Abstract
This study reviewed prostate volumetric-modulated arc therapy (VMAT) plans with intensity-modulated radiotherapy (IMRT) plans after prostate IMRT technique was replaced by VMAT in an institution. Characterizations of dosimetry and radiobiological variation in prostate were determined based on treatment plans of 40 prostate IMRT patients (planning target volume = 77.8–335 cm3) and 50 VMAT patients (planning target volume = 120–351 cm3) treated before and after 2013, respectively. Both IMRT and VMAT plans used the same dose-volume criteria in the inverse planning optimization. Dose-volume histogram, mean doses of target and normal tissues (rectum, bladder and femoral heads), dose-volume points (D99% of planning target volume; D30%, D50%, V30 Gy and V35 Gy of rectum and bladder; D5%, V14 Gy, V22 Gy of femoral heads), conformity index (CI), homogeneity index (HI), gradient index (GI), prostate tumor control probability (TCP), and rectal normal tissue complication probability (NTCP) based on the Lyman-Burman-Kutcher algorithm were calculated for each IMRT and VMAT plan. From our results, VMAT plan was found better due to its higher (1.05%) CI, lower (0.83%) HI and (0.75%) GI than IMRT. Comparing doses in normal tissues between IMRT and VMAT, it was found that IMRT mostly delivered higher doses of about 1.05% to the normal tissues than VMAT. Prostate TCP and rectal NTCP were found increased (1%) for VMAT than IMRT. It is seen that VMAT technique can decrease the dose-volume evaluation criteria for the normal tissues. Based on our dosimetric and radiobiological results in treatment plans, it is concluded that our VMAT implementation could produce comparable or slightly better target coverage and normal tissue sparing with a faster treatment time in prostate radiotherapy.
Key words: Dose-volume histogram, intensity-modulated radiotherapy, normal tissue complication probability, prostate, tumor control probability, volumetric-modulated arc therapy
Introduction
Prostate cancer continues to be one of the most commonly diagnosed cancers in the world. There are many treatment options available for prostate cancer, and radiotherapy is a popular one to provide effective cancer control. In the recent years, significant improvement in the radiation dose delivery technique has been noticed. Treatment planning has now moved from the three-dimensional conformal radiotherapy to intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT). Both IMRT and VMAT are the two most up-to-date technologies used in external beam photon radiotherapy. In short, IMRT delivers the radiation beam without gantry rotation, whereas in VMAT, radiation beam is delivered by the simultaneous adjustments of the gantry speed, multi-leaf collimator (MLC) leaves, and dose rate.[1]
The main objective of radiotherapy is to enhance the tumor control by delivering maximum dose to the target, while reducing dose to the normal tissues. IMRT is one of the advanced treatment techniques that delivers radiation beam either by modulating the beam using continuously moving MLC or by dividing the radiation beam into different segments of various shapes. VMAT is relatively a newer treatment technique which delivers radiation beam by varying the gantry rotational speed, dose rate, and MLC leaf positions at the same time.[2]
VMAT becomes a popular delivery option, taking advantage of shorter delivery time and smaller monitor unit (MU) compared to step-and-shoot IMRT. Several authors have reported treatment planning studies on the comparison of IMRT and VMAT in radical prostate radiotherapy.[2,3,4,5,6] It was also reported that equivalent and even better target dose coverage and normal tissue (e.g. rectum, bladder and femoral heads) sparing can be produced by the VMAT technique than IMRT in patient dosimetry.[7,8,9,10,11,12]
The radiobiological models describe the effects of the radiation treatment on cancer and healthy cells, and the radiobiological effects are generally characterized by the tumor control probability (TCP) and normal tissue complication probability (NTCP).[13,14] Several papers described dose evaluation methods incorporating generalized equivalent uniform dose (gEUD), TCP, and NTCP as radiobiological impact measures and visualized these instead of physical doses.[15,16,17,18,19,20,21,22]
Our previous planning studies were carried out on patient's weight loss, prostate size, internal organ motion, dosimetry and radiobiological model variation that showed VMAT is good in prostate cancer treatment.[23,24,25,26]
Based on the positive dosimetric and radiobiological results from VMAT compared to IMRT through previous retrospective studies,[7,12,23,24,25,26] our institution (Grand River Hospital) started to replace prostate IMRT technique by VMAT since 2013. In this study, the treated prostate VMAT plans were reviewed with the previously treated IMRT plans to justify the effectiveness of our VMAT replacement. For all IMRT and VMAT plans treated, the dosimetry (dose-volume criteria, mean and maximum dose) and prostate TCP, rectal NTCP were calculated using the Lyman-Burman-Kutcher radiobiological model.[27,28,29] The aim of this study is to validate the implementation of prostate VMAT used to replace the prostate IMRT according to their dosimetry and radiobiological parameter variations. The results in this study should provide evidence to medical physicists based on experience, when they are considering replacing the radiation delivery technique from IMRT to VMAT in prostate treatment.
Materials and Methods
Patient data
Forty prostate IMRT patients treated before 2013 and fifty VMAT patients treated afterward in the Grand River Hospital were used in this study. This study received institutional ethics approval. All patients were scanned by the Siemens SOMATOM Sensation Open CT-simulator using the same protocol. The CT-simulations were done with patients in supine position and full bladder. The planning target volume (PTV) was in the range of 77.8 cm3 –335 cm3 for IMRT and 120–351 cm3 for VMAT patients. The PTV, clinical target volume (CTV), rectum, bladder, and femoral heads of all patients were contoured. The gross target volume (GTV) was equal to the CTV (prostate volume), and PTV was created by expansion of the CTV with 1 cm around, except 0.7 cm posteriorly. Dosimetric verifications of VMAT for patients were carried out using the ArcCHECK cylindrical detector array.[30]
Treatment planning
Prostate treatment plans were created by the Eclipse treatment planning system (version 8.5, Varian Medical Systems, Palo Alto, CA, USA) using the progressive resolution optimizer in the RapidArc optimization (Varian Medical Systems), and the Pinnacle3 treatment planning system (Philips Medical Systems, Andover, MA, USA). All treatment planning systems were commissioned for a Varian 21 EX linear accelerator (Varian Medical Systems) with a 120-leaf MLC and 6 MV photon beam. The dose constraints to critical organs, plan objectives, and optimization parameters of prostate plan can be found in our previous work.[23] The prescribed dose was 78 Gy in 39 fractions (2 Gy per fraction). Dose calculations were performed using dose grid resolution set to 0.25 cm, and was prescribed to the median dose (D50%) of the PTV.[23] Prostate VMAT plans were created using the single-arc technique, while a seven-beam technique was used with beam angles equal to 40°, 80°, 110°, 250°, 280°, 310° and 355° for IMRT prostate plans.[32] The dose–volume constraints for the target volumes and critical organs for the inverse planning are shown in Table 1. These constraints were parameters in the optimization cost function. The specific fraction of volume based on the function is allowed to exceed the prescribed dose limit in the case of a critical organ or target, to be less than the prescribed value.[15,32] The same set of constraints as shown in Table 1, and prescription dose for IMRT were utilized for the VMAT prostate plans, in the optimization. The dose delivery of VMAT was carried out using a single 360° photon arc.
Table 1.
Dose-volume constraints of the clinical target volume, planning target volume, rectum, bladder, left and right femoral head used in the 7-beam intensity-modulated radiotherapy and volumetric-modulated arc therapy prostate plans

Average dose-volume histograms (DVHs), mean, minimum, median, and maximum doses of targets (PTV and CTV) and critical organs (rectum, bladder, and femoral heads) were determined. Moreover, mean dose-volume criteria including the D99% of PTV, D30%, D50%, V30 Gy, and V35 Gy of rectum and bladder, and D5%, V14 Gy, and V22 Gy of femoral heads were calculated for both techniques.
Dosimetric evaluation
Dosimetric evaluation of the prostate IMRT and VMAT plans was carried out using the following parameters such as D99%, D95%, D5%, maximum dose (Dmax), mean dose (Dmean), conformity index (CI), homogeneity index (HI) and gradient index (GI) of the PTV for IMRT and VMAT techniques as shown in Table 1. By definition proposed by the radiation therapy oncology group (RTOG) described in the Report 62 of the International Commission on Radiation Units and Measurements,[33] the CI is equal to the volume of the reference dose divided by the target volume. In this study, the reference dose of 98% (i.e. RTOG CI (98)) was used, and CI has an optimal value of 1.[34] HI is defined as the dose received by 5% of the PTV minus the dose received by 95% of the PTV, divided by the mean dose (its optimal value is 0) as shown in Equation 1.[35]

GI is defined as the ratio of volume covered by at least a given percentage of the prescription dose.[36] Mathematically, GI in this study is expressed as:

where V50 is the volume covered by at least 50% of the prescription dose. A value closer to unity embodies a faster dose fall-off in normal tissue, which may indicate a lower dose to critical structure.
Tumor control probability and normal tissue complication probability calculation
The prostate TCP was calculated as follows:

where D is dose, p and q are related to D50 and g50 (normalized slope at the point of 50% probability control), according to Okunieff et al.[37] who summarized clinical data for a variety of tumors that can be related to the slope and dose to control 50% of tumors. Using Equation 3, control probability for the tumorlet with volume and doses, TCP (vi, Di) can be inferred from the TCP for the whole volume by:

where (vi, Di) refers to the differential DVH converted from the cumulated DVH.
Rectal NTCP was calculated using the Lyman-Burman-Kutcher algorithm with the following equations:[27,28,29]

and

where v = V/Vref and TD50 (v) = TD50 (1) v− n, as suggested by Burman et al.[28] TD50 = 80 Gy, n = 0.12, and m = 0.15 were used to calculate the rectal NTCP in this study. Both TCP and NTCP were determined using in-house TCP/NTCP software running on a MATLAB platform (The MathWorks, Natick, MA, USA).[25]
Statistical analysis was performed using SPSS (version 16.0.0, SPSS Inc., Chicago, USA). Independent Student's t-test was used to compare both treatment techniques. A P < 0.05 was considered statistically significant.
Results
Average volumes of the bladder, rectum, right femoral head, and left femoral head were 302 cm3, 75.75 cm3, 207 cm3, and 199 cm3 with IMRT technique, and 311 cm3, 74 cm3, 183 cm3, 173 cm3 with VMAT technique. The average PTV volumes for IMRT and VMAT were 172 cm3 and 194 cm3, respectively.
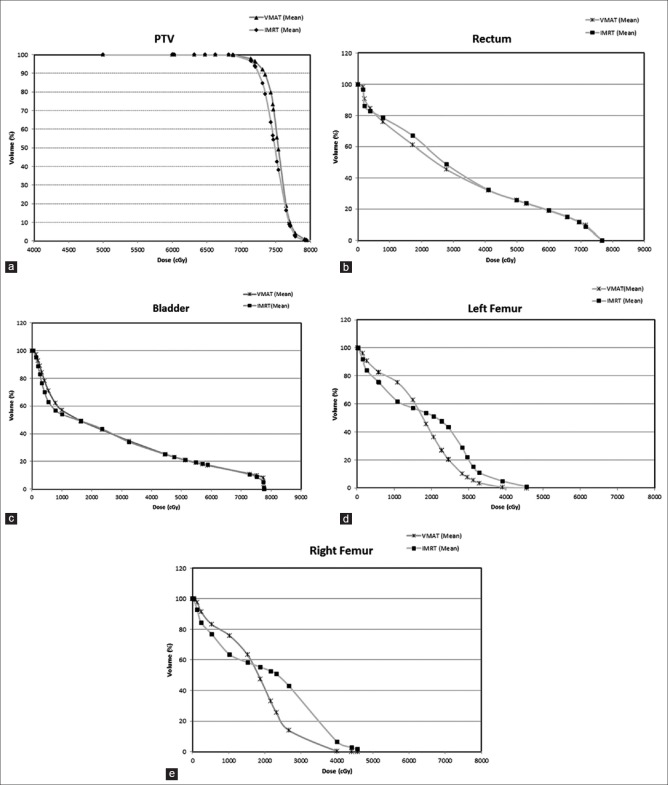
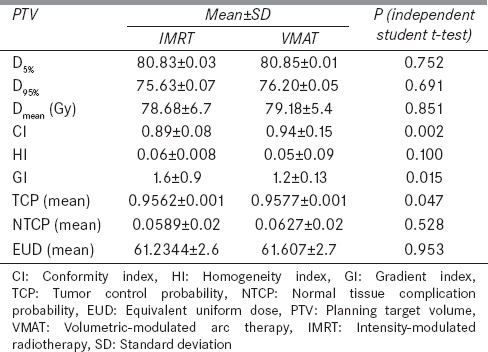
Dose-volume histograms of the PTV, rectum, bladder and femoral heads for all IMRT and VMAT patients are shown in Figure 1a–e, respectively. It is found that VMAT spares the rectum and femoral heads with lower doses compared to IMRT. On the other hands, IMRT spares the bladder with lower doses than VMAT. Dose-volume constraints of the CTV, PTV, rectum, bladder and femoral heads used in the 7-beam IMRT and VMAT prostate plans are shown in Table 1. The Dmean, CI, HI and GI of the PTV were calculated as shown in Table 2. It can be seen from the values of CI for both techniques that VMAT was closer to unity than IMRT. Moreover, lower values of HI and GI were found for VMAT than IMRT.
Figure 1.
Average dose – volume histograms of the (a) planning target volume, (b) rectum, (c) bladder, (d) left femoral head, and (e) right femoral head for the intensity – modulated radiotherapy and volumetric – modulated arc therapy plans
Table 2.
Dosimetric results for planning target volume, prostate tumor control probability, rectal normal tissue complication probability, and rectal equivalent uniform dose
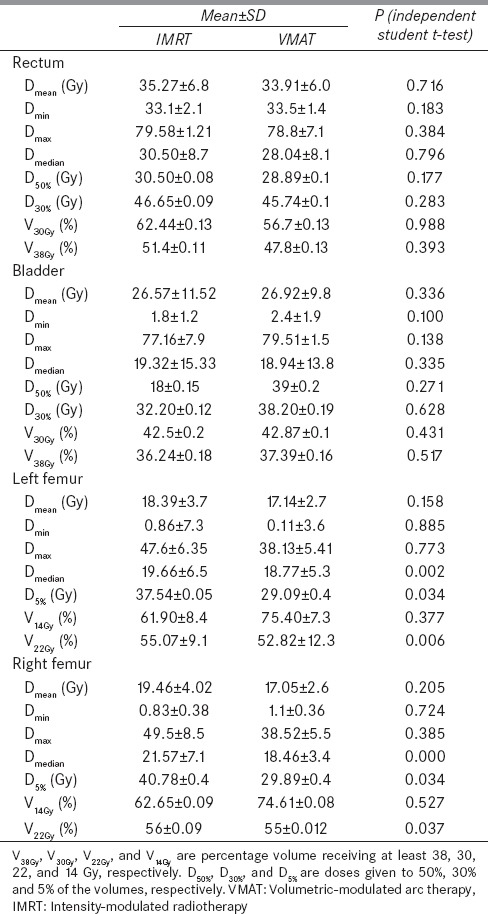
For radiobiological characterizations, prostate TCP, rectal NTCP and rectal EUD were calculated and shown in Table 2. Figures 2–4 show the variation of TCP, NTCP, and EUD with the prostate volume. The Dmean, D30%, D50%, V30% and V38% for rectum and bladder were calculated for both techniques as shown in Table 3. It is found from Table 3 that VMAT spared rectum with a lower dose compared to IMRT, which spared bladder better than VMAT. The doses to the femoral heads were found to be within the acceptable range, and their Dmean, D5%, V14% and V22% were calculated as shown in Table 3. Lower dose-volume values were found for VMAT than IMRT in Table 3.
Figure 2.
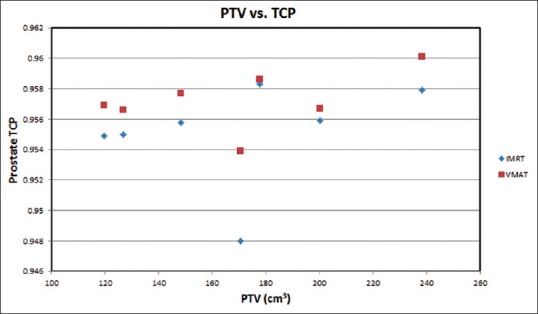
Prostate tumor control probability varying with the prostate planning target volume of the seven patients (having the same volume) based on the intensity-modulated radiotherapy and volumetric-modulated arc therapy prostate plans
Figure 4.
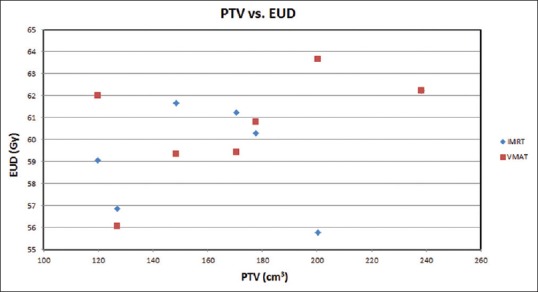
Prostate equivalent uniform dose varying with the prostate planning target volume of the seven patients (having the same volume) based on the intensity-modulated radiotherapy and volumetric-modulated arc therapy prostate plans
Table 3.
Mean dose-volume criteria, average Dmean of the critical organs for volumetric-modulated arc therapy and intensity-modulated radiotherapy plans
Discussion
Dose-volume histogram and dosimetric indices
Figure 1a shows the average DVH of the PTV for all patients planned using the IMRT and VMAT techniques. The dose range in Figure 1a is started from 40 Gy instead of zero to focus on the drop-off region of the curve. For both IMRT and VMAT plans, it is seen that higher doses were accomplished with VMAT. Figure 1b and c show the average DVHs of the rectum and bladder, respectively. It can be seen that percentage volumes receiving given doses (e.g. V30 Gy and V38 Gy) were always lower in VMAT than IMRT. This indicates that VMAT resulted in a better rectum and bladder dose-volume criteria than IMRT. It was also found that VMAT shows lower doses than IMRT to femoral heads. Based on the results in Figure 1a–e, the VMAT technique is found to improve the dose conformity and coverage of the prostate, the rectum, the bladder and the femoral heads than IMRT. An investigation of the dosimetric indices calculated for IMRT and VMAT reveals that VMAT is better to be used as its higher (1.05%) CI, lower (0.83%) HI and (0.75%) GI.
Dose-volume criteria, maximum, minimum, median and mean dose
The important parameters in the treatment plan evaluation are mean dose-volume criteria, maximum and minimum doses. Table 1 shows the mean dose-volume criteria of CTV, PTV, rectum, bladder and femoral head calculated by the treatment planning system. For the PTV, it is seen in Table 2 that the mean dose Dmean of all IMRT patients (78.63 Gy) is less than VMAT (79.05 Gy). VMAT technique delivers higher mean doses of 79.05 Gy, on the other hand, satisfied the evaluation criteria. It delivers 1% higher doses than IMRT, so, it is not too much higher to be pondering upon. For mean D30% and D50% of rectum and bladder satisfied the corresponding dose-volume criteria for both VMAT and IMRT techniques, but in rectum VMAT remains lower than IMRT on average of 1% and in bladder IMRT remains higher on average of 2.16% and 1.18% for D30% and D50% than VMAT plans. Mean doses Dmean and D5% of femoral head were also found higher on the average of 1.05% for IMRT than VMAT. For percentage rectal and bladder volume receiving at least a given dose, VMAT technique shows higher V30 Gy and V38 Gy than IMRT for rectum and vice-versa for bladder.
For further dose-volume comparison beyond the criteria used in the treatment planning quality assurance as shown in Table 1, it is seen in Table 3 that VMAT technique can effectively decrease the dose volume evaluation criteria for the rectum and bladder. However, the effect is increased doses in the femoral head for V14 Gy and decreased for V22 Gy. For average mean, minimum, maximum and median doses of critical organs [Table 3], when using VMAT and IMRT techniques, IMRT delivered higher mean, minimum, and median doses to the bladder and femoral head compared to VMAT.
Prostate tumor control probability, rectal normal tissue complication probability, and rectal equivalent uniform dose
The prostate TCP for the whole treatment (78 Gy/39 fractions) against the PTV is plotted in Figure 2. It is clear in the figure that the prostate TCP for the VMAT is higher (1%) than that of the IMRT technique. For NTCP of critical organs, since the bladder and femoral head NTCP are generally about 1 × 102 and 1 × 105 times smaller than the rectal NTCP, so, only the rectal NTCP is considered in this study.[38,39] It can be extracted from the Figure 3 that IMRT shows higher NTCP for PTVs, (120 cm3–148 cm3, and 150 cm3–230 cm3), whereas in between these PTVs (150 cm3–185 cm3), VMAT shows higher NTCP. For lower rectal dose-volume criteria (D30%, D50%, V30 Gy, and V38 Gy) achieved in the treatment plan, VMAT is still worthwhile to be considered, in spite of the higher rectal NTCP in the limited PTV (150 cm3 -185 cm3) compared to IMRT. Figure 4 demonstrates the relation between EUD and PTV. For the prostate tumor, the average EUD values in the VMAT plans were higher than in the IMRT plans with an average difference of 1.006%.
Figure 3.
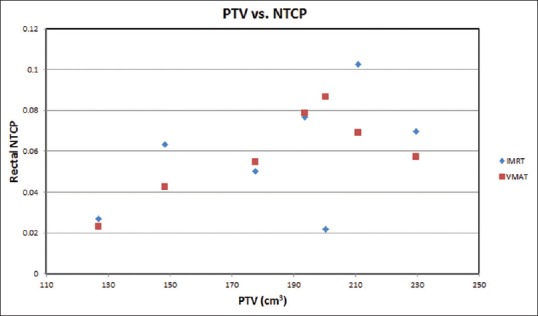
Rectal normal tissue complication probability varying with the prostate planning target volume of the seven patients (having the same volume) based on the intensity-modulated radiotherapy and volumetric-modulated arc therapy prostate plans
Conclusions
Prostate plans have been analyzed for forty IMRT and fifty VMAT patients since VMAT technique was used to substitute IMRT since 2013 in our institution. For these two patient groups before and after the implementation of prostate VMAT, the mean, minimum, maximum and median doses were found comparable between the two techniques. It is therefore concluded that our prostate VMAT implementation was validated based on comparable dosimetric and radiobiological outcomes from the previously used IMRT technique. The patient throughput was increased and the durability of the linear accelerator was enhanced due to the decrease of MU when using VMAT in each cancer treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kopp RW, Duff M, Catalfamo F, Shah D, Rajecki M, Ahmad K. VMAT vs 7-field-IMRT: Assessing the dosimetric parameters of prostate cancer treatment with a 292-patient sample. Med Dosim. 2011;36:365–72. doi: 10.1016/j.meddos.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–7. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 3.Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: An alternative to tomotherapy. Phys Med Biol. 1995;40:1435–49. doi: 10.1088/0031-9155/40/9/004. [DOI] [PubMed] [Google Scholar]
- 4.Webb S, McQuaid D. Some considerations concerning volume-modulated arc therapy: A stepping stone towards a general theory. Phys Med Biol. 2009;54:4345–60. doi: 10.1088/0031-9155/54/14/001. [DOI] [PubMed] [Google Scholar]
- 5.Bzdusek K, Friberger H, Eriksson K, Hårdemark B, Robinson D, Kaus M. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys. 2009;36:2328–39. doi: 10.1118/1.3132234. [DOI] [PubMed] [Google Scholar]
- 6.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: Comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Hardcastle N, Tomé WA, Foo K, Miller A, Carolan M, Metcalfe P. Comparison of prostate IMRT and VMAT biologically optimised treatment plans. Med Dosim. 2011;36:292–8. doi: 10.1016/j.meddos.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guckenberger M, Richter A, Krieger T, Wilbert J, Baier K, Flentje M. Is a single arc sufficient in volumetric-modulated arc therapy (VMAT) for complex-shaped target volumes? Radiother Oncol. 2009;93:259–65. doi: 10.1016/j.radonc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Moret J, Pohl F, Koelbl O, Dobler B. Evaluation of volumetric modulated arc therapy (VMAT) with Oncentra MasterPlan® for the treatment of head and neck cancer. Radiat Oncol. 2010;5:110. doi: 10.1186/1748-717X-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iori M, Cattaneo GM, Cagni E, Fiorino C, Borasi G, Riccardo C, et al. Dose-volume and biological-model based comparison between helical tomotherapy and (inverse-planned) IMAT for prostate tumours. Radiother Oncol. 2008;88:34–45. doi: 10.1016/j.radonc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Happersett L, Hunt M, Jackson A, Zelefsky M, Mageras G. Volumetric modulated arc therapy: Planning and evaluation for prostate cancer cases. Int J Radiat Oncol Biol Phys. 2010;76:1456–62. doi: 10.1016/j.ijrobp.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Wolff D, Stieler F, Welzel G, Lorenz F, Abo-Madyan Y, Mai S, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93:226–33. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Zaider M, Minerbo GN. Tumour control probability: A formulation applicable to any temporal protocol of dose delivery. Phys Med Biol. 2000;45:279–93. doi: 10.1088/0031-9155/45/2/303. [DOI] [PubMed] [Google Scholar]
- 14.Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys Med. 2007;23:115–25. doi: 10.1016/j.ejmp.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Tomé WA. Risk-adaptive optimization: Selective boosting of high-risk tumor subvolumes. Int J Radiat Oncol Biol Phys. 2006;66:1528–42. doi: 10.1016/j.ijrobp.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oinam AS, Singh L, Shukla A, Ghoshal S, Kapoor R, Sharma SC. Dose volume histogram analysis and comparison of different radiobiological models using in-house developed software. J Med Phys. 2011;36:220–9. doi: 10.4103/0971-6203.89971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moiseenko V, Lapointe V, James K, Yin L, Liu M, Pawlicki T. Biological consequences of MLC calibration errors in IMRT delivery and QA. Med Phys. 2012;39:1917–24. doi: 10.1118/1.3692177. [DOI] [PubMed] [Google Scholar]
- 18.Park JY, Lee JW, Chung JB, Choi KS, Kim YL, Park BM, et al. Radiobiological model-based bio-anatomical quality assurance in intensity-modulated radiation therapy for prostate cancer. J Radiat Res. 2012;53:978–88. doi: 10.1093/jrr/rrs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rana S. Radiobiological impact of Acuros XB dose calculation algorithm on low-risk prostate cancer treatment plans created by RapidArc technique. Austral Asian J Cancer. 2012;11:261–9. [Google Scholar]
- 20.Kim Y, Tomé WA. On voxel based iso-tumor control probabilty and iso-complication maps for selective boosting and selective avoidance intensity modulated radiotherapy. Imaging Decis (Berl) 2008;12:42–50. doi: 10.1111/j.1617-0830.2008.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–10. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 22.Rana S, Cheng C. Radiobiological impact of planning techniques for prostate cancer in terms of tumor control probability and normal tissue complication probability. Ann Med Health Sci Res. 2014;4:167–72. doi: 10.4103/2141-9248.129023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow JC, Jiang R. Comparison of dosimetric variation between prostate IMRT and VMAT due to patient's weight loss: Patient and phantom study. Rep Pract Oncol Radiother. 2013;18:272–8. doi: 10.1016/j.rpor.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow JC, Jiang R. Dosimetry estimation on variations of patient size in prostate volumetric-modulated arc therapy. Med Dosim. 2013;38:42–7. doi: 10.1016/j.meddos.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Jiang R, Barnett RB, Chow JC, Chen JZ. The use of spatial dose gradients and probability density function to evaluate the effect of internal organ motion for prostate IMRT treatment planning. Phys Med Biol. 2007;52:1469–84. doi: 10.1088/0031-9155/52/5/016. [DOI] [PubMed] [Google Scholar]
- 26.Chow JC, Jiang R. Prostate volumetric-modulated arc therapy: Dosimetry and radiobiological model variation between the single-arc and double-arc technique. J Appl Clin Med Phys. 2013;14:4053. doi: 10.1120/jacmp.v14i3.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–9. [PubMed] [Google Scholar]
- 28.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–35. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 29.Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991;21:137–46. doi: 10.1016/0360-3016(91)90173-2. [DOI] [PubMed] [Google Scholar]
- 30.Létourneau D, Publicover J, Kozelka J, Moseley DJ, Jaffray DA. Novel dosimetric phantom for quality assurance of volumetric modulated arc therapy. Med Phys. 2009;36:1813–21. doi: 10.1118/1.3117563. [DOI] [PubMed] [Google Scholar]
- 31.Ulmer W, Pyyry J, Kaissl W. A 3D photon superposition/convolution algorithm and its foundation on results of Monte Carlo calculations. Phys Med Biol. 2005;50:1767–90. doi: 10.1088/0031-9155/50/8/010. [DOI] [PubMed] [Google Scholar]
- 32.Chow JC, Jiang R, Markel D. The effect of interfraction prostate motion on IMRT plans: A dose-volume histogram analysis using a Gaussian error function model. J Appl Clin Med Phys. 2009;10:3055. doi: 10.1120/jacmp.v10i4.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Report 62: Prescribing, Recording, and Reporting Photon Beam Therapy (Supplement to ICRU Report 50) Bethesda, Maryland: ICRU; 1999. ICRU (International Commission on Radiation Units and Measurements) [Google Scholar]
- 34.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64:333–42. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Forde E, Kneebone A, Bromley R, Guo L, Hunt P, Eade T. Volumetric-modulated arc therapy in postprostatectomy radiotherapy patients: A planning comparison study. Med Dosim. 2013;38:262–7. doi: 10.1016/j.meddos.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105:194–201. doi: 10.3171/sup.2006.105.7.194. [DOI] [PubMed] [Google Scholar]
- 37.Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys. 1995;32:1227–37. doi: 10.1016/0360-3016(94)00475-z. [DOI] [PubMed] [Google Scholar]
- 38.Luxton G, Hancock SL, Boyer AL. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2004;59:267–84. doi: 10.1016/j.ijrobp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 39.Vlachaki MT, Teslow TN, Amosson C, Uy NW, Ahmad S. IMRT versus conventional 3DCRT on prostate and normal tissue dosimetry using an endorectal balloon for prostate immobilization. Med Dosim. 2005;30:69–75. doi: 10.1016/j.meddos.2005.01.002. [DOI] [PubMed] [Google Scholar]