Abstract
BACKGROUND
Pregnancy-induced hypertension (PIH) causes increased risk of maternal, fetal, and neonatal morbidity and mortality. Identification of risk factors for PIH in early life is central to the development of prevention strategies.
METHODS
A cohort of 703 women aged 25.5–51.3 years from the Bogalusa Heart Study were included. PIH were defined as self-reported hypertension during pregnancy and a blood pressure level <140/90mm Hg without antihypertensive medication (n = 131) at the subsequent examinations. Body mass index (BMI), systolic and diastolic blood pressure, high- and low-density lipoprotein cholesterol, and triglycerides measured during childhood (4–17 years) were considered. General linear models were used to examine differences in childhood between those who did and those who did not develop PIH. Logistic regression models were used to estimate odds ratios for PIH associated with childhood risk factors.
RESULTS
Compared to women who did not develop PIH, those who developed PIH had higher BMI (20.2 vs. 19.2kg/m2, P = 0.0002) and systolic blood pressure (104.1 vs. 103.3mm Hg, P = 0.008) in childhood. After adjustment for other variables, childhood BMI was the only risk factor associated with PIH, with each standard deviation increase in childhood BMI being associated with an odds ratio of 1.35 (95% confidence interval: 1.08–1.68) for PIH. The odds of PIH increased significantly as childhood BMI increased from the bottom quartile to the top quartile (P for trend = 0.006).
CONCLUSIONS
Elevated childhood BMI is a significant risk factor for PIH in adulthood, which underscores the importance of body weight control in childhood for prevention of PIH.
INTRODUCTION
Pregnancy-induced hypertension (PIH) refers to a condition in which high blood pressure (≥140/90mm Hg) develops during pregnancy but blood pressure returns to the nonhypertensive range following delivery.1,2 PIH affects 3–10% of all pregnancies, and leads to increased risk of maternal, fetal, and neonatal morbidity and mortality.2,3 Women with PIH are at increased risk for developing a wide range of chronic conditions, including cardiovascular disease, later in life.4–7 Identification of risk factors for PIH is an essential first step in efforts to reduce the burden of illness due to PIH.
Previous studies have shown that adult cardiovascular risk factors, such as obesity, elevated blood pressure, insulin resistance, and triglyceridemia, before pregnancy are associated with the risk of PIH.2,3,8–11 However, it is not known whether measurements during childhood can predict the risk of subsequent PIH. Such information would be very helpful in understanding the pathogenesis of PIH and efforts to prevent its occurrence.
We used data from the Bogalusa Heart Study (BHS), a long-term, community-based study of natural history of atherosclerosis beginning in childhood,12 to examine childhood risk factors for adult PIH.
METHODS
Study population
The BHS, established in 1973 by Dr Gerald Berenson, is based on a series of long-term studies in a semi-rural community (65% white and 35% black) in Bogalusa, LA to explore the natural history of cardiovascular disease from childhood.12 Between 1973 and 2010, 9 BHS cross-sectional surveys were conducted in children and adolescents aged 4–17 years, and 10 BHS surveys were conducted in adults aged 18–51 years who had participated in earlier BHS surveys as children. We identified 703 women who had been examined at least once during their childhood (aged 4–17 years), had PIH information based on their response to a standardized questionnaire, and were nonhypertensive (blood pressure < 140/90mm Hg and not taking any anti-hypertensive medications) during their subsequent BHS examinations as adults (aged 25.5–51.3 years). The average follow-up time between their first and last BHS examinations was 28.3 (range: 16.2–36.6) years.
All of the adults in this study provided written informed consent at each examination, and consent of a parent/guardian was obtained for those under 20 years of age. Study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center, New Orleans, LA.
Examinations
All of the BHS surveys followed an almost identical protocol for measurement of risk factors for both children (4–17 years old) and adults (>18 years old).12 Participants were instructed to fast for 12 hours prior to each visit. Height and weight were measured twice to within 0.1cm and within 0.1kg, respectively, and the mean values of these measurements were used to estimate body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters). Replicate sitting blood pressure measurements were obtained on the right arm of each participant using the correct size cuff following at least 5 minutes of quiet rest. Systolic and diastolic blood pressure levels were recorded as the first, fourth (in children), and fifth (in adults) Korotkoff phases using a mercury sphygmomanometer. Blood pressure levels were reported as the mean of six replicate readings taken by two randomly assigned trained staff members.
Definition of PIH
PIH, the outcome measure, was defined as self-reported hypertension during pregnancy, based on a “Yes” response to the question “Did you have hypertension in any of your previous pregnancies?” in a standardized questionnaire, and an average blood pressure <140/90mm Hg without treatment for hypertension at the participant’s last available BHS examination (after pregnancy). The requirement of returning to a nonhypertensive level of blood pressure after pregnancy was designed to avoid inclusion of participants with hypertension prior to pregnancy. Those whose blood pressure returned to the nonhypertensive blood pressure range after pregnancy would have been unlikely to be hypertensive before pregnancy. We recognize that the requirement might have led to exclusion of women with PIH who later developed chronic hypertension as PIH women have a much increased risk of developing chronic hypertension.13 In total, 131 (18.6%) of the 703 women studied met the criteria for PIH. For participants who had been examined more than once, we used data in the last survey; the overall agreement for the responses at different occasions was 95.2%.
Laboratory analysis
Between 1973 and 1986 serum cholesterol and triglyceride levels were measured using a Technicon AutoAnalyzer II (Technicon Instrument Corp, Tarrytown, NY) according to the Laboratory Manual of the Lipid Research Clinics Program.14 Between 1986 and 1996 an Abbott VP instrument (Abbott Laboratories, North Chicago, IL) that employed an enzymatic procedure was used to measure these variables.15,16 From 1996 to the present the measurements have been made using a Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN). Serum high-density lipoprotein cholesterol levels were determined using a combination of heparin–calcium precipitation and agar–agarose gel electrophoresis procedure.17 Both the chemical and enzymatic measurements met the performance requirements of the Lipid Standardization Program of the Centers for Disease Control and Prevention (CDC).
Statistical methods
Risk factor measurements during childhood, including BMI, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and systolic and diastolic blood pressure (SBP and DBP), were used as exposures, and PIH as the outcome. For participants with multiple examinations during childhood, we used the average value of their exposure measurements during childhood to increase statistical power. To compare differences in childhood variables between women with and without PIH, general linear models were used with adjustment for race and (average) childhood age. We used general linear models to examine the differences in risk factor variables after pregnancy, adjusting for race and age. We used logistic regression models to examine the association between PIH and childhood risk factor variables that had been standardized to age- and race-specific z-scores and adjusted for race. We also calculated BMI z-scores during childhood and overweight was defined as BMI z-score above the 85th percentile according to CDC growth charts (http://www.cdc.gov/growthcharts/). We performed sensitivity analysis to examine the associations of PIH with risk factor variables measured before (N = 490) or after (N = 665) the age of 12 years. We also examined the interactions between childhood risk factors and race on PIH risk in logistic regression models. All data analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Mean age (±SD, standard deviation) at the participant’s last examination was 38.0±4.9 years, and the average age during childhood was 12.5±2.2 years. In the entire cohort (n = 703), black participants tended to have a higher blood pressure as adults and a better lipid profile in childhood compared to their white counterparts (Table 1).
Table 1.
Characteristics of the cohort (n = 703) by race
| Variable | Whites (n = 481) | Blacks (n = 222) | Pa |
|---|---|---|---|
| Childhoodb | |||
| Age (years) | 12.6±2.3 | 12.5±2.0 | 0.74 |
| BMI (kg/m2) | 19.1±3.3 | 20.1±4.1 | <0.001 |
| Systolic BP (mm Hg) | 103.5±7.7 | 103.5±7.5 | 0.86 |
| Diastolic BP (mm Hg) | 65.2±6.6 | 64.6±6.3 | 0.27 |
| HDL cholesterol (mg/dl) | 59.3±15.2 | 63.5±13.4 | <0.001 |
| LDL cholesterol (mg/dl) | 90.7±21.7 | 93.2±22.5 | 0.16 |
| Triglycerides (mg/dl) | 76.6±28.2 | 62.5±17.1 | <0.001 |
| Adulthoodc | |||
| Age (years) | 38.4±4.7 | 37.0±5.2 | <0.001 |
| BMI (kg/m2) | 28.5±7.1 | 31.8±8.6 | <0.001 |
| Systolic BP (mm Hg) | 110.2±11.0 | 118.4±15.8 | <0.001 |
| Diastolic BP (mm Hg) | 69.8±8.6 | 73.4±10.6 | <0.001 |
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
aP values were adjusted for age and race where appropriate.
bMean values of multiple measurements during childhood are presented.
cMeasured after pregnancy when the questionnaire was administered.
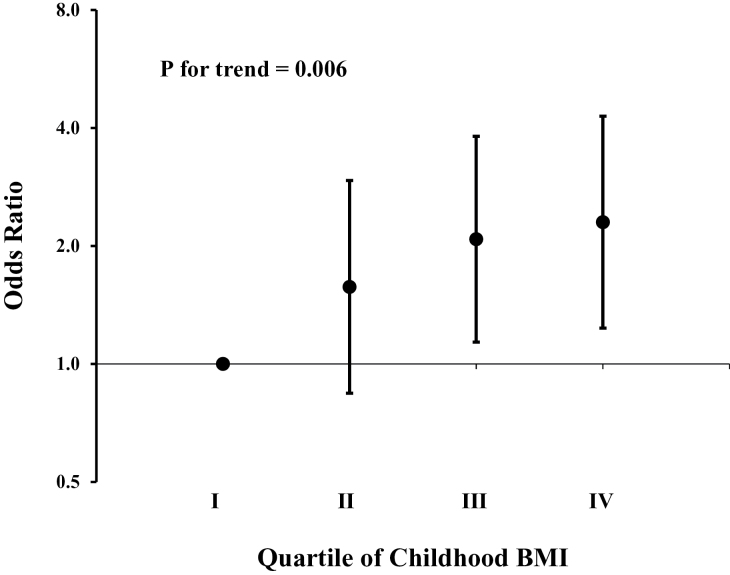
Following adjustment for childhood age and race, BMI (P = 0.0002) and SBP (P = 0.008) were higher during childhood in those who developed compared to their counterparts who did not develop PIH (Table 2), but there were no differences between the other variables measured in childhood. After further adjustment for childhood BMI, childhood SBP was no longer significantly associated with PIH (P = 0.12). In logistic regression analysis, the odds ratio of one BMI standard deviation increase in childhood for PIH was 1.35 (95% confidence interval: 1.08–1.68). When the lowest quartile of BMI during childhood was employed as a reference, having a BMI in the second, third, and highest quartile during childhood was associated with an odds ratio of 1.57, 2.08, and 2.30 for PIH, respectively (P for trend = 0.006) (Figure 1). When age- and race-specific BMI z-scores were replaced with z-scores calculated according to CDC growth charts, the results essentially remained the same (odds ratio: 1.31; 95% confidence interval: 1.05–1.63; P = 0.016).
Table 2.
Characteristics of the study sample by PIH status
| Variable | PIH | ||
|---|---|---|---|
| No (n = 572) | Yes (n = 131) | Pa | |
| Childhoodb | |||
| Age (years) | 12.6±0.1 | 12.1±0.2 | 0.007 |
| BMI (kg/m2) | 19.2±0.1 | 20.2±0.4 | 0.0002 |
| Overweight (%) | 14.5 | 24.4 | 0.006 |
| Systolic BP (mm Hg) | 103.3±0.3 | 104.1±0.7 | 0.008 |
| Diastolic BP (mm Hg) | 65.0±0.3 | 64.8±0.5 | 0.19 |
| HDL Cholesterol (mg/dl) | 61.0±0.6 | 59.2±1.1 | 0.27 |
| LDL Cholesterol (mg/dl) | 91.0±0.9 | 93.5±1.8 | 0.51 |
| Triglycerides (mg/dl) | 71.8±1.1 | 73.9±2.5 | 0.25 |
| Adulthoodc | |||
| Age (years) | 38.1±0.2 | 37.7±0.5 | 0.48 |
| BMI (kg/m2) | 28.8±0.3 | 32.5±0.7 | <0.0001 |
| Systolic BP (mm Hg) | 112.3±0.6 | 115.0±0.9 | 0.03 |
| Diastolic BP (mm Hg) | 70.2±0.4 | 74.0±0.7 | <0.0001 |
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PIH, pregnancy-induced hypertension. Childhood overweight was defined as BMI greater than the 85th percentile according to Centers for Disease Control and Prevention growth charts. Mean ± SE (standard error) is shown.
aP values were adjusted for age and race where appropriate.
bMean values of multiple measurements during childhood are presented.
cMeasured after pregnancy when the questionnaire was administered.
Figure 1.
Odds ratios (ORs) for pregnancy-induced hypertension by quartile of childhood body mass index using the lowest quartile as the reference (OR = 1). P value was adjusted for race, systolic blood pressure, and levels of high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides, in childhood. Error bars show 95% confidence interval.
In sensitivity analysis, odds ratio was 1.16 (95% confidence interval: 0.92–1.48; P = 0.21) for BMI before 12 years of age and 1.51 (95% confidence interval: 1.21–1.90; P = 0.0003) for BMI between 12 and 17 years of age. We did not find significant interaction effects between childhood risk factors and race on PIH risk (P > 0.20 for all risk factor variables considered).
DISCUSSION
In this community-based cohort, we showed that women who experienced PIH in adult life had higher BMI and SBP during childhood than women who did not. However, the observed association between PIH and childhood SBP was largely driven by childhood BMI because after adjustment for childhood BMI, childhood SBP was no longer associated with PIH. One BMI standard deviation increase during childhood was associated with a 35% (95% confidence interval: 8–68%) higher odds of developing PIH. The association between childhood BMI and subsequent PIH was continuous across the entire range of BMI. Noteworthy is that girls who later developed PIH had one unit higher BMI than girls who did not, a difference equivalent to about 5.5 pounds in body weight with average height at age 12 years. Such a difference is clinically significant. Our findings highlight, for the first time to our knowledge, the importance of increased body weight during childhood as a risk factor for the development of PIH in later life.
The underlying mechanisms for the association between childhood BMI and PIH are unclear. It is known that childhood obesity is predictive of adult obesity,18–22 a known risk factor for PIH10,23–25; overweight and obesity before pregnancy are also associated with excessive gestational weight gain, another risk factor for PIH.26 However, we did not have data on adult risk factor variables before pregnancy, which precluded us from exploring the possibility of adult BMI mediating the association between childhood BMI and PIH. Speculatively, the influence of childhood BMI is likely to be mediated, at least in part, by adult BMI before pregnancy, which should be examined in future studies. Other pathways for the link between childhood BMI and PIH may include obesity-associated elevated blood pressure,27–30 insulin resistance,31 inflammation,32–35 and renal and endothelial dysfunction.36
Interestingly, the observed association for BMI measured after puberty was relatively stronger than that before puberty. This is consistent with available evidence that obesity in adolescence is a risk factor for poor pregnancy outcomes.37 Obesity during adolescence may exacerbate insulin sensitivity decrease and lead to sustained insulin resistance and other cardiometabolic risk factors in adult life.37 Lifestyle intervention during adolescence reduces excessive weight gain38 and may ultimately lead to reduced risk of pregnancy-related health outcomes.
Although childhood blood pressure was not significantly associated with subsequent PIH after adjustment for childhood BMI (P = 0.12), we cannot rule out an independent association between childhood blood pressure and PIH due to the relatively small size of our study sample. The association between childhood blood pressure and PIH should be examined in larger studies or in a meta-analysis.
Our observation that childhood BMI was associated with the development of PIH has important implications for prevention and control of PIH, a condition that has serious health consequences. Taken together with other adverse health outcomes of childhood obesity, it is clear that prevention and interventions to manage childhood obesity have multifaceted benefits later in adult life, including, but not limited to, reduced risk of obesity, type 2 diabetes, cardiovascular disease, and now PIH, as indicated by recent data showing that resolution of metabolic syndrome in adult life can substantially mitigate the adverse effects of childhood risk factors on cardiometabolic risk.39
Our study has several strengths. We have consistently and rigorously followed strict quality control protocols in the collection of the BHS observations used in our analysis. The health profile of our participants at baseline and longitudinal nature of the analysis make reverse causation an unlikely explanation for the findings. In addition, those who reported having history of hypertension during pregnancy were unlikely to have had sustained chronic hypertension before their pregnancy because their blood pressure levels at the most recent BHS examination were below 140/90mm Hg based on an average of carefully measured blood pressures obtained by trained nurses.
Several limitations of the study need to be considered. First, PIH in our study was based on a self-report, which might have led to some bias in PIH ascertainment. However, such bias would have led to underestimation of the true association between childhood BMI and PIH because PIH tends to be over reported14 and thus women with self-reported PIH in the current study likely included some without PIH. Second, we did not have information on proteinuria during pregnancy so we cannot separate gestational hypertension from preeclampsia, a severe and yet much less frequent form of PIH. However, PIH is thought to be part of the same continuum and shares many risk factors with preeclampsia.40,41 Increased body weight in childhood may also be a risk factor for preeclampsia. Nevertheless, future studies should specifically examine childhood risk factors for preeclampsia. Third, we did not have information on parity and age at pregnancy, as a result of which we were not able to differentiate the impact of childhood BMI on PIHs at different pregnancies and different ages. The potential confounding effects by age at pregnancy and parity, if present, might be limited because age at pregnancy tends to increase with increasing parity42 and thus age at pregnancy and parity might have opposing effects on PIH risk in the current study. Fourth, we could not examine the influences of central obesity (waist circumference) and glucose and insulin and other potential confounding factors like serum uric acid and renal function during childhood because data on these variables were not available. Thus, we cannot rule out the possibility of residual confounding by unmeasured factors. Finally, the sample size of our study was relatively small and replication studies are needed to confirm the findings from the current study.
In conclusion, increased childhood BMI is associated with elevated risk of PIH. As a result, the current obesity epidemic in children will likely increase the burden of adverse pregnancy-related health conditions. Prevention and control of childhood obesity will have enormous benefits in reducing risk of numerous chronic conditions, PIH included. Promotion of a healthy lifestyle, including increased physical activity and a healthy diet, is of paramount importance.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
The Bogalusa Heart Study is a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. We especially thank the Bogalusa, LA school system, and most importantly, the children and adults who have participated in this work over many years. Shengxu Li was a scholar of the Building Interdisciplinary Research Careers in Women’s Health program, supported by Award Number K12HD043451 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. Shengxu Li is also partly supported by Grant 13SDG14650068 from American Heart Association. We would also like to acknowledge funding support from National Institute of Environmental Health Science (5R01ES021724) and from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (5R01HD069587).
REFERENCES
- 1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1–S22. [PubMed] [Google Scholar]
- 2. Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens 2001; 14:178S–185S. [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy-induced hypertension. Epidemiol Rev 1997; 19:218–232. [DOI] [PubMed] [Google Scholar]
- 4. Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol 2009; 114:961–970. [DOI] [PubMed] [Google Scholar]
- 5. Stekkinger E, Zandstra M, Peeters LL, Spaanderman ME. Early-onset preeclampsia and the prevalence of postpartum metabolic syndrome. Obstet Gynecol 2009; 114:1076–1084. [DOI] [PubMed] [Google Scholar]
- 6. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet 2005; 366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 7. McDonald SD, Yusuf S, Sheridan P, Anand SS, Gerstein HC. Dysglycemia and a history of reproductive risk factors. Diabetes Care 2008; 31:1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, Paul RH. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol 1995; 172:642–648. [DOI] [PubMed] [Google Scholar]
- 9. Odegård RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Risk factors and clinical manifestations of pre-eclampsia. BJOG 2000; 107:1410–1416. [DOI] [PubMed] [Google Scholar]
- 10. Kazemian E, Sotoudeh G, Dorosty-Motlagh AR, Eshraghian MR, Bagheri M. Maternal obesity and energy intake as risk factors of pregnancy-induced hypertension among Iranian women. J Health Popul Nutr 2014; 32:486–493. [PMC free article] [PubMed] [Google Scholar]
- 11. Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology 2011; 22:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berenson GS, Wattigney WA, Bao W, Srinivasan SR, Radhakrishnamurthy B. Rationale to study the early natural history of heart disease: the Bogalusa Heart Study. Am J Med Sci 1995; 310(Suppl 1):S22–S28. [DOI] [PubMed] [Google Scholar]
- 13. Shopen N, Schiff E, Koren-Morag N, Grossman E. Factors that predict the development of hypertension in women with pregnancy-induced hypertension. Am J Hypertens 2016; 29:141–146. [DOI] [PubMed] [Google Scholar]
- 14. Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy lipids related to preterm birth risk: the coronary artery risk development in young adults study. J Clin Endocrinol Metab 2010; 95:3711–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974; 20:470–475. [PubMed] [Google Scholar]
- 16. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 1973; 19:476–482. [PubMed] [Google Scholar]
- 17. Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In Lewis LA. (ed), CRC Handbook of Electrophoresis. v. III. Lipoprotein Methodology and Human Studies. CRC Press: Boca Raton, FL, 1983, pp. 185–204. [Google Scholar]
- 18. Bjerregaard LG, Rasmussen KM, Michaelsen KF, Skytthe A, Mortensen EL, Baker JL, Sorensen TI. Effects of body size and change in body size from infancy through childhood on body mass index in adulthood. Int J Obes (Lond) 2014; 38:1305–1311. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord 2001; 25:735–740. [DOI] [PubMed] [Google Scholar]
- 20. Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 2002; 76:653–658. [DOI] [PubMed] [Google Scholar]
- 21. Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T, Jokinen E, Taittonen L, Raitakari OT, Juonala M. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr 2011; 159:584–590. [DOI] [PubMed] [Google Scholar]
- 22. Li S, Chen W, Srinivasan SR, Xu J, Berenson GS. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: the Bogalusa Heart Study. Am J Epidemiol 2012; 176(Suppl 7):S142—S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health 2005; 95:1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehrenthal DB, Jurkovitz C, Hoffman M, Jiang X, Weintraub WS. Prepregnancy body mass index as an independent risk factor for pregnancy-induced hypertension. J Womens Health (Larchmt) 2011; 20:67–72. [DOI] [PubMed] [Google Scholar]
- 25. Bener A, Saleh NM. The impact of socio-economic, lifestyle habits, and obesity in developing of pregnancy-induced hypertension in fast-growing country: global comparisons. Clin Exp Obstet Gynecol 2013; 40:52–57. [PubMed] [Google Scholar]
- 26. Haugen M, Brantsaeter AL, Winkvist A, Lissner L, Alexander J, Oftedal B, Magnus P, Meltzer HM. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth 2014; 14:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srinivasan SR, Myers L, Berenson GS. Rate of change in adiposity and its relationship to concomitant changes in cardiovascular risk variables among biracial (black-white) children and young adults: the Bogalusa Heart Study. Metabolism 2001; 50:299–305. [DOI] [PubMed] [Google Scholar]
- 28. Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 2001; 108:712–718. [DOI] [PubMed] [Google Scholar]
- 29. Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999; 103:1175–1182. [DOI] [PubMed] [Google Scholar]
- 30. Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes 2000; 49:1042–1048. [DOI] [PubMed] [Google Scholar]
- 31. Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 2002; 51:204–209. [DOI] [PubMed] [Google Scholar]
- 32. Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. Endothelial dysfunction, inflammation, and oxidative stress in obese children and adolescents: markers and effect of lifestyle intervention. Obes Rev 2012; 13:441–455. [DOI] [PubMed] [Google Scholar]
- 33. Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3–16 years. Am J Prev Med 2010; 39:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singer K, Eng DS, Lumeng CN, Gebremariam A, J ML. The relationship between body fat mass percentiles and inflammation in children. Obesity (Silver Spring) 2014; 22:1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siervo M, Ruggiero D, Sorice R, Nutile T, Aversano M, Iafusco M, Vetrano F, Wells JC, Stephan BC, Ciullo M. Body mass index is directly associated with biomarkers of angiogenesis and inflammation in children and adolescents. Nutrition 2012; 28:262–266. [DOI] [PubMed] [Google Scholar]
- 36. Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy 2012; 2012:105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Todd AS, Street SJ, Ziviani J, Byrne NM, Hills AP. Overweight and obese adolescent girls: the importance of promoting sensible eating and activity behaviors from the start of the adolescent period. Int J Environ Res Public Health 2015; 12:2306–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ning Y, Yang S, Evans RK, Stern M, Sun S, Francis GL, Wickham EP., III Changes in body anthropometry and composition in obese adolescents in a lifestyle intervention program. Eur J Nutr 2014; 53:1093–1102. [DOI] [PubMed] [Google Scholar]
- 39. Magnussen CG, Koskinen J, Juonala M, Chen W, Srinivasan SR, Sabin MA, Thomson R, Schmidt MD, Nguyen QM, Xu JH, Skilton MR, Kahonen M, Laitinen T, Taittonen L, Lehtimaki T, Ronnemaa T, Viikari JS, Berenson GS, Raitakari OT. A diagnosis of the metabolic syndrome in youth that resolves by adult life is associated with a normalization of high carotid intima-media thickness and type 2 diabetes mellitus risk: the Bogalusa heart and cardiovascular risk in young Finns studies. J Am Coll Cardiol 2012; 60:1631–1639. [DOI] [PubMed] [Google Scholar]
- 40. Foo L, Tay J, Lees CC, McEniery CM, Wilkinson IB. Hypertension in pregnancy: natural history and treatment options. Curr Hypertens Rep 2015; 17:36. [DOI] [PubMed] [Google Scholar]
- 41. Peters RM, Flack JM. Hypertensive disorders of pregnancy. J Obstet Gynecol Neonatal Nurs 2004; 33:209–220. [DOI] [PubMed] [Google Scholar]
- 42. Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000–2014. NCHS Data Brief 2016;232:1–8. [PubMed] [Google Scholar]