Abstract
This work continues our efforts to improve the diagnostic and radiotherapeutic effectiveness of nanomedicine platforms by developing approaches to reduce the non-target accumulation of these agents. Herein, we developed multi-block HPMA copolymers with backbones that are susceptible to cleavage by cathepsin S, a protease that is abundantly expressed in tissues of the mononuclear phagocyte system (MPS). Specifically, a bis-thiol terminated HPMA telechelic copolymer containing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) was synthesized by reversible addition−fragmentation chain transfer (RAFT) polymerization. Three maleimide modified linkers with different sequences, including cathepsin S degradable oligopeptide, scramble oligopeptide and oligo ethylene glycol, were subsequently synthesized and used for the extension of the HPMA copolymers by thiol–maleimide click chemistry. All multi-block HPMA copolymers could be labeled by 177Lu with high labeling efficiency and exhibited high serum stability. In vitro cleavage studies demonstrated highly selective and efficient cathepsin S mediated cleavage of the cathepsin S-susceptible multi-block HPMA copolymer. A modified multi-block HPMA copolymer series capable of Förster Resonance Energy Transfer (FRET) was utilized to investigate the rate of cleavage of the multi-block HPMA copolymers in monocyte-derived macrophages. Confocal imaging and flow cytometry studies revealed substantially higher rates of cleavage for the multi-block HPMA copolymers containing the cathepsin S-susceptible linker. The efficacy of the cathepsin S-cleavable multi-block HPMA copolymer was further examined using an in vivo model of pancreatic ductal adenocarcinoma. Based on the biodistribution and SPECT/CT studies, the copolymer extended with the cathepsin S susceptible linker exhibited significantly faster clearance and lower non-target retention without compromising tumor targeting. Overall, these results indicate that exploitation of the cathepsin S activity in MPS tissues can be utilized to substantially lower non-target accumulation, suggesting this is a promising approach for the development of diagnostic and radiotherapeutic nanomedicine platforms.
Keywords: Cathepsin S, HPMA, FRET imaging, Mononuclear phagocyte system, Pancreatic cancer, SPECT/CT imaging
1. Introduction
In 2015, pancreatic cancer was estimated to be the 11th and 8th most commonly diagnosed cancer for men and women in the United States, respectively. At the same time, it is estimated to account as the 4th leading cause of cancer related death for both men and women [1]. Pancreatic ductal adenocarcinoma (PDAC), which constitutes over 90% of pancreatic cancers in humans, is a devastating and virtually unexceptionally lethal malignancy [2]. Due to a lack of symptoms in early stages of the disease, PDAC is typically not diagnosed until the disease has progressed to a more advanced state [3, 4]. As a result, despite advances in the field of surgery, chemotherapy, and radiation therapy, the prognosis of PDAC remains extremely poor with a five-year survival rate of less than 5% [5, 6], which is the lowest among common malignancies. Because of the high mortality and rapid progression associated with PDAC, effective detection and accurate staging of pancreatic cancer have become a major challenge in disease management.
Over the years, numerous imaging modalities have been utilized to non-invasively detect cancer. In particular, magnetic resonance imaging [7], positron emission tomography [8], single-photon emission computed tomography (SPECT) [9], computed tomography (CT) [10] and ultrasound [11] have played critical roles in improving the diagnosis and staging of many malignancies. As one of the major imaging techniques used in nuclear medicine today, SPECT is often combined with CT to allow co-registration of the radiotracer and anatomy [12]. Utilizing the dual-modality of SPECT/CT has been shown to yield improved sensitivity and accuracy in cancer imaging [13]. Unfortunately, the potential advantages of SPECT/CT imaging for the detection and staging of PDAC cannot currently be realized due to the lack of a clinically approved imaging agent. As a result, the preclinical development of SPECT imaging agents for pancreatic cancer has been an active area of research [14-16]. These radiotracers have utilized carriers that range from small molecules to macromolecules. To date, the bulk of the macromolecular work has focused on antibodies due to their excellent in vivo targeting and tumor retention properties. Despite the comparable tumor uptake and retention properties, nanomedcines have received relatively little attention in the development of SPECT imaging agents for PDAC and other cancers. This is largely attributable to the high blood retention and uptake of many of these platforms in non-target tissues associated with the mononuclear phagocyte system (MPS) [17]. In order for nanomedicines to become more attractive as a radionuclide carrier, approaches are needed to lower this non-target retention.
During the last two decades, work by Kopeček and other laboratories have demonstrated the potential of poly[N-(2-hydroxypropyl)methacrylamide] (HPMA), a water-soluble, non-immunogenic, non-toxic polymeric platform, as a drug carrier [18]. For cancer, HPMA copolymers have shown great potential as a drug delivery system to enhance the delivery of chemotherapeutics to a variety of tumors through the enhanced permeability and retention (EPR) effect [19]. Although these copolymers exhibited remarkable advantages for drug delivery, the accumulation of this polymeric platform, as with many nanomedicines, in MPS-associated tissues (e.g., blood, liver and spleen) diminishes its potential as a diagnostic agent. Our laboratory has been focused on developing approaches to substantially improve the clearance of radiolabeled HPMA copolymers from MPS-associated, non-target tissues. Our efforts have focused on cathepsin S, a protease that is abundantly expressed in antigen presenting cells (APCs) such as monocytes and macrophages - the primary components of the MPS [20, 21]. Utilizing known peptidic substrates of cathepsin S, we have used these peptides to substantially enhance the clearance of radiolabeled HPMA copolymers from MPS-associated tissues, such as the blood, liver and spleen [22, 23].
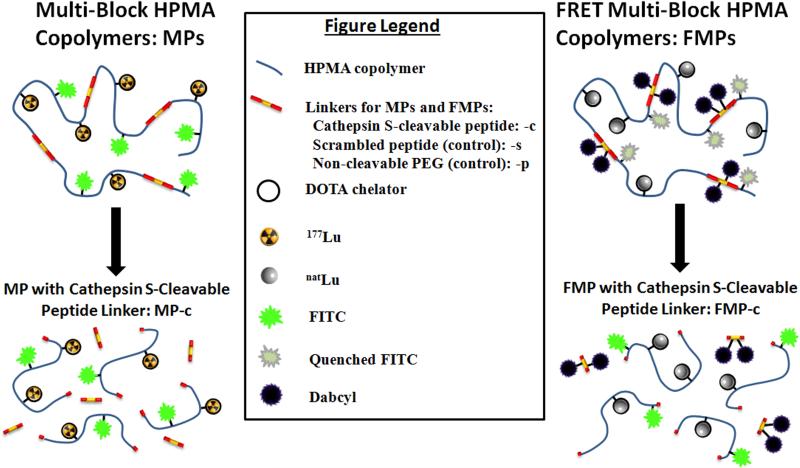
Inspired by the recent work on backbone biodegradable copolymers [24, 25], this manuscript investigates the incorporation of cathepsin S susceptible linkers into the HPMA backbone and its impact on the biological performance of these agents for PDAC imaging. We hypothesized that inclusion of the cathepsin S cleavable, peptidic linker would result in fragmentation of the HPMA copolymers and faster clearance of the copolymer fragments from phagocytic cells (e.g., monocytes and macrophages) and tissues associated with MPS uptake and retention. Furthermore, as a consequence of this fragmentation, the smaller copolymer blocks would be expected to clear more quickly from the body. To investigate, we designed two multi-block HMPA copolymer platforms, depicted in Fig. 1: MPs and FMPs. The MPs, which contains the chelation moiety, were radiolabeled with lutetium-177 (177Lu) and evaluated using various in vitro and in vivo studies. Whereas, the FMPs, which are capable of Förster Resonance Energy Transfer (FRET), were utilized as a tool to investigate in vitro fragmentation kinetics of the biodegradable, multi-block HPMA copolymers. For both the MPs and FMPs, a known cathepsins S peptide substrate (PMGLP) was utilized as the linker as well as controls containing a scrambled peptide analogue and a non-cleavable polyethylene glycol (PEG). Herein, the syntheses, characterization, in vitro cleavage assays and cell trafficking studies for the MPs and FMPs are described. In addition, the biodistribution studies and SPECT/CT imaging results of the MPs in a PDAC mouse model are reported.
Fig. 1.
Schematic design of the cathepsin S-susceptible, multi-block HPMA copolymers. (Left) The 177Lu-labeled biodegradable multi-block HPMA copolymers (MP-c), which could be cleaved by cathepsin S, were evaluated using various in vitro and in vivo studies. (Right) The FRET biodegradable multi-block HPMA copolymers (FMP-c) were designed for studying the intracellular cleavage of the copolymers.
2. Materials and methods
2.1 Materials
Reagent: All solvents used for reaction and silica gel purification were ACS grade and purchased from Fisher Scientific. Acetonitrile was HPLC grade and purchased from Fisher Scientific. Water was deionized by Millipore® Milli Q Biocell Ultrapure Water System before use. CDCl3 and deuterium oxide were obtained from ACROS Organics™. Fluorescein isothiocyanate (FITC), N-Boc-ethylenediamine, N-hydroxysuccinimide (NHS), N,N’-dicyclohexylcarbodiimide (DCC), natLutetium trichloride, 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI), 4,4’-Azobis(4-cyanovaleric acid) (ACVA), thioanisole, triisopropylsilane (TIS), N,N-Diisopropylethylamine (DIEA), hexylamine, sodium acetate, hydrocortisone and cysteine cathepsin S isolated from human spleens were obtained from Sigma-Aldrich. Trifluoroacetic acid (TFA) was purchased from Fisher Scientific. 6-Maleimidohexanoic acid was purchased from Alfa Aesar. Mono-Boc-cystamine hydrochloride and H-S-tert-butylmercapto-L-cysteine was obtained from Chem-Impex. 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate (COMU), Fmoc-protected amino acids and tris(2-carboxyethyl)phosphine (TCEP) were purchased from NovaBiochem. 1,8-Bismaleimido-diethyleneglycol was obtained from Thermo Fisher. N-(2-Hydroxypropyl)methacrylamide was purchased from Polysciences. FITC-APMA and DOTA-APMA were synthesized according to reported methods [26, 27]. Dulbecco's Modified Eagle Medium (DMEM), and phosphate buffered saline (PBS) were obtained from Fisher Scientific. 177Lutetium trichloride was purchased from PerkinElmer. The human pancreatic adenocarcinoma HPAC (CRL-2119) cell line was purchased from American Type Culture Collection. Human monocytes, human AB serum and rhM-CSF were provided by the UNMC Elutriation Core Facility. Matrigel® was obtained from BD Biosciences. L-glutamine, LysoTracker® Red DND-99 and NucBlue® Live ReadyProbe® reagent were purchased from Thermo Fisher Scientific. Cell marker CD 14-FITC and 25F9- eFluor® 660 were purchased from eBioscience. Five weeks old female SCID mice were purchased from Charles River Laboratories. All procedures utilizing animals conform to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center.
Instrumentation: Peptides were synthesized by solid phase peptide synthesis (SPPS) on a Liberty microwave peptide synthesizer from CEM. A Waters e2695 system equipped with a Waters 2489 absorption detector and a Waters Qtof Micro electrospray ionization mass spectrometer was used to perform high performance liquid chromatography/mass spectrometry analyses. 1H-NMR spectrums were recorded on a Bruker Avance-III HD 600 MHz instrument using CDCl3 or deuterium oxide as the solvent. A Phenomenex Jupiter C12 Proteo 250 × 10 mm semiprep column was used for the purification of bulk amounts of peptides. A Viscotek TDA max system equipped with Shodex Asahipak GF-510 HQ GPC column was used for HPMA copolymer molecular weight and size measurements. A Sephadex® LH-20 size exclusion resin obtained from GE HealthCare was used for bulk chromatographic separations. Evaluation and purification of radiolabeled polymer conjugates were performed on a Waters 1515 binary pump equipped with a Waters 2489 absorption detector and a Bioscan Flow Count radiometric detector system using an Agilent PL aquagel-OH MIXED-H Gel Permation Chromatography (GPC) column. Gamma decay detection of 177Lu-labeled, polymer conjugates for biodistribution studies was accomplished using a NaI (Tl) well detector constructed by AlphaSpectra Inc. Fluorescence intensities were measured by a SpectraMax® M5 multimode plate reader. Lab-Tek chambered #1.0 borosilicate coverglass disks (4 well) were used for confocal cell imaging. Confocal microscopy images were taken on a Leica LSM 510 META Microscope equipped with an argon laser. Flow cytometry data were acquired on a Becton Dickinson LSR II, and processed by FlowJo software. Small animal SPECT/CT data acquisition was achieved with the GE Healthcare dual Flex Triumph CT/SPECT instrument. The SPECT/CT images were processed by VivoQuant™ 2.1. Autoradiography studies were accomplished by GE Lifesciences Typhoon 9410 variable mode imager.
2.2 The synthetic procedures and schemes leading to chain transfer reagent (CTA) 1, CTA 2, initiator 3 and peptidic linkers (cleavable peptide linker (CL), scramble peptide linker (SL), Dabcyl conjugated cleavable peptide linker (DCL), Dabcyl conjugated scramble peptide linker (DSL) and Dabcyl conjugated PEG linker (DPL) are reported in supplemental content.
2.3 Synthesis of HPMA polymers
The nomenclature utilized in describing the synthesis of the multi-block HPMA copolymer platforms are described in Schemes 1 and 2.
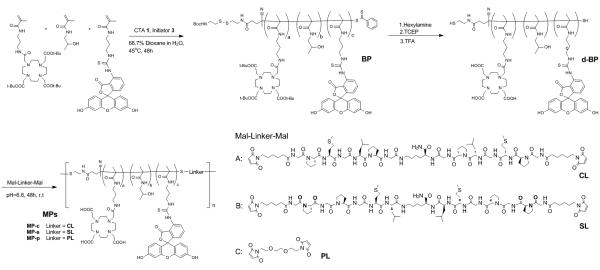
Scheme 1.
Synthesis of MP-c, MP-s and MP-p via RAFT polymerization and thiol-ene click chemistry.
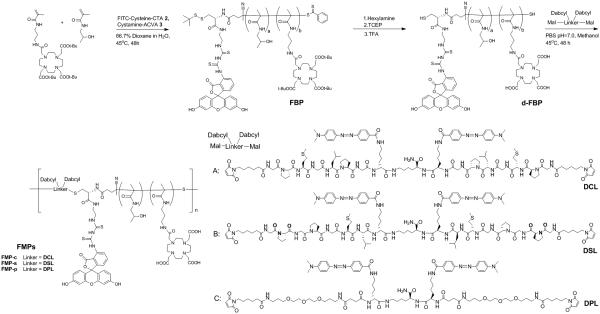
Scheme 2.
Synthesis of FMP-c, FMP-s and FMP-p via RAFT polymerization and thiol-ene click chemistry.
2.3.1 Synthesis of telechelic-block HPMA copolymer (BP) and FRET telechelic-block HPMA copolymer (FBP)
The HPMA copolymers were synthesized by RAFT polymerization. Briefly, for the BPs, CTA 1 and initiator 3 were added to a solution containing 1.0 M HPMA monomer, 10 mM DOTA-APMA and 5 mM FITC-APMA in 500 μl of 33% dioxane with a ratio of 100:1:1.15 (monomer:CTA:initiator). The solution was transferred to an ampoule and purged with nitrogen for 40 minutes. The polymerization was carried out in the sealed ampoule at 45 °C for 48 h and terminated by removal of heat and exposure to oxygen. The mixture was loaded on a LH-20 column to remove the unreacted low molecular weight compounds with methanol as the eluent to give a yellow solid (65 mg, 83.1%). For the FMP copolymer block, the FITC-ended HPMA copolymer was also synthesized by the method described above by using CTA 2 as a chain transfer reagent and polymerizing without FITC-APMA, and obtained as an orange solid (61 mg, 76.4%).
2.3.2 Synthesis of deprotected BP (d-BP) and deprotected FBP (d-FBP)
Copolymer BP and FBP (60 mg) in 1 mL methanol was added in 100 μl of hexylamine. The mixture was stirred for 2 h room temperature, and then the copolymer was recovered by precipitation with cold ether. The yellow precipitate was collected, dried and dissolved in 1 ml DI water, followed by adding in TCEP (100 equiv to the initial copolymer amount) and stirring for 4 h. After reduction, the copolymer was purified and isolated by a LH-20 column. The copolymer was dried and redissolved in 4 ml of 1:1 TFA:DCM to form a light yellow solution which was stirred for 1 h. Finally, the copolymer was purified by LH-20 column to give the resulting deprotected copolymers. Both d-BP and d-FBP were obtained in excellent yield as a yellow solid (47 mg, 86.1%) and orange solid (42 mg, 82.7%), respectively.
2.3.3 General procedure of telechelic extension and fractionation of multi-block HPMA copolymers (MPs and FMPs)
The multi-block HPMA copolymer chain extension was carried out according to the literature method [28] with modification. Briefly, the HPMA copolymer block (10 mg) was dissolved in 150 μl PBS buffer (pH= 6.8) to reach a concentration of 6 mM and added to a solution of peptide linker (10 mM) in 100 μl methanol. The mixture was purged with nitrogen for 30 min, and stirred for 48 h at 50 °C. The mixture was filtered through a 0.22 mm filter, and fractionated on an Asahipak GF-510 HQ column by using 40% acetonitrile in PBS as eluent. The elutes from 6 mL to 7.5 mL was collected, concentrated in vacuo, desalted by using an Amicon ultrafiltration cell with a 10 kDa MWCO membrane, and lyophilized to give MP-c (2.3 mg, 17.1 %), MP-s (2.1 mg, 15.8 %), MP-p (1.7 mg, 13.2 %), FMP-c (1.1 mg, 8.1 %), FMP-p (1.3 mg, 9.6 %) and FMP-p (0.82 mg, 6.1 %).
2.4 Radiolabeling of multi-block HPMA copolymers
The multi-block HPMA copolymers were dissolved in DI water to give a concentration of 2 mg / mL. To 100 μL of the solution was added 1 mCi (37 MBq) of 177LuCl3 or 50 μg of natLuCl3, and the mixture was heated to 90 °C for 45 min. The purification of the radiolabeled multi-block HPMA copolymer was performed by an Agilent PL aquagel-OH MIXED-H GPC column with 40% acetonitrile in PBS as eluent. The radiolabeled HPMA copolymers were identified by signals from a Waters 2489 UV detector (494 nm) and Bioscan flow count radiometric detector. The radiolabeled multi-block HPMA copolymer was collected in a tube containing 5 mg of L-ascorbic acid to prevent radiolysis. Before in vitro or in vivo studies were performed, the radiolabeled HPMA copolymers were simultaneously desalted and concentrated by an Amicon ultrafiltration cell with a 3 kDa molecular weight cut off (MWCO) membrane.
2.5 Cathepsin S-cleavage of multi-block HPMA copolymers
Unlabeled or radiolabeled MPs was added to 100 μL of cleavage buffer, containing sodium acetate (50 mM), DTT (10 mM) and EDTA (1 mM) to make a concentration of 1 mg/mL or 10 μCi/mL (0.37ΜΒq/mL), respectively. To this mixture was added 250 ng of human cathepsin S and incubated for different times at 37 °C. All tests were performed in parallel and the process of the cleavage was monitored by GPC-HPLC or radioactive-GPC-HPLC equipped with Waters 2489 UV detector (494 nm), Bioscan flow count radiometric detector and Malvern TDA system. For cleavage studies of FMPs, the copolymer concentration was 100 μg/mL. The regenerated fluorescence intensity at 519 nm was quantified by using the plate reader with excitation at 495 nm.
2.6 Human serum stability of radiolabeled copolymers
The serum stability of the radiolabeled copolymers was determined by using GPC chromatography. Briefly, 10 μCi (0.37 MBq) of radiolabeled copolymers was incubated with 1 mL of human AB serum at 37°C for 24 h. The mixture was dried under nitrogen flow and was recovered in 50 μL of methanol, which was analyzed by radio-GPC-HPLC system.
2.7 Cell culture
The HPAC cells were cultured in our laboratory, as per ATCC protocols, in DMEM/ Ham’s F12 medium containing 5% FBS, 1.562 nM EGF, 14.3 mM sodium bicarbonate, 2.5 mM L-glutamine, 15 mM HEPES and 0.5 mM sodium pyruvate and supplemented with 0.350 mM insulin, 0.0625 mM transferrin and 0.110 mM hydrocortisone. Cells were incubated at 37 °C at 5% CO2.
Human monocyte-differentiated macrophage were produced according to literature procedures [29]. Briefly, monocytes were plated on borosilicate coverglass disks at a concentration of 1.25×105 cells in 500 μL of macrophage differentiation medium: DMEM containing 10% Human AB serum, 500 U rhM-CSF, 2mM L-glutamine and 1% penicillin/streptomycin. Cells were maintained in a 37 ºC humidified atmosphere with 5% CO2. Media was changed every 3 days for 7 days, at which time, visual confirmation of differentiation led to media replacement with the macrophage maintenance medium (differentiation media without the rhM-CSF). For validation of the differentiation procedure, cells were suspended in PBS containing 0.1% BSA and incubated with markers CD14-FITC and 25F9-eFluor® 660 for 15 min on ice. After washing and fixing of the cells, the flow cytometry studies were carried out for confirmation of the differentiation.
2.8 Confocal microscopy
FMPs and MP-c (positive control) were diluted in macrophage maintenance medium to a concentration of FITC equivalents (6 μM). The cells were incubated with the desired copolymer for 6 and 12 h in a 37 ºC humidified atmosphere with 5% CO2. The cells were washed with fresh media after incubation. LysoTracker Red DND-99 was added to the cells and incubated for 2 h at a concentration of 1 nM. Hoechst 33342 was added in the media to stain the nuclei 15 min prior to imaging. The cells were washed with PBS and images were obtained using an excitation wavelength of 405 nm (blue excitation), 488 nm (green excitation) and 568 nm (red excitation).
2.9 Flow cytometry studies
For assessment of cellular fluorescence regeneration by flow cytometry, HPAC cells and differentiated macrophages were respectively exposed to MPs and FMPs (6 μM) in medium for different time, washed with PBS, trypsinized, and harvested by centrifugation. Thereafter, the cells were resuspended in PBS and fluorescence intensity of FITC in each sample was measured by flow cytometry and analyzed using the FlowJo software package. At least 10,000 live cells from each sample were acquired in linear mode and visualized in logarithmic mode.
2.10 Cell efflux studies
Macrophages (differentiated from 5×105 monocytes) were respectively exposed to 5 μCi of 177Lu-MPs for 24 h. The cells were subsequently washed with room temperature medium to remove the extracellular radioactivity. At 2, 4 and 8 h time points, the medium (2 mL) was withdrawn to measure the externalized radioactivity. At each time point, the media was replaced with fresh medium. The radioactivity remaining in the macrophages was measured after cell lysis by 0.1% SDS solution at the 8 h time point. The efflux percentages in each time point were calculated for each of the 177Lu-MPs.
2.10 Biodistribution studies
Female SCID mice (5 weeks of age) received subcutaneous injections of 5×106 HPAC cells suspended in Matrigel® into the flanks. When the tumor size reached 80 mm3 (two weeks after injection), the mice were randomized into three groups and intravenously injected with 10 μCi (0.37 MBq) of the purified 177Lu-MPs via tail vein. The mice were sacrificed and their tissues excised at 4, 24, 72 and 144 h post-injection (p.i.) time points. The blood, tumor and excised tissues were weighed. The radioactivity for each sample was measured by gamma counter. The percentage injected dose per gram (%ID/g) and the radioactivity ratios between tumor and non-targeted tissues were calculated.
2.11 SPECT/CT imaging
For SPECT/CT imaging, 600 μCi (22.2 MBq) of 177Lu labeled HPMA copolymer was intravenously injected into the bloodstream of HPAC tumor bearing mice. Mice were anesthetized with isoflurane and their body temperature was controlled using warm air during the scanning. Mice were longitudinally imaged at 24, 48, 72 and 144 h post-injection. Five hundred and twelve CT projections for each image were acquired and reconstructed using a Triumph X-O 4.1 at a matrix size of 512 × 512 × 512 with a 0.15mm voxel dimension. Sixty-four SPECT projections for each image were acquired using a Triumph SPECT with a 20% window at 208 keV for 177Lu and reconstructed using Triumph SPECT Reconstruction Application 1.0.8.0 at a matrix size of 64 × 64 × 64 with a 1.14 mm voxel dimension. Co-registration of anatomical CT images and functional SPECT was performed using a VivoQuant 2.1™ software package.
2.12 Autoradiography
At the end of the SPECT/CT imaging studies (i.e., 144 h time point), imaging mice were injected with Hoechst 33342 at a dose of 15 mg/kg. The mice were sacrificed 2 min p.i and dissected. The tumor samples were washed with deionized water, dried and immediately embedded in OCT on dry ice. Cryostat sections (10 μm) of tumor sample were scanned by a fluorescent microscope and exposed to a phosphor plate for 3 days. The phosphor plate was subsequently scanned by a Typhoon system at a 25 mm resolution.
2.13 Statistics
Statistical analyses were performed using analysis of variance. A value of p < 0.05 was considered to be statistically significant.
3. Results and discussion
3.1 Synthesis of the multi-block copolymers
3.1.1 Synthesis of CTAs and initiator
As depicted in Fig. 1, two types of multi-block HPMA copolymers, MPs and FMPs, were targeted for synthesis, synthetic schemes given in Schemes 1 and 2, respectively. The syntheses of the telechelic-block copolymers BP and FBP were envisioned to be carried out by RAFT polymerization [30-32]. In order to carry out this synthesis, chain transfer agents (CTA) and initiators with terminal thiol groups needed to be synthesized for each scheme. The structures of the designed CTAs and initiator are shown in Fig. S1. 4-cyanopentanoic acid dithiobenzoate, a common CTA utilized in RAFT polymerizations, was selected as our starting material. For the synthesis of the BP polymer, the starting material was modified by conjugation to the mono-boc protected cystamine to yield CTA 1 (Scheme S1). In the design of the FBP, FITC was incorporated into the CTA in order to yield HPMA copolymers with terminal FITC moieties, which could be later employed for FRET based studies. In order to accomplish this, a FITC-conjugated CTA was covergently synthesized starting from 4-cyanopentanoic acid dithiobenzoate and FITC (Scheme S2) to yield CTA 2. Initiator 3 was synthesized by conjugation of mono-boc protected cystamine to ACVA, a commonly utilized RAFT initiator (Scheme S3). Overall, the designed compounds were synthesized and purified by silica gel column with high yields except for CTA 2 (total yield < 15%). For the synthesized RAFT agents, structures were confirmed by their respective 1H-NMR spectra and mass spectrometric data (Fig. S2 and S3, correspondingly).
3.1.2 Synthesis of HPMA copolymers (BP and FBP)
The synthesis of the HPMA copolymer blocks, BP and FBP, for both multi-block platforms was accomplished using RAFT polymerization. The structures of these two polymeric blocks are depicted in Scheme 1 and Scheme 2, respectively. In order to be utilized in future chain extension reactions, polymerization reactions utilized CTAs and initiators that, as described previously, were modified to yield polymers with thiols at the terminal ends of the copolymer. All polymerization reactions utilized the synthesized initiator 3, while CTA 1 and 2 was employed for the synthesis of BP and FBP, respectively. The polymerization was carried out in dioxane/H2O (1:2) at 45 °C to accommodate the solubility of both the CTAs and initiator. A constant 1:1.1 ratio of CTA : initiator was found to be optimal in achieving high polymerization yields with narrow molecular weight distributions. The molecular weights, composition, polydispersities and yields of synthesized copolymers are summarized in Table 2. Both the BP and FBP copolymers had molecular weights of approximately 18 kDa with polydispersities less than 1.2. The polymerization yields were 83.1 % and 76.4 % for BP and FBP, respectively, which indicates that the modifications of the CTA and initiator did not affect their polymerization efficiency. The DOTA content for each polymer was calculated based on the 1H-NMR spectrum (Fig. S5).
Table 2.
Characteristics of the synthesized HPMA copolymers.
| Copolymer | Mw (kDa) | PDI | Rha | DOTA (Mol %) | Yield (%) |
|---|---|---|---|---|---|
| BP | 18.6 | 1.06 | 2.96 | 1.41 | 83.1b |
| d-BP | 18.2 | 1.05 | 2.88 | - | 86.1c |
| MP-c | 78.7 | 1.11 | 6.46 | - | 17.1d |
| MP-s | 77.6 | 1.37 | 6.97 | - | 15.8d |
| MP-p | 76.1 | 1.08 | 6.11 | - | 13.2d |
| FBP | 18.7 | 1.15 | 3.02 | 1.39 | 76.4b |
| d-FBP | 18.5 | 1.18 | 2.99 | - | 82.7c |
| FMP-c | 80.3 | 1.21 | 7.05 | - | 8.1d |
| FMP-s | 78.5 | 1.18 | 6.88 | - | 9.6d |
| FMP-p | 79.2 | 1.16 | 6.82 | - | 6.1d |
hydrodynamic radius detected by Viscotek TDAmax system;
yield of RAFT polymerization;
yield of reduction and deprtection by hexylamine, TCEP and TFA;
yield of copolymer chain extension after fractionation.
3.1.3 Synthesis of the linkers
As described earlier, cathepsin S is a protease that is selectively expressed in many phagocytic cell types which are responsible for the non-target uptake and retention of nanomedicines [33, 34]. Our laboratory has previously demonstrated that the cathepsin S-substrate sequence PMGLP, when incorporated into HPMA copolymers, can be readily cleaved by this protease [22, 23]. In this undertaking, our goal was to examine the biological impact of incorporating cathepsin S-cleavable substrates into the backbone of HPMA copolymers. In order to investigate the effect of this structural alteration on biological performance, we designed linkers for both multi-block HPMA copolymer platforms that are cathepsin S-cleavable, scrambled (not highly susceptible to cathepsin S cleavage) and non-cleavable.
The synthesis of the peptides, structures depicted in Schemes 1 and 2, were carried out by SPPS using lysine as a branching point and glycine as a spacer. In addition, the N-termini of the peptides were capped with 6-maleimidohexanoic acid to allow future conjugation using thiol-ene click chemistry. For the MPs, this approach yielded the cathepsin S-cleavable peptidic linker (CL) (Mal-GPMGLPG)2K and the scrambled linker (SL) (Mal-GPGPGML)2K. 1,8-Bismaleimido-diethyleneglycol was utilized as a non-cleavable, PEG linker (PL). In the case of the FMPs, the peptidic linkers needed to accommodate Dabcyl, an effective dye quencher with maximum absorbance wavelength λabs = 472 nm, which, to a large extent, overlaps with the emission spectra of FITC [35]. In order to accomplish this, D-lysine was inserted into the peptide sequences and later conjugated to Dabcyl, as illustrated in Scheme S4. This yielded the cathepsin S-cleavable peptidic linker (DCL) (Mal-GPMGLPG-(D-K-Dabcyl))2K and the scrambled linker (DSL) (Mal-GPGPGML-(D-K-Dabcyl))2K. For the non-cleavable control, a short PEG linker was substituted for the cleavable peptide sequence to give DPL. All linkers were purified by HPLC and characterized by mass spectrometry (Table 1 and Fig. S4).
Table 1.
Characterization of peptidic linkers.
| Linkers | Sequences | Formula | Exact Mass | Mass found | Total yields (%) |
|---|---|---|---|---|---|
| CL | (Mal-GPMGLPG)2K | C80H123N19O21S2 | 1749.86 | 1750.67a | 18.2 |
| SL | (Mal-GPGPGML)2K | C80H123N19O21S2 | 1749.86 | 1750.63a | 15.8 |
| DCL | (Mal-GPMGLPG-D-K(Dabcyl))2K | C122H173N29O25S2 | 2508.26 | 1255.74b | 15.1 |
| DSL | (Mal-GPGPGML-D-K(Dabcyl))2K | C122H173N29O25S2 | 2508.26 | 1255.67b | 13.4 |
| DPL | (Mal-PEG3-D-K(Dabcyl))2K | C82H113N12O17 | 1894.04 | 948.05a | 21.9 |
mass spectrum peak found for [M+H]+;
mass spectrum peak found for [M+2H]2+.
3.1.4 Synthesis of the multi-block HPMA polymers (MPs and FMPs)
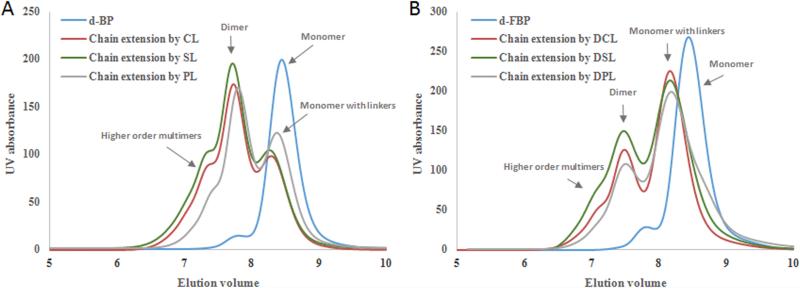
The BP and FBP were successively aminolysized, reduced and deprotected to give d-BP and d-FBP, respectively. Utilizing 1H-NMR, complete deprotection of DOTA was confirmed by disappearance of the t-butyl resonance peaks in both d-BP and d-FBP spectrums (Fig. S5). The chain extension reaction between the deprotected telechelic HPMA copolymers (i.e., d-BP and d-FBP) and the peptidic/PEG linkers was carried out in PBS buffer (pH = 6.8) at 40 °C for 48 h. For d-BP, chain extension with the CL, SL and PL gave the multi-block HPMA copolymers MP-c, MP-s and MP-p, correspondingly. Similarly, extension of the d-FBP with the DCL, DSL and DPL linker groups respectively gave FMP-c, FMP-s and FMP-p. The GPC analysis profiles of the deprotected polymer blocks and the corresponding chain extended copolymers are given in Fig. 2. For both the MPs and FMPs, chain extension reactions resulted in a clear shift to lower elution volumes corresponding to the expected increase in molecular weight. However, under the experimental conditions utilized, the chain extension reaction was significantly more efficient for the MPs compared to the FMPs. We speculate that for the FMPs the increased steric hindrance of the terminal FITC moiety and/or the increased hydrophobic character of the linkers due to inclusion of the Dabcyl were factors that reduced the efficiency of the thiol-ene chain extension. To improve the extension yield, several factors can be considered for future studies, such as reducing steric hindrance of thiol groups, introducing more water soluble maleimide functionalities to the peptide linker and investigating other click conjugation chemistries for copolymer extension [36].
Fig. 2.
GPC profiles of chain extension of HPMA copolymers. (A) GPC profile of deprotected HPMA telechelic block copolymer (d-BP) before and after chain extension by cathepsin S susceptible linker (CL), scramble peptidic linker (SL) and small peg linker (PL); (B) GPC profile of deprotected FITC ended HPMA telechelic block copolymer (d-FBP) before and after chain extension by dabcyl conjugated cathepsin S-susceptible linker (DCL), dabcyl conjugated scrambled peptidic linker (DSL) and Dabcyl conjugated small peg linker (DPL).
Analysis of the molecular weight of the extended copolymers revealed that the majority, with an elution volume of ~ 7.7 mL, were dimers, containing two MP or FMP blocks. However, multi-block HPMA copolymers corresponding to higher order multimers, primarily trimers and tetramers, were observed at elution volumes less than 7.2 min. Fractionation of the MPs and FMPs was performed to collect the bulk of the trimeric and tetrameric constituents. The GPC profiles and characterization data of the resulting multi-block HPMA copolymers are correspondingly given in Fig. 3 and Table 2. Isolated yields of the MPs and FMPs ranged from 13.2 - 17.1 % and 6.1 - 9.6 %, respectively. The molecular weights of both the MP and FMP ranged from 76.1 – 80.3 kDa with calculated polydispersities less than 1.37. The average hydrodynamic radius of these particles was 6.72 ± 0.36 nm.
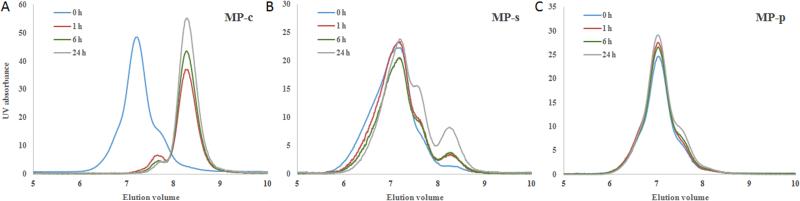
Fig. 3.
GPC profile of multi-block HPMA copolymer fraction and degradation products after incubation with cathepsin S at different time intervals. (A) MP-c, (B) MP-s and (C) MP-p.
3.2 Cleavage studies of MPs
The susceptibility of the MPs to cathepsin S cleavage was examined by incubation of the multi-block copolymers with the protease in an aqueous buffer at 37 °C for 0, 1, 6 and 24 h. At the initial time point (i.e. 0 h), all of the MPs gave an elution volume of approximately 7.2 mL. By the 1 h time point, MP-c was cleaved rapidly by Cathepsin S as demonstrated by the nearly complete shift of the peak towards a higher elution volume of 8.4 mL, which corresponded to a molecular weight of 19.7 kDa, similar to that of a single HPMA copolymer block (Fig. 3A). Conversely, the MP-s demonstrated little cleavage at the same time point. Only after 24 h of incubation did the MP-s show modest amounts (15.6 %) of cathepsin S cleavage (Fig. 3B). Over the course of the time points investigated, MP-p, as expected for the non-cleavable control, demonstrated no signs of size reduction due to cathepsin S degradation (Fig. 3C). These results demonstrate that MP-c, relative to the controls, is substantially more susceptible to cathepsin S cleavage.
When comparing the above studies to our first generation of side chain cleavable HPMA copolymers [23], we found that rate of cleavage for the MP-c to be much faster. Previous studies found that the side chain cleavable HPMA copolymers were only able to achieve a cleavage rate of 25.9% after a 12 h incubation, utilizing a cathepsin S concentration that was 8 fold higher than the above studies. While the side chain cleavable HPMA copolymers were larger (110 kDa) than the constructs used for these studies, the striking difference in cathepsin S degradation is interesting. We hypothesize two reasons for this observation. First, it seems likely that for MP-c the inclusion of the cathepsin S-susceptible linker into the backbone of the HPMA copolymer is significantly less sterically cumbersome compared with side chain functionalization. Second, cleavage of the cathepsin S-labile backbone of the MP-c generates small fragments with significantly less steric hindrance leading to faster and more complete degradation of the multi-block copolymer.
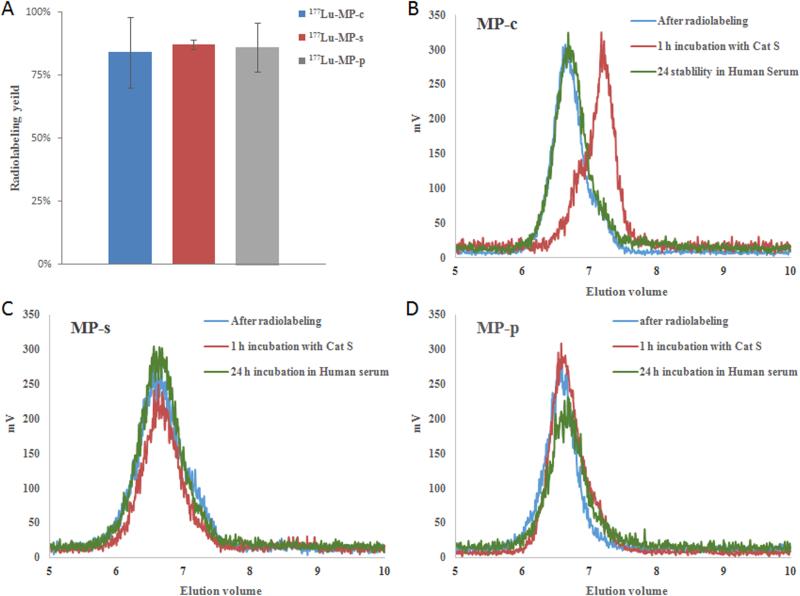
3.3 Radiolabeling and stability study of MPs
The MPs were radiolabeled with 177LuCl3 at 80 °C for 45 min and subsequently purified by radio-GPC-HPLC. High isolated, radiochemical yields were achieved for all MPs, Fig. 4A, demonstrating that sufficient DOTA moieties were attached to the polymer backbone to achieve good specific activities of about 4 µCi/µg (0.148 MBq/µg) of copolymer. As with our non-labeled analogs, exposure of the 177Lu-labeled MP-c to cathepsin S for 1 h yielded rapid cleavage and shifting of the radiolabeled peak to lower molecular weight (Fig. 4B), whereas 177Lu-labeled MP-s and MP-p demonstrated no detectable signs of degradation over the same time period, Fig. 4C and 4D. Incubation of the radiolabeled MPs in serum was utilized to estimate in vivo blood stability. After 24 h of incubation in serum, all of the MPs gave identical radiotraces as their corresponding starting materials indicating that the multi-block HPMA copolymers remained intact.
Fig. 4.
(A) Radiolabeling efficiency of MPs by incubating with 1 mCi of 177LuCl3 at 90 oC for 45 min. (B), (C) and (D) Radio-GPC profiles of 177Lu-MP-c, 177Lu-MP-s and 177Lu-MP-p, correspondingly, before and after incubation with cathepsin S for 1 h and exposure to human plasma for 24 h.
3.4 Evaluation of the FRET effect of FMPs
In order to better understand the intracellular behavior of the multi-block HPMA copolymer, we designed and synthesized the FMPs as FRET probes to monitor proteolytic cleavage in cells. The FRET effect, a non-radiative energy transfer process between an excited donor and a proximal ground state acceptor, has recently been used in pharmaceutical science for a variety of detection purposes [37-39]. In our design, FITC was incorporated at the terminal ends of the HPMA copolymers while Dabcyl, the acceptor, was integrated into the peptidic/PEG linkers. The efficiency of energy transfer for each FRET pair is highly dependent on factors such as transition dipoles, spectral overlap, and most importantly, the distance between the donor and acceptor which typically has to be less than 10 nm (i.e., 1.8 × Förster distance ) [40]. Dabcyl was chosen as the acceptor due to its maximum absorption at 472 nm, which mostly overlaps the emission spectrum of FITC. The linear distances between FITC and Dabcyl in FMP-c/FMP-s and FMP-p were estimated to be 2.3 and 1.6 nm, respectively, which should adequately allow efficient FRET to take place.
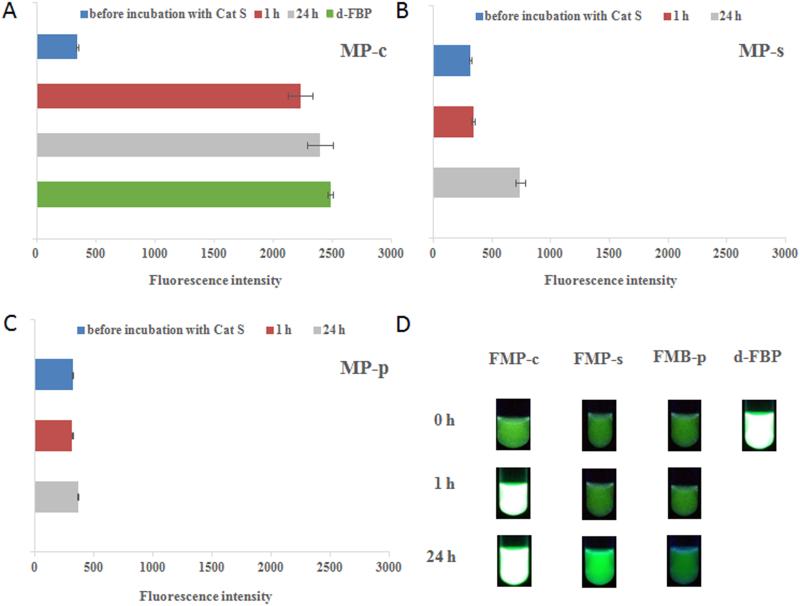
The fluorescence intensities of the FMPs, at a concentration of 100 μg/mL, are depicted in Fig. 6. The fluorescence intensity of the single block HPMA copolymer (d-FMP, green, positive control) before extension is depicted in Fig. 5A. For all of the FMPs, extension with Dabcyl containing linkers substantially reduced the average fluorescence intensities by 86.1 %, consistent with efficient FRET. For the FMP-c, incubation with cathepsin S for 1 h restores 88.2 % of the quenched fluorescence intensity, denoting the rapid and efficient separation of the donor/acceptor pair due to cleavage. Analogous to the cathepsin S cleavage of the MPs, significant amounts of cleavage (19.6 % restoration in fluorescence) for the FMP-s was not observed until the 24 h time point (Fig. 5B), while no cleavage of FMP-p was observed over all time points investigated (Fig. 5C). Visual inspection of the recovery of fluorescence due to the degradation of the FRET effect by cathepsin S cleavage is depicted in Fig. 5D.
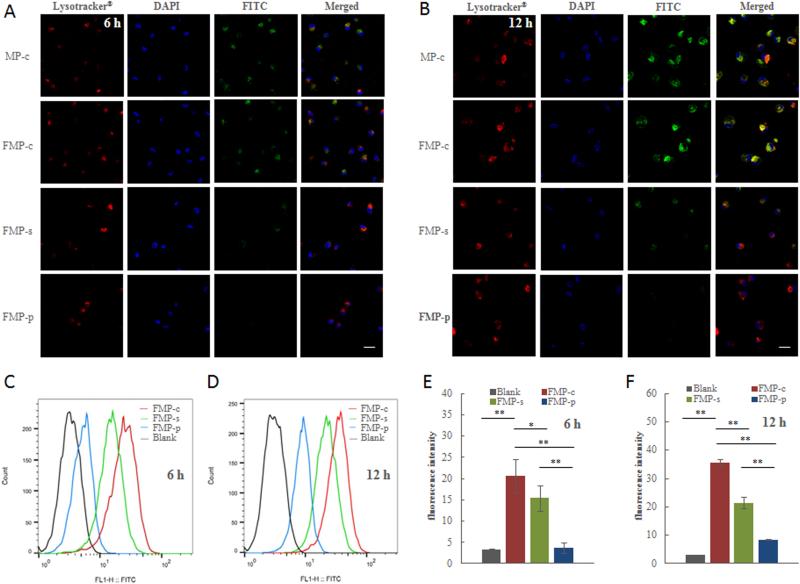
Fig. 6.
Visualization of FITC fluorescence from FMPs in cathepsin S abundant human monocyte-differentiated macrophages by FRET effect. (A) and (B) Representative confocal microscopy images of macrophages incubated with FMP-c, FMP-s, FMP-p and positive control (MP-c) at 6h and 12h, respectively. Lysotracker (red) visualized endolysomal compartments, DAPI (blue) denotes the nucleus and FITC (green) is associated with the copolymer. Scale bar = 20 μm. (C) and (D) Flow cytometry results showing the fluorescence regeneration at 519 nm of FMPs upon intracellular cleavage at 6 h and 12 h. (E) and (F) The median value of fluorescence intensity in macrophages at 6 h and 12 h quantified from flow cytometry. Data are presented as mean ± SD (n = 3). * p < 0.05, ** p < 0.01.
Fig. 5.
Evaluation of FRET effect in FMPs upon cathepsin S cleavage. (A) The fluorescence intensity (519 nm) of d-FBP and FMP-c in the presence of cathepsin S at different incubation times. (B) and (C) The fluorescence intensity (519 nm) of FMP-s and FMP-p upon incubation of cathepsin S at different time intervals, respectively. (D) Fluorescence pictures of FMP-c, FMP-s, FMP-p and d-FBP in cleavage mixture under 365 nm UV light at different time intervals.
3.5 Cell imaging,flow cytometry and cell efflux studies
The cellular constituents of the MPS consists of phagocytic cells, primarily monocytes and macrophages [41]. It has been well established that these phagocytic cells are major contributors to the in vivo sequestration of nanomedicine platforms [42]. In addition, due to their role in antigen presentation, monocytes and macrophages have been shown to express high intracellular levels of cathepsin S [43]. These intersecting findings gave us the basis for the design of cathepsin S-cleavable nanomedicine platforms to circumvent the retention of these agents in MPS-associated tissues. Specifically, in our case, we are seeking to utilize this approach to effectively clear radiolabeled diagnostic and/or therapeutic agents from non-target tissues, particularly the blood, liver and spleen, to improve the clinical potential of these agents.
Using macrophages differentiated from human monocytes as our model of MPS cells, we investigated the uptake of the FMPs and the rate of intracellular cleavage using confocal microscopy and flow cytometry. The differentiation of the monocytes to macrophage was confirmed by flow cytometry studies (Fig. S6). Down-regulation of CD14 and up-regulation of 25F9 were observed after 7 days of incubation with rhM-CSF. These result are in agreement with literature reports regarding the successful differentiation of human monocytes to macrophages [44-46]. For consistency, all FMPs were chelated with non-radioactive lutetium and diluted to equivalent FITC concentrations of approximately 6 µM. Confocal microscopy images of the macrophages incubated with the FMPs for 6 and 12 h are depicted in Fig. 6A and 6B, respectively. In addition to the FITC-containing copolymers, Lysotracker® Red and DAPI were correspondingly utilized to identify the endolysosomal compartments and the nucleus. MP-c, a non-FRET, FITC-containing copolymer with similar structural characteristics to the FMPs, was utilized as a positive control to monitor internalization. At the 6 h time point, MP-c demonstrated significant amounts of internalization. Likewise, macrophages incubated with FMP-c demonstrated similar levels of FITC-associated fluorescence. Substantially weaker FITC-associated fluorescence was recorded for FMP-s while, as expected, no detectable fluorescence of FMP-p was observed. The FITC-associated florescence intensity in FMP-c, FMP-s and the MP-c control significantly increased after 12 h of incubation. However, the same trend continued, with FMP-c demonstrating substantially more FITC-associated activity compared to FMP-s and FMP-p. The co-localization of the FITC and Lysotracker® Red signals confirmed the presence of the copolymers in the endolysomal compartments. This is significant due to the presence of high concentrations of cathepsin S in these subcellular structures [47]. By accumulation of copolymers in the endolysomal compartments, FMPs were continually exposed to the highest subcellular concentrations of cathepsin S which should mediate the cleavage of the cathepsin S-cleavable peptidic linker [48].
The recovery of FITC-associated florescence for the FMPs in macrophages was quantified by flow cytometry at 6 h and 12 h time points, Fig. 6C and 6D, respectively. The time-dependent fluorescence intensity growth in FMP-c and FMP-s groups from 6 to 12 h was due to the increasing concentration of copolymers uptake by the cells as well as the continuous proteolytic cleavage and detachment of the quencher from FITC. The growth in fluorescence intensity for FMP-p is expected to be strictly due to the increased uptake of the multi-block copolymer over time. The corresponding median fluorescence intensities were plotted in Fig. 6E and 6F. The median fluorescence intensity for FMP-c at 6 h was 25.1, which was significantly higher (p < 0.05) than that of FMP-s and FMP-p. Moreover, the disparity of the florescence intensity between FMP-c and the other groups was found to increase at the 12 h time point. These results are in general agreement with the above confocal microscopy studies. In cells, it appears that the FMP-s is cleaved faster than our cathepsin S cleavage studies demonstrated. This is to be expected given the variety of other proteases present in the endolysomal compartments [49]. Despite this, FMP-c demonstrated a clear superiority in the rate in which these multi-block copolymers are cleaved in macrophages. Overall, the results suggested that phagocytic cells, such as macrophages, are capable of rapidly digesting FMP-c upon internalization and thereby mediate the formation of low-molecular weight polymeric blocks, which are anticipated to be more efficiently cleared from non-target tissues. Unlike the efficient uptake in macrophages, relatively little uptake of the MPs was observed in HPAC cells even after 24 h incubation (Fig. S7). This is consistent with our previous findings concerning radiolabeled HPMA copolymers [22, 23].
Efflux studies of the 177Lu-MPs in macrophages revealed the clearance behavior of the copolymers at the cellular level (Fig. S8). At 2 and 4 h time points, the efflux percentages of the copolymers were statistically identical. Although, by the 8 h time point, a higher efflux percentage of the 177Lu-MP-c was observed (74.1 ± 3.50 %) with respect to the controls (68.4 ± 3.99 % for 177Lu-MP-s and 65.8 ± 4.95 % for 177Lu-MP-p). The efflux mechanism of the 177Lu-MPs in macrophage is still unclear. Relative to uptake studies, the literature is relatively sparse in terms of examining the efflux mechanisms of polymeric conjugates. However, the exocytosis of the 177Lu-MPs likely are cleared through the same mechanisms known to efflux nanoparticles systems (e.g., lysosome secretion) [50].
3.6 Biodistribution of 177Lu-MPs
To evaluate the in vivo performance of the 177Lu-MPs, biodistribution of the multi-block copolymers were investigated at 4, 24, 72 and 144 h post-administration in a HPAC xenograft SCID mouse model. The percentage injected dose per gram (%ID/g) in each organ and the total excretion (%ID) of the radioactivity from the mice are presented in Table 3. At all time points investigated, 177Lu-MP-c demonstrated significantly higher overall clearance (p < 0.05) than the other two multi-block copolymers. By the 144 h time point, 77.50 ± 2.94 %ID had cleared for 177Lu-MP-c, while only 68.62 ± 1.58 and 64.36 ± 1.95 had correspondingly cleared for 177Lu-MP-s and 177Lu-MP-p. Given the serum stability of the 177Lu-MPs, this suggests to us that the inclusion of a cathepsin S-cleavable peptide into the backbone of the copolymer was more readily cleaved and cleared, most likely by phagocytic cells, relative to the scrambled and non-cleavable controls. One tissue that seems to be very active in clearance of the 177Lu-MP-c is the blood. By 24 h post-administration, 177Lu-MP-c had achieved a statistically better clearance of 9.05 ± 0.76 %ID/g compared to 14.63 ± 3.37 and 14.93 ± 2.58 for 177Lu-MP-s and 177Lu-MP-p, correspondingly. The divergence in blood clearance substantially increased overtime with 177Lu-MP-c having 0.55 ± 0.10 %ID/g which was 2.8 and 5.3 fold lower than 177Lu-MP-s and 177Lu-MP-p at 144 h p.i.. It is known that monocytes interact with and clear nanomedicine platforms from the blood [51]. However, in this particular case, it is unknown to what extent these phagocytes endocytosed and processed 177Lu-MP-c.
Table 3.
Biodistribution data of the 177Lu-MPs in a HPAC tumor bearing mouse model.
| Tissue (%ID/g) |
4 h | 24 h | 72 h | 144 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MP-c | MP-s | MP-p | MP -c | MP -s | MP -p | MP-c | MP -s | MP-p | MP-c | MP-s | MP-p | |
| Blood | 24.94 ± 4.24 |
36.24 ± 12.13 |
30.99 ± 6.47 |
9.05 ± 0.76 |
14.63 ± 3.37 |
14.93 ± 2.58 |
1.55 ± 0.34 |
3.00 ± 0.36 |
3.51 ± 1.10 |
0.55 ± 0.10 |
1.56 ± 0.16 |
2.93 ± 0.62 |
| Heart | 7.45 ± 2.11 |
10.22 ± 3.60 |
6.42 ± 1.95 |
3.82 ± 0.49 |
5.98 ± 1.39 |
5.75 ± 1.30 |
1.76 ± 0.45 |
2.30 ± 0.90 |
2.34 ± 0.20 |
1.21 ± 0.16 |
1.48 ± 0.36 |
2.11 ± 0.78 |
| Lung | 8.94 ± 1.43 |
10.54 ± 1.31 |
7.96 ± 1.25 |
4.20 ± 0.65 |
5.47 ± 0.82 |
5.89 ± 0.78 |
2.37 ± 1.21 |
2.18 ± 0.56 |
2.38 ± 0.36 |
0.81 ± 0.40 |
2.27 ± 0.42 |
2.40 ± 0.68 |
| Liver | 5.21 ± 0.57 |
6.19 ± 3.07 |
7.17 ± 2.26 |
5.12 ± 0.56 |
6.57 ± 0.24 |
6.45 ± 0.60 |
2.93 ± 0.65 |
5.63 ± 0.81 |
5.94 ± 0.66 |
1.47 ± 0.24 |
2.89 ± 0.29 |
3.86 ± 0.41 |
| Pancreas | 4.61 ± 0.76 |
3.78 ± 1.86 |
5.34 ± 2.29 |
2.74 ± 0.53 |
4.60 ± 2.03 |
4.11 ± 0.32 |
1.24 ± 0.23 |
1.56 ± 0.23 |
1.70 ± 0.24 |
0.62 ± 0.06 |
1.19 ± 0.13 |
1.41 ± 0.34 |
| Stomach | 2.32 ± 1.08 |
2.24 ± 0.47 |
2.12 ± 0.50 |
1.44 ± 0.38 |
1.86 ± 0.79 |
1.70 ± 0.51 |
0.64 ± 0.17 |
0.90 ± 0.19 |
0.88 ± 0.15 |
0.42 ± 0.09 |
0.92 ± 0.17 |
0.94 ± 0.49 |
| Spleen | 22.77 ± 4.73 |
25.01 ± 9.53 |
25.78 ± 10.58 |
11.75 ± 1.11 |
19.64 ± 4.44 |
24.05 ± 6.90 |
7.48 ± 0.84 |
13.83 ± 3.17 |
17.75 ± 2.04 |
4.79 ± 2.16 |
10.40 ± 1.70 |
15.33 ± 2.64 |
| Small int. | 2.00 ± 0.43 |
2.45 ± 0.73 |
2.08 ± 0.41 |
1.22 ± 0.16 |
1.59 ± 0.40 |
1.55 ± 0.29 |
0.57 ± 0.10 |
1.08 ± 0.32 |
0.91 ± 0.16 |
0.40 ± 0.06 |
0.89 ± 0.39 |
0.53 ± 0.22 |
| Large int. | 1.89 ± 0.49 |
3.18 ± 0.55 |
3.01 ± 1.48 |
1.47 ± 0.26 |
2.34 ± 0.57 |
1.82 ± 0.26 |
0.89 ± 0.17 |
0.96 ± 0.15 |
0.90 ± 0.28 |
0.47 ± 0.17 |
0.76 ± 0.19 |
0.90 ± 0.36 |
| Kidney | 5.16 ± 1.01 |
7.79 ± 1.58 |
6.55 ± 2.41 |
2.79± 0.56 |
4.51 ± 1.44 |
5.78 ± 0.47 |
1.70 ± 0.26 |
2.50 ± 0.14 |
3.00 ± 0.18 |
1.29 ± 0.43 |
2.34 ± 0.36 |
2.50 ± 0.62 |
| Muscle | 2.14 ± 0.86 |
2.06 ± 0.40 |
2.09 ± 0.98 |
1.92 ± 1.08 |
2.02 ± 0.93 |
1.82 ± 0.09 |
0.76 ± 0.41 |
0.84 ± 0.15 |
1.31 ± 0.63 |
0.64 ± 0.23 |
0.96 ± 0.15 |
1.11 ± 0.38 |
| Bone | 3.03 ± 0.85 |
3.24 ± 0.49 |
3.75 ± 2.51 |
2.91 ± 1.17 |
3.58 ± 1.34 |
3.05 ± 0.91 |
0.94 ± 0.40 |
1.16 ± 0.33 |
2.22 ± 1.10 |
0.79 ± 0.43 |
1.00 ± 0.26 |
0.99 ± 0.23 |
| Brain | 0.96 ± 0.32 |
1.47 ± 0.41 |
1.31 ± 0.27 |
0.64 ± 0.16 |
0.85 ± 0.14 |
0.78 ± 0.12 |
0.05 ± 0.02 |
0.1 ± 0.05 |
0.29 ± 0.10 |
0.06 ± 0.05 |
0.12 ± 0.02 |
0.09 ± 0.06 |
| Tumor | 5.41 ± 0.94 |
5.82 ± 1.54 |
5.08 ± 2.39 |
5.91 ± 1.11 |
5.31 ± 2.05 |
6.37 ± 1.03 |
5.73 ± 1.32 |
6.13 ± 0.58 |
5.89 ± 1.16 |
4.25 ± 0.70 |
4.75 ± 0.84 |
5.12 ± 1.48 |
|
Excretion
(ID%) |
27.39 ± 3.28 |
18.90 ± 1.72 |
18.89 ± 4.11 |
53.67 ± 6.05 |
39.72 ± 1.15 |
35.99 ± 1.92 |
69.6 ± 2.76 |
59.4 ± 6.64 |
59.21 ± 3.70 |
77.50 ± 2.94 |
68.62 ± 1.58 |
64.36 ± 1.95 |
Data are represented as mean ± SD. (n = 4 - 5).
It has been well established that nanomedicine uptake in the liver, spleen and other MPS-associated tissues (e.g., lung) is due to: 1) uptake by tissue-resident macrophages (e.g., Kupffer cells) and/or 2) a fenestrated vasculature endothelium which allows macromolecules to enter the tissue from the blood stream [52]. Insertion of a cathepsin S-cleavable linker into the multi-block HPMA copolymer was found to substantially reduce the liver and spleen retention over time. For 177Lu-MP-c, the liver retention was 5.12 ± 0.56 %ID/g at 24 h p.i., but had substantially cleared to 1.47 ± 0.24 %ID/g by the 144 h time point. This 7.5 fold reduction is substantially higher than that found for 177Lu-MP-s (2.3 fold) or 177Lu-MP-p (1.7 fold) over the same time period. By the 144 h time point, the liver retention of 177Lu-MP-c was found to be 2.0 and 2.6 fold lower than the scrambled and non-cleavable controls, respectively. Likewise, the spleen uptake for 177Lu-MP-c at 4 h was initially 22.77 ± 4.73 %ID/g, but by 144 h was reduced 4.8 fold to 4.79 ± 2.16 %ID/g. Over the same time span, this longitudinal reduction was significantly lower than either 177Lu-MP-s (2.4 fold) or 177Lu-MP-p (1.7 fold). Comparison amongst the 177Lu-MPs found that the spleen retention of 177Lu-MP-c was 2.2 and 3.2 fold lower than 177Lu-MP-s and 177Lu-MP-p, correspondingly, at the 144 h time point. Undoubtedly, the more rapid blood clearance of 177Lu-MP-c contributed to the lower retention observed in the liver, spleen and other non-target tissues, particularly at early time points (i.e., 4 and 24 h). However, at 72 h and 144 h, the blood activities of 177Lu-MP-c had declined to 6.2 % and 2.2 % of the 4 h time point. The liver and spleen retention of 177Lu-MP-c over the same time period was substantially higher (e.g., at 144 h, liver and spleen retention was 28 and 21 % of the 4 h time point). Since the retention of radioactivity in the liver and spleen did not strongly correlate to the rate of decline in blood retention, this implies that the bulk of the measured radioactivity at these later time points is not associated with the blood, but rather with the tissue itself. Therefore, this suggests that at 72 and 144 h, declines in tissue retention for 177Lu-MP-c largely reflects processing of the cathepsin S-cleavable copolymer by the tissue and not merely a matter of reduced blood retention.
Nanomedicine platforms can passively target a variety of tumors through the EPR effect [53]. This mechanism relies on two factors: 1) the irregular architecture of the tumor vasculature which results in large fenestrations between the endothelial cells of the blood vessel, thereby allowing nanomedicines to enter the tumor tissue and 2) the lack of an efficient lymphatic system to clear the particles from the tumor. The 177Lu-MPs demonstrated maximal uptake values at 24 h (177Lu-MP-c and 177Lu-MP-p) and 72 h (177Lu-MP-s); though, no statistical significant differences (p > 0.3) could be found longitudinally for an individual copolymer or between the various 177Lu-MPs at corresponding time points. Interestingly, despite different blood clearance rates, all three copolymers exhibited statistically indistinguishable tumor retention values, again suggesting, particularly at the later time points, that blood associated radioactivity is not the predominate source of radioactivity in these tissues. Additionally, the statistically identical tumor retention of 177Lu-MP-c relative to the scramble and non-cleavable controls strongly implies that cleavage of the cathepsin S-susceptible linker in the tumor is limited and/or clearance of the resulting single block copolymers from the tumor is ineffective. As a result of the substantial tumor retention and non-target clearance, 177Lu-MP-c demonstrated significantly higher (p < 0.05) T/NT ratios, given in Table 4, in the blood, liver and spleen at 72 p.i.. The maximum T/NT values of the copolymers were achieved at 144 h p.i. when 177Lu-MP-c provided significantly higher contrast in all selected organs (p < 0.05) relative to the other copolymers. The mean T/NT ratios for 177Lu-MP-c were also found to be higher than that of copolymer 177Lu-1 in our first generation copolymers at 72 h (blood (p < 0.02), liver (p < 0.07) and kidney (p < 0.03)) and 144 h (blood (p < 0.05), liver (p < 0.02) [23]. While non-target clearance of the MP-c and our first generation cleavable copolymers were similar, significantly higher retention in the tumors were observed for the MP-c resulting in higher T/NT ratios.
Table 4.
The radioactivity ratio between tumor and non-target organ of 177Lu-MPs in an HPAC tumor bearing mouse model.
| T/NT ratio | 4 h | 24 h | 72 h | 144 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MP-c | MP-s | MP-p | MP -c | MP -s | MP -p | MP-c | MP -s | MP-p | MP-c | MP-s | MP-p | |
| Blood | 0.23 ± 0.05 |
0.15 ± 0.02 |
0.17 ± 0.06 |
0.59 ± 0.13 |
0.36 ± 0.08 |
0.44 ± 0.12 |
3.91 ± 1.37 |
2.06 ± 0.34 |
1.86 ± 0.85 |
7.83 ± 1.03 |
3.06 ± 0.37 |
1.75 ± 0.50 |
| Lung | 0.61 ± 0.05 |
0.54 ± 0.10 |
0.69 ± 0.28 |
1.31 ± 0.36 |
0.97 ± 0.25 |
1.09 ± 0.20 |
3.10 ± 2.04 |
2.96 ± 0.87 |
2.55 ± 0.77 |
6.15 ± 2.83 |
2.16 ± 0.56 |
2.11 ± 0.30 |
| Liver | 1.05 ± 0.17 |
0.80 ± 0.26 |
0.76 ± 0.16 |
1.06 ± 0.28 |
0.82 ± 0.28 |
0.98 ± 0.08 |
2.09 ± 0.77 |
1.11 ± 0.20 |
1.00 ± 0.22 |
2.90 ± 0.14 |
1.65 ± 0.22 |
1.28 ± 0.25 |
| Pancreas | 1.20 ± 0.23 |
2.06 ± 0.93 |
1.04 ± 0.11 |
2.06 ± 0.73 |
1.35 ± 0.68 |
1.56 ± 0.31 |
4.82 ± 1.24 |
4.00 ± 0.85 |
3.56 ± 1.09 |
6.92 ± 0.77 |
4.09 ± 0.28 |
3.70 ± 1.30 |
| Spleen | 0.24 ± 0.04 |
0.25 ± 0.13 |
0.22 ± 0.04 |
0.46 ± 0.11 |
0.28 ± 0.11 |
0.29 ± 0.10 |
0.78 ± 0.17 |
0.46 ± 0.12 |
0.34 ± 0.10 |
1.01 ± 0.39 |
0.47 ± 0.10 |
0.32 ± 0.06 |
| Kidney | 1.07 ± 0.19 |
0.76 ± 0.14 |
0.88 ± 0.30 |
2.01 ± 0.72 |
1.26 ± 0.58 |
1.11 ± 0.20 |
3.44 ± 0.68 |
2.45 ± 0.22 |
1.98 ± 0.47 |
3.50 ± 0.84 |
2.07 ± 0.43 |
2.08 ± 0.68 |
Data are represented as mean ± SD (n = 4 - 5).
3.7 SPECT/CT imaging and autoradiography
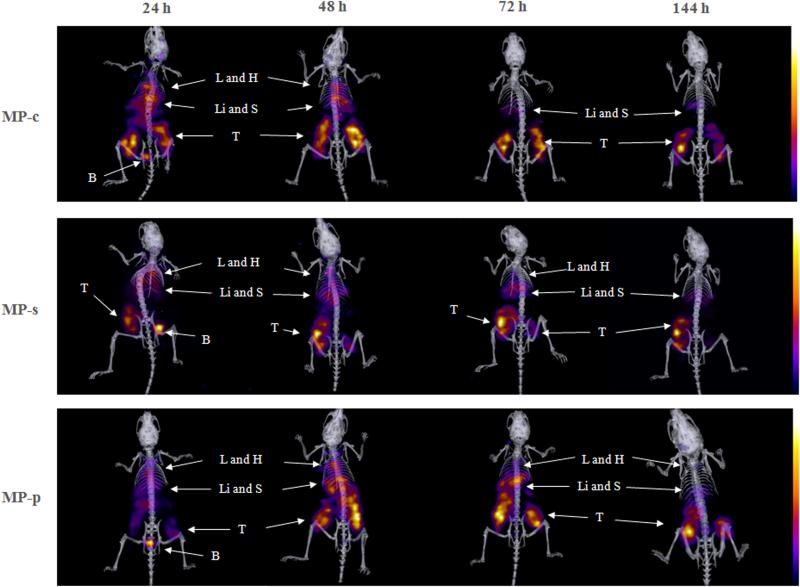
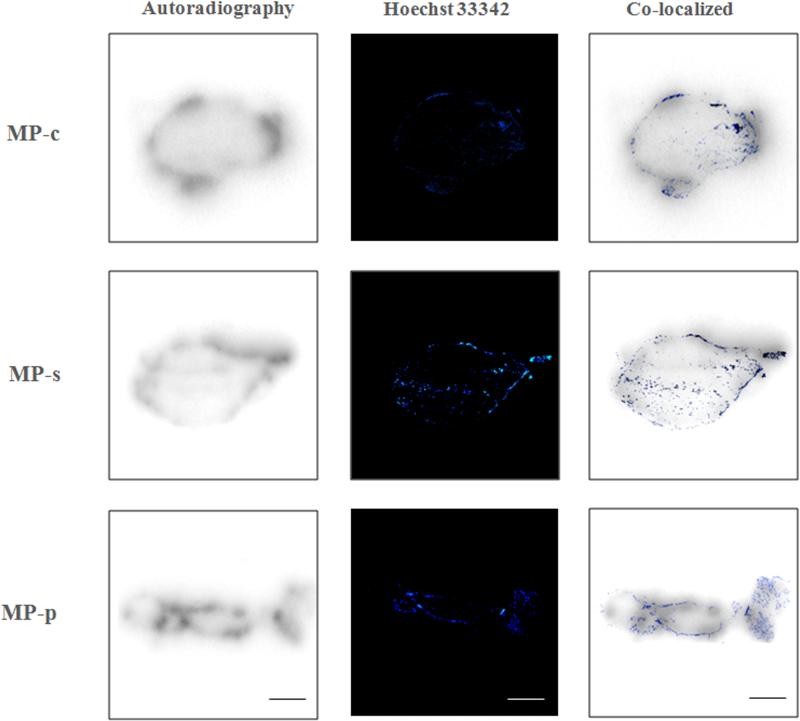
Encouraged by the results from the biodistribution studies, we thereafter tested the in vivo performance of 177Lu labeled copolymers in HPAC xenograft SCID mice using small-animal SPECT/CT imaging. Since no significant differences were observed at the 4 h time point in the biodistribution studies, we performed SPECT/CT acquisitions of the 177Lu-MPs at 24, 48, 72 and 144 h time points, depicted in Fig. 7 and Movie S1. For all 177Lu-MPs, tumors were visualized at 24 h p.i. and became more pronounced at later time points. No doubt, this was the result of improved regional T/NT ratios due to the clearance from non-target tissues, particularly the blood. Autoradiography studies and vessel staining (Hoechst 33342), depicted in Fig. 8, were carried out on the HPAC tumors at the completion of the small-animal SPECT/CT experiments. These studies demonstrated that the bulk of the radioactivity, and typically most of the vasculature, was localized at the perimeter of the tumor. The heterogeneous distribution of the 177Lu-MPs was expected given the limited diffusion range of most nanomedicine platform once it enters the tissue from the vasculature [54]. In the small-animal SPECT/CT imaging studies, pronounced accumulation of the 177Lu-MPs in the thoracic and abdominal regions was observed at 24 h, likely due to retention in the liver, spleen, lungs and heart (blood). For 177Lu-MP-c, the clearance of radioactivity from these non-target tissues outpaced that of the scrambled (177Lu-MP-s) and non-cleavable (177Lu-MP-p) multi-block HPMA copolymers. This leads to 72 h and 144 h images of 177Lu-MP-c with superior tumor contrasting.
Fig. 7.
Representative SPECT/CT images of xenograft tumor bearing SCID mice after being intravenously administrated with 177Lu-MPs at a dose of 600 μCi per mice at 24, 48, 72 and 144 h. Main organs and tumors have been pointed out by arrows (L - liver, H - heart, Li - liver, S - spleen, B – bladder and T – tumor).
Fig. 8.
Autoradiography and the vessel staining by Hoechst 33342 of the tumor. Scale bar = 1 mm.
4. Conclusion
In this study, two groups of backbone cathepsin S-susceptible HPMA copolymers (MPs and FMPs) were synthesized and characterized for in vitro and in vivo evaluation. Utilization of the cathepsin S-susceptible linkers gave multi-block copolymers with rapid in vitro cleavage kinetics for both the MP-c and FMP-c copolymers. This was further confirmed using in vivo biodistribution and SPECT/CT imaging studies in a PDAC mouse model. Relative to controls, 177Lu-MP-c demonstrated substantially higher non-target clearance from MPS-associated tissues without, importantly, affecting the HPAC tumor targeting and retention capabilities of the copolymers. Overall, these findings suggest that exploitation of the cathepsin S activity can be utilized to evade retention in MPS-associated cells thus selectively lowering the non-target retention of macromolecular agents. Given the potential of this approach to improve the clinical efficacy of a wide variety of nanomedicine platforms, we believe that further investigation is warranted.
Supplementary Material
Acknowledgments
We thank Janice A. Taylor and James R. Talaska from the Advanced Microscopy Core Facility at the UNMC for providing assistance with (confocal or super resolution) microscopy; Philip Hexley, Victoria B. Smith and Samantha Wall from the flow cytometry research facility at the UNMC for providing technical support in flow cytometry studies; Li Wu and Na Ly from the Elutriation Core Facility of UNMC for the technical support in cell study; Yuning Zhang from College of Medicine at the UNMC for assistance with SPECT/CT studies. This study was supported by the National Institutes of Health (1R01CA17905901A1) and the National Institute of General Medical Sciences (8 P20 GM10348007). No other potential conflict of interest relevant to this article was reported.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- [2].Biankin AV, Waddell N, Kassahn KS, Gingras M-C, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanada K, Okazaki A, Hirano N, Izumi Y, Teraoka Y, Ikemoto J, et al. Diagnostic strategies for early pancreatic cancer. Journal of gastroenterology. 2015;50:147–54. doi: 10.1007/s00535-014-1026-z. [DOI] [PubMed] [Google Scholar]
- [4].Fokas E, O'Neill E, Gordon-Weeks A, Mukherjee S, McKenna WG, Muschel RJ. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2015;1855:61–82. doi: 10.1016/j.bbcan.2014.12.001. [DOI] [PubMed] [Google Scholar]
- [5].Sahin IH, Iacobuzio-Donahue CA, O'Reilly EM. Molecular signature of pancreatic adenocarcinoma: an insight from genotype to phenotype and challenges for targeted therapy. Expert opinion on therapeutic targets. 2015:1–19. doi: 10.1517/14728222.2016.1094057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- [7].Grimm J, Potthast A, Wunder A, Moore A. Magnetic resonance imaging of the pancreas and pancreatic tumors in a mouse orthotopic model of human cancer. International journal of cancer Journal international du cancer. 2003;106:806–11. doi: 10.1002/ijc.11281. [DOI] [PubMed] [Google Scholar]
- [8].Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Seminars in oncology. 2011;38:55–69. doi: 10.1053/j.seminoncol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim EE. Molecular Anatomic Imaging: PET/CT, PET/MR and SPECT/CT, third edition. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015 [Google Scholar]
- [10].Gurusamy KS, Davidson BR. Diagnostic accuracy of different imaging modalities following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. The Cochrane Library. 2015 doi: 10.1002/14651858.CD011515.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vidal-Jove J, Perich E, del Castillo MA. Ultrasound Guided High Intensity Focused Ultrasound For Malignant Tumors: the Spanish Experience of Survival Advantage in Stage III and IV Pancreatic Cancer. Ultrasonics sonochemistry. 2015 doi: 10.1016/j.ultsonch.2015.05.026. [DOI] [PubMed] [Google Scholar]
- [12].Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Archives of internal medicine. 2009;169:2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buck AK, Nekolla S, Ziegler S, Beer A, Krause BJ, Herrmann K, et al. Spect/Ct. Journal of Nuclear Medicine. 2008;49:1305–19. doi: 10.2967/jnumed.107.050195. [DOI] [PubMed] [Google Scholar]
- [14].Liu Z, Liu H, Ma T, Sun X, Shi J, Jia B, et al. Integrin αvβ6–Targeted SPECT Imaging for Pancreatic Cancer Detection. Journal of Nuclear Medicine. 2014;55:989–94. doi: 10.2967/jnumed.113.132969. [DOI] [PubMed] [Google Scholar]
- [15].Foss CA, Fox JJ, Feldmann G, Maitra A, Iacobuzio-Donohue C, Kern SE, et al. Radiolabeled anti-claudin 4 and anti-prostate stem cell antigen: initial imaging in experimental models of pancreatic cancer. Molecular imaging. 2007;6:131–9. [PubMed] [Google Scholar]
- [16].Bryan RA, Jiang Z, Jandl T, Strauss J, Koba W, Onyedika C, et al. Treatment of experimental pancreatic cancer with 213-Bismuth-labeled chimeric antibody to single-strand DNA. Expert review of anticancer therapy. 2014;14:1243–9. doi: 10.1586/14737140.2014.952285. [DOI] [PubMed] [Google Scholar]
- [17].Olafsen T, Wu AM. Antibody vectors for imaging. Seminars in nuclear medicine: Elsevier; 2010:167–81. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kopeček J, Kopečková P. HPMA copolymers: origins, early developments, present, and future. Advanced drug delivery reviews. 2010;62:122–49. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kopeček J, Kopečková P, Minko T, Lu Z-R. HPMA copolymer–anticancer drug conjugates: design, activity, and mechanism of action. European journal of pharmaceutics and biopharmaceutics. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- [20].Shi GP, Webb AC, Foster KE, Knoll JH, Lemere CA, Munger JS, et al. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem. 1994;269:11530–6. [PubMed] [Google Scholar]
- [21].Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thrombosis and haemostasis. 2011;105:828–36. doi: 10.1160/TH10-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ogbomo SM, Shi W, Wagh NK, Zhou Z, Brusnahan SK, Garrison JC. 177 Lu-labeled HPMA copolymers utilizing cathepsin B and S cleavable linkers: Synthesis, characterization and preliminary in vivo investigation in a pancreatic cancer model. Nuclear medicine and biology. 2013;40:606–17. doi: 10.1016/j.nucmedbio.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shi W, Ogbomo SM, Wagh NK, Zhou Z, Jia Y, Brusnahan SK, et al. The influence of linker length on the properties of cathepsin S cleavable 177 Lu-labeled HPMA copolymers for pancreatic cancer imaging. Biomaterials. 2014;35:5760–70. doi: 10.1016/j.biomaterials.2014.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang R, Luo K, Yang J, Sima M, Sun Y, Janát-Amsbury MM, et al. Synthesis and evaluation of a backbone biodegradable multiblock HPMA copolymer nanocarrier for the systemic delivery of paclitaxel. Journal of Controlled Release. 2013;166:66–74. doi: 10.1016/j.jconrel.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson RN, Chu DS, Shi J, Schellinger JG, Carlson PM, Pun SH. HPMA-oligolysine copolymers for gene delivery: optimization of peptide length and polymer molecular weight. Journal of Controlled Release. 2011;155:303–11. doi: 10.1016/j.jconrel.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Omelyanenko V, Kopečková P, Gentry C, Kopeček J. Targetable HPMA copolymer-adriamycin conjugates. Recognition, internalization, and subcellular fate. Journal of Controlled Release. 1998;53:25–37. doi: 10.1016/s0168-3659(97)00235-6. [DOI] [PubMed] [Google Scholar]
- [27].Jiang Z-X, Feng Y, Yu YB. Fluorinated paramagnetic chelates as potential multi-chromic 19 F tracer agents. Chemical Communications. 2011;47:7233–5. doi: 10.1039/c1cc11150g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pan H, Yang J, Kopečková P, Kopecek J. Backbone degradable multiblock N-(2-hydroxypropyl) methacrylamide copolymer conjugates via reversible addition− fragmentation chain transfer polymerization and thiol− ene coupling reaction. Biomacromolecules. 2010;12:247–52. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zelivyanskaya ML, Nelson JA, Poluektova L, Uberti M, Mellon M, Gendelman HE, et al. Tracking superparamagnetic iron oxide labeled monocytes in brain by high-field magnetic resonance imaging. Journal of neuroscience research. 2003;73:284–95. doi: 10.1002/jnr.10693. [DOI] [PubMed] [Google Scholar]
- [30].Peng ZH, Kopecek J. HPMA Copolymer CXCR4 Antagonist Conjugates Substantially Inhibited the Migration of Prostate Cancer Cells. ACS macro letters. 2014;3:1240–3. doi: 10.1021/mz5006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kopecek J. Polymer-drug conjugates: origins, progress to date and future directions. Advanced drug delivery reviews. 2013;65:49–59. doi: 10.1016/j.addr.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shi J, Schellinger JG, Pun SH. Engineering biodegradable and multifunctional peptide-based polymers for gene delivery. Journal of biological engineering. 2013;7:25. doi: 10.1186/1754-1611-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shi G-P, Webb AC, Foster KE, Knoll J, Lemere CA, Munger JS, et al. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. Journal of Biological Chemistry. 1994;269:11530–6. [PubMed] [Google Scholar]
- [34].Quillard T, Croce K, Jaffer FA, Weissleder R, Libby P. Molecular imaging of macrophage protease activity in cardiovascular inflammation in vivo. Thrombosis and haemostasis. 2011;105:828. doi: 10.1160/TH10-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu H, Wang H, Shi Z, Wang H, Yang C, Silke S, et al. TaqMan probe array for quantitative detection of DNA targets. Nucleic acids research. 2006;34:e4. e. [Google Scholar]
- [36].Nair DP, Podgórski M, Chatani S, Gong T, Xi W, Fenoli CR, et al. The thiol-Michael addition click reaction: a powerful and widely used tool in materials chemistry. Chemistry of Materials. 2013;26:724–44. [Google Scholar]
- [37].Bae B-c, Na K. Self-quenching polysaccharide-based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials. 2010;31:6325–35. doi: 10.1016/j.biomaterials.2010.04.030. [DOI] [PubMed] [Google Scholar]
- [38].Fan W, Wang Y, Dai X, Shi L, Mckinley D, Tan C. Reduction-responsive Crosslinked Micellar Nanoassemblies for Tumor-targeted Drug Delivery. Pharmaceutical research. 2015;32:1325–40. doi: 10.1007/s11095-014-1537-6. [DOI] [PubMed] [Google Scholar]
- [39].Kruger HR, Schutz I, Justies A, Licha K, Welker P, Haucke V, et al. Imaging of doxorubicin release from theranostic macromolecular prodrugs via fluorescence resonance energy transfer. Journal of controlled release : official journal of the Controlled Release Society. 2014;194:189–96. doi: 10.1016/j.jconrel.2014.08.018. [DOI] [PubMed] [Google Scholar]
- [40].Day RN, Davidson MW. Fluorescent proteins for FRET microscopy: monitoring protein interactions in living cells. Bioessays. 2012;34:341–50. doi: 10.1002/bies.201100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nature Reviews Immunology. 2011;11:788–98. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- [42].Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. The FASEB Journal. 2005;19:311–30. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- [43].Petanceska S, Canoll P, Devi LA. Expression of rat cathepsin S in phagocytic cells. Journal of Biological Chemistry. 1996;271:4403–9. doi: 10.1074/jbc.271.8.4403. [DOI] [PubMed] [Google Scholar]
- [44].Gantner F, Kupferschmidt R, Schudt C, Wendel A, Hatzelmann A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-α release by PDE inhibitors. British journal of pharmacology. 1997;121:221–31. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PloS one. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. The American journal of pathology. 2008;172:1112–26. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, et al. Analysis of tumour-and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nature cell biology. 2014 doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rudensky A, Beers C. Cytokines as Potential Therapeutic Targets for Inflammatory Skin Diseases. Springer; 2006. Lysosomal cysteine proteases and antigen presentation; pp. 81–95. [DOI] [PubMed] [Google Scholar]
- [49].Bird PI, Trapani JA, Villadangos JA. Endolysosomal proteases and their inhibitors in immunity. Nature Reviews Immunology. 2009;9:871–82. doi: 10.1038/nri2671. [DOI] [PubMed] [Google Scholar]
- [50].Oh N, Park J-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. International journal of nanomedicine. 2014;9:51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ilinskaya AN, Dobrovolskaia MA. Nanoparticles and the blood coagulation system. Part II: safety concerns. Nanomedicine. 2013;8:969–81. doi: 10.2217/nnm.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van Hinsbergh VW. Endothelial permeability for macromolecules Mechanistic aspects of pathophysiological modulation. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:1018–23. doi: 10.1161/01.atv.17.6.1018. [DOI] [PubMed] [Google Scholar]
- [53].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of controlled release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- [54].Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature biotechnology. 2015;33:941–51. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.