Abstract
In Brief Prospective identification of individuals with diabetes who are at greatest risk for developing complications would have considerable public health importance by allowing appropriate resources to be focused on those who would benefit most from aggressive intervention. Haptoglobin (Hp) is an acute-phase protein that is crucial for the elimination of free hemoglobin and the neutralization of oxidative damage. In the past two decades, associations have been made between polymorphisms in Hp and complications arising from diabetes. Individuals with polymorphism in Hp have been shown to have significantly higher risk of developing cardiovascular disease. This review summarizes the current literature on the role of Hp in health and disease, with a focus on diabetes.
Recent epidemiological studies have showed that the diabetes epidemic affects 415 million people worldwide, and this figure is expected to increase to nearly 642 million people by 2040 (1). Patients afflicted with diabetes often are more susceptible to a host of multi-organ complications that arise as a result of microvascular and macrovascular dysfunction. These complications equate to the leading cause of morbidity and mortality, in the form of accelerated atherosclerotic disease. Dysregulation in glycemic control and subsequent alterations to the vascular endothelium are known to be associated with all grades of atherosclerotic disease (2,3). Accelerated atherosclerosis is prevalent in this patient population, contributing to >75% of the mortality seen among people with diabetes (4).
In the past decade, interventions to obtain stricter glycemic control have been attempted with debatable success. In addition to stricter glycemic control measures, attempts have been made to reduce cardiovascular risk by implementing lifestyle modifications and medication regimens to reduce hyperlipidemia and hypertension. One area of considerable interest in the clinical and research community is the development of improved screening tools to identify and mitigate cardiovascular risk. One such area that has had considerable interest is the predictive value of haptoglobin (Hp) genotype screening in cardiovascular risk.
Polonovski and Jayle first described the biochemical properties of Hp more than 75 years ago (5–7). Since that initial description, numerous important biological functions have been described for Hp. Hp is an acute-phase α2-glycoprotein with a major biological function of binding free hemoglobin (Hb) with very high affinity to prevent the loss of iron following intravascular hemolysis (8,9) and to prevent Hb-mediated renal injury (10–12). The binding of Hp to free Hb forms a stable complex with very high affinity that is cleared from circulation by the reticulocyte system and CD163-positive macrophages, Kupffer cells, and hepatocytes (13,14). In its role as a clearance protein, Hp removes the oxidative potential of the iron contained in the Hb molecule.
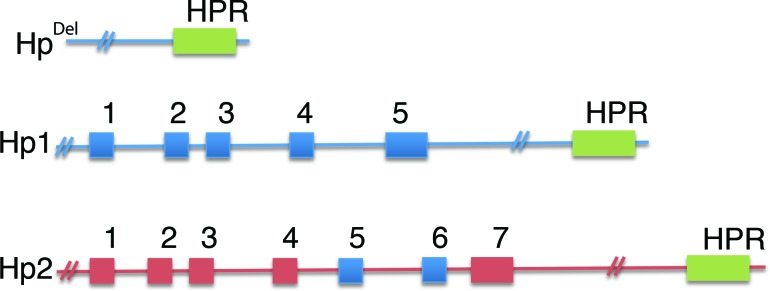
Hp is present in the serum of all mammals, but polymorphism is found only in humans (15). The allelic differences seen in humans arise from a crossover duplication of exons 3 and 4, resulting in an Hp1 allele with 5 exons and an Hp2 allele with 7 exons (Figure 1) (16). Three major genotypes are produced and have been identified by gel electrophoresis: Hp1-1, Hp1-2, and Hp2-2 (17,18). HpDel has also been described in Japanese, Chinese, and Korean populations but has not been found in African or European populations (19–21). Several minor genotypes (Hp1-Johnson, Hp1-M, and Hp0) have also been described (22). The three predominant genotypes display differing structures and biological effects, as described below.
FIGURE 1.
Structural representation of the Hp alleles Del, 1, and 2. Hp exonic sequences are denoted by numbered and shaded boxes. Intronic sequences are denoted by a solid line. Exons 3 and 4 of the Hp1 allele have been duplicated in the Hp2 allele, giving rise to exons 3–6.
Molecular Structure and Regulation
The human gene for Hp is located on chromosome 16q22 and consists of three structural alleles, the products of which are Hp1F, Hp1S, and Hp2 (23). The products of the Hp1F and Hp1S alleles differ by only one amino acid, whereas the Hp2 allele is the result of a fusion of the Hp1F and Hp1S alleles. Transcription and translation occurs as a single polypeptide that is post-translationally processed into a smaller α chain and a larger β chain that are linked by a disulfide bridge (24). However, the presence of the Hp1 and Hp2 alleles in humans gives rise to Hp1-1 dimers, Hp1-2 heterodimers, and Hp2-2 dimers. The estimated frequency of Hp1-1 is 15–18%, Hp2-1 is 46%, and Hp2-2 is 38% (25).
Hp synthesis is principally conducted in the liver, but expression is also seen in the lung, skin, spleen, kidney, and adipose tissue (26–28). The expression of Hp can be increased in the presence of growth hormone, insulin, and bacterial endotoxin and in the expression of macrophage-produced proinflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α) (14,29,30). In addition to induction by cytokines, glucocorticoids, catecholamines, and hypoxia also can activate the expression of Hp (31,32). Despite largely being a serum protein, Hp is also detected in urine, synovial fluid, ascites fluid, cerebrospinal fluid, and pleural fluid (33).
Functions of Hp
The hallmark function of Hp is to facilitate Hb clearance. Hb is the prominent blood protein involved in transporting oxygen in the circulatory system and mediates reactive oxygen and nitrogen species detoxification (34,35). Free Hb released into the blood is a natural phenomenon associated with intravascular hemolysis that occurs during the destruction of senescent red blood cells (RBCs), which occurs at a rate of 2 × 106 RBCs per second (36,37). A variety of severe complications can result from intravascular hemolysis when accompanied by other comorbidities such as diabetes, infectious disease, trauma, and cancer (38,39). Hp forms a strong noncovalent complex with Hb (Hp-Hb complex) that results in its removal via the reticuloendothelial system and receptor-mediated endocytosis via CD163 on hepatocytes, Kupffer cells, and tissue macrophages (40–43).
There is sufficient Hp in circulation (38–208 mg/dL) to bind and clear 3 g of Hb, which would prevent free Hb circulation in the body (44). CD163 expression by these cells is induced by inflammation and the release of cytokines such as IL-1 and IL-6. IL-6 plays an especially important function in that it is important in the stimulation of Hp production and the modulation of CD163 expression on the cell surface (45–47). CD163 is downregulated by TNF-α, IL-4, and interferon γ (48). Regulation of this process is tightly controlled by the release of immunomodulatory molecules.
Hb that is bound with Hp for clearance is internalized, processed, and degraded to release heme for further processing by hemoxygenase-1 to release iron where it can be recycled to construct new Hb proteins. When not bound to Hb, Hp is cleared from the plasma in 3–5 days. When bound to Hb, the average time for removal of the complex is ∼20 minutes (49,50). Hp is not recycled during this clearance process, but rather is degraded, and de novo protein production occurs to resupply the blood and tissue (51). In the absence of a clearance mechanism, free Hb can catalyze the formation of free radicals and mediate the destruction of cellular constituents and extracellular macromolecules and promote the oxidation of LDL cholesterol (37).
Antioxidant Activity
Hp binds to free Hb with perhaps the highest affinity in nature (9,52,53). A variety of unfavorable consequences can arise when free iron is present in the circulation and in tissues. For some time, it has been known that iron overload can contribute to the development of diabetes and atherosclerosis (54–57). The pathophysiology with respect to iron arises from the ability of iron to participate in the generation of powerful oxidant species such as hydroxyl radical (58). Iron participates in the Haber-Weiss reaction to convert reactive oxygen species (ROS) such as superoxide and hydrogen peroxide to more powerful species such as hydroxyl radical (56). Free iron can be a detrimental catalyst in lipid peroxidation because it can both initiate and amplify the process of lipid peroxidation.
The initiation step can be induced by two different iron-dependent mechanisms. The hydroxyl radical-dependent mechanism, which has been adopted by most as the predominant mechanism for lipid peroxidation (59), and an alternate hydroxyl radical-independent mechanism, which has also been proposed and places iron-oxygen complexes rather than hydroxyl radical as the initiator of lipid peroxidation (60). These conversions can have damaging effects to the tissue.
The ability of Hp to reduce the tissue-damaging effects of free radicals is genotype-dependent. Investigations by Melamed-Frank et al. demonstrate that Hp1-1 has a greater capacity to protect against oxidative damage despite the fact that all three genotypes have the same binding affinity (61). The crucial difference is the accessibility of the Hp molecule to the extravascular space. Size differences in the molecules serve as an exclusionary mechanism; consequently, in individuals with Hp2-1 or Hp2-2, free Hb remains in circulation for a protracted period of time and causes greater oxidative stress (61). The antioxidant capacity of Hp in people with diabetes is of special concern. In particular, the Hp2-2 genotype is considered to be a major susceptibility gene for people with type 2 diabetes because it results in reduced antioxidant capacity (62).
Immunoregulatory Activity
The role of Hp as an acute-phase protein brings to light its possible immunoregulatory activity. Dating back as far as 1968 with the work of Nevo and Stutton (63) and, more recently, the work of Langlois and Delanghe (64), individuals of Hp2-2 genotype demonstrated enhanced immune function. For example, Hp2-2 individuals display a greater response to vaccination by producing higher levels of protective antibodies (65). Hp also has been described by Langlois and Delanghe as having inhibitory activity in the synthesis of prostaglandins and, as a result, having anti-inflammatory properties (64).
Hp has been shown to have powerful regulatory activity on lymphocytes. For example, Arredouani et al. (66) and Guetta et al. (67) have demonstrated that Hp plays a role in the balance between T helper-1 (Th1) and T helper-2 (Th2) by heavily promoting a strong Th1 cell response. These studies demonstrated that the Hp1 allele, when complexed to Hb, stimulates the secretion of significantly more IL-6 and IL-10 than the Hp2 allele. A Th1 response is more effective in protecting against intracellular parasites and inhibits the release of Th2 cytokines, which are responsible for defending against extracellular pathogens. These findings suggest that patients with Hp1 alleles are more efficient at protecting against infection.
In the context of immune-mediated diseases, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and systemic lupus erythematosus all have been linked with polymorphisms in Hp. For example, prohaptoglobin-2 (proHp), which is proteolytically cleaved to Hp2 by site-specific cleavage, has been associated with celiac disease and type 1 diabetes (68,69). Recently, studies by Zhang et al. (70) and Moreno-Navarette et al. (71) have suggested that serum levels of proHp are elevated in people with type 2 diabetes and that circulating levels of proHp contribute to insulin resistance in obesity. The elevations in proHp are associated with intestinal permeability, dyslipidemia, inflammation, and insulin resistance.
Angiogenesis and Lymphangiogenesis
Impaired wound healing is a known major complication of diabetes and is caused by apoptosis, cellular infiltration, and reduced angiogenesis. Recent studies have demonstrated reduced lymphangiogenesis and angiogenesis during diabetic wound healing (72–74). Hp is known to play a role in angiogenesis according to Cockerill et al. (75). A study by Oh et al. (76) demonstrated that proHp can upregulate the expression of vascular endothelial growth factor (VEGF) and VEGF receptor 2 and increase endothelial sprouting and branching. These findings suggest that proHp can promote angiogenesis via the VEGF signaling pathway.
Cid et al. (77) observed that sera from patients with systemic vasculitis stimulated angiogenesis in an in vitro model. Serum Hp level in vasculitis patients was shown to correlate with both disease and angiogenic activity. The increased levels of Hp found in chronic inflammatory conditions may play an important role in tissue repair. In systemic vasculitis, for example, Hp might compensate for ischemia by promoting the development of collateral vessels (77).
Neovascularization resulting from angiogenesis may be pathologically important in the context of diabetes. Pathological angiogenesis enhances disease progression and increases macrophage infiltration and vessel wall thickness, leading to hypoxia and interplaque rupture (78,79). Lipid-rich RBC membranes and free Hb are physiologically detrimental in the context of diabetes and atherosclerosis. Neovascularization of the atherosclerotic plaque may be driven by Hp or proHp, leading to greater risk in people with diabetes.
Blockade of Nitric Oxide
Nitric oxide (NO) is a gas produced by a variety of cell types that is involved in several important physiological activities. NO is involved in vascular tone, modulates neurotransmitter function in the central and peripheral nervous systems, and participates in cellular defense and platelet aggregation (80,81). Both free Hb and the Hp-Hb complex inactivate NO, whereas Hp does not affect NO. As a result, an increase in the level of circulating Hb or Hp-Hb complex may result in the depletion of NO and the lack of endothelial relaxation contributing to cardiovascular disease (CVD).
Patients with an Hp1-1 genotype may experience benefits over Hp2-1 or Hp2-2 carriers because the Hp1-1 Hp-Hb complex will be cleared from circulation more efficiently than other Hp complexes. The more rapid clearance enhances the availability of NO to carry out its physiological functions. One illustration of the benefits of one Hp genotype over another was seen in a recent report by Sertorio et al. (82) showing that, in patients with preeclampsia, Hp1-1 played a protective role by reducing NO scavenging. Patients with Hp2-1 and Hp2-2 appeared to have aggravation of preeclampsia, in part because of reduced NO bioavailability.
Disease Implications of Hp Polymorphism
Infectious Disease
Diabetes is a predisposing factor for infections. People with diabetes have a two to four times greater risk of systemic infection than people without diabetes (83,84). Variation in Hp genotype is also implicated as a contribution to mortality from both extracellular and intracellular pathogens. For example, several studies have demonstrated that patients can have a greater susceptibility to malaria and the development of severe complications depending on their Hp genotype (85–88).
Pathogens such as Corynebacterium diptheriae and Staphylococcus aureus, which require iron for growth, are known to take advantage of the Hp-Hb complex for their metabolic activity (89,90). The reduced activity of Hp2 to clear free Hb may allow these bacteria to use this iron source, contributing to enhanced colonization and growth. Pishchany et al. (91) have demonstrated that S. aureus can grow in a manner that is entirely dependent on the ability to bind Hb, extract heme, pass heme through the bacterial cell envelope, and degrade heme in the cytoplasm. The ability of different bacteria to use iron from Hb may allow for the generation of soft tissue infection when free heme is not removed after RBC hemolysis.
People with diabetes are especially susceptible to bacterial infections, especially urinary tract, respiratory, and soft tissue infections. For example, S. aureus is known to cause both respiratory infections and urinary tract infections that can lead to bacterial nephritis. Addtionally, S. aureus is the most common soft tissue infection in people with diabetes (83,92,93). These findings could be especially important in the context of diabetes complications, CVD, surgical interventions, and trauma. In general, host innate immune mechanisms, including Hp, would restrict the accessibility of iron to pathogens, thereby removing one important bacterial growth requirement (94,95).
Finally, Hp2-2 patients also have been found to have higher mortality and poorer outcomes from tuberculosis and Hanson’s disease (leprosy) than other genotypes (88,95–97). Given its role as an acute-phase protein, the participation of Hp in various pathogenic infections is well documented, and the specific genotype that a patient carries has direct bearing on the ultimate outcome of infection.
Hp genotype has also been associated with intracellular pathogens. Diabetes has been recognized as an important risk factor for several intracellular pathogens (98). For example, patients with Hp2-2 have a higher degree of mortality from HIV, with a median survival time of 11 years for people with Hp1-1 or Hp2-1 compared to 7.3 years for those with Hp2-2. Hp2-2 patients also had significantly higher HIV viral titers than those with other genotypes (99). Hp genotype also has been investigated in HIV patients in the development of Kaposi sarcoma (KS). KS is caused by herpes virus 8, an opportunistic infection rarely seen outside of immunocompromised patients. Speeckaert et al. (100) examined the effect of Hp genotype and demonstrated that the Hp1-1 genotype conferred the greatest risk to the development of KS, followed by Hp2-2 and Hp2-1. In contrast, Speeckaert et al. (101) in an earlier study demonstrated that Hp1-1 provided the greatest protection from Epstein-Barr virus, and Hp2-2 has the greatest association as determined by serum titer. In animal models of influenza, there is significant evidence that Hp is upregulated in clinical disease. This has been found in pigs and ferrets, which share a lung physiology similar to that of humans (102,103). Significant observations regarding bovine respiratory disease have been associated with increased Hp production (104). These observations in respiratory infection are especially relevant given the recent report of Hp as part of the human lung surfactant system, in which it co-localizes with surfactant protein B (105). As seen through these few examples, susceptibility to infectious disease is, in part, dependent on Hp genotype, and this is especially important in people with diabetes and Hp polymorphism.
Neurology
Diabetes has been associated with dementia and neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’ disease (PD) (106,107). Although the mechanisms remain unclear, type 2 diabetes can exacerbate and lead to progression of the neurodegenerative process. One possible common mechanism is oxidative stress (108). In addition to this commonality in pathogenesis, there is also evidence that AD itself can promote insulin resistance within the brain (108).
Given the common thread of oxidative stress and the role of Hp in the management of oxidative reactions, several recent studies have demonstrated the relationship of Hp with neurodegenerative pathologies. In fact, Hp genotype has been implicated in greater susceptibility to idiopathic PD (109,110). There are also several reports of Hp involvement in the pathogenesis of AD. The accumulation of β-amyloid in the brain is a driving force for AD pathogenesis. Spagnuolo et al. previously reported that Hp could bind to apolipoprotein E (ApoE) and impair its function in cholesterol homeostasis (111). Immunoprecipitation and immunoblotting has shown that Hp and β-amyloid form complexes in brain tissue from patients with AD. The interaction between ApoE and β-amyloid was shown to be crucial for limiting β-amyloid neurotoxicity and for promoting its clearance. Spagnuolo et al. further demonstrated that Hp, rather than impairing ApoE binding to β-amyloid, promotes the formation of the complex between β-amyloid and ApoE2, ApoE3, or ApoE4 (111). Hence, the suggestion from this study is that the risk of developing AD not only might be linked to the different ApoE isoforms, but also may rely on the level and type of Hp. Song et al. (112) reported a significantly higher level of serum Hp in patients with AD than in healthy control subjects. Their results support the hypothesis that oxidative stress is a key event in the pathogenesis of AD. The specific genotypes were not examined in this study, so a future investigation will be needed to determine the impact of Hp1 versus Hp2 on AD pathogenesis. Brain atrophy, reduced cerebral glucose metabolism, and central nervous system insulin resistance are all features of AD, PD, and type 2 diabetes (113,114).
Finally, several studies have examined the effect of Hp genotype on the outcome and resolution of intracerebral hemorrhage. Murthy et al. (115) recently reported that patients with the Hp2-2 genotype experience worse outcomes after intracerebral hemorrhage. These results were supported by another study by Kantor et al. (116) in the context of subarachnoid hemorrhage, in which patient outcomes were worse in those with the Hp2-2 genotype.
Cardiology
Hp genotype has been consistently associated as a marker and risk factor for CVD. In a study by Chapelle et al. (117), patients with the Hp2-2 genotype had more severe myocardial infarction. Hp was later examined in a prospective study of >342,000 patients over 11.8 years. Hp was shown to be a significant risk factor of acute myocardial infarction (AMI), stroke, and heart failure (HF) (118). Data from this study established an association of Hp in these cardiovascular events that was stronger for men than for women. Hp was almost as predictive as total cholesterol for AMI and about as predictive as total cholesterol of stroke, with a stronger relationship to ischemic disease than to hemorrhagic stroke where a 4.2-fold increase in risk was observed in ischemic disease. Haas et al. (119) likewise established an association with Hp as a potential prognostic biomarker in AMI. In summary, Hp genotype carried as much predictive value as total cholesterol for risk of AMI and stroke.
HF and stroke also have been associated with Hp genotype (118,120). Costacou et al. (120) described a borderline increased risk of stroke in people with type 1 diabetes and the Hp1-1 genotype. In an earlier study (121), this same group described an increased risk for CVD in people with the Hp2-2 genotype compared to those with the Hp1-1 genotype. Staals et al. (122) likewise suggested an increased risk for lacunar stroke depending on patients’ Hp genotype. The aforementioned study by Holme et al. (118) established a twofold increased risk for stroke and a 1.5-fold increased risk for HF. Hp2-2 has been associated with higher total serum and free cholesterol concentrations, reduced graft survival in patients undergoing coronary artery bypass, and a greater risk of restenosis after stent implantation (Hp2-2 36% vs. Hp2-1 31%) (123–125).
Hp2-2 is a clear genetic risk factor for the development of aneurysm, coronary atherosclerosis, and unstable carotid stenosis independent of, or in connection with, many of the classical risk factors, including dyslipidemia, hypertension, diabetes, smoking, and hyperhomocystenemia (12,126,127). In one study involving male patients with diabetes, Lioupis et al. (128) demonstrated that patients had a higher serum level of homocysteine and that those with the Hp2-2 genotype had a higher concentration of iron in the atherosclerotic plaque. These findings suggest that increased intraplaque iron deposition may be responsible for increased oxidative stress and instability of the carotid plaque.
Additionally, Hp is a risk factor for developing refractory hypertension in patients with existing hypertension (129,130). Data demonstrate that patients with the Hp2-2 genotype require more antihypertensive therapy to control blood pressure than do patients with other genotypes. Patients with the Hp2-2 genotype also require more support and follow-up than do patients with alternate Hp genotypes (129,130). Conversely, patients with the Hp1-1 genotype have a lower rate of complications than do hypertensive patients with either the Hp2-1 or the Hp2-2 genotype (130).
The literature strongly supports the hypothesis that the Hp2-2 genotype provides much less protection from oxidative damage to arteries in patients with atherosclerotic plaques. The Hp2-2 genotype also confers additional CVD risk, as well as other complications, including aneurysm, carotid plaque rupture, myocardial infarction, and decreased survival after coronary artery bypass. It has become clear in the past 20 years that Hp can be considered a predictor of susceptibility to cardiovascular disorders and a measure of patients’ prognosis and outcomes.
Diabetes
The generation of ROS has a significant role in the generation of vascular complications in people with diabetes (61). Complications in people with diabetes resulting from HP genotype include cardiovascular events, retinopathy, and nephropathy. In studies of patients on hemodialysis, Levy and others (131,132) have described a protective effect in patients with the Hp1-1 genotype. Two independent studies by Levy et al. (133,134) showed that Hp genotype correlates with vascular complications. For example, the Hp1-1 genotype protects against vascular injury as a consequence of increased antioxidant activity compared to Hp2-1 or Hp2-2. Similar results were described by Szafranek et al. (135), who found that Hp was a major susceptibility gene for diabetic vascular complications. The Hp2 genotype has been reported to be associated with an increased risk for cardiovascular events such as myocardial infarction in individuals with type 2 diabetes (134,136). These studies and others show that Hp2 patients can have as much as a five times greater coronary disease risk and susceptibility to CVD compared to patients with the Hp1-1 genotype.
In addition to cardiovascular risk and complications, Hp genotype also correlates to increased risk for diabetic retinopathy in people with type 2 diabetes. In patients with normal blood pressure, Mogarekar and Hampe (137) showed an increased risk for the development of severe retinopathy in those with the Hp2-2 genotype. When compared to other genotypes, this study showed a graded risk relationship with the number of Hp2 alleles.
Hp genotype determination, therefore, can be valuable in the assessment and management of diabetic retinopathy. Research has clearly demonstrated that blindness from diabetes is preventable with early diagnosis, clarification of risk factors, and timely photocoagulation where appropriate. Hp genotyping can allow for earlier identification of and prompt early intervention for patients who may suffer from diabetic retinopathy.
Diagnostic Implications of Hp
Plasma Hp concentrations have been examined for a variety of pathogenic disorders. Both high and low levels of Hp are indicative of clinical conditions. Elevated plasma concentrations have been described in cases of trauma, burn, inflammation, and cancer because of the acute-phase nature of the protein and its role in the removal of oxidative species from the circulation (33,61,138). Plasma levels are high beginning several days after the inflammatory or traumatic insult and return to normal within several weeks (139). Conditions in which Hp is decreased include malnutrition, hemolysis, hepatic disease, allergic reactions, and seizure disorders (139–142).
Given the involvement of Hp in the pathogenesis of cardiovascular, neurological, infectious, and inflammatory conditions, it would be beneficial to patients to have a diagnostic tool that can quickly identify Hp genotype so proper therapy can be implemented. For example, Hp2-2 is found in ∼36% of the population. For people with this genotype, at least 10 studies examining nearly 175,000 patients collectively have sought to determine the effects of this genotype versus nonhomozygous carriers.
A rapid test can be employed to initiate a therapy such as vitamin E or vitamin C to minimize the progression of CVD by as much as 30–40% (143). Initiation of 400 IU of vitamin E has been shown effective in reducing cardiovascular events (12,144). The antioxidant activity of vitamin E neutralizes the oxidative capacity of free heme and serves as a surrogate for Hp activity (145). Free heme can result in the oxidation of LDL particles and lead to the growth and instability of atherosclerotic plaque. Moderate doses of vitamin C and E can be effective in stabilizing oxidative stress and reducing the levels of circulating free radicals (12,145). The current methodology for determining genotype is gel electrophoresis, which is time-consuming and not optimal for large patient volume or commercial diagnostic purposes. A method developed by Levy et al. (146) uses an enzyme-linked immunosorbent assay (ELISA) to characterize the Hp genotype. This ELISA method is a user-friendly, accurate diagnostic tool for determining Hp genotype in the research environment but has not been employed in a commercial diagnostic capacity.
New methods for high-volume patient genotyping allow for rapid screening and early implementation of alternate antioxidant therapy and provide a tremendous benefit to people with diabetes.
Conclusion
The most thoroughly characterized property of Hp is its capacity to bind free Hb and promote endocytosis via the CD163 scavenger receptor and intracellular degradation of the Hp-Hb complex (9,37,147). In this process, Hp can reduce the loss of free Hb through glomerular filtration and promote the recycling of iron. In addition to its role in clearance, Hp has a protective function in that heme and iron released from free Hb are removed from circulation, thereby preventing the production of ROS.
In the context of diabetes, the role of Hp can be either protective or pathogenic. Patients with the Hp2 allele have a significantly higher risk for cardiovascular, neurological, and infectious complications. Of particular concern in people with diabetes is the significantly increased risk for myocardial infarction, stroke, infection, and kidney disease in those with an Hp2-2 genotype.
Although the risk is higher for those with Hp2-2, there are several ways to reduce this risk back to baseline cardiovascular risk for patients with diabetes. The administration of exogenous antioxidants such as vitamin C and vitamin E can mitigate the deleterious effects of an Hp2-2 genotype (12,134,143,148,149). Thus, it is crucial for the research and diagnostic community to develop new methods of identifying patient genotype and advancing therapeutics for the treatment of people with diabetes who have the Hp2 polymorphism.
Duality of Interest
The authors are employed by MyGenetx, a company that performs diabetes laboratory testing. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–149 [DOI] [PubMed] [Google Scholar]
- 2.Mostaza JM, Lahoz C, Salinero-Fort MA, et al. Carotid atherosclerosis severity in relation to glycemic status: a cross-sectional population study. Atherosclerosis 2015;242:377–382 [DOI] [PubMed] [Google Scholar]
- 3.Ifrim S, Vasilescu R. Early detection of atherosclerosis in type 2 diabetic patients by endothelial dysfunction and intima-media thickness. Rom J Intern Med 2004;42:343–354 [PubMed] [Google Scholar]
- 4.Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol 2004;44:2293–2300 [DOI] [PubMed] [Google Scholar]
- 5.Polonovski M. Biochemistry of haptoglobin and its clinical interpretation. Rend Ist Sup Sanit 1950;13:842–875 [in Italian] [PubMed] [Google Scholar]
- 6.Polonovski M. Influence of plasma globin on the index haptoglobin. Sang 1945;16:496–498 [in French] [PubMed] [Google Scholar]
- 7.Jayle MF, Said I, Gillard P. Action of haptoglobin on peroxidase catalysis of hemoglobin: new theory on the formation of enzymes. Bull Soc Chim Biol (Paris) 1946;28:63–80 [in French] [PubMed] [Google Scholar]
- 8.Lim SK. Consequences of haemolysis without haptoglobin. Redox Rep 2001;6:375–378 [DOI] [PubMed] [Google Scholar]
- 9.Ratanasopa K, Chakane S, Ilyas M, Nantasenamat C, Bulow L. Trapping of human hemoglobin by haptoglobin: molecular mechanisms and clinical applications. Antioxid Redox Signal 2013;18:2364–2374 [DOI] [PubMed] [Google Scholar]
- 10.Lipiski M, Deuel JW, Baek JH, Engelsberger WR, Buehler PW, Schaer DJ. Human Hp1-1 and Hp2-2 phenotype-specific haptoglobin therapeutics are both effective in vitro and in guinea pigs to attenuate hemoglobin toxicity. Antioxid Redox Signal 2013;19:1619–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baek JH, D'Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 2012;122:1444–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenstein H, Levy NS, Levy AP. Haptoglobin genotype and its role in determining heme-iron mediated vascular disease. Pharmacol Res 2012;66:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen MJ, Andersen CB, Moestrup SK. CD163 binding to haptoglobin-hemoglobin complexes involves a dual-point electrostatic receptor-ligand pairing. J Biol Chem 2013;288:18834–18841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Kinzie E, Berger FG, Lim SK, Baumann H. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep 2001;6:379–385 [DOI] [PubMed] [Google Scholar]
- 15.Bowman BH, Barnett DR, Lum JB, Yang F. Haptoglobin. Methods Enzymol 1988;163:452–474 [DOI] [PubMed] [Google Scholar]
- 16.Bowman BH, Kurosky A. Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet 1982;12:189–261, 453–184 [DOI] [PubMed] [Google Scholar]
- 17.Smithies O, Connell GE, Dixon GH. Inheritance of haptoglobin subtypes. Am J Hum Genet 1962;14:14–21 [PMC free article] [PubMed] [Google Scholar]
- 18.Smithies O, Connell GE, Dixon GH. Chromosomal rearrangements and the evolution of haptoglobin genes. Nature 1962;196:232–236 [DOI] [PubMed] [Google Scholar]
- 19.Su YC, Chen YC, Li SC, Lee CC, Tung YT. Detection of Hpdel in healthy individuals and cancer patients in Taiwan. Clin Chem Lab Med 2009;47:745–749 [DOI] [PubMed] [Google Scholar]
- 20.Shimada E, Odagiri M, Chaiwong K, et al. Detection of Hpdel among Thais, a deleted allele of the haptoglobin gene that causes congenital haptoglobin deficiency. Transfusion 2007;47:2315–2321 [DOI] [PubMed] [Google Scholar]
- 21.Park KU, Song J, Kim JQ. Haptoglobin genotypic distribution (including Hp0 allele) and associated serum haptoglobin concentrations in Koreans. J Clin Pathol 2004;57:1094–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai T, Okazaki T, Yanagisawa Y. Analysis of DNA from subjects found to be heterozygous for the haptoglobin-Johnson alpha gene. Exp Clin Immunogenet 1997;14:113–117 [PubMed] [Google Scholar]
- 23.Bensi G, Raugei G, Klefenz H, Cortese R. Structure and expression of the human haptoglobin locus. EMBO J 1985;4:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polticelli F, Bocedi A, Minervini G, Ascenzi P. Human haptoglobin structure and function: a molecular modelling study. FEBS J 2008;275:5648–5656 [DOI] [PubMed] [Google Scholar]
- 25.Wassell J. Haptoglobin: function and polymorphism. Clin Lab 2000;46:547–552 [PubMed] [Google Scholar]
- 26.Yang F, Friedrichs WE, Navarijo-Ashbaugh AL, deGraffenried LA, Bowman BH, Coalson JJ. Cell type-specific and inflammatory-induced expression of haptoglobin gene in lung. Lab Invest 1995;73:433–440 [PubMed] [Google Scholar]
- 27.Friedrichs WE, Navarijo-Ashbaugh AL, Bowman BH, Yang F. Expression and inflammatory regulation of haptoglobin gene in adipocytes. Biochem Biophys Res Commun 1995;209:250–256 [DOI] [PubMed] [Google Scholar]
- 28.Kalmovarin N, Friedrichs WE, O'Brien HV, Linehan LA, Bowman BH, Yang F. Extrahepatic expression of plasma protein genes during inflammation. Inflammation 1991;15:369–379 [DOI] [PubMed] [Google Scholar]
- 29.Smeets MB, Pasterkamp G, Lim SK, Velema E, van Middelaar B, de Kleijn DP. Nitric oxide synthesis is involved in arterial haptoglobin expression after sustained flow changes. FEBS Lett 2002;529:221–224 [DOI] [PubMed] [Google Scholar]
- 30.Yang F, Ghio AJ, Herbert DC, Weaker FJ, Walter CA, Coalson JJ. Pulmonary expression of the human haptoglobin gene. Am J Respir Cell Mol Biol 2000;23:277–282 [DOI] [PubMed] [Google Scholar]
- 31.Oh MK, Park HJ, Kim NH, Park SJ, Park IY, Kim IS. Hypoxia-inducible factor-1alpha enhances haptoglobin gene expression by improving binding of STAT3 to the promoter. J Biol Chem 2011;286:8857–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.do Nascimento CO, Hunter L, Trayhurn P. Regulation of haptoglobin gene expression in 3T3-L1 adipocytes by cytokines, catecholamines, and PPARgamma. Biochem Biophys Res Commun 2004;313:702–708 [DOI] [PubMed] [Google Scholar]
- 33.Javid J. Human haptoglobins. Curr Top Hematol 1978;1:151–192 [PubMed] [Google Scholar]
- 34.Schaer DJ, Alayash AI. Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid Redox Signal 2010;12:181–184 [DOI] [PubMed] [Google Scholar]
- 35.Imai K. The haemoglobin enzyme. Nature 1999;401:437, 439. [DOI] [PubMed] [Google Scholar]
- 36.Ascenzi P, Bocedi A, Visca P, et al. Hemoglobin and heme scavenging. IUBMB Life 2005;57:749–759 [DOI] [PubMed] [Google Scholar]
- 37.Schaer DJ, Vinchi F, Ingoglia G, Tolosano E, Buehler PW. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front Physiol 2014;5:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin DC, Hyen Baek J, Hassell K, et al. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic Biol Med 2015;82:50–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato GJ. Haptoglobin halts hemoglobin's havoc. J Clin Invest 2009;119:2140–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alayash AI, Andersen CB, Moestrup SK, Bulow L. Haptoglobin: the hemoglobin detoxifier in plasma. Trends Biotechnol 2013;31:2–3 [DOI] [PubMed] [Google Scholar]
- 41.Kaempfer T, Duerst E, Gehrig P, et al. Extracellular hemoglobin polarizes the macrophage proteome toward Hb-clearance, enhanced antioxidant capacity and suppressed HLA class 2 expression. J Proteome Res 2011;10:2397–2408 [DOI] [PubMed] [Google Scholar]
- 42.Durnford A, Dunbar J, Galea J, et al. Haemoglobin scavenging after subarachnoid haemorrhage. Acta Neurochir Suppl 2015;120:51–54 [DOI] [PubMed] [Google Scholar]
- 43.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature 2001;409:198–201 [DOI] [PubMed] [Google Scholar]
- 44.Sadrzadeh SM, Bozorgmehr J, Haptoglobin phenotypes in health and disorders. Am J Clin Pathol 2004;121 (Suppl.):S97–S104 [DOI] [PubMed] [Google Scholar]
- 45.Graversen JH, Madsen M, Moestrup SK. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol 2002;34:309–314 [DOI] [PubMed] [Google Scholar]
- 46.Madsen M, Graversen JH, Moestrup SK. Haptoglobin and CD163: captor and receptor gating hemoglobin to macrophage lysosomes. Redox Rep 2001;6:386–388 [DOI] [PubMed] [Google Scholar]
- 47.Buechler C, Eisinger K, Krautbauer S. Diagnostic and prognostic potential of the macrophage specific receptor CD163 in inflammatory diseases. Inflamm Allergy Drug Targets 2013;12:391–402 [DOI] [PubMed] [Google Scholar]
- 48.Gordon S. Homeostasis: a scavenger receptor for haemoglobin. Curr Biol 2001;11:R399–R401 [DOI] [PubMed] [Google Scholar]
- 49.Noyes WD, Garby L. Rate of haptoglobin in synthesis in normal man: determinations by the return to normal levels following hemoglobin infusion. Scand J Clin Lab Invest 1967;20:33–38 [PubMed] [Google Scholar]
- 50.Faulstick DA, Lowenstein J, Yiengst MJ. Clearance kinetics of haptoglobin-hemoglobin complex in the human. Blood 1962;20:65–71 [PubMed] [Google Scholar]
- 51.Van Vlierberghe H, Delanghe J. Haptoglobin polymorphism and iron hemostasis. Clin Chem 2003;49:708–709 [DOI] [PubMed] [Google Scholar]
- 52.McCormick DJ, Atassi MZ. Hemoglobin binding with haptoglobin: delineation of the haptoglobin binding site on the alpha-chain of human hemoglobin. J Protein Chem 1990;9:735–742 [DOI] [PubMed] [Google Scholar]
- 53.Nantasenamat C, Prachayasittikul V, Bulow L. Molecular modeling of the human hemoglobin-haptoglobin complex sheds light on the protective mechanisms of haptoglobin. PLoS One 2013;8:e62996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajapurkar MM, Hegde U, Bhattacharya A, Alam MG, Shah SV. Effect of deferiprone, an oral iron chelator, in diabetic and non-diabetic glomerular disease. Toxicol Mech Methods 2013;23:5–10 [DOI] [PubMed] [Google Scholar]
- 55.Alam SR, Shah AS, Richards J, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging 2012;5:559–565 [DOI] [PubMed] [Google Scholar]
- 56.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care 2007;30:1926–1933 [DOI] [PubMed] [Google Scholar]
- 57.Shah SV, Alam MG. Role of iron in atherosclerosis. Am J Kidney Dis 2003;41(3 Suppl. 1):S80–S83 [DOI] [PubMed] [Google Scholar]
- 58.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev 2012;70:257–265 [DOI] [PubMed] [Google Scholar]
- 59.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qian SY, Buettner GR. Iron and dioxygen chemistry is an important route to initiation of biological free radical oxidations: an electron paramagnetic resonance spin trapping study. Free Radic Biol Med 1999;26:1447–1456 [DOI] [PubMed] [Google Scholar]
- 61.Melamed-Frank M, Lache O, Enav BI, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood 2001;98:3693–3698 [DOI] [PubMed] [Google Scholar]
- 62.Amiri AA, Hashemi-Soteh MB, Haghshenas MR, Daneshvar F, Rastegar A, Farazmand T. Haptoglobin polymorphism in individuals with type 2 diabetic microangiopathy. N Am J Med Sci 2013;5:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nevo SS, Sutton HE. Association between response to typhoid vaccination and known genetic markers. Am J Hum Genet 1968;20:461–469 [PMC free article] [PubMed] [Google Scholar]
- 64.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996;42:1589–1600 [PubMed] [Google Scholar]
- 65.Delanghe JR, Langlois MR, De Buyzere ML. Haptoglobin polymorphism: a key factor in the proatherogenic role of B cells? Atherosclerosis 2011;217:80–82 [DOI] [PubMed] [Google Scholar]
- 66.Arredouani M, Matthijs P, Van Hoeyveld E, et al. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 2003;108:144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guetta J, Strauss M, Levy NS, Fahoum L, Levy AP. Haptoglobin genotype modulates the balance of Th1/Th2 cytokines produced by macrophages exposed to free hemoglobin. Atherosclerosis 2007;191:48–53 [DOI] [PubMed] [Google Scholar]
- 68.Vanuytsel T, Vermeire S, Cleynen I. The role of haptoglobin and its related protein, zonulin, in inflammatory bowel disease. Tissue Barriers 2013;1:e27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011;91:151–175 [DOI] [PubMed] [Google Scholar]
- 70.Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res Clin Pract 2014;106:312–318 [DOI] [PubMed] [Google Scholar]
- 71.Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One 2012;7:e37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saaristo A, Tammela T, Farkkila A, et al. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol 2006;169:1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007;170:1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev 2002;16:773–783 [DOI] [PubMed] [Google Scholar]
- 75.Cockerill GW, Gamble JR, Vadas MA. Angiogenesis: models and modulators. Int Rev Cytol 1995;159:113–160 [DOI] [PubMed] [Google Scholar]
- 76.Oh MK, Park HJ, Lee JH, Bae HM, Kim IS. Single chain precursor prohaptoglobin promotes angiogenesis by upregulating expression of vascular endothelial growth factor (VEGF) and VEGF receptor2. FEBS Lett 2015;589:1009–1017 [DOI] [PubMed] [Google Scholar]
- 77.Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest 1993;91:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreno PR, Purushothaman M, Purushothaman KR. Plaque neovascularization: defense mechanisms, betrayal, or a war in progress. Ann N Y Acad Sci 2012;1254:7–17 [DOI] [PubMed] [Google Scholar]
- 79.Moreno PR, Sanz J, Fuster V. Promoting mechanisms of vascular health: circulating progenitor cells, angiogenesis, and reverse cholesterol transport. J Am Coll Cardiol 2009;53:2315–2323 [DOI] [PubMed] [Google Scholar]
- 80.Gasomediators OlasB. (NO, CO, and HS) and their role in hemostasis and thrombosis. Clin Chim Acta 2015;445:115–121 [DOI] [PubMed] [Google Scholar]
- 81.Suslova TE, Sitozhevskii AV, Ogurkova ON, et al. Platelet hemostasis in patients with metabolic syndrome and type 2 diabetes mellitus: cGMP- and NO-dependent mechanisms in the insulin-mediated platelet aggregation. Front Physiol 2014;5:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sertorio JT, Lacchini R, Amaral LM, et al. Haptoglobin polymorphism affects nitric oxide bioavailability in preeclampsia. J Hum Hypertens 2013;27:349–354 [DOI] [PubMed] [Google Scholar]
- 83.Burekovic A, Dizdarevic-Bostandzic A, Godinjak A. Poorly regulated blood glucose in diabetic patients: predictor of acute infections. Med Arh 2014;68:163–166 [PubMed] [Google Scholar]
- 84.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003;26:510–513 [DOI] [PubMed] [Google Scholar]
- 85.Elagib AA, Kider AO, Akerstrom B, Elbashir MI. Association of the haptoglobin phenotype (1-1) with falciparum malaria in Sudan. Trans R Soc Trop Med Hyg 1998;92:309–311 [DOI] [PubMed] [Google Scholar]
- 86.Mu AK, Bee PC, Lau YL, Chen Y. Identification of protein markers in patients infected with Plasmodium knowlesi, Plasmodium falciparum and Plasmodium vivax. Int J Mol Sci 2014;15:19952–19961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nyakeriga AM, Troye-Blomberg M. Haptoglobin phenotypes and iron status in children living in a malaria endemic area of Kenyan coast. Acta Trop 2013;126:127–131 [DOI] [PubMed] [Google Scholar]
- 88.Quaye IK. Haptoglobin, inflammation and disease. Trans R Soc Trop Med Hyg 2008;102:735–742 [DOI] [PubMed] [Google Scholar]
- 89.Allen CE, Schmitt MP. Utilization of host iron sources by Corynebacterium diphtheriae: multiple hemoglobin-binding proteins are essential for the use of iron from the hemoglobin-haptoglobin complex. J Bacteriol 2015;197:553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tiedemann MT, Pinter TB, Stillman MJ. Insight into blocking heme transfer by exploiting molecular interactions in the core Isd heme transporters IsdA-NEAT, IsdC-NEAT, and IsdE of Staphylococcus aureus. Metallomics 2012;4:751–760 [DOI] [PubMed] [Google Scholar]
- 91.Pishchany G, Haley KP, Skaar EP. Staphylococcus aureus growth using human hemoglobin as an iron source. J Vis Exp 2013;(72):50072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adams N, Johnson MD, Storm DW, Maves RC. Acute focal bacterial nephritis due to methicillin-resistant Staphylococcus aureus in an immunocompetent adult. Infection 2014;42:433–436 [DOI] [PubMed] [Google Scholar]
- 93.Messad N, Prajsnar TK, Lina G, et al. Existence of a colonizing Staphylococcus aureus strain isolated in diabetic foot ulcers. Diabetes 2015;64:2991–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect 2012;14:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shenoy M, Somayajulu GL, Bhaskaran CS. Haptoglobulin phenotypes in leprosy. Lepr India 1983;55:566–569 [PubMed] [Google Scholar]
- 96.Dobler CC, Flack JR, Marks GB. Risk of tuberculosis among people with diabetes mellitus: an Australian nationwide cohort study. BMJ Open 2012;2:e000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre MJ. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health 2012;66:519–523 [DOI] [PubMed] [Google Scholar]
- 98.Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015;144:171–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delanghe JR, Langlois MR, Boelaert JR, et al. Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS 1998;12:1027–1032 [PubMed] [Google Scholar]
- 100.Speeckaert R, Colebunders B, Boelaert JR, et al. Association of haptoglobin phenotypes with the development of Kaposi's sarcoma in HIV patients. Arch Dermatol Res 2011;303:763–769 [DOI] [PubMed] [Google Scholar]
- 101.Speeckaert R, Speeckaert MM, Padalko E, Claeys LR, Delanghe JR. The haptoglobin phenotype is associated with the Epstein-Barr virus antibody titer. Clin Chem Lab Med 2009;47:826–828 [DOI] [PubMed] [Google Scholar]
- 102.Pomorska-Mol M, Markowska-Daniel I, Kwit K, Czyzewska E, Dors A, Rachubik J, Pejsak Z. Immune and inflammatory response in pigs during acute influenza caused by H1N1 swine influenza virus. Arch Virol 2014;159:2605–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinez-Orellana P, Martorell J, Vidana B, et al. Clinical response to pandemic h1n1 influenza virus from a fatal and mild case in ferrets. Virol J 2015;12:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Griebel P, Hill K, Stookey J. How stress alters immune responses during respiratory infection. Anim Health Res Rev 2014;15:161–165 [DOI] [PubMed] [Google Scholar]
- 105.Abdullah M, Goldmann T. Pulmonary haptoglobin (pHp) is part of the surfactant system in the human lung. Diagn Pathol 2012;7;1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis 2015;84:22–38 [DOI] [PubMed] [Google Scholar]
- 107.Sena CM, Pereira AM, Carvalho C, et al. Type 2 diabetes aggravates Alzheimer's disease-associated vascular alterations of the aorta in mice. J Alzheimers Dis 2015;45:127–138 [DOI] [PubMed] [Google Scholar]
- 108.Rosales-Corral S, Tan DX, Manchester L, Reiter RJ. Diabetes and Alzheimer's disease: two overlapping pathologies with the same background: oxidative stress. Oxid Med Cell Longev 2015;2015:985845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Costa-Mallen P, Checkoway H, Zabeti A, et al. The functional polymorphism of the hemoglobin-binding protein haptoglobin influences susceptibility to idiopathic Parkinson's disease. Am J Med Genet B Neuropsychiatr Genet 2008;147B:216–222 [DOI] [PubMed] [Google Scholar]
- 110.Arguelles S, Venero JL, Garcia-Rodriguez S, et al. Use of haptoglobin and transthyretin as potential biomarkers for the preclinical diagnosis of Parkinson's disease. Neurochem Int 2010;57:227–234 [DOI] [PubMed] [Google Scholar]
- 111.Spagnuolo MS, Maresca B, La Marca V, Carrizzo A, Veronesi C, Cupidi C, Piccoli T, Maletta RG, Bruni AC, Abrescia P, Cigliano L. Haptoglobin interacts with apolipoprotein E and beta-amyloid and influences their crosstalk. ACS Chem Neurosci 2014;5:837–847 [DOI] [PubMed] [Google Scholar]
- 112.Song IU, Kim YD, Chung SW, Cho HJ. Association between serum haptoglobin and the pathogenesis of Alzheimer's disease. Intern Med 2015;54:453–457 [DOI] [PubMed] [Google Scholar]
- 113.Priyadarshini M, Kamal MA, Greig NH, et al. Alzheimer's disease and type 2 diabetes: exploring the association to obesity and tyrosine hydroxylase. CNS Neurol Disord Drug Targets 2012;11:482–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson's disease, insulin resistance and novel agents of neuroprotection. Brain 2013;136:374–384 [DOI] [PubMed] [Google Scholar]
- 115.Murthy SB, Levy AP, Duckworth J, et al. Presence of haptoglobin-2 allele is associated with worse functional outcomes after spontaneous intracerebral hemorrhage. World Neurosurg 2015;83:583–587 [DOI] [PubMed] [Google Scholar]
- 116.Kantor E, Bayir H, Ren D, et al. Haptoglobin genotype and functional outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg 2014;120:386–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chapelle JP, Albert A, Smeets JP, Heusghem C, Kulbertus HE. Effect of the haptoglobin phenotype on the size of a myocardial infarct. N Engl J Med 1982;307:457–463 [DOI] [PubMed] [Google Scholar]
- 118.Holme I, Aastveit AH, Hammar N, Jungner I, Walldius G. Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Ann Med 2009;41:522–532 [DOI] [PubMed] [Google Scholar]
- 119.Haas B, Serchi T, Wagner DR, et al. Proteomic analysis of plasma samples from patients with acute myocardial infarction identifies haptoglobin as a potential prognostic biomarker. J Proteomics 2011;75:229–236 [DOI] [PubMed] [Google Scholar]
- 120.Costacou T, Secrest AM, Ferrell RE, Orchard TJ. Haptoglobin genotype and cerebrovascular disease incidence in type 1 diabetes. Diab Vasc Dis Res 2014;11:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes 2008;57:1702–1706 [DOI] [PubMed] [Google Scholar]
- 122.Staals J, Bons JA, van Oostenbrugge RJ, et al. A SELDI-TOF-MS study in lacunar stroke with subsequent haptoglobin phenotyping. Curr Neurovasc Res 2008;5:93–98 [DOI] [PubMed] [Google Scholar]
- 123.Braeckman L, De Bacquer D, Delanghe J, Claeys L, De Backer G. Associations between haptoglobin polymorphism, lipids, lipoproteins and inflammatory variables. Atherosclerosis 1999;143:383–388 [DOI] [PubMed] [Google Scholar]
- 124.Roguin A, Ribichini F, Ferrero V, et al. Haptoglobin phenotype and the risk of restenosis after coronary artery stent implantation. Am J Cardiol 2002;89:806–810 [DOI] [PubMed] [Google Scholar]
- 125.Delanghe J, Cambier B, Langlois M, et al. Haptoglobin polymorphism, a genetic risk factor in coronary artery bypass surgery. Atherosclerosis 1997;132:215–219 [DOI] [PubMed] [Google Scholar]
- 126.Ijas P, Saksi J, Soinne L, et al. Haptoglobin 2 allele associates with unstable carotid plaque and major cardiovascular events. Atherosclerosis 2013;230:228–234 [DOI] [PubMed] [Google Scholar]
- 127.Ruzevick J, Jackson C, Pradilla G, Garzon-Muvdi T, Tamargo RJ. Aneurysm formation in proinflammatory, transgenic haptoglobin 2-2 mice. Neurosurgery 2013;72:70–76 [DOI] [PubMed] [Google Scholar]
- 128.Lioupis C, Barbatis C, Drougou A, et al. Association of haptoglobin genotype and common cardiovascular risk factors with the amount of iron in atherosclerotic carotid plaques. Atherosclerosis 2011;216:131–138 [DOI] [PubMed] [Google Scholar]
- 129.Delanghe JR, Duprez DA, De Buyzere ML, et al. Refractory hypertension is associated with the haptoglobin 2-2 phenotype. J Cardiovasc Risk 1995;2:131–136 [PubMed] [Google Scholar]
- 130.Delanghe JR, Duprez DA, De Buyzere ML, et al. Haptoglobin polymorphism and complications in established essential arterial hypertension. J Hypertens 1993;11:861–867 [DOI] [PubMed] [Google Scholar]
- 131.Burbea Z, Nakhoul F, Rosenberg S, et al. Role of haptoglobin phenotype in end-stage kidney disease. Nephron Exp Nephrol 2004;97:e71–e76 [DOI] [PubMed] [Google Scholar]
- 132.Asleh R, Levy AP. In vivo and in vitro studies establishing haptoglobin as a major susceptibility gene for diabetic vascular disease. Vasc Health Risk Manag 2005;1:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levy AP, Roguin A, Hochberg I, et al. Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med 2000;343:969–970 [DOI] [PubMed] [Google Scholar]
- 134.Levy AP, Hochberg I, Jablonski K, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: the Strong Heart Study. J Am Coll Cardiol 2002;40:1984–1990 [DOI] [PubMed] [Google Scholar]
- 135.Szafranek T, Marsh S, Levy AP. Haptoglobin: a major susceptibility gene for diabetic vascular complications. Exp Clin Cardiol 2002;7:113–119 [PMC free article] [PubMed] [Google Scholar]
- 136.Levy AP, Larson MG, Corey D, Lotan R, Vita JA, Benjamin EJ. Haptoglobin phenotype and prevalent coronary heart disease in the Framingham offspring cohort. Atherosclerosis 2004;172:361–365 [DOI] [PubMed] [Google Scholar]
- 137.Mogarekar MR, Hampe MH. Haptoglobin2-2 phenotype is an additional risk factor of retinopathy in type 2 diabetes mellitus. Indian J Hum Genet 2013;19:154–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang F, Huang W, Li A. Serum haptoglobin suppresses T-lymphocyte functions following burns. Chin Med Sci J 1996;11:180–183 [PubMed] [Google Scholar]
- 139.Burtis CA, Ashwood ER, Bruns DE, Tietz NW (Eds). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 5th ed. St. Louis, Mo, Saunders, 2013 [Google Scholar]
- 140.Piessens MF, Marien G, Stevens E. Decreased haptoglobin levels in respiratory allergy. Clin Allergy 1984;14:287–293 [DOI] [PubMed] [Google Scholar]
- 141.Panter SS, Sadrzadeh SM, Hallaway PE, Haines JL, Anderson VE, Eaton JW. Hypohaptoglobinemia associated with familial epilepsy. J Exp Med 1985;161:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gloria-Bottini F, Lucarelli P, Saccucci P, et al. Genetic polymorphism and idiopathic generalized epilepsy: evidence of interaction between haptoglobin and ACP1 systems. Neuropediatrics 2008;39:357–358 [DOI] [PubMed] [Google Scholar]
- 143.Veiner HL, Gorbatov R, Vardi M, et al. Pharmacogenomic interaction between the haptoglobin genotype and vitamin E on atherosclerotic plaque progression and stability. Atherosclerosis 2015;239:232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blum S, Vardi M, Brown JB, et al. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics 2010;11:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Farid N, Inbal D, Nakhoul N, et al. Vitamin E and diabetic nephropathy in mice model and humans. World J Nephrol 2013;2:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Levy NS, Vardi M, Blum S, et al. An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype. Clin Chem Lab Med 2013;51:1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thomsen JH, Etzerodt A, Svendsen P, Moestrup SK. The haptoglobin-CD163-heme oxygenase-1 pathway for hemoglobin scavenging. Oxid Med Cell Longev 2013;2013:523652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guthrie PA, Abdollahi MR, Gaunt T, et al. Haptoglobin duplicon, hemoglobin, and vitamin C: analyses in the British Women's Heart and Health Study and Caerphilly Prospective Study. Dis Markers 2014;2014:529456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vardi M, Levy NS, Levy AP. Vitamin E in the prevention of cardiovascular disease: the importance of proper patient selection. J Lipid Res 2013;54:2307–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]