Abstract
Frailty is a geriatric syndrome characterized by functional impairments and is associated with poor outcomes; however, the prevalence of frailty and its association with health status in patients treated with percutaneous coronary intervention (PCI) is unknown. To assess the prevalence of frailty and its association with health status in PCI-treated patients, we studied 629 patients ≥65 years old undergoing PCI from October 2005 through September 2008. Frailty was characterised using the Fried criteria: weight loss >10 pounds in the past one year, exhaustion, low physical activity, poor gait speed and grip strength (3 features = frail; 1–2 features = intermediate frailty; 0 features = not frail). Health status was assessed using the Short-Form (SF) 36 and the Seattle Angina Questionnaire (SAQ). Multivariable linear regression models were used to estimate the independent association between frailty and health status. Complete data on 545 patients demonstrated that 19% (n=117) were frail, 47% (n=298) had intermediate frailty, and 21% (n=130) were not frail. Frail patients had more comorbidities and more frequent left main or multivessel disease after adjusting for age and sex (p<0.05 across groups). Multivariable linear regression demonstrated poorer health status in frail patients, as compared to non-frail patients, as evidenced by lower SF-36 scores, lower SAQ scores for physical limitation, and lower SAQ scores for quality of life (p<0.001 for each health status domain). In conclusion, one-fifth of older patients are frail at the time of PCI and have higher comorbid burden, angiographic disease severity, and poorer health status than non-frail adults.
Keywords: frailty, percutaneous coronary intervention, health status
Introduction
While optimizing patients’ health status (their symptoms, function and quality of life) are important therapeutic goals for all patients, they are particularly relevant to older adults.1 Patients’ health status is not only an important outcome in its own right,2 but has also been shown to be associated with clinical events in patients with coronary artery disease (CAD) and quality of life after percutaneous coronary intervention (PCI).3,4 The association between health status and age-associated impairments, such as frailty, are unknown but are needed to better identify opportunities to improve care for the aging population of CAD patients.5,6 Frailty is an emerging concept that is characterized by reduced resilience to stressors and increased physiological vulnerability.7 Fried et al have defined frailty as a clinical syndrome with progressive decline in reserves and physical function, operationalized its assessment, and reported its association with adverse outcomes.8 Although traditional cardiovascular comorbidities in patients with CAD have been studied, the prevalence and correlates of geriatric syndromes, such as frailty, are less understood. With the availability of standardized definitions and valid instruments, the aims of this study were to assess the prevalence of frailty in PCI-treated older patients and to describe the baseline health status measures of frail patients as compared to those without frailty. Finally, we sought to understand the incremental association of frailty to baseline quality of life and disease-specific quality of life after accounting for demographics, comorbidities, and the angiographic severity of patients’ CAD.
Methods
Patients ≥65 years of age undergoing PCI at Mayo Clinic in Rochester, MN and Franciscan Skemp Hospital in LaCrosse, WI from October 2005 to September 2008 and who survived to hospital discharge were prospectively enrolled in a study assessing frailty and health status. A cross-sectional study design was used to administer standardized health status questionnaires and to perform functional assessments of frailty, along with abstraction of clinical comorbidities from medical records. Patients with residual neurological deficits after a stroke, severe Parkinson’s disease, or dementia were excluded from the study given the concern regarding reliability and validity of functional assessments in these patients. The study was approved by the Institutional Review Board of Mayo Clinic and participants provided written informed consent.
We used the Fried and Walston definition of frailty, in which musculoskeletal, neuro-endocrine, and nutritional defects are considered in the determination of frailty.8,9 The five criteria were measured in each study participant, including unintended weight loss (>10 lb in the preceding year), exhaustion, physical activity, gait speed (walking time over 15 feet), and grip strength.8 Exhaustion was measured by the Center of Epidemiologic Studies-Depression’s subscale. The two items in this scale are: how often in the past week did the patient feel the following (a) I felt that everything I did was an effort, and (b) I could not get going. Subjects who answered “a moderate amount of time (3–4 days)” or “most of the time” to either of the statements were categorized as meeting the exhaustion criteria for frailty. Physical activity was measured by the short version of the Minnesota Leisure Time Activity questionnaire. Gait speed was assessed as walking time across a 15-foot distance in an unobstructed, well-lit hallway. Participants walking with an assist device were permitted to do so for the test. Hand-grip strength was tested in the subject’s dominant hand using a Jamar® handgrip dynamometer (Bolingbrook, IL). Participants were told to squeeze as hard as possible and results were scored as kilograms. The details for the thresholds in the five frailty criteria used are provided (Appendix). The assessment for frailty was performed in patients after their PCI procedure. A patient was defined as frail if they were found to have deficits in 3 or more core elements and as intermediately frail if they had deficits in 1 – 2 core elements.8 Patients without any deficits in the core frailty measures were classified as non-frail (i.e., normal).
Comorbidity was assessed using two separate instruments. The Charlson index, a well-validated measure of comorbidity burden, is based on 12 chronic conditions and its corresponding weights were originally determined based on their association with 1-year mortality.10 We used the same procedure to derive a score for the Charlson index in our study participants.10 The CAD-specific index has been compared to the Charlson index and has been shown to perform similarly; however, the CAD-specific index incorporates coronary artery disease severity and left ventricular ejection fraction, in addition to baseline clinical comorbidities.11 Higher values of these indices indicate a greater burden of comorbidities.
Health status assessments were performed after the PCI procedure through administration of the Short-Form 36 (SF-36) for physical component scores (PCS) and mental component scores (MCS) and the disease-specific Seattle Angina Questionnaire (SAQ), instruments that have been shown to be valid and reproducible self-report tools for ascertaining health-related quality of life.12,13 The SF-36 is a generic assessment of patients’ health status using an 8-scale profile of functional health and well-being that form two distinct domains: the PCS and MCS.12 A score of 50 reflects the US population mean and each 10 points represents one standard deviation from that mean. Higher scores indicate better health status. The SAQ quantifies five clinically relevant dimensions of CAD: physical limitation, angina stability, angina frequency, treatment satisfaction, and quality of life. Scales range from 0–100, where higher scores indicate better functioning, fewer anginal symptoms, and better quality of life. A 5-point difference in mean SAQ scores is considered clinically significant.13
Univariate continuous distributions are summarized as median and inter-quartile ranges, unless otherwise specified. Comparisons between groups were tested using linear models with a linear contrast of group means, thus testing for a mean trend from the no frailty to the frail group. Age and sex were added to the linear model to obtain age-sex adjusted p-values. Discrete data, including 30-day outcomes of major cardiovascular events, are summarized as frequency (percentage) and tested across frailty groups with the Armitage trend test. Age-sex adjusted p-values for these comparisons were obtained by modeling the binomial variable as a function of frailty (coded as consecutive integers for the three groups) and age and sex. Partial Spearman correlation coefficients, adjusted for age and sex, were computed to measure the association between frailty, QOL and comorbidity measures.
Multivariable linear regression was used to assess the association between patient demographics, baseline characteristics, frailty, and quality of life measures. Five multiple imputation data sets were created using the aregImpute function from the Hmisc library for S-Plus to allow all observations to be included in the analysis. Regression models were fit within each imputed data set; the 5 sets of estimates were then combined according to Rubin’s rules.14 Box-Cox transformations were created for non-normally distributed variables. However, the results from models with transformed data provided similar conclusions as those without transformed data; for ease of interpretation, the results from untransformed data are presented. Partial residual plots were used to assess the assumptions of linear associations for continuous covariates; no evidence of non-linearity was seen in these plots. Interactions between site, CAD-specific index, and frailty measures were tested.
Results
We screened 1885 patients of whom 629 (33.4%) consented to participate. Slightly more men (69% vs. 63%) and younger patients (74.3±6.4 years vs. 75.8±6.9 years) consented as compared with non-consenting patients. The mean age of participants was 74.8 ± 6.4 years. Among the participants, 117 (18.6%) were frail, 298 (47.4%) had intermediate frailty, and 130 (20.6%) were not frail. Frailty status could not be classified in 84 patients (13.3%) due to incomplete or incorrectly completed forms. The most common frailty measure was slow gait speed (41%) and the least common was unintended weight loss of more than 10 lbs in the preceding year (7.5%).
Characteristics of the study population are shown in Table 1. Patients with features of frailty tended to be older, had higher body mass index, were more likely to be female, and to have more comorbidities, including hypertension, diabetes mellitus, chronic kidney disease, peripheral arterial disease, congestive heart failure, atrial fibrillation, stroke or transient ischemic attack, and myocardial infarction (adjusted p-value <0.05 for comparison across groups). Table 2 describes the procedural and angiographic characteristics of the study cohort. There was a higher frequency of multivessel or left main disease in frail patients (74%) as compared to intermediately frail (68%) or non-frail patients (60%) (p = 0.019) and frailty was found to be associated with the presence of multivessel or left main disease after adjusting for age and sex (p = 0.005). Table 3 describes baseline health-related quality of life stratified by frailty status in patients undergoing PCI. The mean SAQ Physical Limitation score was significantly lower in frail patients as compared to intermediately frail patients and non-frail patients, indicating worse disease-specific physical limitations in patients with frailty. Similarly, the mean SAQ Quality of Life score was significantly lower in frail patients as compared to intermediately frail patients and non-frail patients, suggesting that patients with the frailty phenotype had greater associated quality of life impairments from angina. The SAQ scores for angina frequency and treatment satisfaction were similar across frailty strata. The SF-36 scores for the PCS and MCS domains were lowest in frail patients, as compared with intermediately frail and non-frail patients, suggesting that older adults with frailty undergoing PCI have lower baseline health-related quality of life as compared with non-frail patients. Table 4 shows 30-day outcomes for major cardiovascular events (death, myocardial infarction, and revascularization) by frailty status. Event rates for short-term clinical outcomes were generally low for the cohort overall, with approximately 5% experiencing myocardial infarction 30-days after PCI within each frailty strata. After adjusting for age and sex, there were no statistically significant differences in any of the clinical events by frailty category.
Table 1.
Baseline characteristics of patients by frailty status
| Characteristics | Not Frail (n=130) | Intermediate Frailty (n=298) | Frail (n=117) | p-value | Adj. p-value† |
|---|---|---|---|---|---|
| Age, mean ± SD (years) | 72.6 ± 5.8 | 74.6 ± 6.0 | 77.4 ± 6.8 | <.001 | |
| Men | 106 (82%) | 205 (69%) | 65 (56%) | <.001 | |
| Body mass index (kg/m2) | 29.0 ± 4.2 | 30.1 ± 5.4 | 30.7 ± 6.6 | 0.014 | <.001 |
| Hypertension | 91 (72%) | 244 (82%) | 103 (88%) | 0.001 | 0.008 |
| Current smoker | 4 (3%) | 18 (6%) | 7 (6%) | 0.30 | 0.047 |
| Diabetes mellitus | 20 (16%) | 93 (31%) | 47 (40%) | <.001 | <.001 |
| Chronic kidney disease | 5 (4%) | 39 (13%) | 28 (25%) | <.001 | <.001 |
| Peripheral Arterial Disease | 7 (6%) | 29 (10%) | 25 (22%) | <.001 | <.001 |
| Congestive Heart Failure | 14 (11%) | 47 (16%) | 32 (28%) | <.001 | 0.013 |
| Atrial Fibrillation | 9 (7%) | 41 (14%) | 30 (26%) | <.001 | <.001 |
| Cerebrovascular accident or transient ischemic attack | 11 (9%) | 41 (14%) | 23 (20%) | 0.011 | 0.004 |
| Vascular surgery | 11 (9%) | 42 (14%) | 25 (22%) | 0.003 | 0.005 |
| Myocardial infarction | 29 (23%) | 94 (32%) | 41 (38%) | 0.013 | 0.043 |
| Percutaneous coronary intervention | 38 (29%) | 117 (39%) | 41 (35%) | 0.31 | 0.28 |
| Coronary artery bypass grafting | 21 (16%) | 79 (27%) | 39 (33%) | 0.002 | 0.012 |
| Chronic lung disease or obstructive pulmonary disease | 9 (7%) | 35 (12%) | 31 (26%) | <.001 | <.001 |
| Rheumatologic disease | 45 (35%) | 135 (46%) | 68 (58%) | <.001 | 0.005 |
| Any neoplasm | 32 (25%) | 64 (22%) | 25 (22%) | 0.53 | 0.44 |
| Metastatic solid neoplasm | 13 (10%) | 39 (13%) | 14 (12%) | 0.68 | 0.89 |
| Lymphoma | 0 (0%) | 2 (1%) | 2 (2%) | 0.12 | 0.22 |
| Unexpected fall within 6 months | 5 (4%) | 46 (15%) | 27 (23%) | <.001 | <.001 |
| Fracture | 42 (33%) | 105 (36%) | 36 (31%) | 0.76 | 0.84 |
| Sleep apnea | 16 (13%) | 50 (17%) | 27 (24%) | 0.022 | 0.002 |
| Charlson Index, median (Q1, Q3) | 2.0 (1.0, 3.0) | 2.0 (1.0, 4.0) | 3.0 (2.0, 5.0) | <.001 | <.001 |
| Coronary artery disease-specific Index, median (Q1, Q3) | 1.0 (1.0, 3.0) | 3.0 (1.0, 4.0) | 3.0 (2.0, 6.0) | <.001 | <.001 |
p-values adjusted for age and sex
Table 2.
Angiographic and procedural factors by frailty status
| Characteristics | Not Frail (n=130) | Intermediate Frailty (n=298) | Frail (n=117) | p-value | Adj. p-value† |
|---|---|---|---|---|---|
| Urgency of percutaneous coronary intervention | 0.26 | 0.41 | |||
| Elective | 53 (41%) | 99 (33%) | 35 (30%) | ||
| Urgent | 54 (42%) | 137 (46%) | 62 (53%) | ||
| Emergency | 23 (18%) | 60 (20%) | 20 (17%) | ||
| Myocardial infarction pre-percutaneous coronary intervention | 0.65 | 0.99 | |||
| No myocardial infarction within 24 hrs | 93 (73%) | 193 (66%) | 80 (69%) | ||
| Non-ST elevation myocardial infarction | 18 (14%) | 59 (20%) | 20 (17%) | ||
| ST-elevation myocardial infarction | 17 (13%) | 42 (14%) | 16 (14%) | ||
| Multivessel or left main disease | 74 (60%) | 187 (68%) | 82 (74%) | 0.019 | 0.005 |
| Total number of stents placed | 1.4 ± 0.8 | 1.4 ± 0.9 | 1.6 ± 1.0 | 0.27 | 0.13 |
| Number of segments treated | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.6 ± 0.8 | 0.034 | 0.011 |
| Number of coronary arteries treated | 0.24 | 0.17 | |||
| 1 | 110 (85%) | 252 (85%) | 93 (79%) | ||
| 2 | 19 (15%) | 42 (14%) | 22 (19%) | ||
| 3 | 1 (1%) | 3 (1%) | 2 (2%) | ||
| Percutaneous coronary intervention in native left anterior descending | 69 (53%) | 121 (41%) | 58 (50%) | 0.52 | 0.46 |
| Percutaneous coronary intervention in native right | 39 (30%) | 108 (36%) | 38 (32%) | 0.65 | 0.40 |
| Percutaneous coronary intervention in native left main | 2 (2%) | 9 (3%) | 11 (9%) | 0.002 | 0.003 |
| Percutaneous coronary intervention in native left circumflex | 36 (28%) | 68 (23%) | 31 (26%) | 0.79 | 0.81 |
| Vein graft intervention | 6 (5%) | 35 (12%) | 9 (8%) | 0.36 | 0.97 |
| Use of drug eluting stent | 111 (85%) | 242 (81%) | 94 (80%) | 0.29 | 0.23 |
| Glycoprotein IIb/IIIa use | 78 (60%) | 182 (61%) | 64 (55%) | 0.42 | 0.99 |
| Blood loss requiring transfusion | 1 (1%) | 8 (3%) | 6 (5%) | 0.037 | 0.088 |
| Pseudoaneurysm | 0 (0%) | 0 (0%) | 1 (1%) | 0.13 | 0.94 |
| Hypotension | 8 (6%) | 24 (8%) | 11 (9%) | 0.34 | 0.35 |
| Femoral bleed | 0 (0%) | 3 (1%) | 1 (1%) | 0.41 | 0.47 |
| Hematoma | 2 (2%) | 4 (1%) | 6 (5%) | 0.063 | 0.32 |
| Gastrointestinal bleed | 1 (1%) | 1 (0%) | 2 (2%) | 0.41 | 0.64 |
| Retroperitoneal bleed | 0 (0%) | 0 (0%) | 1 (1%) | 0.13 | 0.94 |
| Central nervous system bleed | 0 (0%) | 0 (0%) | 0 (0%) | --- | --- |
p-values adjusted for age and sex
Table 3.
Health status measures by frailty status
| Characteristics | Not Frail (n=130) | Intermediate Frailty (n=298) | Frail (n=117) | p-value for trend | Adj. p-value† |
|---|---|---|---|---|---|
| SAQ Physical Limitation | 77.6 ± 17.8 | 70.2 ± 20.0 | 59.5 ± 24.5 | <.001 | <.001 |
| SAQ Anginal Frequency | 71.3 ± 24.6 | 73.4 ± 24.6 | 65.9 ± 28.1 | 0.10 | 0.080 |
| SAQ Treatment Satisfaction | 85.6 ± 17.3 | 84.4 ± 17.9 | 82.5 ± 17.2 | 0.16 | 0.17 |
| SAQ Quality of Life Scale | 63.0 ± 23.8 | 59.7 ± 23.9 | 55.1 ± 26.3 | 0.013 | 0.013 |
| Center for Epidemiologic Studies-Depression Score | 5.8 ± 5.8 | 10.5 ± 9.1 | 15.4 ± 10.4 | <.001 | <.001 |
| SF-36 Physical Component Score | 42.9 ± 9.6 | 37.0 ± 9.6 | 29.0 ± 8.3 | <.001 | <.001 |
| SF-36 Mental Component Score | 55.6 ± 7.4 | 52.3 ± 9.7 | 48.8 ± 11.2 | <.001 | <.001 |
P-values adjusted for age and sex.
Abbreviations: SAQ, Seattle Angina Questionnaire; SF-36, short form-36
Table 4.
Short-term (30-day) outcomes by frailty status
| Outcome | Not Frail (n=130) | Intermediate Frailty (n=298) | Frail (n=117) | p-value for trend | Adj. p-value† |
|---|---|---|---|---|---|
| Myocardial infarction | 7 (5%) | 16 (5%) | 6 (5%) | 0.93 | 0.62 |
| Death or myocardial infarction | 7 (5%) | 16 (5%) | 7 (6%) | 0.84 | 0.73 |
| Percutaneous coronary intervention or coronary artery bypass grafting | 3 (2%) | 16 (5%) | 5 (4%) | 0.42 | 0.26 |
| Death, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting | 10 (8%) | 30 (10%) | 11 (9%) | 0.63 | 0.83 |
P-values adjusted for age and sex.
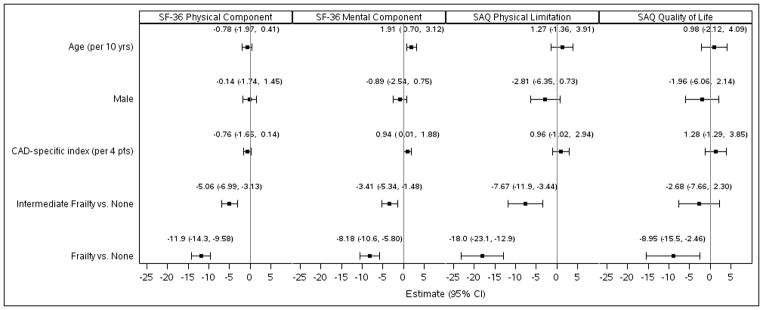
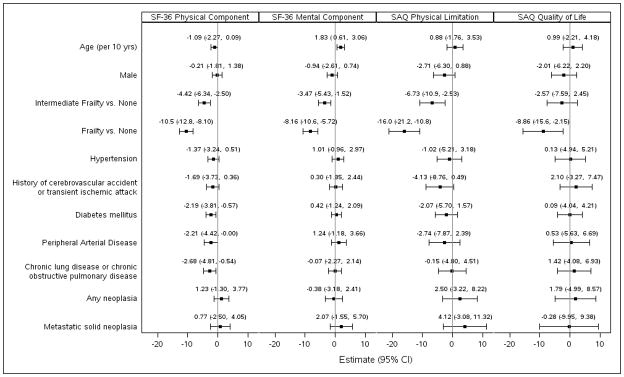
We modeled the estimated effect of frailty classification on health status scores adjusted for age, sex, and the CAD-specific comorbidity index (Figure 1). Intermediately frail patients, as compared to non-frail patients, had significantly lower adjusted scores for the SF-36 PCS (−5.1 points; 95% CI −7.0 to −3.1), SF-36 MCS (−3.4 points; 95% CI −5.3 to −1.5), and the SAQ Physical Limitation score (−7.7 points; 95% CI −12.0 to −3.4). When frailty was compared to non-frail patients, even greater reductions in self-perceived quality of life were found, with lower adjusted scores for the SF-36 PCS (−11.9; 95% CI −14.3 to −9.6; p<0.001), SF-36 MCS (−8.2; 95% CI −10.6 to −5.8; p<0.001), SAQ Physical Limitation score (−18.0; 95% CI −23.1 to −12.9; p<0.001), and SAQ Quality of Life score (−9.0; 95% CI −15.5 to −2.46; p<0.001). To ease the interpretation of frailty as a clinical risk factor, a multivariable linear regression model was performed with adjusting variables rather than the CAD-index. In this model, the frailty phenotype had the greatest association with lower estimated health-related quality of life in physical, mental, and disease-specific domains of physical limitation and quality of life (Figure 2). The magnitude of differences observed for health-related quality of life is in the range of 1.8 to 8.8 times greater than the expected difference for a 10-year increase in age. For example, the estimated SF-36 PCS for a hypothetical 70-year old male with a CAD-specific index of 1 and no frailty is 42.7. However, with intermediate frailty, the SF-36 PCS is predicted to be 37.7 and with frailty to be 30.8. If the person is not frail and lives to 80 with a CAD-specific index of 1, the expected score is 42.0.
Figure 1.
Multivariable linear regression models for health status measures. The parameter estimate and 95% CI for health status measures in intermediate frailty and frailty after adjusting for age, sex, and CAD-specific comorbidity index. Estimates reflect the average mean difference in the endpoint for a given change in the risk factor, given other risk factors remain the same. The estimates for age and CAD-specific index reflects the expected difference for an increase in value equal to the observed interquartile range (IQR). The IQR for age is 10 years; the IQR for the CAD-specific index is 4.
Figure 2.
Multivariable linear regression models for health status measures. The parameter estimate and 95% CI for health status measures in intermediate frailty and frailty after adjusting for age, sex, and comorbidities. The association of frailty and intermediate frailty has a greater estimated reduction in health-related quality of life and disease-specific quality of life as compared to other individual risk factors, adjusted for all other risk factors in the model. Estimates reflect the average mean difference in the endpoint for a given change in the risk factor, given other risk factors remain the same.
Discussion
In adults ≥65 years of age undergoing PCI, nearly one-fifth were frail, as defined by the Fried criteria, and approximately one-half had an intermediate frailty phenotype. Features of frailty are associated with higher comorbid burden and greater angiographic disease severity. Both frailty and intermediate frailty are independently associated with lower health-related quality of life and disease-specific quality of life. Lower quality of life scores for domains of physical limitation and disease-specific quality of life among those with frailty suggests that these patients may have been affected to a greater degree by their angina and that reduction of physical activities may have been one method for minimizing the frequency of their angina.
The prevalence of age-associated impairments, including frailty, increases with age but varies across studies, reflecting differences in population characteristics, selection criteria, and definitions used.15 Patients with age-associated impairments have higher mortality, disability, hospitalization rates, institutionalization rates, and health services utilization.16–19 The present study highlights the prevalence of frailty in patients selected for coronary revascularization by PCI, the majority of whom were receiving treatment for symptomatic CAD. Short term clinical outcomes were similar across categories of frailty, suggesting that despite greater comorbidity, CAD disease severity, and lower disease-specific quality of life, PCI treatment in frail patients results in comparable short term event rates. In the quality of life analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, PCI performed on a background of optimal medical therapy as compared to optimal medical therapy alone resulted in short term improvements in several SAQ domains including physical limitation, angina frequency, angina stability, and quality of life.20 However, benefits were greatest in patients with more severe angina at baseline and, over long term follow up, initial benefits observed with PCI were no longer evident. Within the context of the present study, the observations from COURAGE may suggest that identification of patients that have resorted to reducing physical activity to compensate for limitations related to angina burden may derive short term benefits, albeit modest, from PCI. Whether PCI treatment as compared to medical therapy alone or surgical revascularization improves disease-specific quality of life or long-term outcome in frail older adults requires further study. Our study also illuminates the association between health status across gradations of frailty, including specific quality of life domains affecting patients with CAD. Importantly, associations were identified in frail patients that could impact clinical decision-making in the management of patients with symptomatic CAD. For example, frail status was associated with significantly higher rates of multivessel or left main disease (p = 0.005 across groups) but was not associated with greater anginal frequency per the SAQ scale. Frail adults may experience less angina as they modify their level of physical activity as not to provoke anginal symptoms. Reduction in physical activity, as evidenced by the SAQ Physical Limitation score and SF-36 PCS, support the findings of reduced quality of life in these health domains in frail patients.
Our findings extend the extant literature on frailty in CAD. One prospective study found frailty in 27% of older patients (age ≥70 years) with significant CAD at cardiac catheterization and showed that single-item gait speed was a good indicator of multidimensional frailty.21 Gait speed has been used as a marker of frailty and decline in gait speed over time has been associated with the presence of CAD.21,22 Weakness and exhaustion, characteristics of the frailty phenotype, are shared symptoms with cardiovascular ischemia and heart failure and are well-recognized presentations of myocardial infarction in elderly patients.5,6 Given the degree of overlap between frailty and symptoms of cardiovascular disease, it seems plausible that assessment of age-associated impairments using frailty status measures, such as gait speed and grip strength, may provide reclassification of risk in older adults with known coronary risk factors.
In the present study we used the SF-36, which measures quality of life using physical and mental health status domains, and a disease-specific questionnaire, the SAQ. Frailty was associated with a greater reduction in the health-related quality of life than the CAD-specific comorbidity index,11 an integer score that incorporates comorbid conditions and angiographic disease severity. Given that frailty has been associated with adverse outcomes,8,18,19,21 we compared frailty (referent; no frailty) to other known coronary risk factors in the multivariable regression model and found that frailty was associated with the greatest average reduction in health-related quality of life.
Fried et al previously demonstrated overlap between frailty, comorbidity, and disability.23 Our study extends these observations to include poorer health status measures among frail patients as compared to non-frail adults with angiographically-documented CAD. The implications of our findings are relevant within the context of the prevalence of frailty in a PCI-treated population of Medicare beneficiaries and their associated health status. Frailty has been associated with nearly a 2-fold risk in the odds of death in a population of patients with advanced CAD.21 Reduction in physical activities due to angina may further complicate the downward spiral of the frailty phenotype by diminishing activity, reducing adaptive capacity, and further impairing physiological reserves.
Our study enriches the limited data on the cross-sectional association between frailty and quality of life in the published literature.24,25 Masel et al found that frailty was significantly associated with lower scores on physical and cognitive health related quality of life scales in older Mexican-American individuals.24 In another small study, 24 patients with frailty were noted to have lower quality of life, independent of age, diabetes, macrovascular complication, kidney dysfunction, and depressed mood.25 In a population of older adults referred for cardiac surgery, gait speed frailty offered incremental risk prediction of major morbidity and mortality beyond that predicted from a well-established cardiac risk score.26 The aging of the cardiovascular population further mandates that focus be turned to improve the selection of patients likely to derive the most benefit from PCI by considering the presence or absence of age-associated impairments.
Patients that underwent PCI in our study were from hospitals located in the Midwest U.S. where racial and ethnic characteristics of the patient population may not generalize to settings with different demographic profiles. A large number of patients had to be screened for inclusion in the study due to competing research protocols. This may have influenced the generalizability of our findings. The data describe cross-sectional associations between several aspects of age-associated impairments, frailty and health status, and as such, make difficult inferences regarding causality. However, the cross-sectional study design was selected to better understand prevalence of frailty in a PCI-treated population and its associated comorbid health conditions and health status. Information regarding change in health or frailty status following PCI is not known and would be important to better understand the role of PCI in modifying frailty and health-related quality of life in these patients. Since more conservative management strategies are often selected for frail older patients, by studying a population who underwent PCI, we may have underestimated the association of frailty with health status in a more representative cohort of patients with CAD. However, the greater functional and disease-specific limitations experienced by patients with frailty at baseline may have led operators towards implementing PCI; namely to attempt to improve the health status manifestations of their CAD.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Mayo Foundation. Dr. Gharacholou is a participant in the NIH clinical research loan repayment program (1L30 AG034828-01).
References
- 1.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Vidaillet H, Phillips RS. Health values of hospitalized patients 80 years or older: Hospital Elderly Longitudinal Project (HELP) Investigators. JAMA. 1998;279(5):371–375. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 2.Spertus JA. Evolving applications for patient-centered health status measures. Circulation. 2008;118(20):2103–2110. doi: 10.1161/CIRCULATIONAHA.107.747568. [DOI] [PubMed] [Google Scholar]
- 3.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106(1):43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 4.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110(25):3789–3794. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 5.Alexander KP, Newby LK, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 6.Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 7.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA for the Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83:1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43:576–582. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437–1440. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley; New York: 1987. [Google Scholar]
- 15.Singh M, Alexander K, Roger VL, Rihal CS, Whitson HE, Lerman A, Jahangir A, Nair KS. Frailty and its potential relevance to cardiovascular care. Mayo Clin Proc. 2008;83:1146–1153. doi: 10.4065/83.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh M, Rihal CS, Roger VL, Lennon RJ, Spertus J, Jahangir A, Holmes DR. Comorbid conditions and outcomes after percutaneous coronary intervention. Heart. 2008;94:1424–1428. doi: 10.1136/hrt.2007.126649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. 2002;106:2309–2314. doi: 10.1161/01.cir.0000036598.12888.de. [DOI] [PubMed] [Google Scholar]
- 18.Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45:265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 20.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE for the COURAGE Trial Research Group. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 21.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 24.Masel MC, Graham JE, Reistetter TA, Markides KS, Ottenbacher KJ. Frailty and health related quality of life in older Mexican Americans. Health Qual Life Outcomes. 2009;70:1–7. doi: 10.1186/1477-7525-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanauchi M, Kubo A, Kanauchi K, Saito Y. Frailty, health-related quality of life and mental well-being in older adults with cardiometabolic risk factors. Int J Clin Pract. 2008;62:1447–1451. doi: 10.1111/j.1742-1241.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 26.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. Development and validation of a prognostic index for 4-year mortality in older adults. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.