Abstract
The apple buprestid beetle, Agrilus mali Matsumura, is an invasive pest causing significant damages to rare wild apple forests of Xinjiang. The morphology, abundance and distribution of antennal sensilla in both sexes of this pest were examined. We found that the antennae of A. mali females were longer than those of males. Five types of antennal sensilla were characterized, including trichodea (subtypes Tr.1, Tr.2, and Tr.3), chaetica (subtypes Sc.1, Sc.2, Sc.3, and Sc.4), basiconica (subtypes Ba. 1, Ba. 2, Ba. 3 and Ba.4), Böhm bristles (subtypes BB. 1, and BB. 2), and multiporous grooved sensilla. The most abundant sensilla of Ba.2 tended to occur mainly on flagellomeres 5–8 in both sexes. The last three flagellomeres tended to have the most abundant Tr.1 in both sexes. Overall, the abundance and distribution of these sensilla appeared to be highly conserved in both sexes, and their olfactory organs seemed to cluster on flagellomeres 6–8. However, some sex dimorphisms were also observed. Tr.3 and BB.2 were found only in females. Sensilla of Sc.2 were found on the pedicel and first two flagellomeres only in males. When compared with males, females showed a higher number of Sc.3, but a lower number of Sc.4 on the pedicel. These results indicate that contact cues could be important in intersexual communication in A. mali. The functional roles of these sensilla and their implications in A. mali behaviors are discussed, and further studies of identified chemosensitive sensilla can provide a foundation for developing semiochemical-based management strategies.
Keywords: olfactory sensillum, receptor, sex dimorphism, scanning electron microscopy, semiochemical, contact cue
Many studies have showed that volatile semiochemicals are the principle sensory signals for insects (Crook et al. 2005, Wang et al. 2010, Mamidala et al. 2013). For example, the invasive emerald ash borer (Agrilus planipennis Fairmaire) adults were attracted to volatiles emitted by stressed ash trees, and preferentially oviposited on girdled trees (Rodriguez-Saona et al. 2006) . This beetle might also use unknown sex pheromones (Crook and Mastro 2010). As documented for many Coleopterans, the antennae of beetles can show synergistic responses to pheromones in the presence of host plant volatiles (Lopes et al. 2002, Ploomi et al. 2003, Crook et al. 2008, Giulio et al. 2012, MacKay et al. 2014). Thus, antennae are the primary olfactory organ playing significant roles in host plant selection, ovipositional site search, or intersexual communication of these insects (Zacharuk 1985, Shields 2004, Zhang et al. 2015). Indeed, antennae of these insects have numerous sensory structures called sensilla, where volatiles can be recognized by odorant binding proteins (OBPs), and subsequent signal transduction can lead to the ultimate perception of quality and quantity of odors in the brain (Zacharuk 1980, Pitts and Zwiebel 2006). It was found that the morphology and ultrastructure of anetennal sensilla could be extremely variable among different species of beetles (Scott and Gara 1975; Merivee et al. 1998, 1999, 2002; Volkovitsh 2001; Crook et al. 2008; Ren et al. 2014). However, a few studies have documented antennal sensilla of beetles (e.g., Melanophila sp.) in the Buprestidae, which is a large family with hundreds of species (Scott and Gara 1975, Volkovitsh 2001, Crook et al. 2008).
The apple buprestid, Agrilus mali Matsumura (Coleoptera, Buprestidae), is an important wood-boring beetle causing significant damages to apple trees (Malus Mill.) (Rosaceae) (Cui et al. 2015). It is native to Korea, northern China, and far eastern areas of Russia (Chebanov 1977, Sun et al. 1979; Nikritin 1994, Savotikov and Smetnik 1995). A. mali was considered as a sporadic pest in major apple production areas (e.g., Shaanxi and Shandong) in China, but its occurrence in the Xinjiang area was first discovered in an apple orchard in Xinyuan Co. in 1995 (Cui et al. 2015). Since then, this beetle has rapidly invaded into wild apple [Malus sieversii (Ledeb.) Roem] forests in Yili Valley of Tianshan Mountains, where it has killed thousands of wild apple trees (Cui et al. 2015). In Russia, fumigation and thorough pruning were used to control this pest (Chebanov 1977, Nikritin 1994, Savotikov and Smetnik 1995). But it is difficult to carry out such control measures in the wild fruit forests growing along steep mountain slopes. Detection and monitoring tools are in urgent need for the prevention of rapid spread of this beetle. Characterization of A. mali’s olfactory organs could provide an important starting point and valuable information for further behavioral and physiological studies, which can ultimately lead to developing practical monitoring and management strategies with semiochemicals. Therefore, A. mali beetles were collected from the introduced regions of Xinjiang in China, and the morphology and ultrastructure of antennal sensilla in this beetle was characterized using scanning electron microscopy (SEM). The objectives of this study are to: 1) provide the first detailed fine-structure characterization of antennal sensilla in A. mali; 2) explore the number, distribution and function of these sensilla; and 3) identify sexual differences in antennal sensilla of A. mali.
Materials and Methods
Insects
Apple tree branches (∼0.5 m in length) containing A. mali prepupae were cut from infested trees in the wild fruit forest located in Xinyuan Co., Xinjiang Uygur Autonomous Region, China (43°22′30′′N, 83°34′30′′E) during the summer of 2014. Some collected branches were kept in plastic containers (23 × 27 × 50 cm3) at room temperatures, and checked four times per week for the emergence of adults. Other infested branches were stored in a refrigerator at 4°C in order to delay beetle development until use in the laboratory. Newly emerged adults were fed with fresh foliage of apple (Malus domestica Borkh.) in a plastic container (16 × 25 × 9 cm3), which was covered with wet sand at the bottom (Crook et al. 2008).
Scanning Electron Microscopy
Antennae were carefully excised from the antennal sockets with fine scissors under a stereomicroscope (Nikon SMZ-1500, Nikon Corporation, Tokyo, Japan), and fixed immediately in 2.5% glutaraldehyde at 4°C for 12 h. After washing with 70% ethanol twice, an ultrasonic cleaner was used to clean them for 20 s. Then they were rinsed in a phosphate buffered saline (0.2 M, pH = 7.2) four times for 20 min. After that, the antennae were dehydrated successively for 20 min each in an ethanol series (30, 50, 70, 90, and 100% twice), followed by ethanol: tertiary butanol (TBA) at 3:1, 1:1, and 1:3 for 15 min each. Finally, they are set in the 100% TBA for 30 min. After TBA removal, antennae were dried in a freeze-drying chamber for 3 h. Antennae were then glued onto aluminum pin mounts with double-sided carbon adhesive tape, and sputtered with gold for 30 s. At least 10 beetles per sex were examined using a scanning electron microscope (Hitachi S-3400N, Hitachi Group, Tokyo, Japan). Classification of sensilla in A. mali was based on studies of Schneider (1964), Zacharuk (1980, 1985), and Shields (2004).
Statistical Analysis
Micrographs were first visually inspected for points of reference that could be used to avoid missing areas and counting errors. In order to avoid counting any sensillum more than once, different types of sensilla were marked with color-coded dots using the image processing software Image J 1.50 (U.S. National Institute of Health). Measurements (µm) of length and width for antennomeres (5 replicates per sex) and sensilla types (20 replicates per sex) were made using Photoshop CS6 (Adobe Systems, San Jose, CA, USA). The distribution of each type of sensilla was examined on the dorsal surface of every antennomere, and the numbers were recorded for five individuals per sex. Because SEMs offer a view of only one side of the antenna, we only examined sensilla on the dorsal surface (abundant in sensilla) of each antennum for the purpose of easy comparisons between sexes. Significant differences between females and males for the abovementioned parameters were determined using Student’s t-tests (SPSS Statistics 20.0 for Mac). One-way analysis of variance (ANOVA) was used to identify differences in the numbers of sensilla among antennomeres or among types of sensilla (SPSS Statistics 20.0 for Mac). Separation of means was conducted by using Tukey’s test after significant ANOVA. If necessary, data were log-transformed to meet the requirements of homoscedasticity and normality in these analyses.
Results
General Structure of Antennae in A. mali
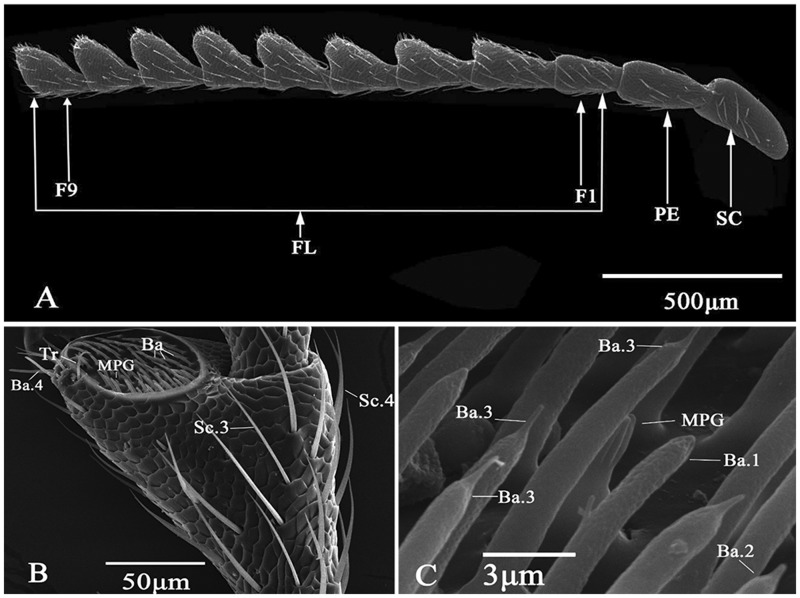
The serrate-shaped antennae of A. mali are located between compound eyes, and attached to the head by the basal segment called scape (Fig. 1A). Distal to the scape are the other two segments, pedicel and flagellum, where the former is the attachment point for the latter. In A. mali, flagellum contains nine distinct flagellomeres. The cross-section shapes of the scape, pedicel and flagellomere 1 are round. Flagellomeres 2–9 are slightly flattened and triangular in shape, such that the antennae of A. mali appear serrated. The basal part of each antennomere is smaller in width, and broadened gradually toward the distal part. Each antennomere has a scaly cuticular surface with various types of sensilla (e.g., chaetica, trichodea, and basiconica) (Fig. 1B and C). The bilaterally flattened flagellomeres each bear a large apical depression (i.e., apical or subapical fossae) where various sensilla can also be housed (Fig. 1B).
Fig. 1.
Morphology of A. mali antennae. (A) Female antennae (SC, Scape; PE, pedicel; FL, flagellum; F1–F9, flagellomere 1–9; (B) Sixth flagellomere in a male; (C) Various sensilla in the apical depression of the eighth flagellomere in a female.
The average lengths of antennae in females (1,751.6 μm) were significantly bigger than those in males (1,658.8 μm) (t = 2.84; P < 0.05) (Table 1). Significant differences in the lengths of the sixth (t = 3.35; P < 0.01), seventh (t = 3.92; P < 0.01) and eighth (t = 3.41; P < 0.01) flagellomere were found between males and females, indicating the three flagellomeres could contribute to the behavioral differences between sexes. No significant differences in the lengths for all the other antennomeres were found between sexes. For both males (F = 21.28; df = 10, 99; P < 0.001) and females (F = 22.71; df = 10, 99; P < 0.001), the scape and pedicel were relatively longer among all antennomeres, whereas flagellomeres 6–8 were relatively shorter. There were no significant differences in the width of each antennomere between males and females. The widths of flagellomeres 4 and 5 were relatively bigger, and those of flagellomere 1 were relatively smaller for both males (F = 2.48; df = 10, 99; P < 0.05) and females (F = 2.91; df = 0, 99; P < 0.01).
Table 1.
Length and width (mean ± SE) of each antennal segment (or sub-segment) in male and female A. mali
| Antennomere | Length (μm) |
Width (μm) |
||
|---|---|---|---|---|
| M | F | M | F | |
| Scape | 189.3 ± 10.9a | 195.6 ± 7.3A | 110.1 ± 6.9ab | 98.8 ± 2.7AB |
| Pedicel | 186.0 ± 5.3a | 177.1 ± 5.0B | 101.8 ± 10.5ab | 87.1 ± 1.9AB |
| Flagellomere 1 | 143.0 ± 2.3cde | 143.0 ± 2.3DE | 86.6 ± 7.5b | 80.8 ± 2.3B |
| Flagellomere 2 | 168.4 ± 4.2ab | 166.4 ± 3.3BC | 112.1 ± 9.5ab | 103.4 ± 8.1AB |
| Flagellomere 3 | 158.7 ± 3.2bc | 162.5 ± 3.1BC | 125.4 ± 10.7ab | 113 ± 9.8AB |
| Flagellomere 4 | 148.0 ± 2.8bcd | 154.6 ± 2.2CD | 133.6 ± 9.9a | 116.9 ± 11.2A |
| Flagellomere 5 | 144.0 ± 2.6cd | 150.9 ± 2.6CD | 133.3 ± 9.2a | 117.4 ± 10.5A |
| Flagellomere 6 | 133.2 ± 2.5*de | 143.4 ± 1.8DE | 127.2 ± 8.6ab | 113.9 ± 8.8AB |
| Flagellomere 7 | 129.2 ± 3.1*de | 143.2 ± 1.8DE | 125.8 ± 10.2ab | 115.8 ± 8.1AB |
| Flagellomere 8 | 120.3 ± 3.0*e | 132.8 ± 2.1E | 118.2 ± 9.7ab | 106.9 ± 6.8AB |
| Flagellomere 9 | 143.0 ± 6.0cde | 143.2 ± 6.1DE | 104.6 ± 9.5ab | 90.1 ± 6.7AB |
| Total | 1658.8 ± 22.1* | 1751.6 ± 24.1 | – | – |
Note: *indicates significant differences between males and females (Student’s t-test, P < 0.05) (highlighted in bold); different lowercase and uppercase letters indicate significant differences among antennomeres for a particular trait of males and females, respectively.
Morphology of Antennal Sensilla in A. mali
Sensilla identified on antennae of A. mali were grouped into five morphological classes, and they were sensilla chaetica (subtype 1–4, Sc.1–Sc.4), sensilla trichodea (subtype 1–3, Tr.1–Tr.3), sensilla basiconica (subtype 1–4, Ba.1–Ba.4), multiporous grooved (MPG) sensilla, and Böhm bristles (subtype 1–2, BB.1–BB.2) (Table 2). Generally, the length of these sensilla tended to follow the order of Sc > Tr > Ba > MPG > BB. No significant differences in length were identified between sexes for any type of the identified sensilla.
Table 2.
Morphological characteristics of each type of sensilla in the antennae of A. mali
| Type | Length (μm) | Diameter (μm) | Wall | Tip | Socket |
|---|---|---|---|---|---|
| Sc.1 F | 38.4 ± 2.4 | 2.86 ± 0.2* | Grooved | Sharp | Wide |
| M | 32.7 ± 5.8 | 2.26 ± 0.1 | |||
| Sc.2 F | 63.9 ± 2.3 | 3.20 ± 0.8 | Grooved | Sharp | Tight |
| M | 65.8 ± 2.6 | 3.53 ± 0.2 | |||
| Sc.3 F | 53.5 ± 2.0 | 3.0 ± 0.2 | Grooved | Sharp | Tight |
| M | 48. 2 ± 2.5 | 2.6 ± 0.1 | |||
| Sc.4 F | 55.1 ± 2.2 | 2.2 ± 0.1 | Grooved | Sharp | Wide, porous |
| M | 56.3 ± 2.2 | 2.0 ± 0.1 | |||
| Tr.1 F | 18.7 ± 0.5 | 1.9 ± 0.1 | Smooth, porous | Branches | Tight |
| M | 24.8 ± 3.0 | 2.4 ± 0.2 | |||
| Tr.2 F | 17.2 ± 0.9 | 1.8 ± 0.1 | Smooth, porous | Branches | Tight |
| M | 15.5 ± 1.5 | 1.8 ± 0.1 | |||
| Tr.3 F | 21.8 ± 0.0 | 1.8 ± 0.1 | Smooth, porous | Sharp | Tight |
| M | – | – | |||
| Ba.1 F | 8.9 ± 0.3 | 2.9 ± 0.1* | Smooth, porous | Blunt | – |
| M | 9.1 ± 0.3 | 2.4 ± 0.1 | |||
| Ba.2 F | 11.5 ± 0.3 | 1.4 ± 0.3* | Smooth, porous | Sharp | – |
| M | 10.8 ± 0.3 | 3.0 ± 0.3 | |||
| Ba.3 F | 11.1 ± 0.2 | 2.8 ± 0.1 | Smooth, porous | Sharp | – |
| M | 11.7 ± 0.4 | 2.9 ± 0.1 | |||
| Ba.4 F | 19.3 ± 0.5 | 2.3 ± 0.1 | Grooved | Blunt, papillae | Tight |
| M | 19.3 ± 0.9 | 2.2 ± 0.1 | |||
| MPG F | 4.7 ± 0.1 | 3.3 ± 0.1 | Smooth | Irregular | – |
| M | 7.7 ± 2.5 | 5.3 ± 2.0 | |||
| BB.1 F | 7.0 ± 0.4 | 1. 6 ± 0.4 | Smooth | Blunt | Tight |
| M | 6.4 ± 0.4 | 1. 4 ± 0.8 | |||
| BB.2 F | 7.2 ± 0.1 | 1.4 ± 0.1 | Smooth | Blunt | Tight |
| M | – | – |
Notes: *indicates significant differences between males and females (t-test, P < 0.05) (highlighted in bold); Tr.3 and BB.2 only found in females; F, females; M, males.
Sensilla chaetica (Sc)
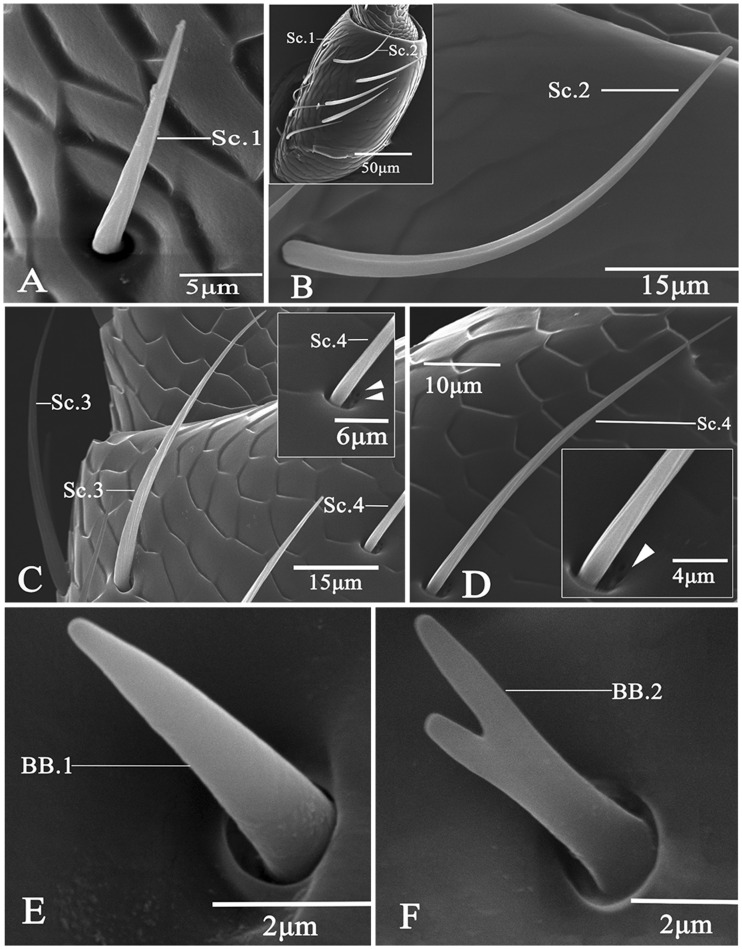
All sensilla chaetica types had a grooved cuticular wall and a sharp tip, and they also have a wide or tight socket (Table 2). Sensilla chaetica subtype 1 (Sc.1) presented a slightly and longitudinally grooved cuticular wall with a wide socket (Fig. 2A); their diameters at the base showed significant differences between males (2.26 μm) and females (2.86 μm) (t = 3.44, P < 0.01); they were 32.7 and 38.4-μm long in males and females, respectively; they were the shortest among all chaetica subtypes. Sensilla of Sc.2 were deeply and longitudinally grooved in cuticular shaft with a tight socket (Fig. 2B); they were the longest among all chaetica types with the mean length being 65.8 μm in males and 63.9 μm in females; the mean basal diameter of these sensilla was 3.53 μm in males and 3.2μm in females. Sensilla of Sc.3 were set into a tight socket, and had obvious longitudinal grooves along the shaft (Fig. 2C; Table 2); they leaned toward the cuticular surface of antennae, and tapered toward the apex; these bristles were 48.2 and 53.5 μm long in males and females, respectively; they were 2.6 and 3.0 μm in the mean basal width in males and females, respectively. Sc.4 were slender with shallow and longitudinal furrows with a sharp tip (Fig. 2D); they were set in a wide and porous socket; they were similar to sensilla basiconica type 3 in size with the mean lengths being 56.3 and 55.1 μm in males and females, respectively; the mean basal diameter was 2.0 μm in males and 2.2 μm in females.
Fig. 2.
Scanning electron micrographs of chaetica and BBs in A. mali. (A) Sensilla chaetica type 1 (Sc. 1); (B) Sensilla chaetica type 2 (Sc. 2) showing the difference in length between Sc.1 and Sc.2; (C) Sensilla chaetica type 3 (Sc. 3); (D) Sensilla chaetica type 4 (Sc. 4) showing a unique open socket; (E) BB type 1 (BB. 1); (F) BB type 2 (BB. 2).
Böhm Bristles
All BBs had a smooth wall and a tight socket, but they appeared different in the apical part (Table 2). Böhm bristles subtype 1 (BB.1) were shorter than basiconic sensilla, and thinner than trichoid sensilla; they were the shortest among all types of sensilla identifed, and had a blunt tip (Fig. 1E); they were about 6.4 (males) to 7.0 (females) μm in length, and 1.4 (males) to 1.6 (females) μm in basal diameter. BB. 2 bristles (Fig. 1F) had a branched apex resembling the letter “V”; both tips in the apical part were cone-shaped, blunt, and short; they had a mean of 7.2 μm in length, and 1.4 μm in width; sensilla of BB.2 were found only in females.
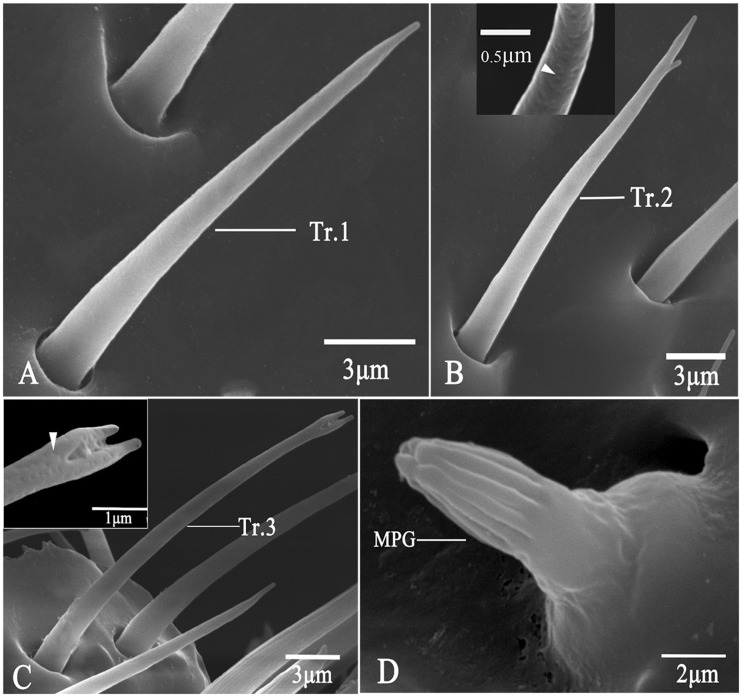
Sensilla Trichodea (Tr)
Trichoid sensilla were smooth-walled and multiparous hairs, and they had a branched or sharp tip and a tight socket (Table 2). They were the second longest among all types of sensilla. Sensilla trichodea subtype 1 (Tr.1) were straight hairs, and they had no branched tips (Fig. 3A); they had a mean length of 24.8 μm in males and 18.7 μm in females; the mean basal diameter was 2.4 μm in males and 1.9 μm in females. Tr.2 had a smooth cuticular surface, and a distinctly bifurcated tip resembling the letter “r” (Fig. 3B); they were measured about 15.5 (males) to 17.2 (females) μm in length, and 1.8 μm in basal diameter. Tr.3 had a v-shaped tip (Fig. 3C); they were similar to Tr.1 and Tr.2 in size (mean length: 21.8 μm; mean diameter: 1.8 μm), but they were found only in females.
Fig. 3.
Scanning electron micrographs of trichodea and MPG sensilla (MPG). in A. mali. (A) Sensilla trichodea type 1 (Tr.1); (B) Sensilla trichodea type 2 (Tr.2); (C) Sensilla trichodea type 3 (Tr.3) showing the unique apex (arrowhead); (D) MPG.
MPG Sensilla
MPG exhibited a uniquely irregular apex, and it had no socket (Table 2; Fig. 3D). These thick pegs had a longitudinally grooved surface in the distal part, but a rugged surface in the basal part (Fig. 3D). The apical part had approximately ten deep longitudinal grooves, which extended about two thirds of the pegs. They had a mean length of 7.7 μm in males 4.7 μm in females. The mean basal diameter was 5.3 and 3.3 μm in males and females, respectively.
Sensilla Basiconica (Ba)
Basiconic sensilla had a blunt or sharp tip without setting in a socket (Table 2). hese pegs were straight, conical, and smooth-walled without longitudinal grooves, and they all had numerous pores on the cuticular surface (Fig. 4). Sensilla basiconica subtype 1 (Ba.1) had a blunt tip looking like a dome (Fig. 4A); they were the shortest among all basiconic sensilla, measuring 8.9 (females) to 9.1 (males) μm in length. The basal diameter of Ba.1 in females (2.9 μm) was significantly bigger than that in males (2.4 μm) (t = 3.70, P < 0.001). Ba.2 was characterized by a short and sharp tip (Fig. 4B), and they were 10.8 (males) to 11.5 (females) μm in length. When compared with females (1.4 μm), males (3.0 μm) showed a significantly higher basal diameter in Ba.2 (t = 4.24, P < 0.001). Sensilla of Ba.3 were pegs with a filament-like long tip (Fig. 4C), and they had a mean length of 11.1μm in females and 11.7 μm in males; their mean width at the base was about 2.8 μm in females and 2.9μm in males. Sensilla of Ba.4 presented a longitudinally and deeply grooved cuticular wall (Table 2; Fig. 4D); these finger-shaped and curved sensilla were set in a tight socket, and they occurred around the apex of the distal eight flagellomeres in both males and females; they possessed a single, oval-shaped pore at the papillatus tip; they had a mean length of 19.3 μm in both sexes, and a mean basal width of 2.2 (males) and 2.3 (females) μm.
Fig. 4.
Scanning electron micrographs of basiconic sensilla in A. mali. (A) Sensilla basiconica type 1 (Ba.1) showing abundant pores over the entire surface (arrowhead); (B) Sensilla basiconica type 2 (Ba.2) showing abundant pores over the entire surface (arrowhead); (C) Sensilla basiconica type 3 (Ba.3); (D) Sensilla basiconica type 4 (Ba.4).
Distribution and Abundance of Antennal Sensilla
Sensilla of BB.1 were located in clusters on the joint between the scape and the head of both sexes. Females and males had 13.0 (±2.4) and 7.0 (±1.7) BB.1 per antennum, respectively, and the difference between sexes in the numbers of BB.1 was significant (t = 2.58, P < 0.05). BB.2 were rare, and they were also found in the first segment of antennae in females.
For chaetica, sensilla of Sc.1 were found only on the “eyelid”-shaped socket between scape and pedicel (Table 3). Males had 5.0 (±0.7) Sc.2 per antennum on the scape, whereas females had 8.0 (±1.6) per antennum; but the difference between sexes was not statistically significant. They were also identified on the pedicel (5.0 per antennum), flagellomere 1 (7.0 per antennum), and flagellomere 2 (10.0 per antennum) in males, but not in females. Sensilla of Sc.3 were found on the pedicel and flagellum of female and male antennae. Higher counts of Sc.3 on the pedicel of females were found than those of males (t = 2.39, P < 0.05). Sensilla of Sc.3 were also identified on every flagellomere in both females and males. When compared with males, higher abundances of Sc.3 in females were found on flagellomere 4 (t = 3.43, P < 0.01), but not on all the other flagellomeres. Sc.3 sensilla were evenly distributed along the entire flagellum for males, but not for females (F = 6.87; df = 9, 40; P < 0.001). Flagellomere 2 had the most numerous Sc.3 among all flagellomeres in females. Sc.4 occurred singly on the sockets of pedicel and all flagellomeres. The abundance of Sc.4 on the pedicel of males was significantly higher than that of females (t = 2.98, P < 0.05). Males presented higher numbers of Sc.4 than females on flagellomere 1 (t = 3.14, P < 0.05), but not on all the other flagellomeres. No significant differences in the number of Sc.4 were found among flagellomeres in males. Flagellomeres 5 and 6 showed more abundant Sc.4 than flagellomere 1 in females (F = 4.03; df = 9, 40; P < 0.01).
Table 3.
Abundance and distribution of different sensilla on the antennae of female and male A. mali
| Gender | Sc.3 | Sc.4 | Ba.1 | Ba.2 | Ba.3 | Ba.4 | MPG | Tr.1 | |
|---|---|---|---|---|---|---|---|---|---|
| F1 | Male | 20.0a | 3.6*a | – | – | – | – | – | – |
| (±1.4) | (±0.4) | – | – | – | – | – | – | ||
| Female | 18.4B | 2.0B | – | – | – | – | – | – | |
| (±1.0) | (±0.3) | – | – | – | – | – | – | ||
| F2 | Male | 23.4a | 4.2a | 4.0a | 24.2d | 3.6a | 4.0d | 1.4b | 3.2c |
| (±0.8) | (±0.4) | (±1.4) | (±2.3) | (±1.4) | (±0.3) | (±0.2) | (±0.6) | ||
| Female | 26.6A | 4.0AB | 5.2A | 26.8C | 4.8A | 4.6D | 1.4C | 2.8D | |
| (±1.2) | (±1.3) | (±0.8) | (±4.7) | (±3.8) | (±0.7) | (±0.2) | (±0.4) | ||
| F3 | Male | 18.8a | 6.0a | 7.6a | 40.0cd | 10.4a | 5.0cd | 1.4*b | 8.8bc |
| (±0.9) | (±0.4) | (±2.6) | (±7.3) | (±3.9) | (±0.4) | (±0.2) | (±0.7) | ||
| Female | 20.2B | 5.0AB | 6.4A | 56.8BC | 6.6A | 4.8CD | 2.4BC | 8.4CD | |
| (±1.6) | (±0.9) | (±1.0) | (±8.1) | (±4.1) | (±0.4) | (±0.2) | (±0.8) | ||
| F4 | Male | 16.2*a | 6.6a | 7.4a | 69.0abc | 4.8a | 4.6d | 2.0ab | 12.0ab |
| (±0.7) | (±0.5) | (±3.4) | (±7.9) | (±2.6) | (±0.2) | (±0.3) | (±0.8) | ||
| Female | 20.4B | 5.6AB | 6.2A | 77.8AB | 10.2A | 4.2CD | 2.8ABC | 11.0BC | |
| (±1.0) | (±0.5) | (±1.5) | (±8.2) | (±6.4) | (±0.2) | (±0.2) | (±1.1) | ||
| F5 | Male | 17.8a | 6.0a | 6.4a | 100.6a | 5.2a | 5.2cd | 2.2ab | 10.8abc |
| (±2.0) | (±1.2) | (±1.5) | (±5.3) | (±2.0) | (±0.4) | (±0.7) | (±0.6) | ||
| Female | 17.8B | 6.0A | 6.6A | 92.4AB | 11.0A | 5.8BC | 3.0ABC | 12.8BC | |
| (±1.4) | (±0.8) | (±1.3) | (±7.5) | (±7.3) | (±0.2) | (±0.3) | (±1.2) | ||
| F6 | Male | 16.8a | 5.0a | 11.2a | 101.6a | 7.6a | 5.6bcd | 2.4ab | 12.8ab |
| (±1.1) | (±0.8) | (±6.0) | (±7.6) | (±2.4) | (±0.6) | (±0.4) | (±1.2) | ||
| Female | 18.6B | 5.8A | 6.6A | 104.4A | 9.4A | 5.4CD | 3.6ABC | 12.0BC | |
| (±1.3) | (±0.7) | (±1.6) | (±9.8) | (±5.3) | (±0.2) | (±0.4) | (±1.4) | ||
| F7 | Male | 15.6a | 5.2a | 6.8a | 102.6a | 8.2a | 7.4bc | 2.8ab | 16.0ab |
| (±1.1) | (±0.6) | (±2.4) | (±8.3) | (±1.8) | (±0.7) | (±1.0) | (±3.4) | ||
| Female | 19.0B | 5.2AB | 6.8A | 108.6A | 12.4A | 6.2BC | 4.4AB | 15.0AB | |
| (±1.1) | (±0.8) | (±2.2) | (±9.7) | (±4.9) | (±0.7) | (±1.2) | (±1.8) | ||
| F8 | Male | 15.4a | 4.4a | 6.6a | 91.0ab | 10.4a | 8.0b | 4.0ab | 14.4ab |
| (±1.1) | (±0.4) | (±1.2) | (±9.5) | (±3.3) | (±0.5) | (±0.6) | (±2.4) | ||
| Female | 17.0B | 4.0AB | 6.6A | 88.4AB | 12.4A | 7.8B | 4.0ABC | 13.6ABC | |
| (±0.5) | (±0.5) | (±1.8) | (±12.7) | (±6.4) | (±0.2) | (±0.8) | (±1.0) | ||
| F9 | Male | 19.0a | 5.2a | 5.6a | 61.2bc | 9.2a | 12.8a | 4.2a | 18.0a |
| (±0.3) | (±0.6) | (±1.6) | (±8.6) | (±3.5) | (±1.0) | (±0.7) | (±2.6) | ||
| Female | 17.0B | 4.0AB | 7.4A | 62.2BC | 12.0A | 10.6A | 5.2A | 19.0A | |
| (±0.5) | (±0.9) | (±1.8) | (±7.3) | (±5.7) | (±1.0) | (±0.6) | (±1.6) | ||
| Total | Male | 175.2f | 50.6g | 55.6g | 590.2e | 59.4g | 52.6g | 20.4g | 96.0fg |
| (±8.1) | (±2.8) | (±18.8) | (±41.2) | (±19.4) | (±1.9) | (±2.7) | (±9.0) | ||
| Female | 193.2F | 43.0G | 51.8G | 617.4E | 78.8G | 48.0G | 26.8G | 94.6FG | |
| (±4.0) | (±5.2) | (±9.1) | (±54.7) | (±43.4) | (±2.7) | (±2.2) | (±5.2) |
Note: F1–F9, flagellomere 1–9; data entries are means (±SE); –, sensilla not found on flagellomere 1; *, significant differences between sexes at α = 0.05 using Student’s t-test (highlighted in bold); the counts of Sc.3 on the pedicel showed significant differences between males (12.0 ± 1.1/antennum) and females (16.0 ± 1.4/antennum) (t = −2.39; P < 0.05); the abundance of Sc.4 on the pedicel of males (4.0 ± 0.5/antennum) was significantly different from that of females (1.0 ± 0.0/antennum) (t = 2.98; P < 0.05); different lowercase and uppercase letters (a to d; A to D) indicate significant differences among flagellomeres for males and females, respectively; different lowercase and uppercase letters (e to g; E to G) indicate significant differences among types of sensilla for males and females, respectively; few sensilla of Sc.1, Sc.2, Tr.2, Tr.3, BB.1, and BB.2 were found, and their numbers and distribution were detailed in the text.
Basiconic sensilla formed extensive fields in apical depressions of the distal eight flagellomeres. No significant differences between sexes were found for the numbers of Ba.1 or Ba.2 on any flagellomere (Table 3). For both males and females, Ba.1 showed no significant differences in abundance among flagellomeres. The most basal and distal flagellomeres tended to have much less Ba.2 than those in the middle for both males (F = 16.55; df = 7, 32; P < 0.001) and females (F = 9.78; df = 7, 32; P = 0.001). Ba.2 tended to be more abundant in flagellomeres 5–8 in both sexes compared to other flagellomeres. Ba.3 usually occurred in small groups on the apical depression of each flagellomere. Abundances of Ba.3 showed no significant differences among flagellomeres in both sexes. Counts of Ba.4 tended to be higher on the three most distal flagellar subsegments, and no significant differences in abundances of Ba.4 on any flagellomere were found between sexes. There tended to have increasing numbers of Ba.4 from the base toward the apex of flagellum, and flagellomere 9 showed the most abundant Ba.4 in both males (F = 25.59; df = 7, 32; P < 0.001) and females (F = 19.77; df = 7, 32; P < 0.001).
MPG were situated along the edge of basiconic sensilla “fields” in the apical depression of each flagellomere, and there were no significant differences between sexes in the abundances of this type of sensilla (Table 3). Flagellomere 9 had significantly more MPG than flagellomere 2 and 3 in both males (F = 3.26; df = 7, 32; P < 0.05) and females (F = 4.01; df = 7, 32; P < 0.01).
There tended to be increasing numbers of trichoid sensilla toward the tip of antennae for both males and females. Tr.1 sensilla were usually situated around Ba.4 on the distal eight flagellar subsegments, and their counts tended to be low on the two most basal flagellomeres. Sensilla of Tr.1 were significantly more abundant in flagellomere 9 than in flagellomere 2 and 3 in both males (F = 6.17; df = 7, 32; P < 0.001) and females (F = 15.20; df = 7, 32; P < 0.001), and the most distal three flagellomeres tended to have the most abundant Tr.1 in both sexes. Few Tr.2 were found on flagellomeres 5, 8, and 9 of males, and flagellomeres 9 and 10 of females. Tr.3 sensilla were very rare, and found on flagellomere 9 of males only.
In both males (F = 109.71; df = 7, 32; P < 0.001) and females (F = 63.35; df = 7, 32; P < 0.001), the most numerous type of sensilla was Ba.2 (Table 3). A total of 601 and 573 Ba.2 per antennum were found in males and females, respectively. Among other types of sensilla, Sc.3 and Tr.1 were relatively more abundant. Sensilla of Sc.3 were significantly more abundant than Sc.4, Ba.1, Ba.3, Ba.4, or MPG in both sexes. The same pattern was found for males and females in terms of the numbers of different types of sensilla.
Discussion
Like other buprestid species (e.g., the emerald ash borer, A. planipennis in Crook et al. 2008), A. mali had apical depressions (i.e., fossae) on all flagellomeres except flagellomere 1. The characteristics of these depressions are believed to be important in studies of systematics and classification on this group of insects (Volkovitsh 2001). In addition, these depressions represented enlarged sensillar fields, where various sensilla (e.g., bristles, hairs and pegs) were found to be housed. In our study, a total of five different classes of sensilla (i.e., chaetica, trichodea, basiconica, MPG, and BB) on the antennae of A. mali were morphologically characterized. These sensilla were further divided into five classes (14 subtypes), and they included four subtypes of chaetica (Sc.1–Sc.4), four subtypes of basiconica (Ba.1–Ba.4), three subtypes of trichodea (Tr.1–Tr.3), two subtypes of Böhm bristles (BB.1–BB.2), and one type of MPG. In contrast, only five subtypes of antennal sensilla were observed in both males and females of the emerald ash borer (A. planipennis), and they included chaetica (one subtype), basiconica (three subtypes) and uniporous sensilla (one subtype) (Crook et al. 2008).
Among all the sensilla identified, the most widespread were basiconica (Ba.1–Ba.4), which accounted for over 64% of the total in both sexes. Ba.2 were found to be the most numerous among the four subtypes of basiconic sensilla, and they contributed about 81% in males and 76% in females to the total number of basiconic pegs. These sensilla are typically lower in height, and have much more wall pores in comparison to trichoid sensilla (Shields 2004). Sensilla of Ba.1 in our study are similar in external morphology to some basiconic pegs found in beetles of Psylliodes chrysocephala (L.), A. planipennis, and Dastarcus helophoroides (Fairmaire) (Bartlet et al. 1999, Crook et al. 2008, Ren et al. 2012). Sensilla of Ba.2 resemble some basiconic pegs of the alfalfa plant bug [Adelphocoris lineolatus (Goeze)] (Sun et al. 2014). Each sensillum of Ba.3 has a long filament, but its functional roles are still not known. Such sensilla were found to respond to host plant odors in GC-SSR (gas chromatography coupled to single cell recording) tests (Lopes et al. 2002, Sun et al. 2014, Zhang et al. 2015). Numerous pores on the cuticular shaft of Ba.1 to Ba.3 in A. mali also suggest their olfactory functions. Each subtype of these basiconic pegs could be tuned such that they were each specialized for a particular set of host and non-host volatiles (Lopes et al. 2002). Sensilla similar to Ba.4 were also found in the emerald ash borer (Crook et al. 2008). The function of such sensilla could be related to their numbers of neurons (e.g., one neuron for mechano-reception and four neurons for contact chemoreception) (Altner and Prillinger 1980, Zacharuk 1985, Merivee et al. 2002). As the most abundant among all sensilla classes in our study, basiconic sensilla represent the major portion of the olfactory receptor repertoire on the antennae of A. mali. Thus, they could be important for long-range chemosensory perception of environmental cues like host plant volatiles in A. mali, and deserve significant attention in future studies.
Chaetica sensilla (Sc.1–Sc.4) were the second most abundant, which accounted for about 20% of the total number of sensilla in both sexes of A. mali. Sensilla similar to Sc.1 of this study are also found in other beetles, such as the hair plate sensilla of P. chrysocephala (Bartlet et al. 1999), and the sensilla chaetica subtype 2 of Tetropium fuscum (F.) (MacKay et al. 2014). Sc.2 in our study could be similar to some chaetoid sensilla in Trogoderma granarium Everts and Trogoderma variabile Ballion in the Dermestidae (Coleoptera) (Wei et al. 2015). Sc. 3 in this study showed similar features of particular chaetica types in A. planipennis and Tetrigus lewisi Candèze (Crook et al. 2005, Ren et al. 2014), as well as in D. helophoroides (Ren et al. 2012). Sc.4 of this study resembled subtype 9 of chaetoid sensilla in the myrmecophilous beetle (Paussus favieri Fairmaire) (Giulio et al. 2012). Groups of the abovementioned bristles with same structures and similar lengths usually occur in the same antennal area with particular functions (Giulio et al. 2012). The aporous long bristles identified in this study (e.g., Sc.2) can be the first to enter into contacts with the substrate, so they could have a mechanosensitive function (e.g., providing the position information for antennae) (Saïd et al. 2003, Shields 2004, Crook et al. 2008). These sensilla could also play a role in mate recognition and perception of specific hydrocarbons on the cuticle of female A. mali that elicit courtship behaviors in this insect according to studies of Silk et al. (2011) and MacKay et al. (2014).
Sensilla trichodea (Tr.1–Tr.3) in our study comprised about 9% of all identified sensilla in both sexes of A. mali. Surprisingly, these trichoid sensilla were not reported in the emerald ash borer (A. planipennis) (Crook et al. 2008). Sensilla of Tr.1 were found to be one of the most abundant types of sensilla and they covered the entire surface of the beetle’s antennal flagellum. Sensilla similar to Tr.1 of this study had been described in beetles of Limonius aeruginosus (Olivier), Bembidion lampros (Hbst.), and D. helophoroides (Merivee et al. 1998, Ploomi et al. 2003, Ren et al. 2012). This kind of sensilla could have multiple wall pores or apical pores based on the abovementioned researches, however we could not identify those pores due to limitations of using SEM micrographs only. Tr.2 and Tr.3 were relatively scarce, and they were merely different in shape when compared to Tr.1. Both of them had a smooth cuticular surface with many wall pores, suggesting their olfactory functions. Indeed, OBPs, such as AgOBP1 and AgOBP3, were broadly expressed in sensilla trichodea of the malaria mosquito (Anopheles gambiae Giles) (Schultze et al. 2013). Such sensilla were demonstrated to be sensitive to sex pheromones and host plant volatiles through experiments of transmission electron microscopy and immunocytochemical labeling (Sun et al. 2014). Some of the identified hairs could have a single sensory neuron innervating the sensillal shaft, indicating a mechanosensory function (Merivee et al. 1998).
MPG sensilla were found to be scarce, which occupied less than 2% of the total number of identified sensilla in both sexes of A. mali. They were similar to some reported sensilla, such as the grooved peg sensilla in P. chrysocephala (L.) and T. fuscum (F.), and the fluted cone sensilla in Dendroctonus valens (Altner and Prillinger 1980, Bartlet et al. 1999, Chen et al. 2010, MacKay et al. 2014). They were sparsely scattered in the apical depression on each flagellomere in our study. They were considered to have a primary function of olfactory receptors (Shields 2004). These sensilla could respond preferentially to more polar (e.g., short-chain carboxylic acids) or apolar (e.g., long-chain aliphatic mono- and sesquiterpenes) stimuli depending on the thickness of their cell wall (Diehl et al. 2003). They could also respond to temperatures (Altner and Prillinger 1980, Altner and Loftus 1985, Bartlet et al. 1999, MacKay et al. 2014).
Sensilla similar to BBs of this study were found in the antennae of other beetle species, such as Semiadalia undecimnotata Schn. (Jourdan et al. 1995), Callosobruchus chinensis (L.) (Hu et al. 2009), and T. lewisi Candèze (Ren et al. 2014). In our study, these bristles occur in low numbers on the joint between the scape and head capsule. As classical mechanoreceptors, BBs are also found in the joint areas between the scape and pedicel, and on the scape and pedicel (Schneider 1964). Their placement on the joint areas between antennal segments in insects indicates a functional role in proprioception of position and movement of antennae, and they might mediate antennal positioning during flight (Jourdan et al. 1995, Merivee et al. 2002, Krishnan et al. 2012).
In this study, the same five classes of sensilla were found on male and female A. mali. Sensilla of Ba.2 are the most abundant type of sensilla in both females and males, indicating that there could be little or no differences in response to plant volatiles between both sexes. The numbers and location for many subtypes of sensilla identified in males (e.g., Ba.1, Ba.2, Ba.3, Ba.4, MPG, and Tr.1) are almost the same as those in females. Thus, the distribution pattern of antennal sensilla appears to be highly conserved in both sexes. However, variations in the numbers of some sensilla were observed between sexes of A. mali. This is not unexpected, because males of the emerald ash borer (A. planipennis) were found to have higher numbers of uniporous sensilla than females of the same species (Crook et al. 2008). In this study, the numbers of Sc.3 and Sc.4 on the pedicel showed significant differences between both sexes. Sensilla of Sc.2 were found on the pedicel and the first two flagellomeres only in males. Tr.3 and BB.2 were both found in females, but not in males. All these sensilla showing sexual differences are believed to be mechano-receptors, suggesting that contact cues could be important for mate recognition and oviposition by females in this beetle. Indeed, it was believed that antennal contact could be important for mate recognition by male A. planipennis, a closely related buprestid beetle (Crook et al. 2008). Future investigations are needed to identify the unique contact cues or signals in A. mali.
This study is the first to characterize the antennal sensilla of the important invasive beetle, A. mali. Sexual differences in the numbers and distribution of various sensilla were also evaluated. This establishes a foundation for future physiological, behavioral and molecular studies aimed at elucidating potential differences in olfaction between both sexes of A. mali using techniques like single sensillum recording and electroantennographic detection. Such studies would clarify the functional roles of various insect sensilla in sensing pheromones, and host and non-host volatiles, as well as the underlying mechanisms, which could ultimately lead to developing efficient semiochemical-based control methods.
Acknowledgments
We would like to thank M. Wang and K.-K. Sun of Northwest A&F University for their lab assistance. We appreciate the field assistance of S.-J. Liao and Z. Xu from Forest Bureau of Yili Prefecture in insect sample collections. This work was supported by Special Fund for Forest Scientific Research in the Public Welfare (Grant No. 201404403) and the National Natural Science Foundation of China (Grant No. U1503102).
References Cited
- Altner H., Prillinger L. 1980. Ultrastructure of invertebrate chemo-, thermo-, and hygro receptors and its functional significance. Int. Rev. Cytol. 67: 139. [Google Scholar]
- Altner H., Loftus R. 1985. Ultrastructure and function of insect thermo- and hygroreceptors. Ann. Rev. Entomol. 30: 273–295. [Google Scholar]
- Bartlet E., Romani R., Williams I. H., Isidoro N. 1999. Functional anatomy of sensory structures on the antennae of Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). Int. J. Insect Morphol. Embryol. 28: 291–300. [Google Scholar]
- Chebanov G. E. 1977. Disinfestation regimes. Zashchita Rastenii. 1: 55–56. [Google Scholar]
- Chen H. B., Zhang Z., Wang H. B., Kong X. B. 2010. Antennal morphology and sensilla ultrastructure of Dendroctonus valens LeConte (Coleoptera: Curculionidae, Seolytinae), an invasive forest pest in China. Micron. 4: 735–741. [DOI] [PubMed] [Google Scholar]
- Crook D. J., Fraser I., Francese J. A., Mastro V. C. 2005. Chemical ecology of the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), in relation to tree volatiles. Emerald Ash Borer Research and Technology Development. 63. [Google Scholar]
- Crook D. J., Kerr L. M., Mastro V. C. 2008. Distribution and fine structure of antennal sensilla in emerald ash borer (Coleoptera: Buprestidae). Ann. Entomol. Soc. Am. 101: 1103–1111. [Google Scholar]
- Crook D. J., Mastro V. C. 2010. Chemical ecology of the emerald ash borer Agrilus planipennis. J. Chem. Ecol. 36: 101–112. [DOI] [PubMed] [Google Scholar]
- Cui X. N., Liu D. G., Liu A. H. 2015. Research progress in integrated management of Agrilus mali. Plant Protection. 41: 16–23. [Google Scholar]
- Diehl P. A., Vlimant M., Guerenstein P., Guerin P. M. 2003. Ultrastructure and receptor cell responses of the antennal grooved peg sensilla of Triatoma infestans (Hemiptera: Reduviidae). Arthropod Struc. Dev. 31: 271–285. [DOI] [PubMed] [Google Scholar]
- Giulio A. D., Maurizi E., Stacconi M. V. R., Romani R. 2012. Functional structure of antennal sensilla in the myrmecophilous beetle Paussus favieri (Coleoptera, Carabidae, Paussini). Micron. 43: 705–719. [DOI] [PubMed] [Google Scholar]
- Hu F., Zhang G. N., Wang J. J. 2009. Scanning electron microscopy studies of antennal sensilla of bruchid beetles, Callosobruchus chinensis (L.) and Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Micron. 40: 320–326. [DOI] [PubMed] [Google Scholar]
- Jourdan H., Barbier R., Bernard J., Ferran A. 1995. Antennal sensilla and sexual dimorphism of the adult ladybird beetle Semiadalia undecimnotata Schn. (Coleoptera: Coccinellidae). Int. J. Insect Morphol. Embryol. 24: 307–322. [Google Scholar]
- Krishnan A., Prabhakar S., Sudarsan S., Sane S. P. 2012. The neural mechanisms of antennal positioning in flying moths. J. Exp. Biol. 215: 3096–3105. [DOI] [PubMed] [Google Scholar]
- Lopes O., Barata E. N., Mustaparta H., Araújo J. 2002. Fine structure of antennal sensilla basiconica and their detection of plant volatiles in the eucalyptus woodborer, Phoracantha semipunctata Fabricius (Coleoptera: Cerambycidae). Arthropod. Struct. Dev. 31: 1–13. [DOI] [PubMed] [Google Scholar]
- MacKay C. A., Sweeney J. D., Hillier N. K. 2014. Morphology of antennal sensilla of the brown spruce longhorn beetle, Tetropium fuscum (Fabr.) (Coleoptera: Cerambycidae). Arthropod. Struct. Dev. 43: 469–475. [DOI] [PubMed] [Google Scholar]
- Mamidala P., Wijeratne A. J., Wijeratne S., Poland T., Qazi S. S., Doucet D., Cusson M., Beliveau C., Mittapalli O. 2013. Identification of odor-processing genes in the emerald ash borer, Agrilus planipennis. PloS ONE. 8: e56555.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merivee E., Rahi M., Bresciani J., Ravn H. P., Luik A. 1998. Antennal sensilla of the click beetle, Limonius aeruginosus (Olivier) (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 27: 311–318. [Google Scholar]
- Merivee E., Rahi M., Luik A. 1999. Antennal sensilla of the click beetle, Melanotus villosus (Geoffroy) (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 28: 41–51. [Google Scholar]
- Merivee E., Ploomi A., Rahi M., Bresciani J., Ravn H. P., Luik A., Sammelselg V. 2002. Antennal sensilla of the ground beetle Bembidion properans Steph. (Coleoptera, Carabidae). Micron. 33: 429–440. [DOI] [PubMed] [Google Scholar]
- Nikritin L. M. 1994. Apple buprestid beetle. Zashchita Rastenii˘ (Moskva). 3: 46. [Google Scholar]
- Pitts R. J., Zwiebel L. J. 2006. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar. J. 5: 26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploomi A., Merivee E., Rahi M., Bresciani J., Ravn H. P., Luik A., Sammelselg V. 2003. Antennal sensilla in ground beetles (Coleoptera: Carabidae). Agron. Res. 1: 221–228. [Google Scholar]
- Ren L., Shi J., Zhang Y., Luo Y. 2012. Antennal morphology and sensillar ultrastructure of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Micron. 43: 921–928. [DOI] [PubMed] [Google Scholar]
- Ren L. L., Wu Y., Shi J., Zhang L., Luo Y. Q. 2014. Antenna morphology and sensilla ultrastructure of Tetrigus lewisi Candèze (Coleoptera: Elateridae). Micron. 60: 29–38. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Saona C., Poland T. M., Miller J. R., Stelinski L. L., Grant G. G., De Groot P., Buchan L., MacDonald L. 2006. Behavioral and electrophysiological responses of the emerald ash borer, Agrilus planipennis, to induced volatiles of Manchurian ash, Fraxinus mandshurica. Chemoecology 16: 75–86. [Google Scholar]
- Saïd I., Tauban D., Renou M., Mori K., Rochat D. 2003. Structure and function of the antennal sensilla of the palm weevil Rhynchophorus palmarum (Coleoptera, Curculionidae). J. Insect Physiol. 49: 857–872. [DOI] [PubMed] [Google Scholar]
- Savotikov Y. F., Smetnik A. I. 1995. Manual of the pests, diseases and weeds of quarantine significance for the territory of the Russian Federation. Nizhnii Novgorod Russia: Arnika. 143–145. [Google Scholar]
- Schneider D. 1964. Insect antennae. Ann. Rev. Entomol. 9: 103–122. [Google Scholar]
- Schultze A., Pregitzer P., Walter M. F., Woods D. F., Marinotti O., Breer H., Krieger J. 2013. The co-expression pattern of odorant binding proteins and olfactory receptors identify distinct trichoid sensilla on the antenna of the malaria mosquito Anopheles gambiae. PLoS One 8: e69412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. W., Gara R. I. 1975. Antennal sensory organs of two Melanophila species (Coleoptera: Buprestidae). Ann. Entomol. Soc. Am. 68: 842–846. [Google Scholar]
- Shields V.D.C. 2004. Ultrastructure of insect sensilla. Encyclopedia of Entomology. Springer Netherlands. 4009–4023. [Google Scholar]
- Silk P. J., Sweeney J., Wu J., Sopow S., Mayo P. D., Magee D. 2011. Contact sex pheromones identified for two species of longhorned beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the subfamily Spondylidinae. Environ. Entomol. 40: 714–726. [DOI] [PubMed] [Google Scholar]
- Sun L., Xiao H. J., Gu S. H., Zhou J. J., Guo Y. Y., Liu Z. W., Zhang Y. J. 2014. The antenna‐specific odorant‐binding protein AlinOBP13 of the alfalfa plant bug Adelphocoris lineolatus is expressed specifically in basiconic sensilla and has high binding affinity to terpenoids. Insect Mol. Biol. 23: 417–434. [DOI] [PubMed] [Google Scholar]
- Sun Y. Z., Liang Y. Y., Sun H. 1979. Studies on the apple buprestid (Agrilus mali Mats.) in Shensi. J. Northwest. Coll. Agri. 2: 47–56. [Google Scholar]
- Volkovitsh M. G. 2001. The comparative morphology of antennal structures in Buprestidae (Coleoptera): evolutionary trends, taxonomic and phylogenetic implications (Part 1). Acta Musei Moraviae-Scientiae Biologicae (Brno). 86: 43–169. [Google Scholar]
- Wang X. Y., Yang Z. Q., Gould J. R., Zhang Y. N., Liu G. J., Liu E. S. 2010. The biology and ecology of the emerald ash borer, Agrilus planipennis, in China. J. Insect Sci. 10: 128.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Ren B., Chen X., Zhou X., Wang W., Wang Z. 2015. Scanning electron microscope observations on the antennal sensilla of two stored grain pests Trogoderma granarium and Trogoderma variabile (Coleoptera: Dermestidae). Fla. Entomol. 98: 140–148. [Google Scholar]
- Zacharuk R. Y. 1980. Ultrastructure and function of insect chemosensilla. Ann. Rev. Entomol. 25: 27–47. [Google Scholar]
- Zacharuk R. Y. 1985. Antennae and sensilla. Compr. Insect Physiol. Biochem. Pharmacol. 6: 1–69. [Google Scholar]
- Zhang Y., Ren L., Zhang L., Luo Y. 2015. Ultrastructure of antennal and posterior abdominal sensilla in Chlorophorus caragana females. Micron 75: 45–57. [DOI] [PubMed] [Google Scholar]