Abstract
Parasitological examination of marine perciform fishes belonging to four species of Carangoides, i.e. C. chrysophrys, C. dinema, C. fulvoguttatus and C. hedlandensis (Carangidae), from off New Caledonia revealed the presence of nematodes. The identification of carangids was confirmed by barcoding of the COI gene. The eight nematode species found were: Capillariidae gen. sp. (females), Cucullanus bulbosus (Lane, 1916) (male and females), Hysterothylacium sp. third-stage larvae, Raphidascaris (Ichthyascaris) sp. (female and larvae), Terranova sp. third-stage larvae, Philometra dispar n. sp. (male), Camallanus carangis Olsen, 1954 (females) and Johnstonmawsonia sp. (female). The new species P. dispar from the abdominal cavity of C. dinema is mainly characterised by the body length (5.14 mm), the lengths of markedly unequal spicules (163 and 96 μm) and gubernaculum (102 μm long) provided with a dorsal protuberance and a small, reflexed dorsal barb on its posterior portion. The finding of C. bulbosus represents the first record of this parasite a century after its discovery; the first study of this species by scanning electron microscopy (SEM) enabled detailed redescription. The finding of Johnstonmawsonia sp. in C. fulvoguttatus is the first record of a rhabdochonid nematode from a host belonging to the Carangidae family. Johnstonmawsonia africana Moravec & Puylaert, 1970 and J. campanae Puylaert, 1973 are transferred to Prosungulonema Roytman, 1963 as P. africanum (Moravec & Puylaert, 1970) comb. n. and P. campanae (Puylaert, 1973) n. comb.
Keywords: Parasitic nematode, New species, Marine fish, New Caledonia, South Pacific
Abstract
L’examen parasitologique de poissons perciformes marins appartenant à quatre espèces de Carangoides, C. chrysophrys, C. dinema, C. fulvoguttatus et C. hedlandensis (Carangidae) de Nouvelle-Calédonie a révélé la présence de nématodes. L’identification des carangidés a été confirmée par barcoding du gène COI. Les huit espèces de nématodes trouvées étaient: Capillariidae gen. sp. (femelles), Cucullanus bulbosus (Lane, 1916) (mâles et femelles), Hysterothylacium sp. (larves de troisième stade), Raphidascaris (Ichthyascaris) sp. (femelles et larves), Terranova sp. (larves de troisième stade), Philometra dispar n. sp. (mâle), Camallanus carangis Olsen, 1954 (femelles) et Johnstonmawsonia sp. (femelle). La nouvelle espèce P. dispar, de la cavité abdominale de C. dinema, se caractérise principalement par la longueur du corps (5.14 mm), les longueurs des spicules sensiblement inégales (163 et 96 μm) et un gubernaculum (102 μm de long) montrant une protubérance dorsale et un petit ardillon dorsal orienté vers l’arrière sur sa partie postérieure. La trouvaille de C. bulbosus représente la première mention de ce parasite, un siècle après sa découverte; la première étude de cette espèce par MEB a permis une redescription détaillée de l’espèce. La découverte de Johnstonmawsonia sp. chez C. fulvoguttatus est la première mention d’un nématode Rhabdochonidae chez un hôte appartenant à la famille Carangidae. Johnstonmawsonia africana Moravec & Puylaert, 1970 et J. campanae Puylaert, 1973 sont transférés vers Prosungulonema Roytman, 1963 comme P. africanum (Moravec & Puylaert, 1970) n. comb. et P. campanae (Puylaert, 1973) n. comb.
Introduction
Carangoides Bleeker (Carangidae, Perciformes) is a genus comprising at present 21 species of marine fishes that inhabit the tropical and subtropical regions of the Indian, Pacific and Atlantic Oceans [14]. In 2009 and 2010, during extensive studies of the parasites of marine fishes in New Caledonian waters, specimens of four species of Carangoides were examined. Since no data on the parasites of Carangoides spp. from off New Caledonia were available, the newly obtained helminthological material has provided the first information from this zoogeographically interesting region.
Based on this material, digeneans [4, 9–12] and trypanorhynch cestodes [8] have already been recorded. Regarding the parasitic nematodes, Moravec & Justine [40] mentioned the finding of the unidentified capillariid female, Capillariidae gen. sp., from C. dinema Bleeker (erroneously reported as C. oblongus (Cuvier) – see Bray & Justine [12]), and Shamsi et al. [64] recorded four ascaridoid larval types, Anisakis type I, Raphidascaris type and Terranova types I and II, in five Carangoides spp. Results of the evaluation of nematodes collected from four species of congeneric hosts from off New Caledonia are presented herein.
Materials and methods
Fish and their identification
Fish were purchased from the fish market in Nouméa, New Caledonia. Most fishes from the fishmarket were taken with mackerel nets within a few miles off Nouméa and were very fresh. All carangids were relatively young specimens, far from the maximum lengths reported for these species [67]. The following fish species were examined: Carangoides chrysophrys (Cuvier) (n = 3), C. dinema (n = 7), C. fulvoguttatus (Forsskål) (n = 10) and C. hedlandensis (Whitley) (n = 2) (Table 1). Fish were identified by their morphology, and confirmation of identification, from photographs of specimens, was sought from experts in ichthyology (Ronald Fricke, Bernard Séret and Samuel Iglésias). Fish DNA was extracted from tissue samples using the NucleoSpin 96 Tissue kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s instructions. Sequences were obtained by amplification and sequencing the 5′ region of the cytochrome oxidase subunit I (COI) mitochondrial gene using the primers FishF1 (5′-TCAACYAATCAYAAAATYGGCAC-3′) and FishR1 (5′-TGATTYTTYGGYCACCCRGAAGT-3′) [70]. Standard PCRs were carried out in a total volume of 20 μL, containing about 30 ng of DNA, 1 × 10× PCR buffer, 2 mM MgCl2, 200 μM mix dNTPs, 150 nM of each primer and 1 unit of Taq polymerase (Qiagen, Hilden, Germany). After an initial denaturation of 3 min at 95 °C, the mitochondrial DNA was amplified through 39 cycles of 15 s at 95 °C, 20 s at 48 °C and 40 s at 72 °C, with a terminal elongation for 5 min at 72 °C. PCR products were purified and sequenced in both directions on a 3730xl DNA Analyser 96-capillary sequencer (Applied Biosystems, Waltham, MA, USA). Sequences were edited using CodonCode Aligner software (CodonCode Corporation, Dedham, MA, USA), compared with the GenBank database content using BLAST and deposited in GenBank under Accession Numbers KX712506–KX712510. Species identification was confirmed using the BOLD identification engine [59] and BLAST in GenBank. The fish nomenclature adopted follows FishBase [18].
Table 1.
Specimens of fish positive for nematodes, their characteristics and COI barcoding sequences, and nematodes found.
| Species | MHNN JNC # | Date | Fork length (mm) | Weight (g) | COI sequence | Nematodes |
|---|---|---|---|---|---|---|
| Carangoides chrysophrys | JNC3212 | 21 July 2010 | 265 | 398 | KX712510 | Camallanus carangis |
| Carangoides dinema | JNC3184 | 4 June 2010 | 315 | 708 | KX712509 | Raphidascaris (Ichthyascaris) sp. |
| JNC2880 | 13 March 2009 | 310 | 624 | – | Capillariidae gen. sp 3 | |
| JNC3224 | 26 August 2010 | 320 | 650 | – | Raphidascaris (Ichthyascaris) sp. | |
| Philometra dispar n. sp. | ||||||
| JNC3225 | 26 August 2010 | 305 | 592 | – | Capillariidae gen. sp 3 | |
| Raphidascaris (Ichthyascaris) sp. | ||||||
| Carangoides fulvoguttatus | JNC3176 | 28 May 2010 | 270 | 340 | KX712507 | Cucullanus bulbosus |
| Hysterothylacium sp. | ||||||
| Raphidascaris (Ichthyascaris) sp. | ||||||
| Terranova sp. | ||||||
| Johnstonmawsonia sp. | ||||||
| JNC3180 | 3 June 2010 | 295 | 430 | KX712508 | Capillariidae gen. sp 3 | |
| Cucullanus bulbosus | ||||||
| Hysterothylacium sp. | ||||||
| Raphidascaris (Ichthyascaris) sp. | ||||||
| Carangoides hedlandensis | JNC3172 | 27 May 2010 | 245 | 341 | KX712506 | Camallanus carangis |
Nematodes
Parasites were collected using a “wash” method [25]. The nematodes were fixed in hot 4% formalin or 70% ethanol. For light microscopic examination, they were cleared with glycerine. Drawings were made with the aid of a Zeiss drawing attachment. Specimens used for scanning electron microscopy were postfixed in 1% osmium tetroxide (in phosphate buffer), dehydrated through a graded acetone series, critical-point-dried and sputter-coated with gold; they were examined using a JEOL JSM-7401F scanning electron microscope at an accelerating voltage of 4 kV (GB low mode). All measurements are in micrometres unless indicated otherwise. The classification system of the Ascaridoidea adopted follows Keys to the Nematode Parasites of Vertebrates [1, 19].
Molecular identification of fish
Carangoides chrysophrys. The single sequence (GenBank KX712510) obtained from fish JNC3212 was 99.23–100% identical to sequences of C. chrysophrys included in BOLD and/or GenBank.
Carangoides dinema. The single sequence (GenBank KX712509) obtained from fish JNC3184 was 99.85–100% identical to sequences of C. dinema included in BOLD and/or GenBank.
Carangoides fulvoguttatus. The two sequences (GenBank KX712507, KX712508) obtained from fish JNC3176 and JNC3180 were identical. They were 99.65–100% identical to sequences of C. fulvoguttatus included in BOLD and/or GenBank.
Carangoides hedlandensis. The single sequence (GenBank KX712506) obtained from fish JNC3172 was 99.53–100% identical to sequences of C. hedlandensis included in BOLD and/or GenBank.
In all these cases, we consider that our morphological identifications were confirmed by high similarity (>99%) or identity (100%) to sequences registered under the same taxon name in databases.
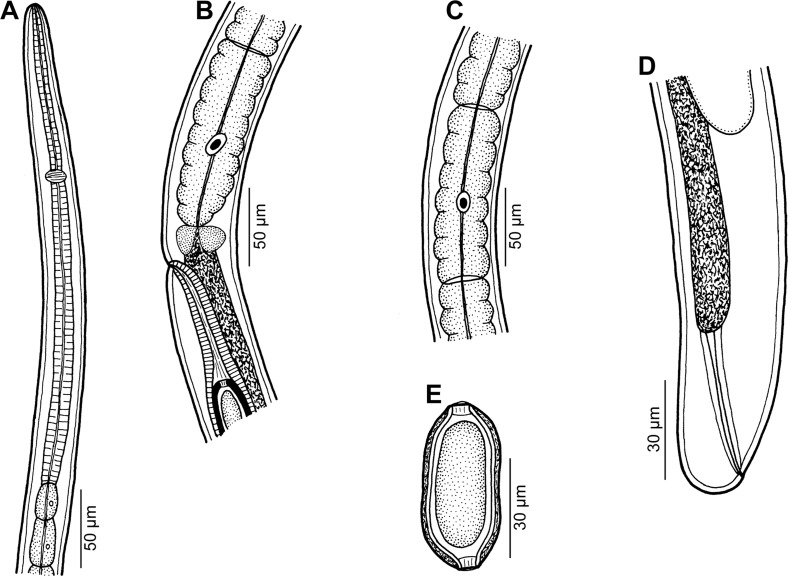
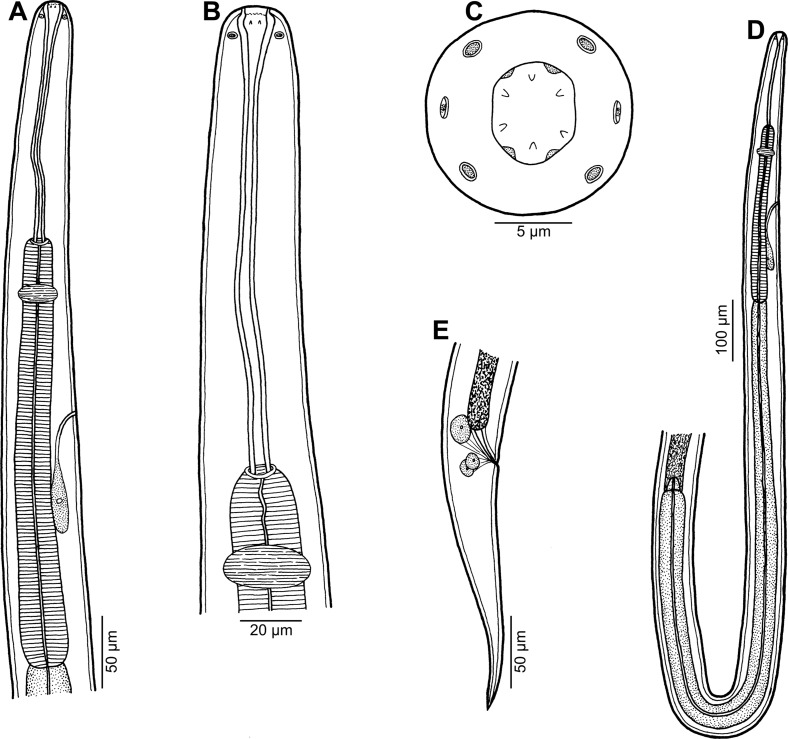
Capillariidae gen. sp. 3 of Moravec & Justine, 2010 (Fig. 1)
Figure 1.
Capillariidae gen. sp., gravid female from Carangoides dinema. A: Anterior end of body. B: Region of vulva, lateral view. C: Stichocyte from middle part of stichosome. D: Posterior end of body, lateral view. E: Egg.
Fam. Capillariidae Railliet, 1915
Hosts: Shadow trevally Carangoides dinema and yellowspotted trevally C. fulvoguttatus (both Carangidae, Perciformes).
Site of infection: Digestive tract.
Locality: Fish market, Nouméa, New Caledonia (JNC2880, collected 13 March 2009; JNC3180, 3 June 2010; JNC3225, 26 August 2010).
Prevalence and intensity: in 2 of 7 C. dinema and in 1 of 10 C. fulvoguttatus examined; 1 nematode per fish.
Deposition of voucher specimens: Muséum National d’Histoire Naturelle, Paris (MNHN JNC2880, JNC3225, C. dinema; MNHN JNC3180D, C. fulvoguttatus).
Description
Female (three gravid specimens): Medium-sized filiform nematodes. Anterior end of body narrow; cephalic papillae indistinct (Fig. 1A). Length of body 11.75–14.55 mm, maximum width 60–69. Two lateral, fairly wide bacillary bands extending along almost whole body length; their width at region of posterior end of oesophagus 18–24. Length of entire oesophagus 6.84–7.40 mm, representing 51–61% of body length. Muscular oesophagus 207–354 long (Fig. 1A). Stichosome consisting of single row of elongate stichocytes subdivided usually (mainly in its middle and posterior parts) into many (8–15) transverse annuli (Figs. 1B, 1C); nuclei of stichocytes large. Length of stichosome 6.63–7.05 mm; stichocytes approximately 50 in number. Nerve ring encircling muscular oesophagus at about its one third, 81–120 from anterior extremity (Fig. 1A). Two small wing-like cells present at oesophago-intestinal junction (Fig. 1B). Vulva located 6.92–7.53 mm from anterior end of body (at 52–61% of body length), 13–132 posterior to level of oesophago-intestinal junction (Fig. 1B); vulval lips not elevated or anterior lip slightly elevated. Vagina short, muscular. Eggs in anterior part of uterus arranged in single row, more distant eggs in two rows. Eggs oval, usually somewhat narrowed equatorially, with slightly protruding polar plugs (Fig. 1E). Egg wall appearing as two-layered; inner layer hyaline, outer layer thicker, with fine superficial net-like sculpture. Eggs including polar plugs 57–60 × 24–30, thickness of egg wall 3–4; polar plugs 6 long and 6 wide. Content of fully developed eggs uncleaved. Caudal end rounded; anus subterminal, length of tail 6–12. Rectum formed by hyaline tube 60–66 long (Fig. 1D).
Male: Not known.
Remarks
Available female specimens cannot be identified to generic level, because conspecific males are absent. These nematodes are characterised mainly by a relatively short body length and muscular oesophagus, stichocytes with distinct transverse annuli, a subterminal anus and especially by the shape, structure and size of eggs. To date, three species of capillariids have been reported from marine fishes of the family Carangidae: Pseudocapillaria carangi (Parukhin, 1971), P. decapteri (Luo, 2001) and Capillaria gracilis (Bellingham, 1840) [29, 35]. Whereas C. gracilis is a parasite mainly of gadiform fishes and its record in the carangid Trachinotus carolinus (Linnaeus) may well be a misidentification, P. carangi and P. decapteri, both inadequately described, are reported only from members of Carangidae. Therefore, it is probable that our specimens also belong to Pseudocapillaria Freitas, 1959.
Parukhin [51–53] reported P. carangi from 12 species of carangid fishes (including two Carangoides spp.) from the western part of the Indian Ocean (Monar Bay, Arabian Sea near Oman, Gulf of Aden, Red Sea, off southeastern coast of Africa), whereas P. decapteri was recorded from the North Pacific Ocean near Japan [29]. Although specimens of the present material may belong to one of these two species (which, however, may be identical to each other), their poor original descriptions and principally the absence of a male in our material do not allow us to assign the New Caledonian specimens to a species.
The finding of one female specimen of this species, reported as Capillariidae gen. sp. 3, in New Caledonian waters was recorded by Moravec & Justine [40]; however, the host reported as Carangoides oblongus (Cuvier) was in fact C. dinema [12].
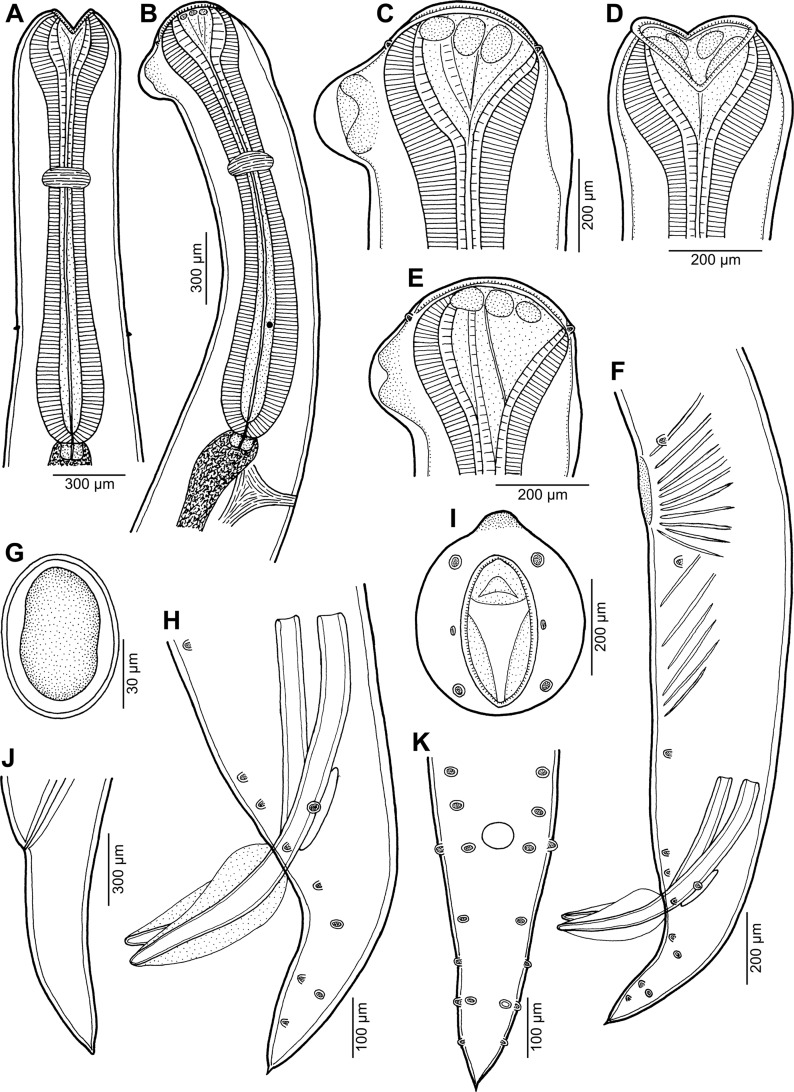
Cucullanus bulbosus (Lane, 1916) Barreto, 1918 (Figs. 2, 3)
Figure 2.
Cucullanus bulbosus (Lane, 1916) from Carangoides fulvoguttatus. A, B: Anterior end of gravid female, dorsoventral and lateral views, respectively. C, D: Cephalic end of gravid female, lateral and ventral views, respectively. E: Cephalic end of male, lateral view. F: Posterior end of male, lateral view. G: Egg. H: Caudal end of male, lateral view. I: Cephalic end of male, apical view. J: Tail of gravid female, lateral view. K: Caudal end of male, ventral view.
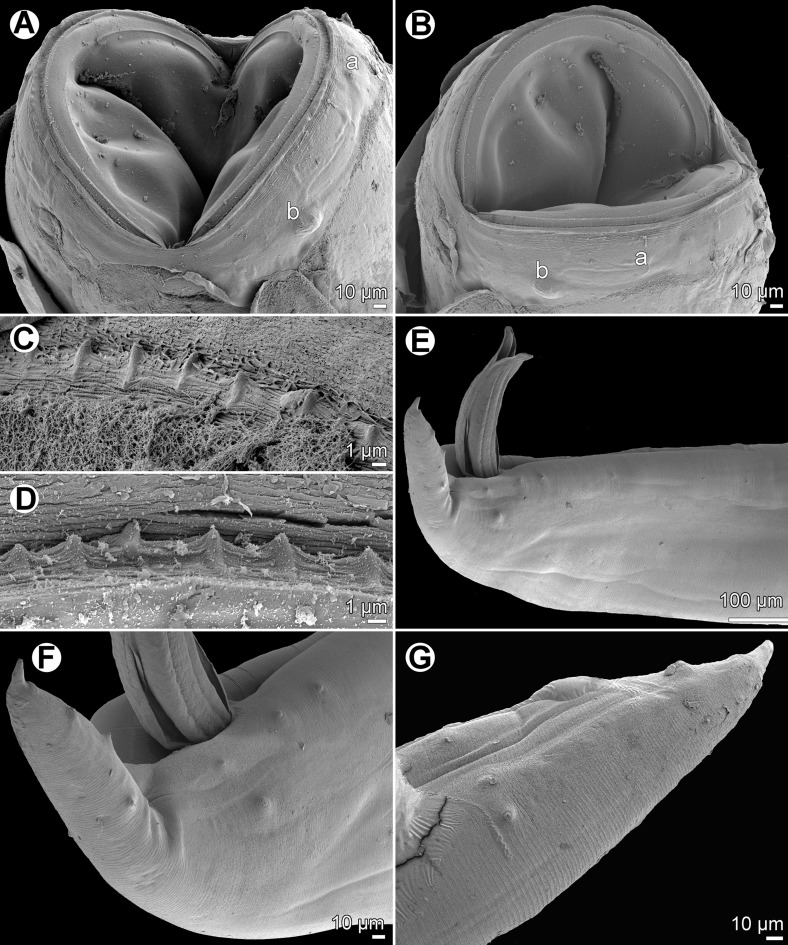
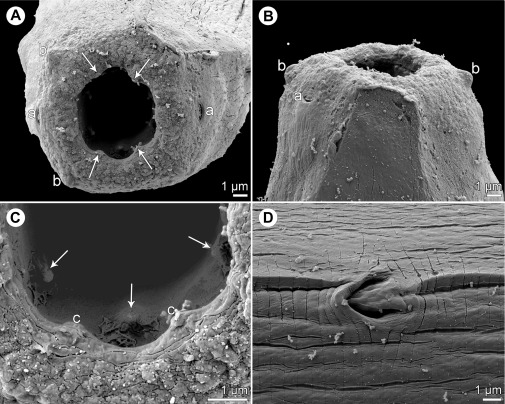
Figure 3.
Cucullanus bulbosus (Lane, 1916), scanning electron micrographs. A, B: Cephalic end of nongravid female, ventral and lateral views, respectively. C, D: Peribuccal teeth of male and nongravid female, respectively. E: Posterior of male, lateral view. F: Tail of male, lateral view. G: Posterior part of male tail, lateral view. Abbreviations: a, amphid; b, cephalic papilla.
Syn.: Bulbodacnitis bulbosa Lane, 1916.
Fam. Cucullanidae Cobbold, 1864
Host: Yellowspotted trevally Carangoides fulvoguttatus (Carangidae, Perciformes).
Site of infection: Digestive tract.
Locality: Fish market, Nouméa, New Caledonia (JNC3176, collected 28 May 2010; JNC3180, collected 3 June 2010).
Prevalence and intensity: in 2 of 10 C. fulvoguttatus examined; 1 and 2 nematodes.
Deposition of voucher specimen: Muséum National d’Histoire Naturelle, Paris (MNHN JNC3176).
Description
General: Medium-sized nematodes. Body whitish, elongate, with anterior end somewhat curved dorsally. Cuticle slightly transversely striated. Lateral alae absent. Cephalic end somewhat asymmetrical in lateral view, with conspicuous large dorsal hemispherical elevation at level of pseudobuccal capsule (Figs. 2B, 2C and 2E). Oral aperture dorsoventrally elongate, surrounded by raised narrow membranous ala (collarette) supported by row of minute basal teeth (Figs. 2D, 2I and 3A–3D). Four submedian cephalic papillae and pair of lateral amphids present (Figs. 2C, 2E, 2I, 3A and 3B). Oesophagus muscular, somewhat expanded at anterior end to form rather large pseudobuccal capsule (oesophastome); posterior part of oesophagus also expanded, slightly narrower than pseudobuccal capsule (Figs. 2A and 2B); cuticular lining of oesophastome consists of complex set of thickened cuticularised pieces separated by sutures (Figs. 2A–2E, 2I, 3A and 3B). Oesophagus opens into intestine through large valve. Nerve ring encircles oesophagus at distance representing 39–41% of oesophageal length. Deirids small, hooked, at short distance anterior to end of oesophagus (Figs. 2A and 2B). Postdeirids not found. Excretory pore slightly posterior to oesophago-intestinal junction (Fig. 2B). Tail conical, with sharply pointed tip.
Male (1 specimen): Length of body 16.19 mm, maximum width 571; width at region of oesophastome including dorsal elevation 476, at region of middle of oesophagus 381. Length of entire oesophagus 1.97 mm, representing 12% of body length; length of oesophastome 354, its width 367; minimum width of oesophagus 163; maximum width of posterior part of oesophagus 313. Distance from nerve ring to anterior extremity 726, representing 39% of oesophageal length. Deirids and excretory pore 1.63 and 2.15 mm, respectively, from anterior end of body. Posterior end of body curves ventrally. Ventral region of cloacal opening not elevated. Spicules equal, 830 long, their distal parts provided with markedly wide dorsal and ventral alae; maximum width of spicules including alae 136 (Figs. 2F, 2H, 3E and 3F). Gubernaculum well sclerotised, narrow in lateral view, 218 long. Ventral sucker and oblique muscle bands well developed (Fig. 2F); former situated 1.22 mm from cloacal aperture. Preanal papillae 5 subventral pairs; adanal papillae 1 subventral pair and 1 lateral pair; postanal papillae 3 subventral and 2 lateral pairs (Figs. 2F, 2H, 2K, 3E and 3F). Length of tail 435.
Female (1 gravid and 1 nongravid specimen; measurement of latter in parentheses): Length of body 18.39 (12.77) mm, maximum width 666 (490); width at region of oesophastome including dorsal elevation 585 (408), at region of middle of oesophagus 462 (286). Length of entire oesophagus 2.07 (1.74) mm, representing 11 (14)% of body length; length of oesophastome 381 (367), its width 367 (326); minimum width of oesophagus 150 (136); maximum width of posterior part of oesophagus 326 (231). Distance from nerve ring to anterior extremity 816 (707), representing 39 (41)% of oesophageal length. Deirids and excretory pore 1.84 (1.40) and 2.48 (2.11) mm, respectively, from anterior end of body. Vulva postequatorial, 13.74 (8.61) mm from anterior extremity, at 75 (67)% of body length; vulval lips elevated. Vagina directed anteriorly from vulva. Uteri opposed. Eggs numerous (eggs absent in nongravid specimens); fully developed eggs oval, thin-walled, with contents uncleaved or cleaved at most into several blastomeres (Fig. 2G); eggs 82 long, 54 wide. Length of tail 462 (422) (Fig. 2J).
Remarks
Lane [28] described a new cucullanid species, Bulbodacnitis bulbosa, from the bluefin trevally Caranx melampygus Cuvier off Sri Lanka and established the new genus Bulbodacnitis to accommodate it, because he considered the presence of the dorsal hemispherical cephalic elevation in this species to be of generic importance. However, Barreto [5, 6] considered Bulbodacnitis Lane, 1916 a junior synonym of Cucullanus Müller, 1777, to which he transferred Lane’s species. Nevertheless, Smedley [66] and Simon [65] described two new species of Bulbodacnitis from North American salmonids, while other authors [7, 15, 58, 71] did not recognise Bulbodacnitis as an independent genus. Subsequently, Maggenti [31] re-erected Bulbodacnitis for the cucullanids with the oral aperture dorsally oblique to the longitudinal body axis (see also [21, 48]). However, Petter [54] pointed out that this feature is not found in B. bulbosus, the type species of Bulbodacnitis, and, consequently, she retained Bulbodacnitis as a synonym of Cucullanus and established a new genus Truttaedacnitis Petter, 1974 for the species with the distinctly oblique oral aperture. According to Moravec [34], Truttaedacnitis should be considered as a subgenus of Cucullanus.
The present specimens from C. fulvoguttatus correspond, more or less, to the description of C. bulbosus, both these forms were collected from carangid fishes, and C. melampygus, the type host of C. bulbosus, also occurs in New Caledonian waters [18]. Therefore, the New Caledonian nematodes undoubtedly belong to C. bulbosus.
Cucullanus bulbosus has not been recorded since its description by Lane [28], making the New Caledonian specimens the first finding of this species after a century. The original description of Bulbodacnitis bulbosa (= C. bulbosus) is relatively good (a somewhat modified description, based on the original one, was published by Baylis [7]). The present study, including the first scanning electron microscopy (SEM) examination, confirmed some previously reported morphological features in this species, showed some new characters (presence of circumoral spines and ventral oblique muscle bands in the male) and provided more exact observations of the cephalic structures and male caudal papillae. The finding of C. bulbosus in C. fulvoguttatus from off New Caledonia represents new host and geographical records.
Hysterothylacium sp. (Figs. 4A–4C)
Figure 4.
Ascaridoid larvae from Carangoides spp. A–C: Hysterothylacium sp. third-stage larva from Carangoides fulvoguttatus (A: anterior end of body; B: cephalic end; C: tail; all lateral views). D–F: Raphidascaris (Ichthyascaris) sp. third-stage larva from Carangoides fulvoguttatus (D: anterior end of body; E: cephalic end; F: tail; all lateral views). G–I: Raphidascaris (Ichthyascaris) sp. fourth-stage larva from Carangoides fulvoguttatus (G: anterior end of body; H: cephalic end; I: tail; all lateral views). J, K: Terranova sp. third-stage larva from Carangoides fulvoguttatus (J: anterior end of body; K: tail; both lateral views).
Fam. Anisakidae Railliet & Henry, 1912
Host: Yellowspotted trevally Carangoides fulvoguttatus (Carangidae, Perciformes).
Site of infection: Digestive tract.
Locality: Fish market, Nouméa, New Caledonia (JNC3176, collected 28 May 2010; JNC3180, collected 3 June 2010)
Prevalence and intensity: in 2 of 10 C. fulvoguttatus examined; 1 nematode per fish.
Deposition of voucher specimens: Muséum National d’Histoire Naturelle, Paris (MNHN JNC3180, JNC3180D).
Description
Third-stage larva (1 specimen): Body length 2.94 mm, maximum width 82. Cephalic end truncated, with anlagen of lips and ventral tooth (Fig. 4B). Lateral alae absent. Oesophagus 345 long, maximum width 24. Ventriculus oval, 36 long, 27 wide. Posterior ventricular appendix 267 long, 21 wide. Nerve ring and excretory pore 132 and 135, respectively, from anterior end of body. Intestine straight. Anterior intestinal caecum short, 87 long, 21 wide (Fig. 4A). Length ratio of caecum and ventricular appendix 1:3. Tail conical, 93 long, provided with several small cuticular spikes at tip (Fig. 4C); length of spines ca. 3.
Remarks
The genus Hysterothylacium Ward & Magath, 1917 includes many species, which are gastro-intestinal parasites mostly of marine fishes belonging to different families and orders. To date, only three species of Hysterothylacium have been recorded from New Caledonian waters: H. alatum Moravec & Justine, 2015 from Plectropomus laevis (Lacépède) (Serranidae), H. cenaticum (Bruce & Cannon, 1989) from Kajikia audax (Philippi) (Istiophoridae) and H. sphyraenae Moravec & Justine, 2015 from Sphyraena qenie Klunzinger (Sphyraenidae) [39, 43]. In addition, unidentified larvae of Hysterothylacium have been reported off New Caledonia from several fish species of the Balistidae, Clupeidae, Lethrinidae, Nemipteridae, Scombridae, Serranidae, Sphyraenidae and Trichiuridae [23, 24, 64].
The life cycles and larval morphogenesis of Hysterothylacium spp. remain mostly unknown, making species identification of the larvae of this genus from fishes, based on morphological features, impossible. Shamsi et al. [63, 64] distinguished 14 morphotypes of larval Hysterothylacium spp. (types I–XIV) from marine fishes in Australian and New Caledonian waters, of which types VI, XIII and XIV were recorded from fishes from off New Caledonia [64]. However, it is necessary to note that the “sinusoidal” or “serpengenous” patterns of the intestine, reported to be characteristic of the larval types VI and XIII, were in fact the coils of the developing genital tract, as is evident from the respective microphotographs (Figs. 2A and 2C of Shamsi et al. [64]).
Based on their morphology, the present Hysterothylacium larvae from C. fulvoguttatus cannot be assigned to any of the congeneric larval types of Shamsi et al. [63, 64], all of which were reported from non-carangid fishes. It is not clear whether the present Hysterothylacium larvae may attain full maturity in C. fulvoguttatus, serving thus as the definitive host, or whether this fish is only utilised as the paratenic host. The only two species reported from carangid fishes are H. chorinemi (Parukhin, 1966), recorded from Atule mate (Cuvier), Caranx sexfasciatus Quoy & Gaimard and Scomberoides lysan (Forsskål) (all Carangidae) in the South China, Arabian and Red Seas and off the southeastern coast of Africa [50, 53], and H. carangis (Kalyankar, 1971), described from Carangoides malabaricus (Bloch & Schneider) off India [13, 26].
Raphidascaris (Ichthyascaris) sp. (Figs. 4D–4I)
Fam. Anisakidae Railliet & Henry, 1912
Hosts: Shadow trevally Carangoides dinema and yellowspotted trevally C. fulvoguttatus (both Carangidae, Perciformes).
Site of infection: Digestive tract.
Locality: Fish market, Nouméa, New Caledonia (collected 28 May, 3 and 4 June and 26 August 2010).
Prevalence and intensity: C. dinema: 3 of 7 fish examined infected; 1–2 nematodes per fish. C. fulvoguttatus: 2/10; 6 and 9 nematodes.
Deposition of voucher specimens: Muséum National d’Histoire Naturelle, Paris (C. fulvoguttatus, JNC3176, JNC3180C; C. dinema, JNC3184, JNC3224, JNC3225).
Description
Female (one body fragment of posterior end of gravid specimen from C. dinema): Length of body fragment 1.50 mm, maximum width 231. Tail conical, 159 long, with many small papilla-like cuticular projections at tip. Uterus containing several thin-walled, almost spherical eggs 39–42 in diameter with uncleaved content.
Female fourth-stage larva (two specimens from C. dinema and C. fulvoguttatus): Body length 4.45–5.70 mm, maximum width 163–245. Cephalic end with well-developed lips (Fig. 4H); lips 36–51 long. Narrow lateral alae united anteriorly close to ventrolateral lips on ventral side of body present (Fig. 4H). Oesophagus 462–680 long, maximum width 82–122. Ventriculus transverse-oval, 36–54 long and 63–95 wide. Posterior ventricular appendix 249–313 long, 33–41 wide. Nerve ring and excretory pore 190–272 and 190–272, respectively, from anterior end of body (Fig. 4G). Vulva, still covered by cuticle, located 911–1,346 from anterior extremity, i.e. at 20–24% of body length (Fig. 4G). Vagina directed posteriorly from vulva; many coils of developing genital tract present in body posterior to vulva. Tail conical, 204–218 long, pointed, without any caudal projections at tip (Fig. 4I).
Advanced third-stage larva (5 specimens from C. fulvoguttatus): Body length 3.79–4.58 mm, maximum width 136–163. Cephalic end without lips, bearing distinct ventral larval tooth (Fig. 4E). Lateral alae not observed. Oesophagus 408–517 long, maximum width 60–68. Ventriculus transverse-oval, 33–36 long and 51–66 wide. Posterior ventricular appendix 231–309 long, 27–45 wide. Nerve ring and excretory pore 135–165 and 135–176, respectively, from anterior end of body (Fig. 4D). Vulva and vagina absent. Body with many coils of developing genital tract. Tail conical, sharply pointed, 82–190 long (Fig. 4F).
Remarks
All the above-mentioned forms are considered to represent one and the same species of Raphidascaris Railliet & Henry, 1915, which attains full maturity in Carangoides spp. The presence of characteristic, anteriorly united lateral alae in fourth-stage larvae shows that this currently undescribed species belongs to the subgenus Ichthyascaris Wu, 1949.
To date, 10 species of Raphidascaris (Ichthyascaris) are known as parasites of marine fishes [72]. Of these, five species were reported from the South Pacific Ocean in the Australian region: R. (I.) fisheri (Hooper, 1983), R. (I.) gymnocraniae (Bruce, 1990) and R. (I.) sillagoides (Bruce, 1990) in Australian waters and R. (I.) etelidis Moravec & Justine, 2012 and R. (I.) nemipteri Moravec & Justine, 2005 from off New Caledonia [13, 39, 41]. However, none of the Raphidascaris (Ichthyascaris) spp. has so far been described from fishes of the family Carangidae. Therefore, it can be assumed that the nematodes parasitising Carangoides spp. in New Caledonian waters belong to a new species.
Ascaridoid larvae designated as “Raphidascaris larval type” were reported from Carangoides chrysophrys from off New Caledonia by Shamsi et al. [64].
Terranova sp. (Figs. 4J, 4K)
Fam. Anisakidae Railliet & Henry, 1912
Host: Yellowspotted trevally Carangoides fulvoguttatus (Carangidae, Perciformes).
Site of infection: Digestive tract.
Locality: Fish market, Nouméa, New Caledonia (collected 28 May 2010).
Prevalence and intensity: in 1 of 10 C. fulvoguttatus examined; 4 nematodes.
Deposition of voucher specimens: Muséum National d’Histoire Naturelle, Paris (MNHN JNC3176).
Description
Third-stage larva (four specimens): Body length 6.61–9.02 mm, maximum width 204–286. Cephalic end truncated, with anlagen of lips and distinct ventral tooth. Oesophagus 802–952 long, maximum width 68–95. Ventriculus large, oval, 340–435 long, 109–136 wide. Nerve ring 231–286 from anterior end of body. Excretory pore just posterior to larval tooth. Anterior intestinal caecum 612–748 long, 41–68 wide (Fig. 4J). Tail conical, 109–122 long, pointed (Fig. 4K).
Remarks
Larvae of this type, designated as Terranova type II, were already reported from off New Caledonia by Shamsi et al. [64], who had recorded them from fishes of the families Carangidae (including C. fulvoguttatus), Chirocentridae, Monodactylidae, Scombridae, Serranidae, Sphyraenidae and Trichiuridae.
Species of Terranova Leiper & Atkinson, 1914 are parasites of the digestive tract of fishes and reptiles. Many species of teleost fishes serve only as paratenic hosts of larvae, which is apparently the case of carangids.
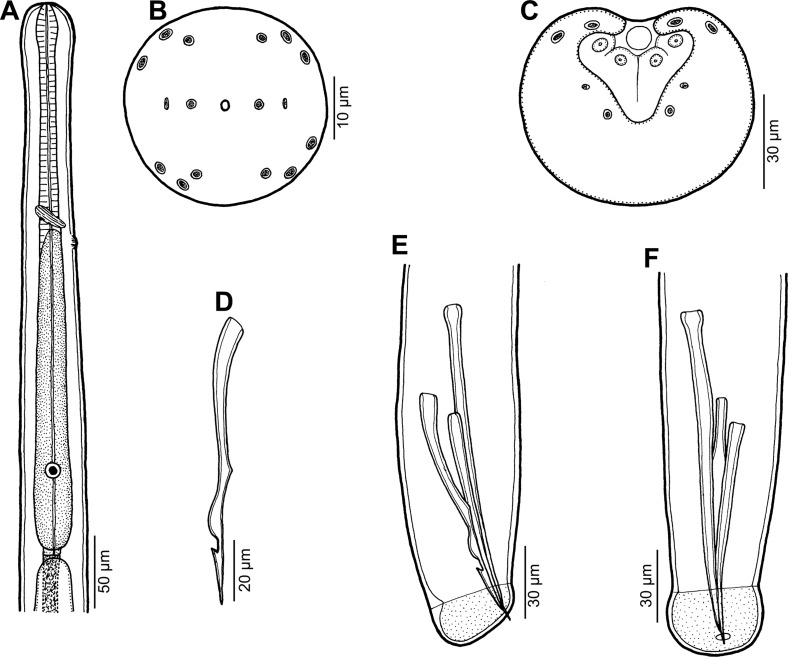
Philometra dispar n. sp. (Figs. 5, 6)
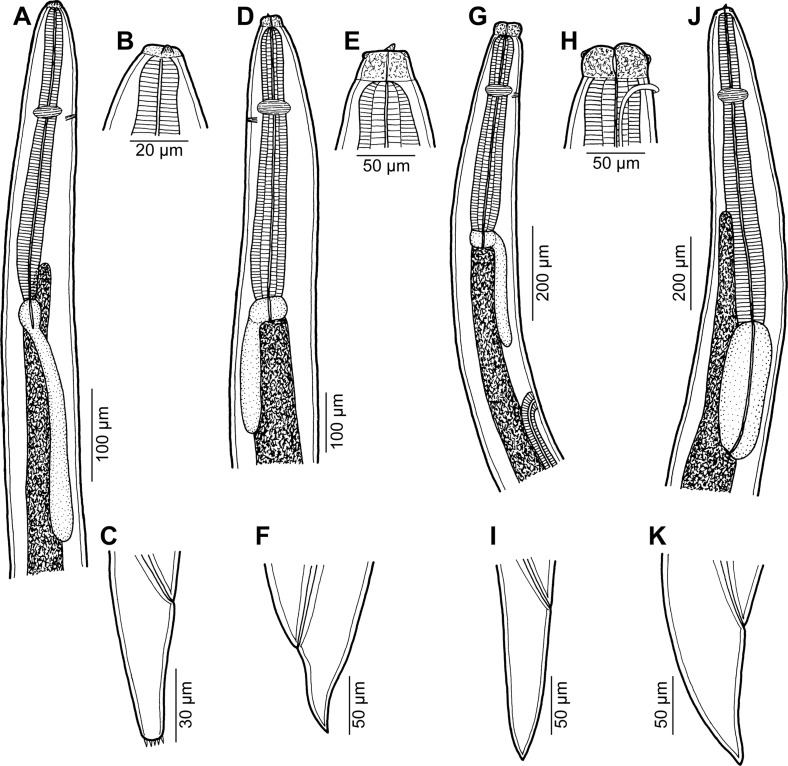
Figure 5.
Philometra dispar n. sp. from Carangoides dinema, male. A: Anterior end of body, lateral view. B: Cephalic end, apical view. C: Caudal end, apical view. D: Gubernaculum, lateral view. E, F: Posterior end, lateral and ventral views.
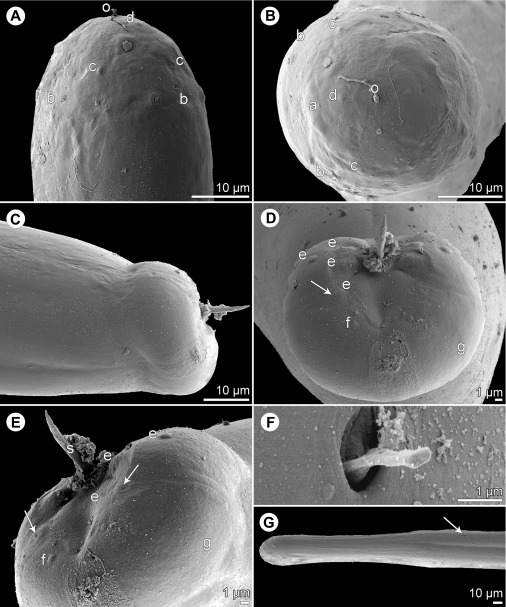
Figure 6.
Philometra dispar n. sp. from Carangoides dinema, scanning electron micrographs of male. A, B: Cephalic end, dorsoventral and apical views, respectively. C, D: Caudal end, lateral and apical views, respectively (arrow indicates phasmid). E: Caudal end, subdorsal view (arrows indicate phasmids). F: Deirid. G: Anterior end of body, dorsoventral view (arrow indicates location of deirid). Abbreviations: a, amphid; b, submedian pair of cephalic papillae of external circle; c, submedian cephalic papilla of internal circle; d, lateral cephalic papilla of internal circle; e, caudal papillae in region of cloacal aperture; f, caudal papilla of last postanal pair; g, caudal mound; o, oral aperture.
Fam. Philometridae Baylis & Daubney, 1926
urn:lsid:zoobank.org:act:C4134134-E130-48D7-AE28-EA4B5F3CC288
Type host: Shadow trevally Carangoides dinema (Carangidae, Perciformes); JNC3224 (see Table 1); Fork length 320 mm, weight 650 g.
Site in host: Probably abdominal cavity (found in wash).
Type locality: Off Nouméa, New Caledonia (collected 26 August 2010).
Prevalence and intensity: 1 of 7 fish examined infected; 1 nematode.
Deposition of type specimen (holotype mounted on SEM stub): Helminthological Collection of the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic (Cat. No. N-1118).
Etymology: The specific name dispar (= unequal, disparate) is a Latin adjective and relates to the characteristic feature of this species, i.e. unequally long spicules.
Description
Male (1 specimen, holotype): Body whitish, filiform, 5.14 mm long, maximum width at middle 63; anterior part of body slightly narrower just posterior to cephalic end (Figs. 5A and 6G); body width at this narrowed part 39. Maximum width/body length 1:82; width of cephalic end 45, that of posterior end 36. Cuticle smooth. Cephalic end rounded. Oral aperture small, oval, surrounded by 14 cephalic papillae arranged in two circles: external circle formed by four submedian pairs of papillae; internal circle formed by four submedian and two lateral papillae (Figs. 5B, 6A and 6B). Small lateral amphids just posterior to lateral papillae of internal circle in lateral views (Figs. 5B, 6A, 6B). Small deirids present at middle oesophageal region (Fig. 6F, 6G). Oesophagus 504 long, maximum width 27, forming 10% of body length, slightly inflated at anterior end; posterior part of muscular oesophagus overlapped by well-developed oesophageal gland with large cell nucleus situated somewhat posterior to its middle (Fig. 5A); anterior oesophageal inflation 24 long and 16 wide. Small ventriculus present. Nerve ring, excretory pore and oesophageal nucleus 183, 222 and 429, respectively, from anterior extremity. Testis reaching almost to posterior end of oesophagus (Fig. 5A). Posterior end of body blunt, forming distinct tail 9 long, with broad, U-shaped mound extending laterally (Figs. 5C, 6C–6E). Four pairs of very flat, hardly visible caudal papillae situated on sides of cloacal aperture and one pair of postanal papillae located more posteriorly on caudal mound (Figs. 5C, 6D and 6E). Pair of small phasmids present at about middle of each mound arm (Figs. 5C, 6D and 6E). Spicules slender, needle-like, very unequal, with somewhat expanded proximal and sharply pointed distal tips (Figs. 5E, 5F, 6C–6E); length of right spicule 123, comprising 2.3% of body length, that of left spicule 96; length ratio of spicules 1:1.28. Gubernaculum narrow, 102 long, with anterior portion slightly bent dorsally; length of anterior bent part 57, representing 56% of entire gubernaculum length; posterior portion of gubernaculum with distinct dorsal protuberance followed by small, reflexed barb located 27 from distal tip (Fig. 5D–5F). Length ratio of gubernaculum and longer (right) spicule 1:1.21. Spicules and gubernaculum well sclerotised; spicules brown-coloured, gubernaculum colourless.
Female: Not known.
Remarks
To date, eight nominal species belonging to four philometrid genera (Buckleyella Rasheed, 1963, Caranginema Moravec, Montoya-Mendoza & Salgado-Maldonado, 2008, Philometra Costa, 1845 and Philometroides Yamaguti, 1935) are known to parasitise carangid fishes [42]. Four of these are known solely from their females, whereas males have been described only for Caranginema americanum Moravec, Montoya-Mendoza & Salgado-Maldonado, 2008 from subcutaneous tissues of Caranx hippos (L.) in the Gulf of Mexico, Philometra austropacifica Moravec & Justine, 2014 from the ovary of Alepes vari (Cuvier) off New Caledonia, P. selaris Moravec & Justine, 2014 from the abdominal cavity (?) of Selar crumenophthalmus (Bloch) off New Caledonia and P. tauridica Ivashkin, Kovaleva & Khromova in Ivashkin et al., 1971 from the abdominal cavity of Trachurus mediterraneus (Steindachner) in the Black Sea [21, 38, 42].
By the body length 5.1 mm, the present male resembles only that of P. selaris (5.3–5.5 mm), whereas the males of other three species are distinctly shorter (1.5–3.3 mm). Moreover, both P. dispar sp. n. and P. selaris possess a dorsal reflexed barb at the tip of the gubernaculum, which is absent in other species. However, the new species differs from P. selaris in having conspicuously unequal spicules (length ratio of spicules 1:1.28 vs. 1:1.03–1.04), a different shape and structure of the gubernaculum (presence vs. absence of a dorsal protuberance) and a more posterior location of the oesophageal cell nucleus; in addition, it was collected from a fish belonging to a different genus (Carangoides vs. Selar). Males of the remaining four philometrid species from carangids are not known and, consequently, cannot be compared with P. dispar; however, these species can be separated based on the different genus of their type host and their geographical distribution. The allocation of the new species to Philometra is provisional; present philometrid genera are mostly based on the morphology of gravid and subgravid females, whereas males of some genera (e.g. Caranginema, Philometra and Philometroides) are unidentifiable to genus [42].
By the very unequally long spicules, P. dispar n. sp. differs from all other philometrid species from marine fishes, for which males are known, except for Philometra katsuwoni Petter & Baudin-Laurencin, 1986 and P. gymnosardae Moravec, Lorber & Konečný, 2007, both gonad-infecting parasites of tuna fishes (Scombridae) in the Atlantic and Indian Oceans, respectively [16, 45, 55]. However, differences in the lengths of spicules of P. katsuwoni and P. gymnosardae are much more conspicuous as compared with that in P. dispar.
The authors are aware of the fact that this new species is being described from a single specimen, a procedure that cannot be generally recommended; however, in this case, the new species appears to be readily recognisable and, therefore, they consider it more reasonable and useful to give it a specific name rather than to report it only as Philometra sp.
Camallanus carangis Olsen, 1954
Fam. Camallanidae Railliet et Henry, 1915
Syns.: Camallanus marinus Schmidt & Kuntz, 1969; C. paracarangis Velasquez, 1980.
Hosts: Longnose trevally Carangoides chrysophrys and C. hedlandensis (both Carangidae, Perciformes).
Site of infection: Digestive tract.
Localities: New Caledonia (JNC3172, collected 27 May 2010; JNC3212, 21 July 2010)
Prevalence and intensity: C. chrysophrys: 1 of 3 fish examined infected; 1 nematode. C. hedlandensis: 1/2; 1.
Deposition of voucher specimens: Muséum National d’Histoire Naturelle, Paris (JNC3172 (C. hedlandensis), JNC3212 (C. chrysophrys)).
Remarks
Only two ovigerous female specimens, 11.06 mm and 18.43 mm long, were collected. The tail tip of both of them bears three small caudal projections. Since the general morphology of these specimens corresponds to that of C. carangis, as redescribed by Moravec et al. [44], they are assigned to this species.
Camallanus carangis was originally described by Olsen [49] from Caranx sp. in Fiji. At present, this species is known to occur in carangids and fishes belonging to some other families in Hawaii, French Polynesia, the Philippines and in the Arabian, Arafura, South China and Red Seas [27, 51, 60, 62, 69]. Moravec et al. [44] reported C. carangis from Nemipterus furcosus (Valenciennes) (Nemipteridae), Parupeneus ciliatus (Lacépède) and Upeneus vittatus (Forsskål) (both Mullidae) from off New Caledonia. The present findings of C. carangis in C. chrysophrys and C. hedlandensis represent new host records.
Moravec et al. [44] observed that the tail tip of young nongravid and small subgravid (ovigerous) females of C. carangis possess three small caudal projections, whereas these are totally absent from conspecific gravid (larvigerous) females. This is confirmed by the present study.
Johnstonmawsonia sp. (Figs. 7, 8)
Figure 7.
Johnstonmawsonia sp. from Carangoides fulvoguttatus, nongravid female. A: Anterior end of body, lateral view. B: Same, larger magnification. C: Cephalic end, apical view. D: Oesophageal portion of body, lateral view. E: Tail, lateral view.
Figure 8.
Johnstonmawsonia sp. from Carangoides fulvoguttatus, scanning electron micrographs of nongravid female. A, B: Cephalic end, apical and dorsoventral views, respectively (arrows indicate sublabia). C: Detail of mouth, apical view (arrows indicate inner prostomal teeth). D: Excretory pore, ventral view. Abbreviations: a, amphid; b, submedian cephalic papilla; c, sublabium.
Fam. Rhabdochonidae Travassos, Artigas et Pereira, 1928
Host: Yellowspotted trevally Carangoides fulvoguttatus (Carangidae, Perciformes); JNC3176 (see Table 1); Fork length 270 mm, weight 340 g.
Site in host: Probably abdominal cavity (found in wash).
Locality: Off Nouméa, New Caledonia (collected 28 May 2010).
Prevalence and intensity: 1 of 10 fish examined infected; 1 nematode.
Voucher specimen: Not maintained (used for SEM).
Description
Female (1 nongravid specimen): Small, slender whitish nematode. Cuticle thin, finely transversely striated (Fig. 8D). Body 6.35 mm long, maximum width 69. Cephalic end rounded. Oral aperture hexagonal, surrounded by 4 submedian papillae and pair of lateral amphids (Figs. 7C, 8A, 8B). Pseudolabia absent. Four small submedian sublabia present (Figs. 7C, 8A, 8C). Deirids not found. Vestibule (stoma) long, with distinct funnel-shaped prostom at anterior end (Fig. 7A, 7B and 7D); prostom without anterior, subterminal teeth, but with 6 (1 dorsal, 1 ventral and 2 lateral on either side) minute denticles extending anteriorly from inner wall of prostom more posteriorly (Figs. 7A–7C, 8A and 8C); length of vestibule including prostom 171; prostom 24 long, 15 wide. Muscular oesophagus 318 long, maximum width 33, somewhat expanded towards its posterior end, well separated from glandular oesophagus; glandular oesophagus 1.13 mm long, maximum width 45, opens into intestine through large valve; length ratio of both oesophageal portions 1:3.55 (Figs. 7A, 7B and 7D). Length of vestibule and entire oesophagus represents 25% of body length. Intestine narrow, pale-coloured. Nerve ring encircles muscular oesophagus approximately at its first seventh; excretory pore located somewhat anterior to mid-length of muscular oesophagus (Figs. 7A, 7D and 8D). Nerve ring and excretory pore 213 and 300, respectively, from anterior extremity. Vulva situated 3.60 mm from anterior end of body, i.e. at 57% of body length. Vagina short. Uterus little developed, empty, formed by narrow tube provided with reflexed ovaries at both ends. Three distinct unicellular rectal glands present dorsally from rectum. Tail conical, 183 long, with pointed tip (Fig. 7E).
Remarks
In having a hexagonal oral aperture, no pseudolabia and a long vestibule (stoma) forming a distinct funnel-shaped prostom at its anterior end, the single available female specimen evidently belongs to the thelazioid family Rhabdochonidae. At present, this family consists of 11 genera, of which Trichospirura Smith & Chitwood, 1967 contains several species parasitising tetrapod vertebrates (amphibians, reptiles and mammals) [2], whereas the nematodes belonging to all other genera are parasites of fishes [36].
Of these, representatives of three genera, Rhabdochona Railliet, 1916, Prosungulonema Roytman, 1963 and Beaninema Caspeta-Mandujano, Moravec & Salgado-Maldonado, 2001, are parasites of freshwater fishes; whereas Rhabdochona contains more than 100 species (all intestinal parasites) distributed in all main zoogeographical regions [37], Prosungulonema and Beaninema are represented by a few species parasitic in the host’s intestine, liver, gall-bladder or swimbladder in eastern Asia, Africa and Mexico [17, 36]. On the contrary, the remaining seven genera, Johnstonmawsonia Campana-Rouget, 1955, Hepatinema Rasheed, 1964, Heptochona Rasheed, 1965, Vasorhabdochona Martin & Zam, 1967, Pancreatonema McVicar & Gibson, 1975, Fellicola Petter & Køie, 1993, and Megachona Mejía-Madrid & Pérez-Ponce de León, 2007, contain only several species (most of these genera are monotypic) that are parasites in the digestive tract and associated glands, bloodstream and body cavity of marine and brackish-water fishes in tropical and subtropical regions [3, 33, 45, 47].
In having the nerve ring encircling the muscular oesophagus, a well-developed funnel-shaped prostom and no anterior prostomal teeth, the available nematode specimen from C. fulvoguttatus distinctly differs from species of Beaninema, Fellicola, Hepatinema, Heptochona, Pancreatonema, Prosungulonema, Rhabdochona, Megachona and Trichospirura [36]. From the monotypic Vasorhabdochona, it can be differentiated by the didelphic (vs. monodelphic) female genital tract and an almost equatorial location of the vulva (at 53% vs. 8–19% of body length in Vasorhabdochona) [20, 32, 47]. Therefore, the present New Caledonian specimen is provisionally assigned to Johnstonmawsonia.
At present, the genus Johnstonmawsonia is represented by the following four species: J. campanarougetae Machkovskiy & Parukhin, 1979, J. coelorhynchi (Johnston & Mawson, 1945) (type species), J. muraenophidis Campana-Rouget, 1955, and J. porichthydis Tanzola & Gigola, 2002, all parasites of the digestive tract and pancreatic ducts of marine fishes [14, 22, 30, 68]. Two additional species of Johnstonmawsonia were described from freshwater fishes in Africa [46, 57], but, with respect to the papers by Roytman and Ivanova [61] and Moravec et al. [47], these should be transferred to Prosungulonema as P. africanum (Moravec & Puylaert, 1970) n. comb. and P. campanae (Puylaert, 1973) n. comb.
All species of Johnstonmawsonia were described to have no teeth in the prostom. The present specimen has no anterior prostomal teeth, but its prostom is provided with six minute, more posteriorly located denticles, which are visible only with the use of SEM. However, it should be remarked that none of the Johnstonmawsonia spp. has so far been studied by SEM (except for the poor quality SEM micrograph of the cephalic end of J. porichthydis [68]). Therefore, it is not currently clear whether the presence of small posterior prostomal denticles is a generic feature in Johnstonmawsonia or it is only a character of an apparently undescribed congeneric species parasitising Carangoides fulvoguttatus.
The presence of posteriorly located prostomal teeth, as found in the present specimen of Johnstonmawsonia, is not exceptional among rhabdochonids. Megachona chamelensis Mejía-Madrid & Pérez-Ponce de León, 2007, a parasite of intestinal caecae of Kyphosus ocyurus (Jordan & Gilbert) (Kyphosidae) off the Pacific coast of Mexico, possesses many large anterior prostomal teeth and numerous smaller teeth with an irregular arrangement located more posteriorly [33]. On the other hand, the anterior prostomal teeth are absent in Fellicola longispiculus Petter & Køie, 1993, a parasite of the gall-bladder of Coryphaenoides rupestris Gunnerus (Macrouridae) from off the Faroes, North Atlantic, but its prostom is internally lined with six longitudinal thickenings (four lateral simple and two median bilobed) appearing anteriorly as small denticles [56]. In Beaninema nayaritense Caspeta-Mandujano, Moravec & Salgado-Maldonado, 2001, a parasite of the gall-bladder of Cichlasoma beani (Jordan) (Cichlidae) in Mexico, the anterior prostomal teeth are absent, but there are six large conical teeth in the posterior half of the prostom [17]. Both B. nayaritense and F. longispiculus markedly differ from Johnstonmawsonia spp. in having the nerve ring that encircles the vestibule instead of the muscular oesophagus.
Since no rhabdochonid nematodes were previously recorded from carangid hosts, it is almost certain that the recorded specimen of Johnstonmawsonia sp. from C. fulvoguttatus belongs to an undescribed species. However, because only a single nongravid female was available, we refrain from describing this new taxon.
Conflict of Interest
The Editor-in-Chief of Parasite is one of the authors of this manuscript. COPE (the Committee on Publication Ethics, http://publicationethics.org), to which Parasite adheres, advises special treatment in these cases. In this case, the peer review process was handled by an Invited Editor, Jérôme Depaquit.
Acknowledgments
We thank Bernard Marchand and Isabelle Mary for help with the parasitological survey, and Ronald Fricke, Bernard Séret and Samuel Iglésias for help in identifying carangids. Thanks are also due to the staff of the Laboratory of Electron Microscopy, Institute of Parasitology, BC CAS, in České Budějovice for their technical assistance, and to Blanka Škoríková of the same Institute for help with the illustrations. This study was partly supported by the Institute of Parasitology (with institutional support RVO 60077344) and the Czech Science Foundation (Project No. P505/12/G112).
Cite this article as: Moravec F, Gey D & Justine J-L: Nematode parasites of four species of Carangoides (Osteichthyes: Carangidae) in New Caledonian waters, with a description of Philometra dispar n. sp. (Philometridae). Parasite, 2016, 23, 40.
References
- 1. Anderson RC, Chabaud AG, Willmott S. 2009. Keys to the nematode parasites of vertebrates: archival volume. CABI: Wallingford. [Google Scholar]
- 2. Bain O, Junker K. 2013. Trichospirura aethiopica n. sp. (Nematoda: Rhabdochonidae) from Malacomys longipes (Rodentia: Muridae) in Gabon, first record of the genus in the Ethiopian Realm. Parasite, 20, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bain O, Mutafchiev Y, Junker K. 2014. Order Spirurida, in Handbook of Zoology – Gastrotricha, Cycloneuralia and Gnathifera. Volume 2: Nematoda, Schmidt-Rhaesa A, Editor De Gruyter: Berlin, Boston: p. 661–732. [Google Scholar]
- 4. Bakhoum AJS, Quilichini Y, Justine J-L, Bray RA, Bâ CT, Marchand B. 2015. Ultrastructural study of sperm cells in Acanthocolpidae: the case of Stephanostomum murielae and Stephanostomoides tenuis (Digenea). PeerJ, 3, e744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barreto AL. 1918. Notas helmintológicas III. Cucullanus pulcherrimus n. sp. de nematódeo. Brasil Medico, 18, 137–138. [Google Scholar]
- 6. de Barreto BAL. 1922. Revisão da familia Cucullanidae Barreto, 1916. Memórias do Instituto Oswaldo Cruz, 14, 68–87. [Google Scholar]
- 7. Baylis HA. 1936. The Fauna of British India, Including Ceylon and Burma. Nematoda II (Filarioidea, Dioctophymoidea and Trichinelloidea). Taylor & Francis: London. [Google Scholar]
- 8. Beveridge I, Bray RA, Cribb TH, Justine J-L. 2014. Diversity of trypanorhynch metacestodes in teleost fishes from coral reefs off eastern Australia and New Caledonia. Parasite, 21, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray RA, Cribb TH, Justine J-L. 2010. Alcicornis haroldi n. sp. (Digenea: Bucephalidae) from the yellowspotted trevally Carangoides fulvoguttatus (Forsskål) (Carangidae) from off New Caledonia. Systematic Parasitology, 77, 35–43. [DOI] [PubMed] [Google Scholar]
- 10. Bray RA, Justine J-L. 2011. Acanthocolpidae (Digenea) of marine fishes off New Caledonia, with the descriptions of two new species. Folia Parasitologica, 58, 35–47. [DOI] [PubMed] [Google Scholar]
- 11. Bray RA, Justine J-L. 2012. Further reports of Acanthocolpidae Lühe, 1906 (Digenea) from fishes off New Caledonia, with descriptions of two new species. Systematic Parasitology, 83(1), 39–50. [DOI] [PubMed] [Google Scholar]
- 12. Bray RA, Justine J-L. 2013. Three species of opisthomonorchiine monorchiids (Digenea) in Carangoides spp. (Perciformes: Carangidae) from off New Caledonia, with a description of Opisthomonorchis dinema n. sp. Systematic Parasitology, 85(2), 147–156. [DOI] [PubMed] [Google Scholar]
- 13. Bruce NL, Adlard RD, Cannon LRG. 1994. Synoptic checklist of ascaridoid parasites (Nematoda) from fish hosts. Invertebrate Systematics, 8(3), 583–674. [Google Scholar]
- 14. Campana-Rouget Y. 1955. Sur deux nouveaux genres de Spirurides parasites de poissons: discussion systématique des genres voisins. Annales de Parasitologie Humaine et Comparée (Paris), 30, 346–362. [PubMed] [Google Scholar]
- 15. Campana-Rouget Y. 1957. Parasites de poissons de mer ouest-africains récoltés par J. Cadenat. Nématodes (4e note). Sur quelques espèces de Cucullanidae. Révision de la sous-famille. Bulletin de l’Institut Fondamental d’Afrique Noire, Série A, 19, 417–465. [Google Scholar]
- 16. Cárdenas MQ, Moravec F, Kohn A. 2009. First record of Philometra katsuwoni (Nematoda, Philometridae), a parasite of skipjack tuna Katsuwonus pelamis (Perciformes, Scombridae), off South American Atlantic coast. Biota Neotropica, 9(2), 263–266. [Google Scholar]
- 17. Caspeta-Mandujano JM, Moravec F, Salgado-Maldonado G. 2001. Two new species of rhabdochonids (Nematoda: Rhabdochonidae) from freshwater fishes in Mexico, with a description of a new genus. Journal of Parasitology, 87(1), 139–143. [DOI] [PubMed] [Google Scholar]
- 18. Froese R, Pauly D, Editors 2016. FishBase. World Wide Web electronic publication; www.fishbase.org. [Google Scholar]
- 19. Gibbons LM. 2010. Keys to the nematode parasites of vertebrates: supplementary volume, Vol. 10 CABI. [Google Scholar]
- 20. González-Solís D. 2004. A new host record for Vasorhabdochona cablei (Nematoda, Rhabdochonidae) in the Pacific coast of Mexico. Acta Parasitologica, 49(1), 87–88. [Google Scholar]
- 21. Ivashkin VM, Sobolev AA, Khromova LA. 1971. Camallanata of animals and man and the diseases caused by them. Osnovy nematodologii, 22, 1–388 (in Russian). [Google Scholar]
- 22. Johnston T, Mawson R. 1945. Parasitic nematodes. Reports B.A.N.Z. Antarctic Research Expeditions 1929–1931, 5, 73–159. [Google Scholar]
- 23. Justine J-L, Beveridge I, Boxshall GA, Bray RA, Miller TL, Moravec F, Trilles J-P, Whittington ID. 2012. An annotated list of fish parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda, Nematoda) collected from Snappers and Bream (Lutjanidae, Nemipteridae, Caesionidae) in New Caledonia confirms high parasite biodiversity on coral reef fish. Aquatic Biosystems, 8(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Justine J-L, Beveridge I, Boxshall GA, Bray RA, Moravec F, Trilles J-P, Whittington ID. 2010. An annotated list of parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda and Nematoda) collected in groupers (Serranidae, Epinephelinae) in New Caledonia emphasizes parasite biodiversity in coral reef fish. Folia Parasitologica, 57, 237–262. [DOI] [PubMed] [Google Scholar]
- 25. Justine J-L, Briand MJ, Bray RA. 2012. A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitology Research, 111(1), 341–351. [DOI] [PubMed] [Google Scholar]
- 26. Kalyankar SD. 1971. Thynnascaris carangis sp. n., a new nematode (Nematoda, Stomachidae, Raphidascaridinae) from an Indian fish Caranx malabaricus Day. Acta Parasitologica Polonica, 19, 147–150. [Google Scholar]
- 27. Kataytseva TV. 1975. Fauna of nematodes of the suborder Camallanata from some marine food fishes of the tropical part of the Pacific Ocean. Izvestiya Tikhookeanskogo Nauchno-Issledovatelskogo Instituta Rybnovo Khozyaystva I Okeanografii (TINRO), 98, 218–221 (in Russian). [Google Scholar]
- 28. Lane C. 1916. The genus Dacnitis Dujardin 1845. Indian Journal of Medical Research, 4, 93–104. [Google Scholar]
- 29. Luo DM. 2001. Notes on nematodes of fishes from Taiwan Strait I (Nematoda: Trichocephalida: Capillariidae; Spirurida: Dracunculidae). Acta Zootaxonomica Sinica, 2, 154–161 (in Chinese with English abstract). [Google Scholar]
- 30. Machkevskiy VK, Parukhin AM. 1979. A new nematode species of the genus Johnstonmawsonia and some comments to its taxonomy. Zoologitcheskii Zhurnal, 58, 1225–1227 (in Russian). [Google Scholar]
- 31. Maggenti AR. 1971. A review of the family Cucullanidae Cobbold, 1864 and the genus Bulbodacnitis Lane, 1916 with a description of Bulbodacnitis ampullastoma sp. n. (Nematoda: Cucullanidae) from Salmo gairdnerii Richardson. Proceedings of the Helminthological Society of Washington, 38(1), 80–85. [Google Scholar]
- 32. Martin WE, Zam SG. 1967. Vasorhabdochona cablei, gen. et sp. n. (Nematoda) from blood vessels of the marine fish, Gillichthys mirabilis Cooper. Journal of Parasitology, 53, 389–391. [PubMed] [Google Scholar]
- 33. Mejía-Madrid H, Pérez-Ponce de León G. 2007. A new rhabdochonid from the blue striped chub Sectator ocyurus (Osteichthyes: Kyphosidae) in Chamela Bay, Mexico. Journal of Parasitology, 93(1), 166–170. [DOI] [PubMed] [Google Scholar]
- 34. Moravec F. 1976. Occurrence of the encysted larvae of Cucullanus truttae (Fabricius, 1794) in the brook lamprey, Lampetra planeri (Bl.). Scripta Facultatis Scientiarum Naturalium Universitatis Purkynianae Brunensis. Biologia, 1(6), 17–20. [Google Scholar]
- 35. Moravec F. 2001. Trichinelloid nematodes parasitic in cold-blooded vertebrates. Academia: Praha: p. 432. [Google Scholar]
- 36. Moravec F. 2007. Some aspects of the taxonomy and biology of adult spirurine nematodes parasitic in fishes: a review. Folia Parasitologica, 54(4), 239–257. [DOI] [PubMed] [Google Scholar]
- 37. Moravec F, Adlard R. 2016. Redescription of Rhabdochona papuensis (Nematoda: Thelazioidea), a parasite of rainbow fishes (Melanotaenia spp.); the first record of the species of Rhabdochona in Australia. Acta Parasitologica, 61(4), 820–827. [DOI] [PubMed] [Google Scholar]
- 38. Moravec F, Bakenhaster M. 2012. New observations on philometrid nematodes (Philometridae) in marine fishes from the northern Gulf of Mexico and the Indian River Lagoon of Florida (USA), with first description of the male of Caranginema americanum. Journal of Parasitology, 98(2), 398–403. [DOI] [PubMed] [Google Scholar]
- 39. Moravec F, Justine J-L. 2005. Two anisakid nematodes from marine fishes off New Caledonia, including Raphidascaris (Ichthyascaris) nemipteri n. sp. from Nemipterus furcosus. Systematic Parasitology, 62(2), 101–110. [DOI] [PubMed] [Google Scholar]
- 40. Moravec F, Justine J-L. 2010. Some trichinelloid nematodes from marine fishes off New Caledonia, including description of Pseudocapillaria novaecaledoniensis sp. nov. (Capillariidae). Acta Parasitologica, 55, 71–80. [Google Scholar]
- 41. Moravec F, Justine J-L. 2012. Raphidascaris (Ichthyascaris) etelidis n. sp (Nematoda, Anisakidae), a new ascaridoid nematode from lutjanid fishes off New Caledonia. Zoosystema, 34(1), 113–121. [Google Scholar]
- 42. Moravec F, Justine J-L. 2014. Philometrids (Nematoda: Philometridae) in carangid and serranid fishes off New Caledonia, including three new species. Parasite, 21, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moravec F, Justine J-L. 2015. Anisakid nematodes (Nematoda: Anisakidae) from the marine fishes Plectropomus laevis Lacépède (Serranidae) and Sphyraena qenie Klunzinger (Sphyraenidae) off New Caledonia, including two new species of Hysterothylacium Ward & Magath, 1917. Systematic Parasitology, 92(3), 181–195. [DOI] [PubMed] [Google Scholar]
- 44. Moravec F, Justine J-L, Rigby MC. 2006. Some camallanid nematodes from marine perciform fishes off New Caledonia. Folia Parasitologica, 53(3), 223–239. [DOI] [PubMed] [Google Scholar]
- 45. Moravec F, Lorber J, Konečný R. 2007. Two new species of parasitic nematodes from the dogtooth tuna Gymnosarda unicolor (Pisces) off the Maldive Islands. Journal of Parasitology, 93(1), 171–178. [DOI] [PubMed] [Google Scholar]
- 46. Moravec F, Puylaert FA. 1970. On Johnstonmawsonia africana sp. n. (Nematoda: Rhabdochonidae) from the freshwater fish Haplochromis shwetzi, of Angola. Revue de Zoologie et Botanique Africaines, 82, 306–314. [Google Scholar]
- 47. Moravec F, Salgado-Maldonado G, Cabanas-Carranza G. 2001. New observations on Vasorhabdochona cablei (Nematoda: Rhabdochonidae) with remarks to the family Rhabdochonidae. Helminthologia, 38, 231–235. [Google Scholar]
- 48. Mudry DR, McCart P. 1974. Bulbodacnitis alpinus sp. nov. (Nematoda: Cucullanidae) from Arctic char, Salvelinus alpinus L., with notes on other species of Bulbodacnitis. Canadian Journal of Zoology, 52(4), 441–446. [DOI] [PubMed] [Google Scholar]
- 49. Olsen LS. 1954. A new species of Camallanus (Nematoda) from a Fijian marine fish. Transactions of the American Microscopical Society, 73, 258–260. [Google Scholar]
- 50. Parukhin AM. 1966. On the study of the helminth fauna of fishes of the family Carangidae from the South China Sea, in Gel’mintofauna zhivotnykh yuzhnykh morey, Vodyanitskiy VA, Editor Naukova Dumka: Kiev: p. 80–96 (in Russian). [Google Scholar]
- 51. Parukhin AM. 1971. Nematodes from fishes of the Red Sea and the Indian Ocean, in Voprosy Ekologii Ryb Yuzhnikh Morey. Biologiya Morya 23. Naukova Dumka: Kiev: p. 177–193 (in Russian). [Google Scholar]
- 52. Parukhin AM. 1973. Nematodes of fishes of the southern seas. Biologiya Morya, 31, 162–177 (in Russian). [Google Scholar]
- 53. Parukhin AM. 1976. Parasitic worms of food fishes of the southern Seas. Naukova Dumka: Kiev: p. 183 (in Russian). [Google Scholar]
- 54. Petter AJ. 1974. Essai de classification de la famille des Cucullanidae. Bulletin du Muséum National d’Histoire Naturelle, 3e série. Zoologie, 177, 1469–1491. [Google Scholar]
- 55. Petter AJ, Baudin-Laurencin F. 1986. Deux espèces du genre Philometra (Nematoda, Dracunculoidea) parasites de thons. Bulletin du Muséum National d’Histoire Naturelle, 8(4), 769–775. [Google Scholar]
- 56. Petter AJ, Køie M. 1993. Fellicola longispiculus gen. nov., sp. nov. (Nematoda, Rhabdochonidae) from the gall bladder of the marine fish Coryphaenoides rupestris. Annales de Parasitologie Humaine et Comparée, 68(5–6), 226–228. [Google Scholar]
- 57. Puylaert FA. 1973. Rhabdochonidae parasites de poissons africains d’eau douce et discussion sur la position systématique de ce roupe (Vermes - Nematoda). Revue de Zoologie et Botanique Africaines, 87, 647–665. [Google Scholar]
- 58. Rasheed S. 1968. The nematodes of the genus Cucullanus Mueller, 1777, from the marine fish of Karachi coast. Anales de la Escuela Nacional de Ciencias Biologicas, México, 15, 23–59. [Google Scholar]
- 59. Ratnasingham S, Hebert PDN. 2007. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molecular Ecology Notes, 7(3), 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rigby MC, Adamson ML, Deardorff TL. 1998. Camallanus carangis Olsen, 1954 (Nematoda: Camallanidae) reported from French Polynesia and Hawai’i with a redescription of the species. Journal of Parasitology, 84, 158–162. [PubMed] [Google Scholar]
- 61. Roytman V, Ivanova G. 1973. On the validity of Prosungulonema Roytman, 1963 (Rhabdochonidae: Prosungulonematinae). Trudy Gel’ mintologicheskoy Laboratorii Akademii Nauk SSSR, 23, 129–135 (in Russian). [Google Scholar]
- 62. Schmidt GD, Kuntz RE. 1969. Nematode parasites of Oceanica. V. Four new species from fishes of Palawan, P. I., with a proposal for Oceanicucullanus gen. nov. Parasitology, 59(2), 389–396. [PubMed] [Google Scholar]
- 63. Shamsi S, Gasser R, Beveridge I. 2013. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitology International, 62(3), 320–328. [DOI] [PubMed] [Google Scholar]
- 64. Shamsi S, Poupa A, Justine J-L. 2015. Characterisation of ascaridoid larvae from marine fish off New Caledonia, with description of new Hysterothylacium larval types XIII and XIV. Parasitology International, 64(5), 397–404. [DOI] [PubMed] [Google Scholar]
- 65. Simon JR. 1935. A new species of nematode, Bulbodacnitis scotti, from the trout, Salmo lewisi (Girard). University of Wyoming Publications, 2(2), 11–15. [Google Scholar]
- 66. Smedley EM. 1933. Nematode parasites from Canadian marine and fresh-water fishes. Contributions to Canadian Biology and Fisheries, 8(1), 169–179. [Google Scholar]
- 67. Smith-Vaniz WF. 1999. Carangidae, in FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 4. Bony fishes part 2 (Mugilidae to Carangidae), Carpenter KE, Niem VH, Editors FAO: Rome: p. 2659–2756. [Google Scholar]
- 68. Tanzola RD, Gigola G. 2002. Johnstonmawsonia porichthydis n. sp. (Nematoda: Rhabdochonidae) from Porichthys porosissimus (Pisces: Batrachoidiformes). Helminthologia, 39, 99–102. [Google Scholar]
- 69. Velasquez C. 1966. Some parasitic helminths of Philippine fishes. University of the Philippines Research Digest, 5, 23–29. [Google Scholar]
- 70. Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. 2005. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society of London B Biological Sciences, 360(1462), 1847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yamaguti S. 1961. Systema Helminthum: Nematodes of vertebrates (in 2 pts.). Interscience Publishers: New York, London: p. 699. [Google Scholar]
- 72. Yooyen T, Moravec F, Wongsawad C. 2011. Raphidascaris (Ichthyascaris) arii sp. n. (Nematoda: Anisakidae), a new ascaridoid nematode from marine catfishes in the Gulf of Thailand. Helminthologia, 48(4), 262–267. [Google Scholar]