Abstract
Changes in synaptic strength on ventral tegmental area (VTA) dopamine neurons are thought to play a critical role in the development of addiction-related behaviors. However, it is unknown how a single injection of cocaine at different doses affects locomotor activity, behavioral sensitization, and glutamatergic synaptic strength on VTA dopamine neurons in mice. We observed that behavioral sensitization to a challenge cocaine injection scaled with the dose of cocaine received one day prior. Interestingly, the locomotor activity after the initial exposure to different doses of cocaine corresponded to the changes in glutamatergic strength on VTA dopamine neurons. These results in mice suggest that a single exposure to cocaine dose-dependently affects excitatory synapses on VTA dopamine neurons, and that this acute synaptic alteration is directly associated with the locomotor responses to cocaine and not to behavioral sensitization.
Keywords: VTA, behavioral sensitization, plasticity
Introduction
Although the direct pharmacological effects of abused drugs are well established, less is known regarding the changes that occur in the brain after drug exposure. Many studies provide evidence of an interaction between the midbrain dopamine system and addictive drugs, in that drugs not only affect the firing (Appel et al., 2003; Fisher et al., 1998; French, 1997) and N-methyl-D-aspartate receptor (NMDAR) currents (Schilstrom et al., 2006) of VTA dopamine neurons in vitro, but also elevate dopamine levels in the nucleus accumbens in vivo (Di Chiara and Imperato, 1988). How acute drug-induced neurochemical changes lead to drug-related behaviors is unknown, but numerous lines of evidence suggest the VTA is a critical nucleus for the development of these behaviors (Kalivas and Alesdatter, 1993; Kalivas and McFarland, 2003; Kalivas and Weber, 1988; McFarland et al., 2004; McFarland and Kalivas, 2001). For example, behavioral sensitization, which is the enhanced locomotor response to an addictive drug after previous drug exposure, requires NMDAR activation in the VTA (Kalivas and Alesdatter, 1993) and can also be elicited by direct drug infusions into the VTA (Kalivas and Weber, 1988; Vezina, 1996; Vezina and Stewart, 1989). Behavioral sensitization can persist for up to a year (Paulson et al., 1991), and is thought to be comparable to long-lasting drug craving in human addicts (Boileau et al., 2006; Evans et al., 2006; Kauer, 2004). Many groups report robust behavioral sensitization after multiple drug injections (Borgland et al., 2004; Kalivas and Weber, 1988; Vezina, 1996; Vezina and Stewart, 1989). However, less is known regarding sensitization from a single drug exposure (Jackson and Nutt, 1993; Robinson et al., 1982), which is important to addiction research since minimal prior drug experience can enhance the acquisition of drug self-administration behaviors (Schenk and Partridge, 2000).
In addition to its behavioral effects, in vivo drug exposure also induces long-lasting synaptic changes on VTA dopamine neurons (> 5 days), which is observed as an increase in the ratio of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) to the NMDAR currents (AMPAR/NMDAR) (Borgland et al., 2004; Melis et al., 2002; Saal et al., 2003; Ungless et al., 2001). However, it is unexplored how a single injection of cocaine at different doses affects (i) the degree of behavioral sensitization, (ii) the changes in synaptic strength as detected by alterations in the AMPAR/NMDAR, and (iii) whether changes in the AMPAR/NMDAR are associated with the behavioral sensitization or with the motor activity after initial cocaine exposure in mice.
Materials and Methods
Behavioral sensitization
All procedures conformed to National Institutes of Health and Ernest Gallo Clinic and Research Center policies for animal care. 3–4 wk-old C57BL/6 mice (Charles River, Hollister, CA) were given mock injections for 2 days prior to behavioral sensitization experiments, similar to a previous study (Jackson and Nutt, 1993). On Day 1, activity was monitored (MED Associates, Inc., St. Albans, VT) after intra-peritoneal (i.p. at 10 ml/kg) injections of cocaine or saline. Mice received an injection 3 hrs later in the home cage of either saline for context-dependent mice, or cocaine (40 mg/kg i.p.) for context-independent mice. On Day 2, activity was recorded after an injection of cocaine (15 mg/kg, i.p). No difference existed between the distance traveled in the first 15 min of the session on Day 2 between context-independent mice (40 mg/kg cocaine on Day 1, 6444 ± 794 cm, n = 9) and saline-injected mice (4973 ± 639 cm traveled, n = 8, p > 0.05 by unpaired Students t-test); therefore, subsequent experiments explored only context-dependent sensitization as context was critical for its development. Activity data are averaged into 5-min bins and presented as average ± SEM. Due to computer malfunctions, some files were not collected, accounting for the different number of animals between Day 1 and Day 2 in certain treatment groups. Significance was determined with one-way ANOVAs with post-hoc Dunnett’s tests.
Electrophysiology
Horizontal brain slices (170 μm) of mice injected with saline or cocaine 24 hr prior were prepared as described previously (Ungless et al., 2001). Solutions were saturated with 95% O2 – 5% CO2. Brains were sliced in a chilled solution containing, in mM: 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 75 sucrose. Slices recovered for ~1 hr at 32°C in an artificial cerebral spinal fluid (aCSF), at 295–305 mOsm and contained, in mM: 126 NaCl, 2.5 KCl, 1.1 NaH2PO4, 1.4 MgCl2, 2.4 CaCl2, 11 d-glucose, and 26 NaHCO3. aCSF (~32°C) with 100 μM picrotoxin, was perfused at ~2.0 mL/min over slices. Whole cell patch-clamp recordings were made with an internal solution containing: 117 mM cesium methanesulfonate, 20 mM HEPES, 0.4 mM EGTA, 2.8 mM NaCl, 5 mM TEA-Cl, 2.5 mg/mL Mg-ATP, and 0.25 mg/mL Mg-GTP at pH = 7.2 – 7.4 and 275 – 285 mOsm. Dopamine neurons were identified by the presence of Ih (Grace and Bunney, 1983; Wanat et al., 2008). Excitatory postsynaptic currents (EPSCs) were elicited from a bipolar stimulating electrode (0.1 Hz, 100 – 300 μm rostral to neuron). The AMPAR/NMDAR was calculated by averaging 12 EPSCs at +40 mV before and after application of AP-5 (50 μM). NMDAR responses were calculated by subtracting the average response in the presence of AP-5 (AMPAR only) from that seen in its absence. The peak AMPAR EPSC was divided by the peak NMDAR EPSC to yield the AMPAR/NMDAR. Drugs were purchased from Sigma (St. Louis, MO).
Results
A single injection of cocaine can elicit behavioral sensitization (Jackson and Nutt, 1993), however, it is unknown if the dose of cocaine administered correlates with the degree of sensitization. On Day 1, locomotor activity was recorded after injections of saline or cocaine (0.8 – 80 mg/kg) in mice (Fig. 1A). There was a significant effect between treatment groups (F (5, 49) = 12.4, P < 0.01, Fig. 1B), where saline-injected mice traveled 9332 ± 534 cm (n = 8), and cocaine-injected mice traveled 10100 ± 805 cm (0.8 mg/kg, n = 11), 13110 ± 779 cm (4 mg/kg, n = 11), 16560 ± 967 cm (8 mg/kg, n = 10), 29440 ± 3702 cm (40 mg/kg, n = 9), and 17340 4913 cm (80 mg/kg, n = 6). Although there was a trend for increased activity with escalating cocaine doses up to 40 mg/kg, only the 40 mg/kg cocaine-injected mice exhibited significantly higher activity relative to saline-injected mice (p < 0.01 by post-hoc Dunnett’s test). Interestingly, higher doses of cocaine did not significantly increase locomotion, which may be due to the anesthetic or deleterious properties of cocaine (Reith et al., 1985). Stereotypic counts were also monitored during the session and we observed a significant effect between treatment groups (F (5, 49) = 13.6, P < 0.05, Table 1) with only the 8 mg/kg and 40 mg/kg cocaine doses producing significant increases in stereotypic counts relative to saline-injected mice (Table 1).
Fig. 1. Locomotor activity following a single injection of saline or cocaine in mice.
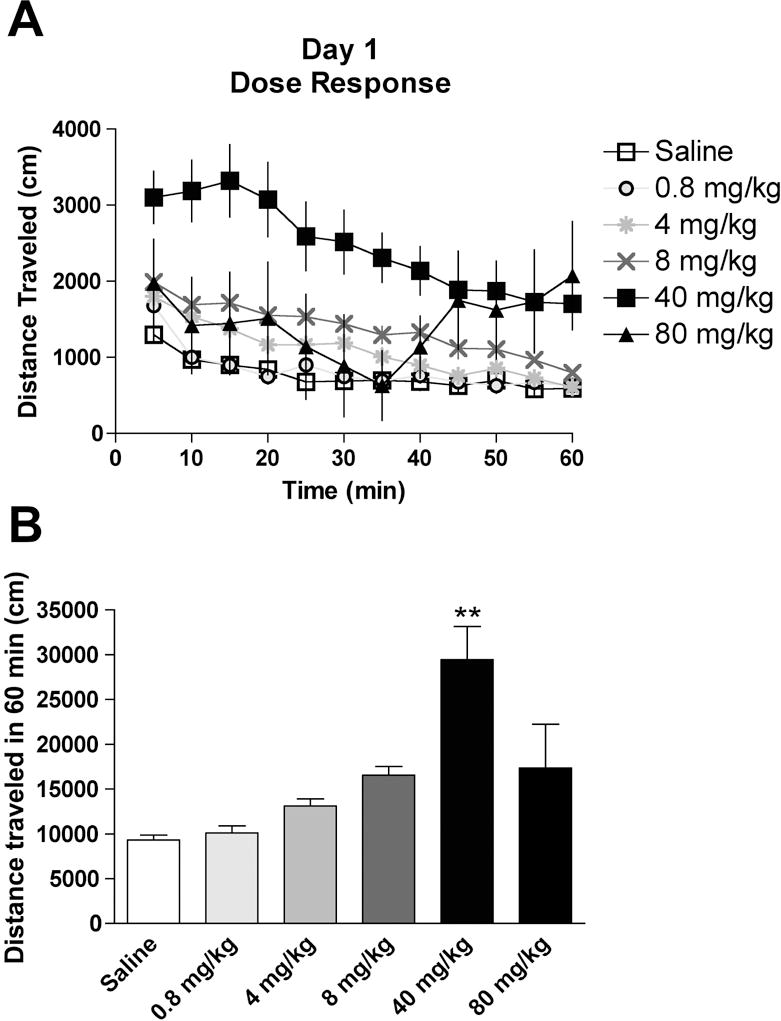
(A) The time-course of activity for 1 hr after a single injection of saline or cocaine (0.8 – 80 mg/kg). (B) Total distance traveled during the 1 hr in the activity chamber for each treatment group. ** = P < 0.01 by post-hoc Dunnett’s test compared to saline group.
Table 1.
Stereotypic counts after saline or cocaine injections.
| Day 1 (dose response) | Day 2 (challenge) for full session | Day 2 (challenge) for first 15 min | |
|---|---|---|---|
| Saline | 2628 ± 127 | 2893 ± 157 | 750 ± 39 |
| 0.8 mg/kg Cocaine | 2691 ± 115 | 3105 ± 84 | 847 ± 21 |
| 4 mg/kg Cocaine | 2996 ± 178 | 2883 ± 138 | 767 ± 39 |
| 8 mg/kg Cocaine | 3399 ± 152* | 3172 ± 162 | 823 ± 42 |
| 40 mg/kg Cocaine | 3501 ± 183* | 3204 ± 78 | 773 ± 41 |
| 80 mg/kg Cocaine | 2379 ± 482 | 2737 ± 222 | 624 ± 73 |
= P < 0.01, 0.05 by post-hoc Dunnett’s test compared to saline group.
On Day 2, we assayed for the development of behavioral sensitization after a 15 mg/kg challenge injection of cocaine (Fig. 2A). No differences in activity were observed between groups during the full hour (P = 0.51 between groups; saline: 22480 ± 3064 cm, n = 8; 0.8 mg/kg cocaine: 26470 ± 2814 cm, n = 12; 4 mg/kg cocaine: 29320 ± 2976 cm, n = 13; 8 mg/kg cocaine: 25730 ± 3415 cm, n = 12; 40 mg/kg cocaine: 28170 ± 2867 cm, n = 9; 80 mg/kg cocaine: 32380 ± 3379 cm, n = 6, Fig. 2B). However, apparent differences existed between treatment groups during the first 15 min that are obscured by analyzing the activity over the whole hour (Fig. 2A). Since habituation to activity chambers prevents sensitization to a single cocaine injection (Jackson and Nutt, 1993), and context-dependent effects of cocaine are likely most salient early in the session, we instead analyzed the activity for 15 min after cocaine challenge and observed a significant effect between treatment groups (F (5, 54) = 5.0, P < 0.001; saline: 4973 ± 639 cm, n = 8; 0.8 mg/kg cocaine: 6004 471 cm, n = 12; 4 mg/kg cocaine: 8049 ± 887 cm, n = 13; 8 mg/kg cocaine: 8295 ± 1046 cm, n = 12; 40 mg/kg cocaine: 9867 ± 759 cm, n = 9; 80 mg/kg cocaine: 11250 ± 926 cm, n = 6, Fig. 2C). Behavioral sensitization was observed with mice receiving cocaine injections on Day 1 of 8 mg/kg (P < 0.05), 40 mg/kg (P < 0.01), and 80 mg/kg (P < 0.01) compared to Day 1-injected saline mice (Fig. 2C, significance by post-hoc Dunnett’s test). When examining the stereotypic counts on Day 2, there were no differences between treatment groups during the full hour (P = 0.19), but there was a significant group effect during the first 15 min of the session (F (5, 54) = 6.8, P < 0.05), though none of the treatments were significantly different from saline-injected mice with post-hoc analysis (Table 1).
Fig. 2. Locomotor activity following a challenge injection of cocaine in mice that received an injection of either saline or cocaine at various doses on the previous day.
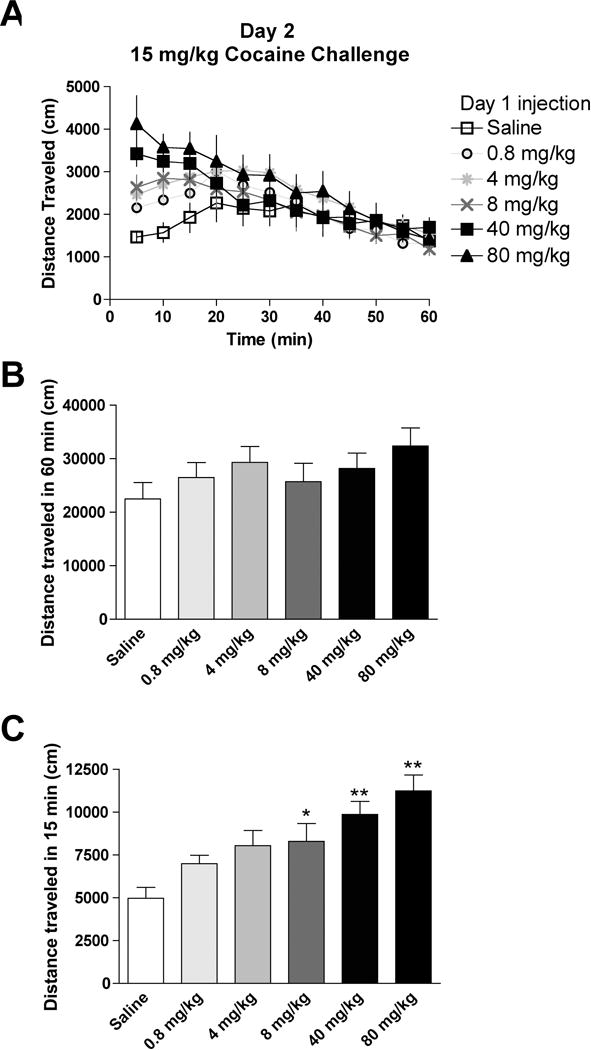
(A) The time-course of activity for 1 hr after the challenge injection of cocaine (15 mg/kg) in mice that received either saline or cocaine (0.8 – 80 mg/kg) one day prior. (B) Total distanced traveled during the 1 hr in the activity chamber for each treatment group. (C) Distanced traveled during the first 15 min in the activity chamber for each treatment group. *, ** =P < 0.05, 0.01 by post-hoc Dunnett’s test compared to saline group.
In a separate group of animals, we next examined the effect of a single cocaine injection at different doses on the excitatory synaptic strength in VTA dopamine neurons. Identical to the dose-dependent effects of cocaine on initial activity (Fig. 1B), there was a significant difference between treatment groups (F (5, 44) = 3.2, P < 0.05, Fig. 3), and a trend for a dose-dependent increase in the AMPAR/NMDAR with escalating cocaine doses (0.8 mg/kg: 0.42 ± 0.05, n = 6; 4 mg/kg: 0.54 ± 0.04, n = 8; 8 mg/kg: 0.56 ± 0.05, n = 7). However, only the 40 mg/kg cocaine-injected group produced a significant increase in the AMPAR/NMDAR (0.60 ± 0.05, n = 8) when compared to saline-injected mice (0.40 ± 0.04, n = 13, P < 0.01 by post hoc Dunnett’s test, Fig. 3). Surprisingly, mice injected with 80 mg/kg of cocaine were not different from the saline treatment group in both initial locomotor activity (Fig. 1), and the observed AMPAR/NMDAR (0.46 ± 0.05, n = 8, P > 0.05, Fig. 3). These findings demonstrated that changes in the glutamatergic synaptic strength on VTA dopamine neurons elicited by different doses of cocaine correspond to the initial activity after cocaine, and not to the development of behavioral sensitization.
Fig. 3. The AMPAR/NMDAR on VTA dopamine neurons from mice receiving an injection of either saline or cocaine at various doses on the previous day.
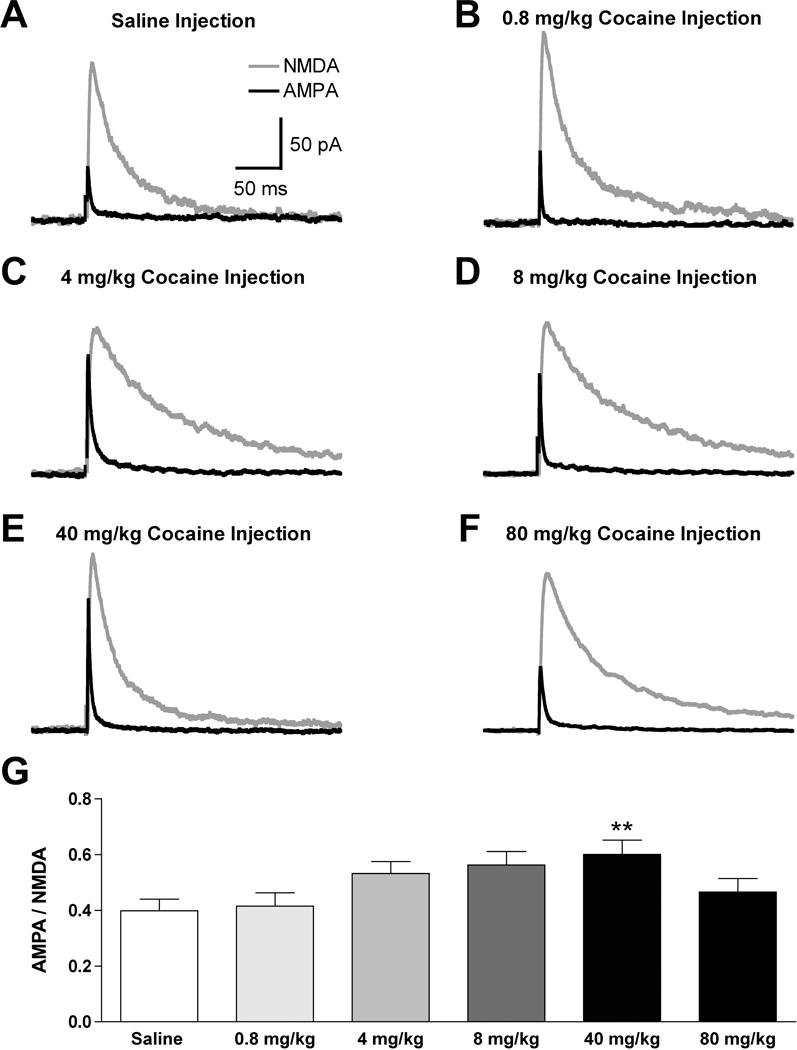
Example traces demonstrating the NMDA component (blue) and AMPA component (red) from mice that received a saline injection (A), or a cocaine injection of 0.8 mg/kg (B), 4 mg/kg (C), 8 mg/kg (D), 40 mg/kg (E), or 80 mg/kg (F), on the previous day. (G) The average AMPAR/NMDAR for all treatment groups. ** = P < 0.01 by post-hoc Dunnett’s test compared to saline group.
Discussion
In this study, we first characterized the locomotor activity after a single exposure to cocaine at various doses. Next, we identified a dose-dependent relationship between the initial dose of cocaine administered and the behavioral sensitization after a challenge injection of cocaine. Finally, we found that a single cocaine injection elicited changes in the AMPAR/NMDAR in VTA dopamine neurons that were associated with the activity after the initial drug exposure, and not to the observed behavioral sensitization.
A single exposure to cocaine can elicit context-dependent behavioral sensitization in a similar paradigm (Jackson and Nutt, 1993), but the doses capable of producing sensitization by a single injection have not been identified. Although we observed a trend for increased activity in response to the 4 mg/kg and 8 mg/kg doses of cocaine, only the 40 mg/kg dose of cocaine significantly augmented locomotor activity. We also found that cocaine injections of 8 mg/kg and 40 mg/kg cocaine significantly enhanced stereotypy. The 80 mg/kg cocaine injection did not affect activity or stereotypy, which may result from the anesthetic (Reith et al., 1985) or seizure-inducing properties of cocaine at high doses (George, 1991). In support, we observed a rebound in the activity of the 80 mg/kg cocaine-treated mice ~ 40 min after injection (Fig. 2A), which is to be expected since i.p. administered cocaine at moderate doses has a half-life of 16 min in both the brain and plasma of mice (Benuck et al., 1987).
We next examined the development of behavioral sensitization with a challenge injection of 15 mg/kg cocaine on the following day. There was no significant effect on activity over the full hour, though it was apparent that the activity profiles between groups converged after the first 15 min post-injection (Fig. 2A). The context-dependent effects of the prior cocaine exposure are likely most relevant during the early portion of the locomotor session since mice habituated to activity chambers do not exhibit behavioral sensitization under a similar paradigm (Jackson and Nutt, 1993). In this regard, we analyzed the first 15 minutes of the session after the challenge injection of cocaine and found a significant increase in activity in mice that were given injections of cocaine at doses of 8 mg/kg, 40 mg/kg, and 80 mg/kg on the previous day. Although there was sensitization of locomotor activity, there was no significant difference in the stereotypic counts between the cocaine-injected and saline-injected mice after the challenge injection. It is interesting to note that even though there was no significant effect on activity when mice received an 80 mg/kg cocaine injection, the following day those mice exhibited the greatest degree of behavioral sensitization, which highlights that previous locomotor activity to cocaine exposure does not necessarily predict the extent of observed sensitization.
Exposure to abused drugs can alter the synaptic properties of VTA dopamine neurons (Borgland et al., 2004; Liu et al., 2005; Melis et al., 2002; Nugent et al., 2007; Saal et al., 2003; Ungless et al., 2001), which has been hypothesized to play an important role in drug-related behaviors (Kauer, 2004). In this regard, we examined how a single cocaine injection at various doses affected the AMPAR/NMDAR in VTA dopamine neurons and whether the changes in synaptic strength were associated with drug-induced behavior. We observed a trend for a dose-dependent increase in the AMPAR/NMDAR with escalating cocaine doses, but only the 40 mg/kg cocaine-injected group produced a significant increase in the AMPAR/NMDAR, which mirrored our results characterizing the motor activity after the initial cocaine exposure. Furthermore, even though treatment with 80 mg/kg cocaine produced the largest behavioral sensitization, there was no effect of this treatment on initial motor activity or to alterations in the AMPAR/NMDAR on VTA dopamine neurons. Together, this suggests that changes in synaptic strength on dopamine neurons may not only require drug exposure, but also drug-induced increases in activity. In addition, our results complement previous work demonstrating that the AMPAR/NMDAR on dopamine neurons correlated with the activity to the initial exposure of cocaine at a single dose in rats receiving a week-long regimen of daily cocaine injections (Borgland et al., 2004). Therefore, the motor activity from the initial cocaine exposure corresponds with changes in glutamatergic synaptic strength on VTA dopamine neurons, whether due to individual variations to a single cocaine dose (Borgland et al., 2004), or due to different cocaine doses, as was described in the present study.
The VTA is critical for the development of behavioral sensitization (Kauer, 2004), since local antagonism of NMDARs blocks sensitization (Kalivas and Alesdatter, 1993) and direct intra-VTA injections of dopamine receptor agonists (Pierce et al., 1996) or psychostimulants (Kalivas and Weber, 1988; Vezina, 1996) are sufficient for the initiation of sensitization. However, the current findings, coupled with those previously reported (Borgland et al., 2004), indicate that changes in the AMPAR/NMDAR of VTA dopamine neurons do not mediate the development of behavioral sensitization, as was originally hypothesized (Kauer, 2004). Rather, changes in the AMPAR/NMDAR on dopamine neurons could be important for other drug-related behaviors such as drug self-administration (Kauer, 2004). In summary, we established the dose-dependent effect of a single injection of cocaine on behavioral sensitization, but found that the changes in the glutamatergic synaptic strength on VTA dopamine neurons correspond instead to the activity after initial drug exposure in mice.
Acknowledgments
This research was supported by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (AB) and the National Institute on Drug Abuse R01 DA016782-04 (AB), F31 DA21464-01 (MW). We also thank Stephanie Borgland for her critical input and Lisa G. Daitch for proofreading the manuscript.
References
- Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther. 2003;306(2):437–446. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- Benuck M, Lajtha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243(1):144–149. [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63(12):1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24(34):7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, Brooks DJ, Lees AJ, Piccini P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59(5):852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Pidoplichko VI, Dani JA. Nicotine modifies the activity of ventral tegmental area dopaminergic neurons and hippocampal GABAergic neurons. Journal of physiology, Paris. 1998;92(3–4):209–213. doi: 10.1016/s0928-4257(98)80012-0. [DOI] [PubMed] [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neuroscience letters. 1997;226(3):159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- George FR. Cocaine toxicity: genetic evidence suggests different mechanisms for cocaine-induced seizures and lethality. Psychopharmacology (Berl) 1991;104(3):307–311. doi: 10.1007/BF02246028. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–1. Identification and characterization. Neuroscience. 1983;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Nutt DJ. A single preexposure produces sensitization to the locomotor effects of cocaine in mice. Pharmacol Biochem Behav. 1993;45(3):733–735. doi: 10.1016/0091-3057(93)90533-y. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267(1):486–495. [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245(3):1095–1102. [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437(7061):1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24(7):1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22(6):2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446(7139):1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103(4):480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental area administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. J Pharmacol Exp Ther. 1996;278(1):384–392. [PubMed] [Google Scholar]
- Reith ME, Meisler BE, Lajtha A. Locomotor effects of cocaine, cocaine congeners, and local anesthetics in mice. Pharmacol Biochem Behav. 1985;23(5):831–836. doi: 10.1016/0091-3057(85)90078-4. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain research. 1982;253(1–2):231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37(4):577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine’s reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66(4):765–770. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26(33):8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411(6837):583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16(7):2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain research. 1989;499(1):108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. The peptide CRF increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. The Journal of physiology. 2008 doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]


