Abstract
Alveolar macrophages have been investigated for years by approaches involving macrophage extraction from the lung by bronchoalveolar lavage, or by cell removal from lung tissue. Since extracted macrophages are studied outside their natural milieu, there is little understanding of the extent to which alveolar macrophages interact with the epithelium, or with one another to generate the lung’s innate immune response to pathogen challenge. Here, we review new evidence of macrophage-epithelial interactions in the lung and we address the emerging understanding that the alveolar epithelium plays an important role in orchestrating the macrophage driven immune response.
The lung protects against inhaled pathogens by activating defensive epithelial mechanisms including release of anti-bacterial secretions and induction of protective inflammatory responses. Although lung defense can be brought into play throughout the airway, here we focus on immune responses in the alveolar compartment, which comprises the bulk of the lung’s epithelial surface. Alveolar host-pathogen interactions can damage the alveolar epithelium, causing loss of critical barrier properties that normally protect the alveolar gas space against fluid entry from the adjoining vascular compartment. The resulting alveolar edema is clinically recognized as Acute Lung Injury (ALI), a condition that predisposes to the Acute Respiratory Distress Syndrome (ARDS) in which multi-organ failure causes high mortality and morbidity[1]. Since specific therapies for ALI/ARDS are not available, better mechanistic understanding of pathogen-induced alveolar inflammatory mechanisms may lead to the development of new therapeutic strategies.
Important players in alveolar inflammation are resident alveolar macrophages, which are pathogen sensors and promote immune cell recruitment in alveoli. Another important player is the alveolar epithelium, which protects alveolar homeostasis and normally inhibits entry of immune cells into the alveolar space. We review here the emerging understanding that that cell-cell communication between these immune significant alveolar cell types might be important for physiological alveolar homeostasis as well as for alveolar host-pathogen interactions.
Resident alveolar macrophages
Resident alveolar macrophages, which are constitutively present in the lung, differ from recruited macrophages, which enter alveoli 2-3 days after onset of the primary inflammatory response[2, 3]. Recruited macrophages initially promote and then later suppress inflammation through multiple processes, including conversion of the macrophages from the inflammatory (M1) to the anti-inflammatory (M2) phenotype, induction of efferocytosis (phagocytosis of apoptotic cells) for neutrophil clearance, and secretion of cytokines such as IL-10 and IL-1R antagonist [4, 5]. The biology of recruited macrophages has been discussed recently [2-4] and will not be further considered here.
Resident alveolar macrophages are embryonically derived [6-9], originating as erythromyeloid progenitors (EMPs) in the yolk sac on embryonic day (E) 8.5. EMPs colonize the fetal liver by E10.5, then give rise to fetal monocytes that migrate to the lung around E12.5[6, 8]. The newly developed fetal vascular system, which develops at about this embryonic age may provide conduits for migration of fetal monocytes to the lung, although cues that guide the migration are not known. The maturation of fetal monocytes to alveolar macrophages occurs in the presence of granulocyte-monocyte colony stimulating factor (GM-CSF) and is complete by the third postnatal day.
A popular methodological approach for studying alveolar macrophages is to recover macrophages from the bronchoalveolar lavage (BAL) on the assumption that BAL macrophages represent alveolar macrophages. However this assumption may not be entirely correct. Although the mouse lung possesses approximately 4 × 106 alveoli[10], BAL macrophage numbers tend to be in the order of 105. Hence BAL macrophage numbers are at least an order of magnitude less than the numbers expected if macrophages were present in all alveoli and if BAL recovered all alveolar macrophages. This discrepancy is partly explained by the lung morphological data. As indicated in the Table, the number of alveolar macrophages factored by the morphologically estimated alveolar surface area[11] gives a spatial distribution ratio of 0.3, suggesting one macrophage is present for every three alveoli. This prediction was recently confirmed to a remarkable degree of similarity by optical imaging of macrophage distribution in live alveoli[12]. We may expect therefore, that the mouse lung contains approximately a million alveolar macrophages of which the BAL accesses a small fraction.
TABLE.
| Reported data | ||
| 1. No. of alveoli per lung | 4.2 × 106 | Mercer 1994[10] |
| 2. Surface area of an alveolus (sq microns) |
5.7 × 103 | Mercer 1994[10] |
| 3. Alveolar surface area per macrophage (square microns) |
18 × 103 | Stone et al 1992[11] |
| Calculated data | ||
| 4. Alveolar surface area per lung1 (μ2) |
23.9 × 109 | |
| 5. No. of macrophages per lung2 |
1.3 × 106 | |
| No. of macrophages per alveoli3 |
0.3 |
Row #1 multiplied by row #2;
row #4 divided by row #3;
row #5 divided by row #1
The possibility that BAL macrophages differ from alveolar macrophages is supported by optical studies of live alveoli, which indicate that alveolar macrophages are sessile and adherent to the alveolar epithelium to the extent that BAL fails to remove these macrophages[12]. Hence, BAL macrophages might represent a macrophage subset that populates lung regions other than the alveoli, for example conducting airways that might be subject to high shear during lavage. However some authors believe airway resident macrophages are also sessile and resist washout by BAL[13]. It is possible that BAL accesses macrophages that bind weakly to the epithelium. Although mechanisms that underlie adhesion of alveolar macrophages to the epithelium remain unclear, possibilities are that receptor proteins such as CD200R that bind ligands expressed on the epithelium[14] subserve macrophage-epithelial adhesion. Interestingly, lung stretch induced by high tidal volume ventilation rapidly decreases BAL macrophage numbers[15]. This finding suggests the intriguing, but untested possibility that an optimal level of alveolar stretch facilitates macrophage adhesion to the epithelium, decreasing macrophage washout in the BAL.
The question of how resident alveolar macrophages carry out sentinel function against inhaled pathogens has been of interest. According to one possibility, resident macrophages continually patrol the alveolar surface. Recent evidence even indicates that alveolar macrophages could serve as antigen carriers transporting pathogens to lung draining lymph nodes[16]. Although these findings support the notion that under pathogen challenge a subset of alveolar macrophages might migrate to lymph nodes, there is presently no direct evidence that macrophages patrol alveoli. In fact live optical studies suggest the opposite in that indicate sessile alveolar macrophages do not migrate from their home alveoli despite the presence of bacteria in adjoining macrophage-free alveoli[12].
Alveolar homeostasis
Immune modulation by sessile alveolar macrophages might be attributable to unique alveolar properties. Alveoli are cup-like structures in which the broad-rimmed opening formed by the mouth of the cup opens into alveolar ducts that arise from terminal bronchioles. Flat septa formed by squamous type 1 (AT1) cells account for 90% of the alveolar surface. Cuboidal AT2 cells lie in the alveolar dome, most often in niches formed by meeting points of adjacent alveolar septa[17]. We consider below unique features of macrophage-epithelial interactions that might determine alveolar immunity.
1. Surfactant clearance
Alveolar surfactant, a mixture of phospholipids and surfactant proteins, forms a surface-active layer adjoining the alveolar epithelium[18]. AT2 cells secrete surfactant, which floats and spreads across the alveolar surface in the aqueous alveolar wall liquid (AWL). Alveolar surfactant opposes the air-liquid interfacial tension, a surface-active force that acts on the aqueous layer adjacent to the alveolar epithelium, tending to oppose alveolar expansion and promote alveolar collapse. Hence, surfactant protects alveolar patency and gas exchange. The surfactant layer varies in thickness from 1-3 micrometers, establishing a fluid barrier that minimizes direct contact between inhaled pathogens with the air-facing surface of the alveolar epithelium. Surfactant contains proteins A and D that bind bacteria disabling negative bacterial interactions with the epithelium[19].
AT2 cells and resident macrophages clear alveolar surfactant to prevent phospholipid build up in alveoli. Defective surfactant clearance causes alveolar proteinosis, a disease in which large volumes of surfactant rich liquid accumulate in alveoli, causing respiratory failure. Interestingly, airway instillation of bone marrow-derived macrophages effectively removes the accumulated alveolar liquid[20], attesting to the critical role of macrophages in clearance of alveolar surfactant.
Although each alveolus contains AT2 cells and therefore has the capacity to produce surfactant, as we discuss above, not all possesses a macrophage. Hence spatial relations between AT2 cells and resident macrophages of the alveolar region are likely to be important in regulating surfactant clearance. Although this morphologic understanding is lacking, alveolar macrophages probably do not lie in close proximity to AT2 cells. Such an arrangement could impair alveolar surfactant spreading as macrophages could prematurely scavenge freshly secreted surfactant. However, mechanisms underlying delivery of surfactant from sites of secretion at AT2 cells to surfactant scavenging macrophages are not well understood.
2. Liquid secretion by the alveolar wall
The alveolar wall liquid (AWL) lining the alveolar wall defends against inhaled pathogens by providing an aqueous layer at the airepithelial interface. Low-temperature microscopy of the lung indicates that the AWL is distributed throughout the epithelial surface as an uninterrupted liquid layer[21]. Lindert et al carried out real time studies of the AWL by means of two-photon microscopy[22]. Microinjection of the water-soluble, membrane impermeant fluorescent dye, FITC-dextran in the AWL resulted in spontaneous loss of FITC fluorescence from the alveolar lining, indicating that the AWL is formed by alveolar water secretion. The secretion was absent in mice lacking the gene for the cystic fibrosis transmembrane activator receptor (CFTR), indicating CFTR-dependent Cl− secretion across the alveolar epithelium causes AWL formation.
Lindert et al propose that the flow of the AWL occurs in an outward direction from the alveolus such that the AWL flows from alveoli towards the alveolar duct and then to the terminal bronchiolar epithelium that might absorb the liquid. Since all alveoli secrete AWL, it is conceivable that this flow establishes a liquid stream that delivers alveolar pathogens to sessile alveolar macrophages located downstream from sites of liquid secretion. Impairment of AWL flow may decrease pathogen sensing by sessile macrophages, promoting alveolar pathology[22].
3. Alveolar gap junctional channels (GJCs)
AT1 and AT2 cells communicate Ca2+ signals through GJCs. The communication coordinates mechanical stretch imposed on AT1 cells by lung expansion with Ca2+-induced surfactant secretion by AT2 cells[23]. Recent findings indicate that approximately 40% of sessile alveolar macrophages communicate Ca2+ signals to the alveolar epithelium through GJCs[12]. These findings raise the possibility that pathogen-induced Ca2+ increases in alveolar epithelial cells might be GJC communicated to activate sessile macrophages. Pathogen-induced Ca2+ increases in the alveolar epithelium could occur in response to activation of receptors such as tumor necrosis factor receptor type 1 (TNFR1) that are well expressed on alveolar epithelium[24-26]. Ligation of TNFR1 by Staphylococcus aureus increases epithelial levels of cytosolic Ca2+ [26]. Other pathogen-sensitive receptors that may induce GJC transmitted Ca2+ signals from the epithelium include toll-like receptors-2 and -4, which are expressed on alveolar epithelium and Club cells (also called Clara cells) of the distal airway[27, 28]. However it is not known whether Club cells establish GJCs with alveolar epithelium or alveolar macrophages. Although further studies are required, the presence of alveolar GJCs suggests that non-migratory alveolar macrophages might achieve sentinel function by taking advantage of interconnectivity amongst alveolar epithelia and macrophages.
4. Other modes of macrophage-epithelial crosstalk
Increasing evidence implicates microparticles in cell-cell communication. Microparticles enclose cytoplasmic constituents from the originating cell [29-31]. In pneumonia or ARDS, microparticles of epithelial, platelet and neutrophil origin are found in the BAL [29, 32, 33]. In vitro studies have shown that microparticles may regulate phagocytosis of bacteria[31]. Alveolar macrophages secrete microparticles containing the anti-inflammatory proteins, SOCS1 and SOCS3[34]. Uptake of these macrophage-derived microparticles by the alveolar epithelium provides a mechanism by which activated macrophages may inhibit alveolar inflammation.
Alveolar inflammation
The development and resolution of alveolar inflammation depend on the interplay between pathogen-activated pro- and anti-inflammatory signaling mechanisms. Alveolar pathogens are likely to encounter sessile alveolar macrophages as the first set of immunity inducing host cells. This critical proinflammatory role is evident in that inhibition of TLR4 signaling in alveolar macrophages by cell-specific knockout of the TLR4 adapter, MyD88 blocks LPS-induced lung inflammation[12]. New understanding indicates that concomitant with proinflammatory induction, macrophages initiate an anti-inflammatory response. Although these signaling mechanisms remain inadequately understood for macrophages in situ, here we summarize understanding derived from in vitro macrophage studies that might apply.
Proinflammatory signaling pathways
Since alveolar macrophages face the inhaled air, inhaled pathogens can interact directly with macrophage pattern-recognition molecules, such as TLRs. The immune response initiates as receptor-mediated mechanisms activate macrophage secretion of cytokines such as TNFα. TNFα ligates its receptor, TNFR1 that is constitutively expressed on the luminal aspect of the alveolar epithelium[24], resulting in proinflammatory signaling between the alveolar epithelium and the adjoining capillary endothelium. This proinflammatory crosstalk is rapid, initiating in minutes as shown in studies in which TNFα was directly instilled in alveoli[24], or in which the alveolar epithelium was injured by pore formation[35].
Another important mediator of acute lung injury is macrophage inflammatory protein-1 alpha (MIP-1α). Alveolar macrophages secrete MIP-1α after LPS-stimulation that in turn seems to be related to the production of TNFα. The secretion of MIP-1 alpha leads to increased neutrophil recruitment and a worsening of ALI[36, 37]. Initially thought to be a T-cell cytokine of the adaptive immune system, macrophage migration inhibitory factor (MIF) promotes innate and adaptive immune responses through macrophage activation[38]. LPS stimulates MIF release from macrophages [39]. Treatment with MIF-specific antibody was shown to rescue mice from sepsis and MIF-deficient mice were protected form LPS-induced sepsis[38].
The proinflammatory signaling cascade induced in alveolar macrophages has been addressed in the context of the prototypic TLR4 ligand, lipopolysaccharide (LPS) that gram-negative bacteria express on the outer membrane. This signaling involves myeloid differentiation factor 88 (MyD88), or TIR-domain-containing adapter-inducing interferon-beta (TRIF). MyD88 is a central adapter protein shared by almost all TLRs. The association of TLR4 and MyD88 recruits members of the interleukin-1 receptor associated kinase (IRAK) family and tumor necrosis factor receptor-associated factor 6 (TRAF 6)[40]. While some IRAKs may activate inflammation, IRAK-M may negatively regulate TLR-mediated signaling[41]. The association of TLR4, MyD88, IRAK and TRAF6 finally leads to the activation of NFkB or activating protein-1 (AP-1) that regulate inflammatory responses by inducing proinflammatory cytokines[40].
The TLR4-mediated pathway through TRIF is dependent on TRIF-related adapter molecule (TRAM, also known as TICAM2), which may be a required adapter linking TLR4 and TRIF[40]. Macrophages of MyD88-deficient mice fail to produce proinflammatory cytokines in response to LPS. However, activation of NFkB and mitogen-activated protein (MAP) kinase family may still takes place and LPS may induce IFNβ production by macrophages and dendritic cells independently of MyD88[42]. In this context, TRIF may activate a MyD88-independent TLR4 signaling pathway[43, 44]. Since production of several inflammatory cytokines other than IFNβ was reduced in TRIF deficient mice[43], it is suggested that both MyD88- and TRIF-dependent pathways are required for a maximal induction of inflammatory cytokines in response to LPS[40]. However in models of ALI induced by airway instillation of LPS, secretion of pro-inflammatory cytokines, protein leak and neutrophil recruitment to the lung are abrogated in mice deficient for the adapter molecules MyD88 and Toll/Interleukin-1 receptor (TIR)-domain-containing adapter protein (TIRAP) but independent of TRIF[45]. Further, mice lacking MyD88 specifically in alveolar macrophages fail to develop LPS induced ALI[12]. These findings indicate that induction of the MyD88 pathway in alveolar macrophages is critical for TLR4-induced lung injury.
LPS may also trigger acute lung injury through other receptors such as the ligation of purinergic receptors. For example, CD14 dependent ligation of purinergic receptor P2×7 (P2×7R) induces calcium influx and ATP depletion in alveolar macrophages leading to macrophage necrosis. The necrotic macrophages then release pro-interleukin-1 alpha (IL-1a) that induces IL-1R-MyD88 signaling pathways on lung vascular epithelium[46].
Anti-inflammatory signaling pathways
LPS-induced alveolar inflammation may be inhibited by several mechanisms. The mouse macrophage cDNA library contains a cDNA encoding for soluble TLR4[47], suggesting that release of soluble TLR4 may provide a decoy receptor to mitigate direct LPS ligation on the cell membrane. Recombinant soluble TLR4 inhibits LPS-induced NFkB activation and TNFα production by macrophages in vitro[47]. TLR4 signaling is inhibited by activation of the deubiquitinase, A20 that negatively regulates the TLR4 adapter, TNFα-receptor associated factor-6 (TRAF6)[48]. TLR4 ligation induces expression of micro-RNAs such as miR-146a and miR-21 in macrophages[49, 50]. MiRs may inhibit lung inflammation in response to LPS by downregulating receptor expression of TLR4 as shown for the let7-miRNA family, or they may alter cytokine production profiles to negatively regulate pro-inflammatory responses[51]. Amongst the best characterized are miR-146a and miR-21. MiR-146a targets IRAK and TRAF6 to negatively regulate the MyD88-NFkB signaling pathway following bacterial infection[52]. MiR-21 promotes anti-inflammatory responses by facilitating the production of IL-10[49].
Proteins involved in the TLR4 signaling cascade may have a dual function working through negative feedback to mitigate inflammation. Members of the IRAK family have such dual effects. Although required for MyD88-dependent proinflammatory signaling, some IRAKs inhibit inflammation. IRAK M for instance is predominantly expressed in monocytes and macrophages[53] and exerts an anti-inflammatory effect by inhibiting the association of IRAK-1 with TRAF-6[41]. Another major regulatory feedback loop could be induced through the activation of cellular kinases such as phosphatidylinositol 3′-kinases (PI3Ks). PI3Ks belong to a family of signal-transducing enzymes that are divided into 3 classes based on their structural characteristics and substrate specificity[54]. In vitro, class I PI3Ks could specifically inhibit the effects of LPS-mediated activation of NFkB and cytokine production in RAW cells, which is a monocyte-macrophage cell line[55].
The role of cell Ca2+
Macrophage-epithelial interactions in alveoli involve Ca2+ communication through GJCs. The extent to which the communicated Ca2+ signal modulates alveolar inflammation is of interest. Multiple lines of evidence attest to the proinflammatory effect of Ca2+. Thus in lung epithelium, cytosolic Ca2+ increases activate proteases such as calpain[26] that deplete intercellular junctional proteins such as E-cadherin, facilitating alveolar influx of immune cells across the diminished blood-gas barrier. Increase of cytosolic Ca2+ may increase mitochondrial Ca2+, causing increase of mitochondrial reactive oxygen species (ROS)[56], hence ROS-induced mitochondrial damage and apoptosis. Increases of cytosolic Ca2+ levels in the macrophage can induce release of proinflammatory cytokines such as TNFα.
However, new understanding reveals that Ca2+ can also be counter inflammatory. In LPS-treated lungs, transmission of periodic Ca2+ waves between sessile alveolar macrophages and alveolar epithelium induces anti-inflammatory mechanisms[12]. The communication occurs through connexin 43 (Cx43)-containing GJCs. Blocking the communication, for example by deleting Cx43 in macrophages, worsens lung inflammation, indicating that Ca2+-induced processes in the alveolar epithelium inhibit proinflammatory signaling.
The Ca2+-induced anti-inflammatory effect occurs through a signaling pathway in which increases of the cytosolic Ca2+ lead to binding of Ca2+ to the Ca2+-receptor protein, calmodulin (CAM). The Ca2+-CAM complex can activate multiple enzymes of the CAM-kinase (CAMK) family, including CAMK-kinase (CAMKK), which occurs as the α and β isoforms (CAMKK1 and CAMKK2, respectively). Interest has focused largely on CAMKKβ, which is ubiquitously expressed in tissues and is capable of activating the metabolic maser regulator, AMP-kinase (AMPK)[57, 58]. Although the effects of CAMKKβ in relation to lung immunity remain inadequately understood, the consequences of CAMKKβ-mediated AMPK activation are of potential interest. Alveolar epithelial AMPK can be activated, for example by high CO2 levels[57]. In a bacterial model of ALI, AMPK activation in the lung was shown to be immunosuppressive, hence protective against ALI[59]. Hence, the extent to which the Ca2+-CAM → CAMKKβ → AMPK activation cascade promotes lung repair in ALI requires further consideration.
The significance of the Ca2+-CAM cascade also relates to its downstream effects through Akt phosphorylation. Akt is a serine/threonine kinase occurring in three isoforms all of which contain three major functional domains: the N-terminal pleckstrin homology domain, a serine/threonine catalytic domain, and a C-terminal regulatory domain. The canonical pathway of Akt activation occurs through ligation of growth receptors that induce membrane translocation of Akt. Subsequently, Akt phosphorylation at threonine 308 and serine 473 takes place through mechanisms involving PI3K activation [60]. This pathway of Akt activation promotes neutrophil accumulation in LPS-treated lungs[61], and is implicated in the susceptibility of COPD patients to influenza[62] and in the activated PI3K-δ syndrome characterized by repeated respiratory infections[63].
The immune significance of Akt activation lies in downstream effects involving nuclear factor κB (NFκB). Inhibitory kappa-B (IκB) proteins sequester dimers of the NFκB proteins, c-Rel, p65, and p50 in the cytosol. The IκB kinase (IKK) complex comprises IKKα, IKKβ, and IKKγ (NEMO). Activated IKKβ causes phosphorylation of IκB proteins leading to their proteasomal degradation, releasing the sequestered proteins. Released NFκB dimers, p50/p65 and p50/c-Rel migrate to the nucleus to initiate gene transcription. Ca2+-induced activation of NFκB is attributed to activation of the Ca2+-CAM receptor protein, CAMKII[64].
The extent to which Akt modulates the NFκB pathway seems to depend on the extent of Akt activation. Chen et al addressed this issue in the kidney cell line, HEK293 in which IL-1β phosphorylated IKKβ to activate NFκB. However, overexpression of Akt blocked NFκB activation. The authors suggest that Akt might inhibit IL-1β-induced NFκB activation by a negative feedback loop. Critical findings of this study were that Ca2+ mobilizing agents such as ionomycin or thapsigargin inhibited IL-1β-induced NFκB activity, and that overexpression of dominant negative CAMKK reversed the inhibition. These findings are evidence that Ca2+-induced signaling mechanisms acting through CAMKK negatively regulate NFκB.
A paradigm changing report by Yano et al indicates that CAMKKα causes non-canonical Akt activation[65]. In this study, induced increases of cytosolic Ca2+ in the neuroblastoma cell line, NG108, or the fibroblast cell line, COS-1 caused CAMKKα dependent Akt activation. Importantly, the effect was not inhibited by wortmannin, indicating that the canonical pathway through PI3K was not involved. Further, the Akt activation was inhibited through expression of dominant negative CAMKK, establishing the CAM-activated pathway as the causal mechanism. A major downstream effect of Akt activation was serine phosphorylation of the pro-apoptotic Bcl2 family member, BAD, resulting in sequestration of BAD to protein 14-3-3. Accordingly, apoptosis of NG108 cells induced by serum withdrawal was blocked to a major extent by exposing the cells to Ca2+ mobilizing agents[66]. Together these findings indicate that the anti-apoptotic effect of Akt can be induced by Ca2+-induced activation of CAMKKα.
Recent findings by Westphalen et al reveal new understanding of the role of CAMKKα in lung immunity[12]. These authors confirmed that wild type lungs express CAMKKα and Akt. LPS treatment increased the association of CAMKKα with Akt, which was phosphorylated on threonine 308, a marker of Akt activation. However, inhibition of GJC channels between sessile alveolar macrophages and the alveolar epithelium blocked phospho-Akt expression in alveolar epithelium. CAMKKα knockdown by short interfering RNA decreased Akt phosphorylation and enhanced IκB degradation and NFκB activation, thereby enhancing BAL leukocyte counts. These findings indicate that in contrast to the proinflammatory, PI3K-Akt pathway, Ca2+ communication between sessile alveolar macrophages and the alveolar epithelium activates the CAMKKα-Akt pathway, inhibiting NFκB activation and protecting against ALI.
Effects of macrophage depletion
Cell depletion protocols have been applied to delineate specific functional roles for alveolar macrophages. However, findings are confusing. Depletion of alveolar macrophages by airway instillation of clodronate liposomes protected against ventilator- and ischemia-perfusion-induced ALI[15, 67], supporting a pro-injury role for alveolar macrophages. By contrast in the context of LPS or FAS challenge, clodronate had no effect on the lung inflammatory response[68], or it exacerbated[69, 70], or decreased the response[71, 72]. In Staphylococcus-challenged lungs clodronate increased mortality, but failed to increase neutrophil counts in the BAL[73]. The clodronate studies imply that alveolar macrophages may modulate alveolar inflammation differently for bacterial versus physical damage to the alveolar epithelium.
Cell depletion studies have been carried out using the diphtheria toxin (DT) strategy. The DT receptor (DTR) of primates, but not mice binds DT. Hence, transgenic mice can be generated in which cell-specific promoters control DTR expression. DT treatment of DTR expressing mice causes specific depletion of DTR-expressing cells. Accordingly, DTR has been expressed in alveolar macrophages under control of the CD11c or the LySM promoter[74, 75]. Findings indicate that depletion of resident alveolar macrophages by these strategies mechanistically impacts severity of lung inflammation. Thus, macrophage depletion is reported to increase mortality[74], lung inflammation [75, 76], and lung bacterial load[73], consistent with the anti-inflammatory effect of alveolar macrophages. However, macrophage depletion decreased BAL levels of the proinflammatory cytokine IL-23[77], revealing a proinflammatory macrophage effect. Concerns have been expressed that the DTR strategy might be associated with non-specific immune effects attributable to depletion of cells other than macrophages[75, 76, 78]. For example, for strategies using the CD11c promoter, the possibility that CD11c-expressing dendritic cells might also be depleted confounds macrophage-related interpretations.
Conclusions and future directions
The availability of genetic reagents that allow expression of fluorescent proteins in alveolar macrophages has enabled their direct in situ visualization by optical microscopy. The new understanding indicates that in lung, sessile alveolar macrophages induce proinflammatory responses to LPS challenge, while a subset of sessile macrophages communicate immunosuppressive Ca2+ signals to the alveolar epithelium through GJCs (Figure). In future research, we need to focus more strongly on these functional aspects, especially in regard to mechanisms regulating pro- and anti-inflammatory roles of sessile macrophages, their turnover and survival during the immune response, and their interactions with recruited macrophages in facilitating lung injury resolution. In addition, there is scope to further develop novel investigative approaches for in situ understanding, as for example in human lungs that are increasingly available from brain dead donors for mechanistic studies of ALI[79]. Better understanding of the biology of sessile macrophages in their natural environment will lead to new therapy for immune diseases of the lung.
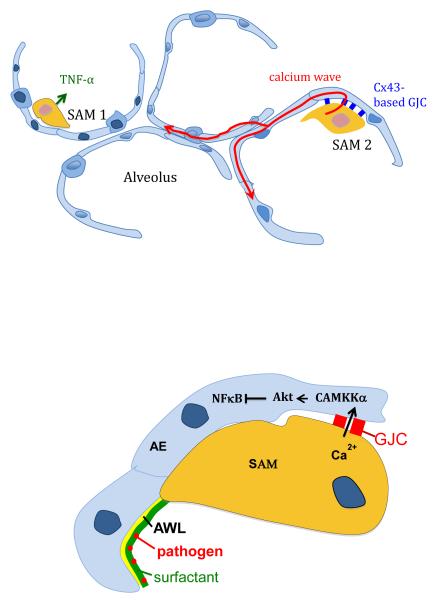
Figure.
Functional heterogeneity in sessile alveolar macrophages (SAMs). The upper figure shows a macrophage that forms gap junctional channels (GJCs) with the alveolar epithelium (right) and one that does not (left). LPS challenge induces secretion of proinflammatory cytokines from the macrophages, initiating alveolar inflammation. Ca2+ waves (red arrows) originate from the GJC-forming macrophage, then diffuse in the adjoining alveolar epithelium. The lower figure shows that GJC transmitted Ca2+ activates CAMKKα-induced signaling, inhibiting inflammatory signaling in the alveolar epithelium (AE). Pathogen sensing by sessile macrophages takes place as the alveolar wall liquid (AWL) convectively transports pathogens caught in alveolar surfactant towards the macrophages.
Acknowledgments
We are grateful to Drs. Sunita Bhattacharya, Chris Schindler and M. Naeem Islam for their help with comments and manuscript preparation. JB was supported by NIH grants HL36024, HL57556, and HL122730.
References
- [1].Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- [2].Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. American journal of respiratory and critical care medicine. 2011;184(5):547–60. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35(2):227–35. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- [4].Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution, American journal of physiology. Lung cellular and molecular physiology. 2014;306(8):L709–25. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. American journal of respiratory and critical care medicine. 2011;183(10):1380–90. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- [6].Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. The Journal of experimental medicine. 2013;210(10):1977–92. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–51. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ginhoux F. Fate PPAR-titioning: PPAR-gamma 'instructs' alveolar macrophage development. Nat Immunol. 2014;15(11):1005–7. doi: 10.1038/ni.3011. [DOI] [PubMed] [Google Scholar]
- [9].Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- [10].Mercer RR, Russell ML, Crapo JD. Alveolar septal structure in different species. J Appl Physiol (1985) 1994;77(3):1060–6. doi: 10.1152/jappl.1994.77.3.1060. [DOI] [PubMed] [Google Scholar]
- [11].Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6(2):235–43. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- [12].Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506(7489):503–6. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Geiser M, Serra AL, Cruz-Orive LM, Baumann M, Im Hof V, Gehr P. Efficiency of airway macrophage recovery by bronchoalveolar lavage in hamsters: a stereological approach. Eur Respir J. 1995;8(10):1712–8. doi: 10.1183/09031936.95.08101712. [DOI] [PubMed] [Google Scholar]
- [14].Goulding J, Godlee A, Vekaria S, Hilty M, Snelgrove R, Hussell T. Lowering the threshold of lung innate immune cell activation alters susceptibility to secondary bacterial superinfection. J Infect Dis. 2011;204(7):1086–94. doi: 10.1093/infdis/jir467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. American journal of physiology. Lung cellular and molecular physiology. 2006;291(6):L1191–8. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- [16].Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol. 2009;183(3):1983–9. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol (1985) 2007;103(3):1037–44. doi: 10.1152/japplphysiol.00160.2007. [DOI] [PubMed] [Google Scholar]
- [18].Clements JA. Lung surfactant: a personal perspective. Annu Rev Physiol. 1997;59:1–21. doi: 10.1146/annurev.physiol.59.1.1. [DOI] [PubMed] [Google Scholar]
- [19].Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- [20].Suzuki T, Arumugam P, Sakagami T, Lachmann N, Chalk C, Sallese A, Abe S, Trapnell C, Carey B, Moritz T, Malik P, Lutzko C, Wood RE, Trapnell BC. Pulmonary macrophage transplantation therapy. Nature. 2014;514(7523):450–4. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bastacky J, Lee CY, Goerke J, Koushafar H, Yager D, Kenaga L, Speed TP, Chen Y, Clements JA. Alveolar lining layer is thin and continuous: low-temperature scanning electron microscopy of rat lung. J Appl Physiol (1985) 1995;79(5):1615–28. doi: 10.1152/jappl.1995.79.5.1615. [DOI] [PubMed] [Google Scholar]
- [22].Lindert J, Perlman CE, Parthasarathi K, Bhattacharya J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am J Respir Cell Mol Biol. 2007;36(6):688–96. doi: 10.1165/rcmb.2006-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ashino Y, Ying X, Dobbs LG, Bhattacharya J. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. American journal of physiology. Lung cellular and molecular physiology. 2000;279(1):L5–13. doi: 10.1152/ajplung.2000.279.1.L5. [DOI] [PubMed] [Google Scholar]
- [24].Kuebler WM, Parthasarathi K, Wang PM, Bhattacharya J. A novel signaling mechanism between gas and blood compartments of the lung. J Clin Invest. 2000;105(7):905–13. doi: 10.1172/JCI8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Patel BV, Wilson MR, O'Dea KP, Takata M. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol. 2013;190(8):4274–82. doi: 10.4049/jimmunol.1202437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ahn DS, Parker D, Planet PJ, Nieto PA, Bueno SM, Prince A. Secretion of IL-16 through TNFR1 and calpain-caspase signaling contributes to MRSA pneumonia. Mucosal Immunol. 2014;7(6):1366–74. doi: 10.1038/mi.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tomita T, Sakurai Y, Ishibashi S, Maru Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene. 2011;30(31):3429–39. doi: 10.1038/onc.2011.53. [DOI] [PubMed] [Google Scholar]
- [28].Thorley AJ, Grandolfo D, Lim E, Goldstraw P, Young A, Tetley TD. Innate immune responses to bacterial ligands in the peripheral human lung--role of alveolar epithelial TLR expression and signalling. PLoS One. 2011;6(7):e21827. doi: 10.1371/journal.pone.0021827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guervilly C, Lacroix R, Forel JM, Roch A, Camoin-Jau L, Papazian L, Dignat-George F. High levels of circulating leukocyte microparticles are associated with better outcome in acute respiratory distress syndrome. Crit Care. 2011;15(1):R31. doi: 10.1186/cc9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27(1):31–9. doi: 10.1016/j.blre.2012.12.002. [DOI] [PubMed] [Google Scholar]
- [31].Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163(8):4564–73. [PubMed] [Google Scholar]
- [32].Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. American journal of physiology. Lung cellular and molecular physiology. 2009;297(6):L1035–41. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mutschler DK, Larsson AO, Basu S, Nordgren A, Eriksson MB. Effects of mechanical ventilation on platelet microparticles in bronchoalveolar lavage fluid. Thromb Res. 2002;108(4):215–20. doi: 10.1016/s0049-3848(03)00005-7. [DOI] [PubMed] [Google Scholar]
- [34].Bourdonnay E, Zaslona Z, Penke LR, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Peters-Golden M. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. The Journal of experimental medicine. 2015;212(5):729–42. doi: 10.1084/jem.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Westphalen K, Monma E, Islam MN, Bhattacharya J. Acid contact in the rodent pulmonary alveolus causes proinflammatory signaling by membrane pore formation. American journal of physiology. Lung cellular and molecular physiology. 2012;303(2):L107–16. doi: 10.1152/ajplung.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Quintero PA, Knolle MD, Cala LF, Zhuang Y, Owen CA. Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice. J Immunol. 2010;184(3):1575–88. doi: 10.4049/jimmunol.0900290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shanley TP, Schmal H, Friedl HP, Jones ML, Ward PA. Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J Immunol. 1995;154(9):4793–802. [PubMed] [Google Scholar]
- [38].Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. The Journal of experimental medicine. 1994;179(6):1895–902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13(5):816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- [41].Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- [42].Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- [43].Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- [44].Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- [45].Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho FR, Vasseur V, Erard F, Ryffel B, Couillin I, Moser R. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol. 2007;88(6):387–91. doi: 10.1111/j.1365-2613.2007.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dagvadorj J, Shimada K, Chen S, Jones HD, Tumurkhuu G, Zhang W, Wawrowsky KA, Crother TR, Arditi M. Lipopolysaccharide Induces Alveolar Macrophage Necrosis via CD14 and the P2×7 Receptor Leading to Interleukin-1alpha Release. Immunity. 2015;42(4):640–53. doi: 10.1016/j.immuni.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165(12):6682–6. doi: 10.4049/jimmunol.165.12.6682. [DOI] [PubMed] [Google Scholar]
- [48].Yuk JM, Kim TS, Kim SY, Lee HM, Han J, Dufour CR, Kim JK, Jin HS, Yang CS, Park KS, Lee CH, Kim JM, Kweon GR, Choi HS, Vanacker JM, Moore DD, Giguere V, Jo EK. Orphan Nuclear Receptor ERRalpha Controls Macrophage Metabolic Signaling and A20 Expression to Negatively Regulate TLR-Induced Inflammation. Immunity. 2015;43(1):80–91. doi: 10.1016/j.immuni.2015.07.003. [DOI] [PubMed] [Google Scholar]
- [49].Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11(2):141–7. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- [50].O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11(3):163–75. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- [51].Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, Margioris AN, Tsichlis PN, Tsatsanis C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31(2):220–31. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274(27):19403–10. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- [54].Lang T, Mansell A. The negative regulation of Toll-like receptor and associated pathways. Immunol Cell Biol. 2007;85(6):425–34. doi: 10.1038/sj.icb.7100094. [DOI] [PubMed] [Google Scholar]
- [55].Kuo CC, Lin WT, Liang CM, Liang SM. Class I and III phosphatidylinositol 3'-kinase play distinct roles in TLR signaling pathway. J Immunol. 2006;176(10):5943–9. doi: 10.4049/jimmunol.176.10.5943. [DOI] [PubMed] [Google Scholar]
- [56].Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, Bhattacharya S, Bhattacharya J. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest. 2011;121(5):1986–99. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest. 2008;118(2):752–62. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264(30):17907–12. [PubMed] [Google Scholar]
- [59].Liu Z, Bone N, Jiang S, Park DW, Tadie JM, Deshane J, Rodriguez CA, Pittet JF, Abraham E, Zmijewski JW. AMP-activated protein kinase and Glycogen Synthase Kinase 3beta modulate the severity of sepsis-induced lung injury. Mol Med. 2015 doi: 10.2119/molmed.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Risso G, Blaustein M, Pozzi B, Mammi P, Srebrow A. Akt/PKB: one kinase, many modifications. Biochem J. 2015;468(2):203–14. doi: 10.1042/BJ20150041. [DOI] [PubMed] [Google Scholar]
- [61].Kumar S, Xu J, Kumar RS, Lakshmikanthan S, Kapur R, Kofron M, Chrzanowska-Wodnicka M, Filippi MD. The small GTPase Rap1b negatively regulates neutrophil chemotaxis and transcellular diapedesis by inhibiting Akt activation. The Journal of experimental medicine. 2014;211(9):1741–58. doi: 10.1084/jem.20131706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hsu AC, Starkey MR, Hanish I, Parsons K, Haw TJ, Howland LJ, Barr I, Mahony JB, Foster PS, Knight DA, Wark PA, Hansbro PM. Targeting PI3K-p110alpha Suppresses Influenza Virus Infection in Chronic Obstructive Pulmonary Disease. American journal of respiratory and critical care medicine. 2015;191(9):1012–23. doi: 10.1164/rccm.201501-0188OC. [DOI] [PubMed] [Google Scholar]
- [63].Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debre M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers ER, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–71. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ling H, Gray CB, Zambon AC, Grimm M, Gu Y, Dalton N, Purcell NH, Peterson K, Brown JH. Ca2+/Calmodulin-dependent protein kinase II delta mediates myocardial ischemia/reperfusion injury through nuclear factor-kappaB. Circ Res. 2013;112(6):935–44. doi: 10.1161/CIRCRESAHA.112.276915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396(6711):584–7. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- [66].Yano S, Morioka M, Kuratsu J, Fukunaga K. Functional proteins involved in regulation of intracellular Ca(2+) for drug development: role of calcium/calmodulin-dependent protein kinases in ischemic neuronal death. J Pharmacol Sci. 2005;97(3):351–4. doi: 10.1254/jphs.fmj04007x5. [DOI] [PubMed] [Google Scholar]
- [67].Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. American journal of physiology. Lung cellular and molecular physiology. 2006;291(5):L1018–26. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- [68].Dhaliwal K, Scholefield E, Ferenbach D, Gibbons M, Duffin R, Dorward DA, Morris AC, Humphries D, MacKinnon A, Wilkinson TS, Wallace WA, van Rooijen N, Mack M, Rossi AG, Davidson DJ, Hirani N, Hughes J, Haslett C, Simpson AJ. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. American journal of respiratory and critical care medicine. 2012;186(6):514–24. doi: 10.1164/rccm.201112-2132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bem RA, Farnand AW, Wong V, Koski A, Rosenfeld ME, van Rooijen N, Frevert CW, Martin TR, Matute-Bello G. Depletion of resident alveolar macrophages does not prevent Fas-mediated lung injury in mice, American journal of physiology. Lung cellular and molecular physiology. 2008;295(2):L314–25. doi: 10.1152/ajplung.00210.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Elder A, Johnston C, Gelein R, Finkelstein J, Wang Z, Notter R, Oberdorster G. Lung inflammation induced by endotoxin is enhanced in rats depleted of alveolar macrophages with aerosolized clodronate. Exp Lung Res. 2005;31(6):527–46. doi: 10.1080/019021490944223. [DOI] [PubMed] [Google Scholar]
- [71].Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, Blackwell TS, Christman JW, Schlondorff D, Seeger W, Lohmeyer J. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. American journal of physiology. Lung cellular and molecular physiology. 2002;282(6):L1245–52. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- [72].Koay MA, Gao X, Washington MK, Parman KS, Sadikot RT, Blackwell TS, Christman JW. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am J Respir Cell Mol Biol. 2002;26(5):572–8. doi: 10.1165/ajrcmb.26.5.4748. [DOI] [PubMed] [Google Scholar]
- [73].Martin FJ, Parker D, Harfenist BS, Soong G, Prince A. Participation of CD11c(+) leukocytes in methicillin-resistant Staphylococcus aureus clearance from the lung. Infect Immun. 2011;79(5):1898–904. doi: 10.1128/IAI.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Saini Y, Wilkinson KJ, Terrell KA, Burns KA, Livraghi-Butrico A, Doerschuk CM, O'Neal WK, Boucher RC. Neonatal Pulmonary Macrophage Depletion Coupled to Defective Mucus Clearance Increases Susceptibility to Pneumonia and Alters Pulmonary Immune Responses. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Roberts LM, Ledvina HE, Tuladhar S, Rana D, Steele SP, Sempowski GD, Frelinger JA. Depletion of alveolar macrophages in CD11c diphtheria toxin receptor mice produces an inflammatory response. Immun Inflamm Dis. 2015;3(2):71–81. doi: 10.1002/iid3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chapman TJ, Georas SN. Adjuvant effect of diphtheria toxin after mucosal administration in both wild type and diphtheria toxin receptor engineered mouse strains. J Immunol Methods. 2013;400-401:122–6. doi: 10.1016/j.jim.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bosmann M, Grailer JJ, Russkamp NF, Ruemmler R, Zetoune FS, Sarma JV, Ward PA. CD11c+ alveolar macrophages are a source of IL-23 during lipopolysaccharide-induced acute lung injury. Shock. 2013;39(5):447–52. doi: 10.1097/SHK.0b013e31828f9c92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141(3):398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]