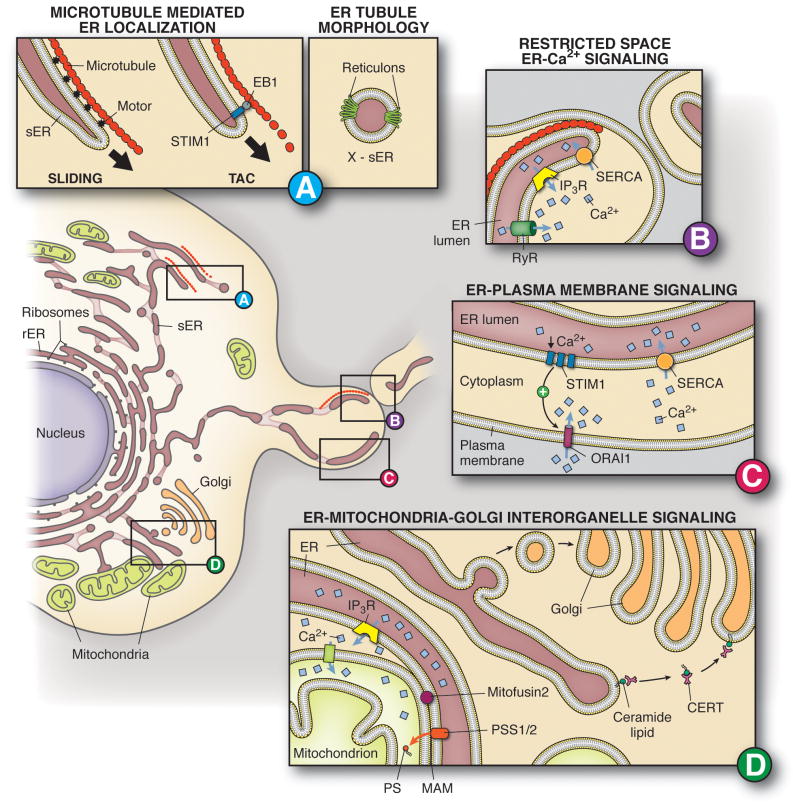
The ER consists of a membranous network of tubules and sheets extending throughout the cytoplasm (Figure 1A) with the extent of the organelle dependent on the cell's function. For example, secretory cells like pancreatic acinar cells have large amounts of rER and little sER (Palade, 1956). Furthermore, the ER in a specific cell type can remodel based on changing cellular needs. When B-lymphocytes detect an antigen and transition to become plasma cells and secrete antibodies, their rough ER network is greatly upregulated, as measured by increases in ER resident proteins (van Anken et al., 2003). Interestingly, this large peripheral network exhibits independent, dynamic changes in tubule formation and degradation as part of its normal physiology (Lee and Chen, 1988). The specialized design of the peripheral ER in this manner (Figure 1A) allows for localized signaling within specific, restricted areas of the cell. There are a number of polarized or localized molecules in the ER [(i.e., calcium related proteins (Figure 1B, C), lipid synthases (Figure 1D)] that have integral roles in signal transduction to and from the organelle. Specific localization of these molecules maintains normal cellular homeostasis, primarily by influencing lipid synthesis, trafficking and imparting strict spatial and temporal control over intracellular calcium ([Ca2+]i). The close apposition of ER to other cellular organelles, including mitochondria and the Golgi apparatus (Figure 1D), promotes lipid and ion flux between cellular compartments. In addition to its essential roles in lipid and calcium homeostasis, peripheral ER networks may provide convenience in localized protein translation. Based on these observations, the ER is central to cellular signaling microdomains.
Figure 1. Endoplasmic reticulum localization and signaling in microdomains.
A. Examples of microtubule (red circle) mediated ER trafficking, (1) ER sliding and (2) Tip Attachment Complexes (TAC). Reticulons mediate ER tubule curvature by insertion into the membrane in a hairpin fashion. B. Spatially restricted calcium signaling that occurs from the ER. This is characterized by expression of specific receptors (IP3R, RyR) and cytoskeletal elements (microtubules). C. ER-PM signaling as illustrated by STIM1-Orai1 interactions to refill the ER. D. Interorganelle signaling occurs in a restricted space. (1) ER to Golgi transfer of lipids can occur via an unconventional, non-vesicular (Ceramide Transfer Protein, CERT) method. (2) The mitochondrial associated membrane (MAM) is a distinct section of the ER membrane tethered to the mitochondria by proteins and is enriched with IP3R and phospholipid synthases (Phosphatidylserine synthase 1 and 2, PSS 1/2) to facilitate ER-mitochondria signaling.
This review discusses the processes and molecules that comprise ER-mediated signaling and highlights those that occur in restricted spaces of the cell. For our purposes, we have defined a restricted cellular space as an anatomical area of the cell that is distinctly separate from the cytoplasm of the rest of the cell, usually encompassing a small projection of a cell or nanometer domains between organelle membranes. These cellular microdomains are identified by expression of specific proteins, receptors and/or ion channels (Billaud et al., 2014; Caceres et al., 1984; Cerqua et al., 2010). Numerous examples can be found throughout an organism; we will focus on dendritic spines of neurons (Bourne and Harris, 2008), plasma membrane-sarcoplasmic reticulum (SR) junctions in myocytes (Wang et al., 2001), and myoendothelial junctions in endothelial cells (Billaud et al., 2014). The ER is further able to form unique signaling microdomains in close contact with Golgi, mitochondrial, and plasma membranes, which are integral to calcium and lipid homeostasis (Figure 1C, D).
Regulation of ER function and morphology
Physiology dictates that structure facilitates function. An understanding of ER tubules and sheets, their formation and how the ER subsequently projects into restricted cellular spaces is essential to understanding signaling in localized areas. The specialized nature of peripheral ER structural organization defies standard energetically favorable organelle formation (Terasaki et al., 2013; Voeltz et al., 2006) (e.g., a spheroid shape), so there must be some reasoning for the sheets and tubules to form. Some disagreement exists, but it is typically thought that the rER is found in sheet form while the sER is comprised mostly of tubules (Shibata et al., 2010; Shibata et al., 2006). This may be due to the canonical views of each as hubs for protein manufacturing and calcium signaling, respectively. Moreover, it is likely that the functions of the ER are segregated based on the presence or absence of ribosomes, as the highly curved tubules may not be able to accommodate ribosomal complexes. Based on the preponderance of ribosomes and their central function in protein translation, the primary function of rER has been ascribed to protein synthesis. Conversely, peripheral ER tubules, characterized as sER, primarily control calcium signaling, manufacturing of lipids and often reside in close proximity to other organelles, thus promoting interorganelle signaling (Hu et al., 2011).
The ability of ER to signal in restricted cellular spaces is dependent on the structural flexibility of the ER membrane. For a theoretical model of how this occurs, see (Shemesh et al., 2014).] In peripheral ER tubules, there is enriched expression of reticulons (Figure 1A) and receptor expression enhancing proteins (also referred to as DP1 and REEPs), ER membrane proteins (Bjork et al., 2013) that interact to promote ER membrane curvature and tubule formation (Hu et al., 2008; Voeltz et al., 2006). The caveolin proteins that form curved caveolae signaling domains in some plasma membranes are analogous in function. Mechanistically, the curvature occurs due to the proteins' two hydrophobic transmembrane segments of ∼30 amino acids that insert into the outer leaflet of the ER membrane to form “hairpin”-like segments (Shibata et al., 2010; Voeltz et al., 2006). The hairpin segments do not make up the entirety of the tubule, but are stable and oligomerized (Shibata et al., 2008) in a scaffold or arc-like fashion, thereby only required to make up 10 percent of the tubule membrane surface (Hu et al., 2011). Without reticulons, cells express ER predominantly in the form of sheets; overexpression of reticulons leads to more cells with tubular ER (Voeltz et al., 2006). The hairpin domains of reticulons and REEPs are highly conserved across species (Hu et al., 2011), further underscoring the importance of ER tubule formation. Once formed, these tubules can fuse to make three-way junctions with each other (Park and Blackstone, 2010) that expand the ER network.
Junctions are important for movement or equilibration of ions and other molecules with the entirety of the cellular ER network. Tubular fusion to create the junctions is mediated by a group of proteins known as atlastins, which also interact with reticulons and REEPs (Hu et al., 2009). Atlastins are integral ER membrane GTPases which require GTP binding to promote tubule fusion. Overexpression or depletion of atlastins leads to long unbranched ER tubules and less three way tubular junctions, respectively (Hu et al., 2009). The transmembrane protein Lunapark1 (Lnp1) is also localized to three way junctions in order to dynamically regulate the ER network. Without Lnp1 in yeast cells, the ER network collapses into a more sheet-like morphology and Lnp1 expression at the tubular ER junctions is dependent on the yeast homolog of atlastin (Sey1p) (Chen et al., 2012). This translates to higher organisms as the same researchers determined that Lnp1 helps maintain ER structure in mammalian cells. Only 50% of junctions expressed Lnp1, but those that had Lnp1 were less dynamic (Chen et al., 2015), indicating that junctions can form without Lnp1 but that they are less stable.
Recent work has shown ADP-ribosylation factor-like 6 interacting protein 1 (Arl6IP1), a protein that binds to atlastin, is similar to the reticulons and localizes to the ER membrane in the same hairpin fashion. This could provide another mechanism to bend the ER membrane beyond the reticulons Indeed, overexpression of Arl6IP1 led to an excess of ER tubule formation and promoted tubule stability, similar to what has been reported with reticulons (Yamamoto et al., 2014).
While there may be more molecules involved in ER structure and maintenance, Rab5, a Rab GTPase, is also essential for normal tubule formation and its deletion from C. elegans shows similar effects on ER morphology as deletion of reticulons and REEPs (Audhya et al., 2007). However, in contrast to the role of atlastin in binding to reticulons and REEPs, Rab5 was not shown to have direct interaction with either. Rab5 is localized on endosomal membranes, and its ability to contact the ER in this manner may account for its effects on ER formation (Audhya et al., 2007). This observation begins to highlight the importance of interorganelle contacts for ER distribution as well as signaling.
The cytoskeleton provides assistance with ER distribution as the microtubules of the cell facilitate localization of ER tubules in restricted spaces via several different routes. The first is the use of microtubule motors like kinesin (Feiguin et al., 1994; Wozniak et al., 2009), dynein (Wozniak et al., 2009) and myosin-Va (Bridgman, 1999) that promote ER tubule movement and localization along acylated microtubules (Figure 1A). This has been shown to occur in small spaces such as neuronal axons and dendritic spines (Wagner et al., 2011). Movement along established microtubules, regardless of motor presence, is termed “ER sliding” (Waterman-Storer and Salmon, 1998). Other organelles such as mitochondria have been shown to utilize this same method of localization (Friedman et al., 2010), a possible explanation for why ER is often localized with mitochondria in the periphery of the cell. The second route relies on formation of tip attachment complexes (TAC) between the ER and the plus end of growing microtubules, which can make up approximately one fourth of the ER tubule extension that occurs in cells (Grigoriev et al., 2008). TAC relies on the direct interaction of the cytosolic C terminus of an integral ER membrane protein, stromal interaction molecule 1 (STIM1; a calcium sensor in the ER) and a protein found on the plus end of microtubules, EB1 (Grigoriev et al., 2008). This was discovered via live cell imaging of the two fluorescently tagged proteins, which showed STIM1 localized with the end of the growing microtubule in a “comet-like” manner (Grigoriev et al., 2008).
Like STIM1, the integral ER membrane protein cytoskeleton-linking membrane protein, (climp) 63, has a cytosolic domain that can bind to microtubules (Klopfenstein et al., 1998) but unlike STIM1, is restricted to ER sheets. Formation of sheets is increased with expression of climp 63, which is restricted from the tubules (Shibata et al., 2010) and nuclear envelope due to its alpha helical segment that protrudes into the ER lumen (Klopfenstein et al., 2001). When climp 63 is depleted, the morphology of the sheets is disrupted and the luminal space becomes smaller. This interaction between climp 63 and microtubules seems to be more for stabilization and holding the ER in place, rather than a method of ER movement. However, depolymerization of microtubules and actin with a variety of pharmacological compounds still results in a diffuse ER network in Xenopus egg extracts (Dreier and Rapoport, 2000). The functional effect of this was not fully investigated, so the resultant network may have impaired signaling. In addition to ER-microtubule interactions, receptors in the ER membrane like the inositol 1, 4, 5-triphosphate receptor (IP3R) have been shown to associate with actin filaments (Grayson et al., 2004). Actin may serve a similar function as the microtubules and helps to localize the IP3R in regions that are deficient in microtubules. Despite the evidence given above for the ER reliance on the cytoskeleton, it appears peripheral ER tubules may still form and travel without the cytoskeleton, possibly with other cellular components or signals.
The maintenance of ER structure and promotion of its localization to small spaces also relies on the lipid content of the membrane. There are only nominal concentrations of cholesterol present in the ER membrane (Lange et al., 1999); the ratio of cholesterol to phospholipid composition is 0.15 compared to 1.0 in the plasma membrane (van Meer et al., 2008; Zambrano et al., 1975). When cholesterol levels increase, membrane fluidity decreases (Ikonen, 2008), so this differential composition is likely advantageous for the complex folding and tubulation, plus the dynamic extension and retraction exhibited by the ER. An accumulation of cholesterol in the ER membranes of macrophages activates the unfolded protein response and leads to apoptosis (Feng et al., 2003), supporting the evidence that amounts of cholesterol should be kept at low levels in the ER membrane. Sphingolipids are an important group of membrane lipids synthesized in the ER, but present in low quantities in the ER membrane. Their presence and packing decreases membrane fluidity (van Meer and Lisman, 2002) similar to cholesterol and higher concentrations may hinder the normal formation of ER tubules and sheets.
Lipid synthesis, trafficking and signaling centered around the ER
The roles of cellular lipids can be grouped into several diverse functions. Lipids in the form of lipid droplets, among other functions, store energy for the organism (Farese and Walther, 2009). Amphipathic lipids such as phospholipids are responsible for providing compartmentalization of organelles and individual cells. Additionally, bioactive lipids and regional membrane composition of lipids create membrane domains that further facilitate signaling, especially in the plasma membrane.
Lipids have a symbiotic relationship with the ER. Inositol 1,4,5 triphosphate (IP3) initiates calcium release by binding to its specific receptor on the ER membrane. Phosphatidic acid can make glycerophospholipids such as phosphatidylserine, phosphatidylcholine, phosphatidylinositol and phosphatidylethanolamine in the ER (Lev, 2012). Cholesterol, a vital component of cell membranes, is made by the ER membrane localized HMG-CoA reductase (Liscum et al., 1985). The de novo pathway of sphingolipid synthesis occurs within the ER as well (Fagone and Jackowski, 2009). Once lipids are synthesized, the ER can export them to other organelles or the plasma membrane via a non-vesicular process.
Lipid transfer proteins like ceramide transfer protein (CERT) are a part of the non-vesicular pathway. Ceramide is a sphingolipid, itself central to sphingolipid metabolism and synthesized in the ER. It requires transportation to the Golgi in order to be metabolized into sphingomyelin or more complex glycosphingolipids. The structure of CERT is divided into three domains: one domain targets the ER membrane with an FFAT motif (Loewen et al., 2003), another targets the trans Golgi membrane, and the C domain, containing a steroidogenic acute regulatory protein transport (StART) site (Ponting and Aravind, 1999), solubilizes ceramide so that it may be transported between the organelles (Figure 1D). CERT is very specific for various chain lengths of ceramide; sphingosine and sphingomyelin are not transported despite their ability to be converted to ceramide (Kumagai et al., 2005). In lieu of a vesicular mechanism, ceramide is taken out of the ER membrane by CERT and moved to the trans Golgi (Hanada et al., 2003).
The reasons why ceramide needs CERT to be trafficked this way out of the ER are not clear, but it has been hypothesized that an excess of ceramide leads to a stiffer cell membrane, thereby impeding ceramide transport in vesicles (Levine, 2004). If ceramide traveled to the cis Golgi via vesicles, it could be glycosylated by ceramide glucosyltransferase (Levine, 2004) into a more complex sphingolipid, thus complicating its metabolism back to sphingomyelin in the trans Golgi. By limiting the vesicular transfer and targeting the distal portion of the Golgi, CERT may be mitigating other homeostatic processes in the cell.
LY-A cells, a mutant Chinese hamster ovarian cell line, have a defect in the transfer of ceramide to the Golgi, resulting in lower sphingomyelin levels that cannot be explained by differences in synthesis or sphingomyelin precursor levels. This was determined to be due to a mutation in the LY-A CERT protein (Fukasawa et al., 1999). Additionally, ceramide is not the only lipid that utilizes non-vesicular mechanisms to leave the ER. Phosphatidylinositol and phosphatidylcholine are chaperoned by phosphatidylinositol transfer proteins (PITPs) which relocate them to the PM, or any other membrane deficient in either lipid species (Cockcroft, 2001).
Sterol regulatory element binding proteins (SREBP) and cholesterol synthesis
Sterol regulatory element-binding proteins are transcription factors that are the gatekeepers for activation of genes responsible for cholesterol and fatty acid synthesis. In order to maintain lipid homeostasis, SREBPs are inhibited by high levels of sterols. The membrane sterols change the protein conformation of a SREBP-associated molecule, the SREBP cleavage activating protein (SCAP) (Brown et al., 2002). When more sterol synthesis is required, SCAP becomes a chaperone for SREBP to the Golgi apparatus and the two are “sorted” into budding COPII vesicles (characterized by the expression of vesicular stomatitis virus glycoprotein). In order to leave the ER, SCAP complexes with the vesicular COPII proteins [for example, Sec23/24 and potentially Sar1B, which is known to mediate export of SREBP-2 from the ER (Fryer et al., 2014)]. Increased sterols physically inhibit the interaction to prevent Golgi transport and continued sterol synthesis (Espenshade et al., 2002). There seems to be a number of ways to regulate this signaling, as the ER proteins Insig-1 and 2 negatively regulate the SCAP/SREBP complex by binding to SCAP and keeping the complex retained in the ER membrane under the presence of high cholesterol levels in the cell (Yabe et al., 2002; Yang et al., 2002). Furthermore, the flavonoid known as xanthohumol can inhibit SREBP by preventing SCAP binding to the Sec23/24 COPII proteins and decrease expression of genes that promote cholesterol synthesis (Miyata et al., 2015). Importantly, these molecular events can have whole animal physiological consequences. Mice fed a high fat diet supplemented with xanthohumol were significantly less obese and had less fat accumulation in their livers (Miyata et al., 2015).
Once SCAP and SREBP are transferred to the Golgi, they encounter Site 1 and 2 serine proteases S1P and S2P anchored in the Golgi apparatus (Bartz et al., 2008; Nohturfft et al., 1999; Sakai et al., 1998). Their active sites are within the Golgi lumen, where they cleave the nSREBP domain from its precursor. Once the nSREBP is cleaved, it travels to the nucleus to activate transcription of lipogenic genes. During mitosis, the spatial separation between ER and the Golgi prevents SREBP from becoming activated and turning off lipogenic gene transcription (Bartz et al., 2008). SREBPs upregulate the synthesis of HMG-CoA reductase, another ER membrane resident protein and the rate-limiting enzyme for cholesterol synthesis. Thus, the control of lipid synthesis and proper transfer or trafficking outside the ER can occur in a restricted manner within the cell and is important for overall cellular homeostasis.
Molecules responsible for ER calcium signaling in cellular microdomains
A central tenet of the restricted space definition is the localized expression of specific receptors, ion channels, ion pumps and proteins that occurs in the ER and on the ER membrane. The IP3R is one of these molecules important for ER signaling modulation, as it is a tetrameric calcium channel with six transmembrane domains present in the ER membrane. Its ligand, IP3, is a well-characterized second messenger formed after the hydrolysis of phosphatidylinositol 4,5-bisphosphate by phospholipase C. In one important study, vesicles with IP3R were incorporated into planar lipid bilayers and membrane potential was recorded after addition of calcium and IP3. This data confirmed the IP3R is only activated upon addition of IP3 and the current from the channel increases with an increase in [IP3]. Although the IP3R is dependent on IP3, the presence of calcium is needed, as IP3 cannot activate the channel alone. The activity of the channel (in the presence of IP3) was low at both low and high concentrations of calcium. It seems there is a ideal concentration of calcium (∼0.25uM) that correlates with max channel activity, represented in a bell curve manner (Bezprozvanny et al., 1991). In restricted spaces, this property can allow the IP3R to regulate its response more selectively to respond to physiological levels of calcium.
There are three isoforms of the IP3 receptors, known for localized ER expression but it is not known if this is a cause or result of the heterogeneity of calcium stores that has been proposed to exist in the ER (Montero et al., 1997; Vermassen et al., 2004). There is also marked heterogeneity of expression between cell types (Wojcikiewicz, 1995). Within the three isoforms, addition of molecules like ATP can enhance IP3R activity and calcium mobilization to an extent. Addition of ATP increased calcium signaling in only the first and third isoforms of IP3R, suggesting differential regulation of activity between isoforms (Miyakawa et al., 1999). The isoforms also have different sensitivities, or tendency to release calcium in response to low concentrations of IP3; IP3R2 has the highest sensitivity, followed by IP3R1 & IP3R3. Subtype co-localization leads to an additive sensitivity to IP3 and one isoform becomes more dominant in response to ATP or calcium (Miyakawa et al., 1999). This is important for specific localization of isoforms, as they can have differential effects on the calcium signal or response to IP3 in the restricted space.
The location of the IP3R has been suggested to be mobile within the ER membrane; depending on the calcium status of the cell, the various isoforms can move within the lipid bilayer to cluster in specific areas (Vermassen et al., 2004). Clusters of IP3R have been observed upon increases in [IP3], relevant for the local calcium signaling in microdomains. As [IP3] increases, the number of IP3R opening also increases, which results in sequentially larger calcium signals into the cytoplasm. However, this paradigm of IP3R mobility within the ER membrane has been questioned. Instead, clusters of IP3R may be pre-localized (rather than moving to that location upon increases in IP3) in order to quickly release calcium (Smith et al., 2009). Ultimately, keeping the increases in IP3 restricted to a specific area prevents the signal from propagating throughout the cell and tissue. Cells can use inositol polyphosphate-5-phosphatase to compartmentalize calcium release by degradation of IP3 to IP2 (Hansen et al., 1987; Mitchell et al., 1989) and the phosphatase activity depends on the amount of both IP3 and calcium (Sims and Allbritton, 1998).
There is more than one way to elicit calcium release from the ER or sarcoplasmic reticulum (SR); IP3 is not the only second messenger in this physiological process. Extracellular calcium, magnesium, ATP and a number of adenine nucleotides (e.g., ADP, AMP, cAMP, adenosine) that enter the cell are able to interact with ryanodine receptors (RyR), calcium gated calcium channels on the ER and SR membrane (Lanner et al., 2010). RyR are the largest known ion channels and at ∼2MDa, are roughly twice the size of IP3R. This extraordinary size facilitates a much higher amount of calcium released per opening (Bezprozvanny, 1996) and allows more space for interaction with regulatory and modulatory proteins. For example, RyR are part of a macromolecular complex in the SR that includes voltage gated calcium channels Cav1.1/Cav1.2, protein kinase alpha, FKBP12 and 12.6, triadin, junction, and calsequestrin (Lanner et al., 2010). Both the RyR and IP3R have specific calmodulin and calcium-calmodulin kinase II binding sites (Bezprozvanny, 1996), suggesting similar patterns of regulation.
Despite their large size and multitude of modulatory proteins, the RyR can be localized in myocytes to areas of the SR in close contact with the sarcolemma. The resultant localized calcium signals from RyR have been called sparks, due to their appearance with calcium indicators, and in order to distinguish them from other localized calcium signals that are caused via IP3R opening. The enrichment of RyR facilitates the localized sparks that occur in cardiomyocytes (Cheng et al., 1993) and vascular smooth muscle (Nelson et al., 1995) and can be blocked with micromolar concentrations of ryanodine. The IP3R and RyR are two of the more important channels regulating calcium signaling in restricted cellular spaces and have adapted to do this in a number of cell types.
ER-mediated calcium signaling in restricted spaces
Cytosolic concentrations of calcium are physiologically kept at nanomolar levels, but the sequestered ER calcium concentration can create localized calcium microdomains into a specific area of the cell. Typically these areas consist of an ER projection into a cellular extension or the presence of the ER membrane within tens of nanometers from the PM. The structure and specific localization of proteins facilitate the restricted calcium signaling that occurs in these small spaces and this signaling has important physiological consequences (Billaud et al., 2014). Elementary calcium release events occur in the microdomains, but can be coupled together, as in the case of cardiomyocytes, to produce a more significant effect on the cell.
Structure of restricted spaces involved in calcium signaling
There are a number of cell types that utilize restricted spaces to localize signaling and concentrate ER expression in these areas. For example, neurons in many areas of the brain are more likely to exhibit dendrites that have spines, including the hippocampus, cerebellum, and cortex. There are a number of advantages to the addition of spines to dendrites. They make more connections with other neurons, promote plasticity within the brain and prolong calcium signals (Park et al., 2008), indicating the importance of the ER that is localized to the dendritic spine (Emptage et al., 1999; Wagner et al., 2011). They can be differently shaped depending on the head versus neck diameter of the projection (i.e. mushroom-like, branched, stubby, thin) (Bourne and Harris, 2008), which could cause differential signaling by slowing or speeding up calcium diffusion.
This spine-like morphology is not limited to the nervous system. Endothelial cells, which are polarized like neurons with an apical and basal side, exhibit similar projections which occur primarily in the walls of small resistance arteries (Heberlein et al., 2009). They send projections basally across the internal elastic lamina to make contact with the opposing smooth muscle cells, creating signaling domains known as myoendothelial junctions (MEJ) that have ER projections (Isakson, 2008; Isakson et al., 2007; Ledoux et al., 2008; Toussaint et al., 2015). There is evidence for microtubular presence in the MEJ, important for trafficking protein-mRNA complexes (Heberlein et al., 2012), but this may also be a mechanism of ER localization to the MEJ. An example of ER at MEJs is demonstrated in Figure 2.
Figure 2. An example of endoplasmic reticulum in restricted spaces, the myoendothelial junction.
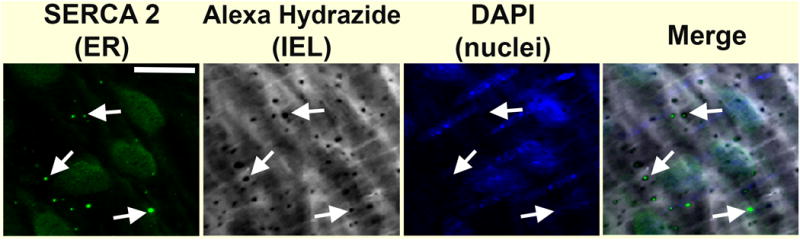
An antibody against SERCA was used as a marker for endoplasmic reticulum (ER). In this example, thoracodorsal arteries were removed from a mouse, cut longitude to the resistance artery, and viewed en face. The arteries were immunolabelled for SERCA, isoform 2 (green), and counter stained with Alexa Hydrazide 647 to mark the internal elastic lamina (IEL) between endothelium and smooth muscle and reveal holes where myoendothelial junctions reside (grey). DAPI staining was used to mark endothelial and smooth muscle nuclei (blue). Arrows point to examples of ER in holes of the IEL, and scale bar is 10 μm.
Localized expression of calcium-handling proteins
A common theme, despite differences in cell type and physiological output, is the specific localization of proteins and receptors involved in calcium signaling to the restricted spaces. This expression primarily occurs due to a protein's specific association with the ER and confers the ability to initiate or terminate calcium signaling, which is vital to segregate signaling from the cytoplasm. The IP3R is one of the best examples of proteins involved in localized ER signaling as it has been shown to be localized in nerve terminals (Takei et al., 1992), dendritic spines (Wagner et al., 2011), at the MEJ (Isakson, 2008; Toussaint et al., 2015), and the nuclear envelope of cardiomyocytes (Bare et al., 2005). Because the IP3R is a transmembrane ER protein, it is also strategically located to interact and influence luminal protein localization.
Chaperone proteins within the lumen of the ER or SR can be highly localized to restricted spaces to participate in calcium signaling and buffering. At triadic junctions, where localized calcium signaling occurs in myocytes, calsequestrin is enriched, along with the ER transmembrane proteins triadin and junctin, and the three molecules form a macromolecular complex with the RyR (Terentyev et al., 2007). Calreticulin, an ER resident protein and calcium buffer (Michalak et al., 2009), is present in the MEJ (Biwer et al., 2014). This illustrates the capacity of calcium buffers to localize to specific parts of the ER lumen and potentially increase calcium storage within the reticular network. This in turn can modulate specific processes involved in calcium release. Ultimately, the combination of calcium release, calcium uptake, calcium sensing and calcium storage in restricted spaces is conferred by the localized expression of specific proteins.
Signaling processes: implications for function
Calcium as a signaling molecule has a broad range of actions: fertilization, proliferation, secretion, metabolism. However, in specific localized cellular regions, the effects of calcium signaling seem to be more limited to events that control contraction (cardiomyocytes, smooth muscle cell) and synaptic plasticity (neurons).
Calcium induced calcium release (CICR) is the foundation of excitation-contraction coupling and is a physiological process that is founded on signaling in a restricted space. It occurs in a gap between the SR and the plasma membrane that is roughly 12 nanometers wide. When an L-type calcium channel opens upon cellular depolarization, the extracellular calcium influx into this area activates roughly four RyR located on the SR. Using calcium imaging via a patch clamp technique combined with confocal microscopy, the opening of the calcium channel on the PM looks like a “sparklet”, a very small calcium relase signal, while the calcium leaving the SR appears as “sparks”, a localized but more robust calcium release (Wang et al., 2001). Combining the rich source of extracellular calcium with the sequestered SR source is a perfect design to spatially manage the calcium response within the cardiomyocyte, and ultimately the contractility of the heart.
CICR is not limited to myocytes. When a hippocampal pyramidal cell is stimulated with an electrode to depolarize the membrane, ionotropic NMDA receptors are activated by glutamate and glycine, triggering an excitatory, post synaptic calcium transient that is restricted to the dendritic spine. Cyclopentazoic acid, an inhibitor of the sarcoplasmic-endoplasmic reticulum ATP-ase, greatly diminished this transient, suggesting that the calcium transient needs a small calcium influx via NMDA activation to subsequently trigger calcium release from the ER (Emptage et al., 1999).
In a different pathway but with similar physiological effects, parallel fibers synapse on cerebellar Purkinje neurons, releasing glutamate that activates local metabotropic glutamate receptors (mGluR) located on the dendritic spines. Smooth ER localized here exhibits the presence of IP3R, which releases calcium in response to the IP3 generated via mGluR activation. The IP3R activation is confirmed as a delayed calcium response (it appeared after the CICR calcium signal) using confocal microscopy and inhibited by addition of heparin (Finch and Augustine, 1998). Spread of the signal was restricted, due to the short half life of IP3 combined with the limited physical space. The combination of depolarization and parallel fiber signaling, with ER calcium release is a hallmark of long term depression. The importance of the depolarization/mGluR generation of IP3 and calcium release from the ER signaling cascade in long term depression and synaptic plasticity is underscored by no changes in the excitatory post synaptic current or potentials of Purkinje neurons (e.g., no long term depression) of mGluR1 knockout mice (Aiba et al., 1994), inhibition of IP3R with heparin (Khodakhah and Armstrong, 1997) and in cerebellar neurons of IP3R1 knockout mice (Inoue et al., 1998).
Thus, restricted neuronal expression of ER and calcium signaling is important for structural plasticity of the brain, which relies on long term depression. Structural plasticity occurs when the architecture of the brain is physically changed as a result of learning (e.g., synapses move and strengthen). In the hippocampus, this can be directly related to learning and memory, while in the cerebellum it is related to motor learning and physical coordination.
Similar to structural plasticity in dendritic spines, repeated and restricted calcium signals in the vascular wall have important physiological outcomes and facilitate cell-cell communication. The MEJ is a small projection, approximately 0.5 μm wide and long, so localized calcium release from IP3R had been difficult to image for many years. Using a unique mouse model that has a calcium biosensor in the endothelium (GCaMP2) and small mesenteric resistance arteries, holes in the internal elastic lamina were, for the most part, associated with calcium pulsars, IP3R mediated calcium release event from the ER. These pulsars occur on a regular basis (about 270ms apart) and in the same locations (i.e. the holes in the IEL). In comparison to other restricted calcium signals in vascular smooth muscle, measurable pulsars are slower and have smaller amplitude. Pharmacological inhibition of mediators in the IP3-calcium release pathway showed that there is a basal level of IP3 in EC that can cause calcium pulsars (Ledoux et al., 2008). Other researchers have used pressurized rat mesenteric arteries with Oregon Green 488 BAPTA-AM dye to show that the ER mediates spontaneous calcium events at the MEJ (Kansui et al., 2008) and after smooth muscle stimulation with phenylephrine (Kansui et al., 2008). The exact mechanism and pathway for the ER-mediated signals is still under investigation: calcium/calmodulin kinase II has been suggested as one mediator of IP3R pulsars (Toussaint et al., 2015). ER calcium signals at the MEJ are an important way to allow communication between endothelium and smooth muscle. It is possible the physiological output of ER-mediated calcium release could be activation of calcium-activated potassium channels (e.g., (Dora et al., 2008; Sandow et al., 2006) and subsequent vasodilation. More work on this topic regarding receptor localization and function, especially in regards to the ER (e.g., (Billaud et al., 2014)) is certainly required.
ER signaling involving cross talk with other organelles
The ER is dispersed throughout the cell and as such makes connections with a number of other organelles (Toulmay and Prinz, 2011). These connections, typically separated by a 10-40nm distance, are purposefully designed to take advantage of other cellular resources and are likely sER due to a lack of ribosomes (Hu et al., 2011).
Mitochondria (Mitochondrial Associated Membrane)
Mitochondria are close partners to the ER and the two organelles can make contact at the mitochondrial associated membrane (MAM), which acts as a bridge between ER and mitochondria. It is estimated that 5-20 percent of the mitochondrial surface consists of MAM (Rizzuto et al., 1998) and that areas of mitochondrial division within the cell are concomitant with ER tubule presence (Friedman et al., 2011). Lipid and calcium transfer are the prime reasons for their association. The ER makes a number of lipid species [phosphatidylserine (Shiao et al., 1995), for example] that the mitochondria must import. Furthermore, increases or decreases in mitochondrial uptake of calcium via its uniporter can be a trigger for autophagy or apoptosis, respectively. The IP3R is clustered in this membrane (Olson et al., 2010) and a slight increase in calcium near the mitochondria can increase mitochondrial metabolism, causing increased synthesis of ATP. Mitochondria can also buffer the calcium signal caused by ER calcium release, which can lead to an increase in IP3R activity (Olson et al., 2010).
Another important function of the MAM is for phospholipid synthesis (Vance, 1990). A number of enzymes that synthesize lipids are located in this region, one specifically being phosphatidylserine synthase (Stone and Vance, 2000). Once phosphatidylserine is made in the MAM, it can easily be transported to the mitochondria nearby (Figure 1D). In contrast to the vesicular transfer of lipids described earlier, the MAM is able to transport lipids in a nonvesicular fashion, likely via movement within the membrane to the mitochondria.
Recent research has focused on proteins that play central roles in linking the membrane of the two organelles. One of them is trichoplein/mitofusin which interacts with Mitofusin 2, a GTPase localized to both the outer mitochondrial membrane and the ER, indicating its presence in the MAM (Cerqua et al., 2010; de Brito and Scorrano, 2008). In cells that lack mitofusin 2, calcium coupling between the ER and mitochondria is decreased, probably due to an increased distance between the organelles. Interestingly, these experiments indicated mitofusin 2 also regulates the structure of the ER network as the knockout cells exhibited fragmented ER (de Brito and Scorrano, 2008). Presenilin proteins 1 and 2 are found at the interface of the MAM and mutations in their structure are implicated in Alzheimer's disease. The second isoform is responsible in part for tethering ER to mitochondria and interorganelle calcium transfer (Zampese et al., 2011), so it is evident that the proteins controlling these interactions are important for normal cellular homeostasis.
Plasma Membrane
The ER-plasma membrane junction with a separation of 7-30nm was first described in excitable cells in regards to calcium flux and these observations remain highly relevant today (Carrasco and Meyer, 2011). Not only is the signaling that occurs between the ER-plasma membrane localized in a small space, but the junctions themselves could be polarized to a specific region of the cell, as only 5% of the PM has these junctions (Wu and Bers, 2006). Lipid transfer can occur between the membranes as well, but this has been shown solely in drosophila and yeast cells; its relevance to mammalian cell signaling and physiology is unclear.
Store operated calcium entry (SOCE) is calcium signaling that occurs in the space between the ER and plasma membrane, originating from the ER (Figure 1C). It is the main method of calcium entry in non-excitable cells but it was not until the past ten years that the proteins responsible, STIM 1/2 and Orai 1-3, were understood and integrated as part of the process. When ER calcium is depleted, by any means (IP3R-mediated, experimentally with thapsigargin or otherwise), SOCE occurs and extracellular calcium enters the cell. This is reflected in the emergence of the inward calcium release activating current (ICRAC) (Hoth and Penner, 1992). This current is due to the CRAC channel being permeable to calcium. The Orai gene encodes the CRAC channel and its proteins make up the pore of the channel that is responsible for the calcium influx. STIM1 is localized to the ER membrane as a single pass transmembrane protein, where it can make a physical interaction with the N and C termini of Orai1 (Park et al., 2009).
The mechanism by which SOCE occurs begins with the depletion of ER calcium and the ability of STIM1 to sense this deficit and oligomerize, thus setting off a signaling cascade in order to refill the ER calcium level (Liou et al., 2005). Its structure contains an EF hand, a structural domain that is known to bind calcium, in its ER-lumen located N-terminus. STIM1 translocates to an area of the ER near the PM (punctates corresponding to STIM1 appear within 10-25 nm of the PM) to subsequently activate the calcium release activated calcium (CRAC) current. When STIM1 is expressed alone and calcium is depleted, STIM1 punctates still appear localized near the PM. However, when GFP-tagged Orai1 is expressed alone and thapsigargin is added to the cell, no punctates are seen until both STIM1 and Orai1 are expressed together (Park et al., 2009).
The tubule design of the peripheral ER can be further appreciated in this context, as it was shown that deletion of reticulon 4 caused ER tubules to morph into sheets (Jozsef et al., 2014; Voeltz et al., 2006) and this affected the cellular SOCE but none of the other important ER functions (Jozsef et al., 2014).
Conclusion
Microdomains within the cell allow for compartmentalized ER signaling that can be separate from overall cell function or be integrated into a larger physiological response. Although energetically unfavorable, the formation of the diffuse tubules of the peripheral ER network allows for localization into restricted spaces of cells, such as neuronal dendritic spines and for contact with other organelles (Figure 1). The purpose of ER-mediated signaling within a small space permits molecules with vulnerability to degradation or modification, like IP3 or ceramide, to have a greater impact. Localized ER signaling saves energy and time as proteins, lipids and ions do not have to traverse large distances, allowing a faster, more efficient effect. The extensive and unique properties of the ER allow it to localize and enhance otherwise weak signals, which can have important functional effects on physiological processes within an organism.
Acknowledgments
This work was supported by National Institutes of Health grants HL088554 (B.E.I.), HL107963 (B.E.I.). The American Heart Association and National Institutes of Health training grant (HL007284) provided predoctoral (LB) fellowships that supported this work. We thank Jerome D. Biwer and Anita Impagliazzo for the illustration. We would also like to thank the Stiftelsen Nordisk Fysiologi (SNF) for generous support for the AP Symposium on “Endothelium-dependent hyperpolarizations 2015.”
Footnotes
Conflict of Interest: There is no conflict of interest to declare.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Audhya A, Desai A, Oegema K. A role for Rab5 in structuring the endoplasmic reticulum. The Journal of cell biology. 2007;178:43–56. doi: 10.1083/jcb.200701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. The Journal of biological chemistry. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- Bartz R, Sun LP, Bisel B, Wei JH, Seemann J. Spatial separation of Golgi and ER during mitosis protects SREBP from unregulated activation. The EMBO journal. 2008;27:948–955. doi: 10.1038/emboj.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. Inositol (1,4,5)-trisphosphate receptors: functional properties, modulation, and role in calcium wave propagation. Society of General Physiologists series. 1996;51:75–86. [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacological reviews. 2014;66:513–569. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biwer L, Straub A, Isakson B. Subcellular enrichment of calreticulin at myoendothelial junctions (664.2) The FASEB Journal. 2014;28:664–662. [Google Scholar]
- Bjork S, Hurt CM, Ho VK, Angelotti T. REEPs are membrane shaping adapter proteins that modulate specific g protein-coupled receptor trafficking by affecting ER cargo capacity. PloS one. 2013;8:e76366. doi: 10.1371/journal.pone.0076366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annual review of neuroscience. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. The Journal of cell biology. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Molecular cell. 2002;10:237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain research. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annual review of biochemistry. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqua C, Anesti V, Pyakurel A, Liu D, Naon D, Wiche G, Baffa R, Dimmer KS, Scorrano L. Trichoplein/mitostatin regulates endoplasmic reticulum-mitochondria juxtaposition. EMBO reports. 2010;11:854–860. doi: 10.1038/embor.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Desai T, McNew JA, Gerard P, Novick PJ, Ferro-Novick S. Lunapark stabilizes nascent three-way junctions in the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:418–423. doi: 10.1073/pnas.1423026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Novick P, Ferro-Novick S. ER network formation requires a balance of the dynamin-like GTPase Sey1p and the Lunapark family member Lnp1p. Nature cell biology. 2012;14:707–716. doi: 10.1038/ncb2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Phosphatidylinositol transfer proteins couple lipid transport to phosphoinositide synthesis. Seminars in cell & developmental biology. 2001;12:183–191. doi: 10.1006/scdb.2000.0235. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of Endothelial Cell KCa3.1 Channels During Endothelium-Derived Hyperpolarizing Factor Signaling in Mesenteric Resistance Arteries. CircRes. 2008 doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier L, Rapoport TA. In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. The Journal of cell biology. 2000;148:883–898. doi: 10.1083/jcb.148.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- Espenshade PJ, Li WP, Yabe D. Sterols block binding of COPII proteins to SCAP, thereby controlling SCAP sorting in ER. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11694–11699. doi: 10.1073/pnas.182412799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. Journal of lipid research. 2009;50(Suppl):S311–316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F, Ferreira A, Kosik KS, Caceres A. Kinesin-mediated organelle translocation revealed by specific cellular manipulations. The Journal of cell biology. 1994;127:1021–1039. doi: 10.1083/jcb.127.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature cell biology. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. The Journal of cell biology. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer LG, Jones B, Duncan EJ, Hutchison CE, Ozkan T, Williams PA, Alder O, Nieuwdorp M, Townley AK, Mensenkamp AR, Stephens DJ, Dallinga-Thie GM, Shoulders CC. The endoplasmic reticulum coat protein II transport machinery coordinates cellular lipid secretion and cholesterol biosynthesis. The Journal of biological chemistry. 2014;289:4244–4261. doi: 10.1074/jbc.M113.479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa M, Nishijima M, Hanada K. Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells. The Journal of cell biology. 1999;144:673–685. doi: 10.1083/jcb.144.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson TH, Haddock RE, Murray TP, Wojcikiewicz RJ, Hill CE. Inositol 1,4,5-trisphosphate receptor subtypes are differentially distributed between smooth muscle and endothelial layers of rat arteries. Cell Calcium. 2004;36:447–458. doi: 10.1016/j.ceca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr, Hoogenraad CC, Akhmanova A. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Current biology : CB. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hansen CA, Johanson RA, Williamson MT, Williamson JR. Purification and characterization of two types of soluble inositol phosphate 5-phosphomonoesterases from rat brain. The Journal of biological chemistry. 1987;262:17319–17326. [PubMed] [Google Scholar]
- Heberlein KR, Han J, Straub AC, Best AK, Kaun C, Wojta J, Isakson BE. A novel mRNA binding protein complex promotes localized plasminogen activator inhibitor-1 accumulation at the myoendothelial junction. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1271–1279. doi: 10.1161/ATVBAHA.112.246371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation. 2009;16:307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hu J, Prinz WA, Rapoport TA. Weaving the web of ER tubules. Cell. 2011;147:1226–1231. doi: 10.1016/j.cell.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, Rapoport TA, Prinz WA. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–561. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nature reviews Molecular cell biology. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5366–5373. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. Journal of cell science. 2008;121:3664–3673. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circulation research. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- Jozsef L, Tashiro K, Kuo A, Park E, Skoura A, Albinsson S, Rivera-Molina F, Harrison KD, Iwakiri Y, Toomre D, Sessa WC. Reticulon 4 Is Necessary for Endoplasmic Reticulum Tubulation, STIM1-Orai1 Coupling, and Store-operated Calcium Entry. The Journal of biological chemistry. 2014;289:9380–9395. doi: 10.1074/jbc.M114.548602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakhah K, Armstrong CM. Induction of long-term depression and rebound potentiation by inositol trisphosphate in cerebellar Purkinje neurons. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14009–14014. doi: 10.1073/pnas.94.25.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. The EMBO journal. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Klumperman J, Lustig A, Kammerer RA, Oorschot V, Hauri HP. Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha-helical segment. The Journal of cell biology. 2001;153:1287–1300. doi: 10.1083/jcb.153.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K. CERT mediates intermembrane transfer of various molecular species of ceramides. The Journal of biological chemistry. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- Lange Y, Ye J, Rigney M, Steck TL. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. Journal of lipid research. 1999;40:2264–2270. [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harbor perspectives in biology. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Chen LB. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- Lev S. Nonvesicular lipid transfer from the endoplasmic reticulum. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T. Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends in cell biology. 2004;14:483–490. doi: 10.1016/j.tcb.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current biology : CB. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L, Finer-Moore J, Stroud RM, Luskey KL, Brown MS, Goldstein JL. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. The Journal of biological chemistry. 1985;260:522–530. [PubMed] [Google Scholar]
- Loewen CJ, Roy A, Levine TP. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. The EMBO journal. 2003;22:2025–2035. doi: 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. The Biochemical journal. 2009;417:651–666. doi: 10.1042/BJ20081847. [DOI] [PubMed] [Google Scholar]
- Mitchell CA, Connolly TM, Majerus PW. Identification and isolation of a 75-kDa inositol polyphosphate-5-phosphatase from human platelets. The Journal of biological chemistry. 1989;264:8873–8877. [PubMed] [Google Scholar]
- Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. The EMBO journal. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Inoue J, Shimizu M, Sato R. Xanthohumol Improves Diet-induced Obesity and Fatty Liver by Suppressing Sterol Regulatory Element-binding Protein (SREBP) Activation. The Journal of biological chemistry. 2015;290:20565–20579. doi: 10.1074/jbc.M115.656975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Alvarez J, Scheenen WJ, Rizzuto R, Meldolesi J, Pozzan T. Ca2+ homeostasis in the endoplasmic reticulum: coexistence of high and low [Ca2+] subcompartments in intact HeLa cells. The Journal of cell biology. 1997;139:601–611. doi: 10.1083/jcb.139.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nohturfft A, DeBose-Boyd RA, Scheek S, Goldstein JL, Brown MS. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:11235–11240. doi: 10.1073/pnas.96.20.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ML, Chalmers S, McCarron JG. Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. The Journal of biological chemistry. 2010;285:2040–2050. doi: 10.1074/jbc.M109.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. The endoplasmic reticulum. The Journal of biophysical and biochemical cytology. 1956;2:85–98. doi: 10.1083/jcb.2.4.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Choi YM, Kang YK, Petersen OH. The endoplasmic reticulum as an integrator of multiple dendritic events. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2008;14:68–77. doi: 10.1177/1073858407305691. [DOI] [PubMed] [Google Scholar]
- Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca(2+) pool: visualization of rapid Ca(2+) movements and equilibration. The EMBO journal. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Blackstone C. Further assembly required: construction and dynamics of the endoplasmic reticulum network. EMBO reports. 2010;11:515–521. doi: 10.1038/embor.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends in biochemical sciences. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Sakai J, Rawson RB, Espenshade PJ, Cheng D, Seegmiller AC, Goldstein JL, Brown MS. Molecular identification of the sterol-regulated luminal protease that cleaves SREBPs and controls lipid composition of animal cells. Molecular cell. 1998;2:505–514. doi: 10.1016/s1097-2765(00)80150-1. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? JAnat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh T, Klemm RW, Romano FB, Wang S, Vaughan J, Zhuang X, Tukachinsky H, Kozlov MM, Rapoport TA. A model for the generation and interconversion of ER morphologies. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E5243–5251. doi: 10.1073/pnas.1419997111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. The Journal of biological chemistry. 1995;270:11190–11198. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA. Mechanisms determining the morphology of the peripheral ER. Cell. 2010;143:774–788. doi: 10.1016/j.cell.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Voeltz GK, Rapoport TA. Rough sheets and smooth tubules. Cell. 2006;126:435–439. doi: 10.1016/j.cell.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Voss C, Rist JM, Hu J, Rapoport TA, Prinz WA, Voeltz GK. The reticulon and DP1/Yop1p proteins form immobile oligomers in the tubular endoplasmic reticulum. The Journal of biological chemistry. 2008;283:18892–18904. doi: 10.1074/jbc.M800986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims CE, Allbritton NL. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate by the oocytes of Xenopus laevis. The Journal of biological chemistry. 1998;273:4052–4058. doi: 10.1074/jbc.273.7.4052. [DOI] [PubMed] [Google Scholar]
- Smith IF, Wiltgen SM, Shuai J, Parker I. Ca(2+) puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Science signaling. 2009;2 doi: 10.1126/scisignal.2000466. ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. The Journal of biological chemistry. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- Takei K, Stukenbrok H, Metcalf A, Mignery GA, Sudhof TC, Volpe P, De Camilli P. Ca2+ stores in Purkinje neurons: endoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:489–505. doi: 10.1523/JNEUROSCI.12-02-00489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M, Shemesh T, Kasthuri N, Klemm RW, Schalek R, Hayworth KJ, Hand AR, Yankova M, Huber G, Lichtman JW, Rapoport TA, Kozlov MM. Stacked endoplasmic reticulum sheets are connected by helicoidal membrane motifs. Cell. 2013;154:285–296. doi: 10.1016/j.cell.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Vedamoorthyrao S, Oduru S, Gyorke I, Williams SC, Gyorke S. Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. The Journal of physiology. 2007;583:71–80. doi: 10.1113/jphysiol.2007.136879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Current opinion in cell biology. 2011;23:458–463. doi: 10.1016/j.ceb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint F, Charbel C, Blanchette A, Ledoux J. CaMKII regulates intracellular Ca(2+) dynamics in native endothelial cells. Cell calcium. 2015;58:275–285. doi: 10.1016/j.ceca.2015.06.005. [DOI] [PubMed] [Google Scholar]
- van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- van Meer G, Lisman Q. Sphingolipid transport: rafts and translocators. The Journal of biological chemistry. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. The Journal of biological chemistry. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biology of the cell / under the auspices of the European Cell Biology Organization. 2004;96:3–17. doi: 10.1016/j.biolcel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Wagner W, Brenowitz SD, Hammer JA., 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nature cell biology. 2011;13:40–48. doi: 10.1038/ncb2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Current biology : CB. 1998;8:798–806. doi: 10.1016/s0960-9822(98)70321-5. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. The Journal of biological chemistry. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- Wozniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ. Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells. Journal of cell science. 2009;122:1979–1989. doi: 10.1242/jcs.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circulation research. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12753–12758. doi: 10.1073/pnas.162488899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Yoshida A, Miyazaki N, Iwasaki K, Sakisaka T. Arl6IP1 has the ability to shape the mammalian ER membrane in a reticulon-like fashion. The Biochemical journal. 2014;458:69–79. doi: 10.1042/BJ20131186. [DOI] [PubMed] [Google Scholar]
- Yang T, Espenshade PJ, Wright ME, Yabe D, Gong Y, Aebersold R, Goldstein JL, Brown MS. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- Zambrano F, Fleischer S, Fleischer B. Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochimica et biophysica acta. 1975;380:357–369. doi: 10.1016/0005-2760(75)90104-6. [DOI] [PubMed] [Google Scholar]
- Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2777–2782. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]