Abstract
This study was carried out to evaluate radioprotective effects of hesperidin (HES) administration before the irradiation on the cardiac oxidative stress and histopathological changes in an experimental rat model. The cardiovascular complications of radiation exposure cause morbidity and mortality in patients who received radiotherapy. HES, an antioxidant flavonoid found in citrus fruits, suggests the protection against the tissue damage. Fifty-eight rats were divided into four groups: Group 1 received phosphate buffered saline (PBS) and sham radiation; Group 2, HES and sham radiation; Group 3, PBS and radiation; and Group 4, HES and radiation. The rats were exposed to single dose of 18 Gy of 6 MV X-ray. One hundred milligrams per kilogram doses of HES was administered for 7 days before irradiation. The estimation of superoxide dismutase (SOD), malondialdehyde (MDA), and histopathological analyses was performed at 24 h and 8 weeks after radiation exposure. The irradiation of chest area resulted in an elevated MDA level and decreased SOD activity. Moreover, long-term pathological lesions of radiation were inflammation, fibrosis, the increased number of mast cells and macrophages, and development of plaque, vascular leakage, myocardial degeneration, and myocyte necrosis. Although the administration of HES decreases inflammation, fibrosis, mast cell and macrophage numbers, and myocyte necrosis, it did not result in reduced thrombus, myocardium degeneration, and vascular leakage. In conclusion, these results suggest that HES can perform a radioprotection action. The protective effect of HES may be attributable to its immunomodulatory effects and free radical-scavenging properties.
Key words: Cardiomyopathy, cardiotoxicity, hesperidin, radioprotector
Introduction
Today, the indications of the use of radiotherapy (RT) for cancer treatment are increased and the management of side effects of cancer therapy is more important than in the past. Several studies have shown that heart disease is a common disorder among patients who have undergone RT or population who were exposed to ionizing radiation (IR). Cardiovascular diseases are among the most important late side effects of radiation therapy for thoracic and chest wall tumors. RT is a common modality for treatment of many different types of cancers, including breast, lung, mediastinum, chest, and Hodgkin's disease.[1] In these cases, it is possible that whole or a part of the heart is located within the treatment field. Survivors of breast cancer and Hodgkin's disease have a longer survival as compared to other mentioned cancers. On the other hand, they face a greater risk of the radiation-induced heart disease (RIHD).[2] Moreover, younger people are at higher risk because RIHD generally occurs with a long latent period of several years after treatment.[3] A study by the American Society of Clinical Oncology has shown that RIHD is at 10% and 30% by 5-10 years after treatment among cancer survivors.[4] Studies of atomic bomb survivors have also shown an excess risk for cardiovascular disease among people who were exposed.[5,6,7]
The most important manifestations of RIHD among these patients are acute and chronic pericarditis, myocarditis, coronary artery diseases (including fibrosis, accelerated atherosclerosis, and thickening of intima layer), pericardial and myocardial fibrosis, and valvular disorders. The possible mechanisms of initiation of RIHD are inflammation and inflammatory cell infiltration, oxidative stress, increase in the production of superoxide in the microvessels, endothelial damage, increase in the development of macrophage-rich atherosclerotic lesions, and mast cell hyperplasia.[8,9]
The IR can increase the reactive oxygen species (ROS) and disrupt the structure of chemical bonds. The absorbed energy of IR causes the ionization of different atoms and molecules, including water and essential macromolecules such as DNA and membrane lipids.[10,11] Lipid peroxidation is a reason of cell membrane destruction and one of the main factors associated with tissue damage by ROS.[12] The endpoint of lipid peroxidation is malondialdehyde (MDA) which is cytotoxic and prohibits the function of antioxidant enzymes.[13,14] The deleterious effect of IR is generally due to ROS, including superoxide radical (O2 °−), hydroxyl radical (OH°), and hydrogen peroxide (H2 O2) generated by the decomposition of water.[15] Serious damaging potential of ROS causes the cells to depend on the antioxidant defense system, both enzymatic and nonenzymatic components.[16] The essential anti-oxidative enzymes are superoxide dismutases (SODs), catalase (CAT), and glutathione peroxidase (GSH-Px). It is a proven fact that SOD plays a key role in cellular defenses against oxidative damage. In addition, SOD catalyzes the dismutation of O2 °− into H2 O2. CAT and GSH-Px enzymes can transform H2 O2 into H2 O and O2.[15,17,18,19]
Because of the importance of this problem, it is crucial to explore ways of reducing cardiac toxicities following radiotherapy treatment. It seems that the use of antioxidants and immunomodulators can alleviate the changes induced by oxidative stress and inflammatory cell infiltration. Hesperidin (HES) (C28 H34 O15) is an inexpensive antioxidant flavonoid found in citrus fruits. Some observations have demonstrated that HES can provide an antioxidant protection against the damaging effects induced by oxidative stress.[20] Besides, it is reported that HES can modulate inflammatory targets, including nuclear factor-kB, nitric oxide synthase, and cyclooxygenase (COX)-2, and ameliorate signs of chronic inflammation.[21] Cardioprotective effects of HES, including increase in capillary resistance and permeability, have previously been reported.[22]
This study was carried out to evaluate radioprotective effects of HES administration before the irradiation on oxidative damages, inflammation and long-term pathological changes of X-radiation in an experimental rat model.
Materials and Methods
Chemicals and animals
All chemicals including HES (CAS registry number: 520-26-3), phosphate buffered saline (PBS) tablet, thiobarbituric acid (TBA), trichloroacetic acid, 1,1,3,3 tetraethoxypropane, ethylenediaminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, mannitol, and sucrose were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Healthy adult male Sprague–Dawley rats were purchased from Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences (SUMS), Shiraz, Iran. Rats weighing 220 ± 5 g were housed in accordance to the principles, outlined in “The Guide for The Care and Use of Laboratory Animals” prepared by SUMS in the university animal house. These principles include characteristics of animal's natural life in captivity situation, using spacious cage, preparing appropriate ventilation and light, handling with care, given standard pellet diet and water ad libitum, etc., All animals were kept under controlled conditions of temperature (23 ± 2°C), humidity (55 ± 5%), and light (12 h of light and dark cycle). Four animals were housed together in polypropylene cages containing sterile husk bedding throughout the experiments. In the end, this study was exactly performed according to the ethical committee instructions of SUMS.
Irradiation of animals
Before radiation exposure, the rats received anesthesia using ketamine 10% (Alfasan, Woerden, Holland) at a dose of 80 mg/kg and xylazine 2% (Alfasan, Woerden, Holland) at a dose of 5 mg/kg with an intraperitoneal injection. The rats were immobilized in the supine position by taping the extremities on a well-ventilated plexiglas container. Animals were irradiated with a 6 MV X-ray linear accelerator machine (Elekta Compact 6 MV, China) at a source-to-surface distance of 100 cm. A single dose of 18 Gy was delivered to the thorax area at a dose rate of 350 monitor unit in the department of RT, Nemazee Teaching Hospital, Shiraz, Iran. This dose was selected based on the data reported by Lauk et al. - they have discussed that after local irradiation of the rat heart with X-ray doses of over 10 Gy (single dose), animals develop the symptoms of radiation-induced heart disease and Gürses et al. - they have discussed that a single dose of 18 Gy significantly induces heart injury.[23,24]
Administration of hesperidin
HES was dissolved in PBS (pH 7.6) and administered orally using a ball-tipped needle for 7 consecutive days before exposure to irradiation. It should be pointed that the drug was freshly prepared each day. To obtain optimum radiation protective effect of HES, the dose of 100 mg/kg was selected for this study based on the reports by Hosseinimehr et al. and Pradeep et al.[25,26] They have shown that as compared to the other doses of HES, this dose has better protective effect against IR-induced damage. To prepare this dose, 22 mg of HES was dissolved in 2 ml of PBS.
Experimental design
Male rats were randomly divided into four groups. Group 1 (control): Fourteen rats served as controls only received PBS for 7 days. Group 2 (HES): Eight rats were treated with HES for 7 days. Group 3 (radiation): Eighteen rats received PBS for 7 days and then exposed to X-ray 1 h after the last dose of PBS. Group 4 (HES + R): Eighteen rats were treated with HES for 7 days and then exposed to X-ray 1 h after the last dose of HES. After the last administration of HES or PBS on the 7th day, the animals in control and HES groups were anesthetized as similar to rats in Radiation and HES + R groups. Eight animals in each group were sacrificed 24 h after RT for biochemical assay and acute histopathological evaluation. Remaining animals were sacrificed 8 weeks after RT for chronic histopathological evaluation.
Supernatant preparation
After 24 h of the last dose of the specific treatment, animals were anesthetized with ketamine and xylazine, and then a laparotomy was performed, thus the chest was opened. The heart was perfused in situ through the right ventricle of the heart with sodium chloride 0.9% and diced with scissors. The perfused tissue was cleared from any red blood cells and clot with PBS, at pH 7.4. Furthermore, to determine the SOD activity, 0.3 g of the heart tissue was homogenized in 1.5 ml of cold 20 mM HEPES buffer, pH 7.2, (containing 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose). The crude homogenate was centrifuged 1500 ×g for 5 min at 4°C. Moreover, with determining the total MDA concentrations, 0.2 g of the heart tissue was homogenized in 1 ml of cold buffer (50 mM PBS, pH 7, containing 1 mM EDTA). The crude homogenate was centrifuged 1500 ×g for 10 min at 4°C. Homogenate was prepared using an IKA T 10 basic ULTRA-TURRAX (Germany) homogenizer. And then, the clear supernatant was used for biochemical analysis.
Biochemical assay
SOD activity was assayed using commercial assay kits (Cayman, USA) as per the manufacturer's instructions. The SOD activity was measured using a tetrazolium salt to detect the O2 °− s generated by xanthine oxidase and hypoxanthine which produced a yellow color that was consequently measured by absorbance at 440 nm. The MDA levels were assayed for products of lipid peroxidation according to TBARS method.[27] MDA content in samples and standards reacted with TBA at 95°C for 20 min, incubated at 25°C for 5 min, and then centrifuged with torque of 5000 rpm at 4°C for 15 min. MDA concentration was spectrophotometrically read at 532 nm and determined in comparison with predetermined MDA standard curve. The SOD activity and MDA concentration are expressed in U/ml and nM, respectively.
Histopathological evaluation
Rats were anesthetized with ketamine and xylazine. After removal of the heart from the chest, heart were fixed, inflated with 10% neutral buffered formalin introduced through the airways, and then embedded in paraffin. Whole-mount sections of the heart were cut (5 μm), processed, and stained with H and E (H and E), Masson's trichrome (MTC), and Ziehl–Neelsen acid fast (AF). All histopathological studies were performed at the unit of pathology, Faghihee Teaching Hospital, Shiraz, Iran. The blinded histopathological evaluation was performed under the light microscope (Olympus BX41TF, Japan) using a semiquantitative scoring system for the severity and extent of histological parameters, in which left and right ventricles were separately examined according to the three layers of the heart (endocardium, myocardium, and epicardium).
Inflammation, fibrosis, mast cells, macrophages, myocyte necrosis, plaque, thrombus, myocardial degeneration, vascular damage, and vascular leakage were also the items used for the description of heart radiation injury. The degree of fibrosis and mast cells for each layer of the ventricles was scaled from G0 to G3: G0 indicates no injury, G1 - mild, G2 - moderate, and G3 - severe. Myocyte necrosis was graded as (GX0) no necrosis, (GX1) single cell necrosis, and (GX2) more than one cell. In addition, the description of myocardial inflammation, macrophages, thrombus, plaque, myocardial degeneration, vascular damage, and vascular leakage was quantified by a graded scale from G0 to G3: 0 (no change), 1 (mild), 2 (moderate), and 3 (severe). Sections were stained using H and E for general tissue characterization.[28] Moreover, total collagen accumulation was determined by preparing tissue sections with MTC stain.[29,30] Mast cells were evaluated using AF stain.[31,32] The heart index was calculated as follows: Heart index = (heart weight/body weight) ×100.
Statistical analysis
First and foremost, data were analyzed using a commercially available statistics software package (SPSS® for Windows version 19, Chicago, USA). Biochemical assay was analyzed by ANOVA test and post hoc Tukey honest significant difference (HSD). Besides, histopathological evaluations were analyzed by the Pearson Chi-square test and a pair-wise comparison with the Mann–Whitney. A Pearson correlation was run to determine the relationship between left and right ventricles using the bivariate correlation (Pearson) test. A one-way ANOVA test with post hoc Tukey HSD was also performed for evaluating the body weight, heart weight, and heart index. Meanwhile, survival rate was evaluated with Kaplan–Meier method. In this study, the results are presented as mean ± standard deviation; P < 0.05 was considered statistically significant difference.
Results
Effect of hesperidin on the survival rate of rats
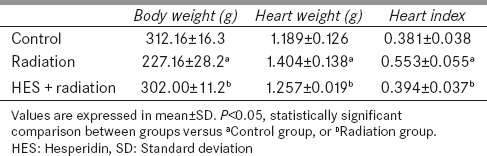
The results of the survival rate, body weights, heart weights, and heart indexes of control and experimental rats evaluated 8 weeks after local-thorax irradiation are shown in Figure 1 and Table 1. Rats were preadministered with 100 mg/kg/day of HES for 7 consecutive days before irradiation and monitored daily for 60 days. The results indicated that the rats, received 18 Gy radiation, showed the signs of discomfort which are characterized by the decreased physical activity and the reduced uptake of food and water. The radiation group also exhibited some signs of radiation sickness such as irritability, ruffling of hair, weight loss, emaciation, and epilation with a median survival period of 55 days. On the other hand, the rats that were preadministered with HES reduced the signs of radiation sickness and significantly improved the physical activity, body weight, and survival rate.
Figure 1.
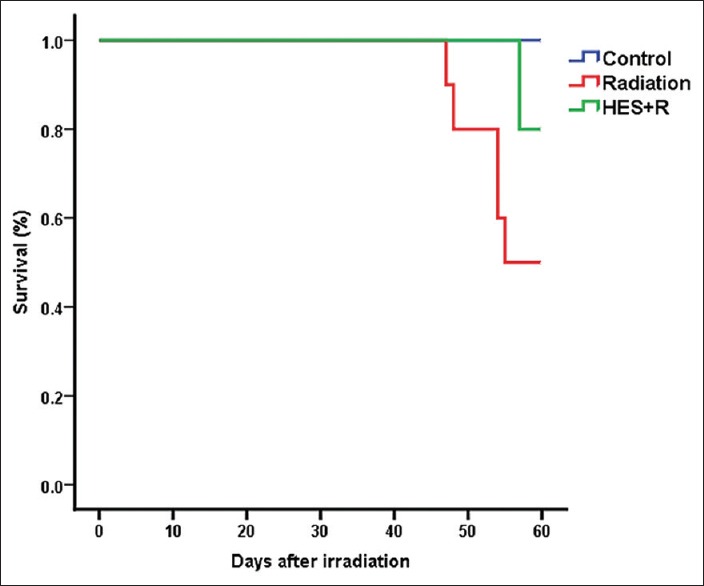
Dose-dependent effect of hesperidin on the survival rate of rats observed for an experimental duration of 60 days. Control group: Six rats survived; radiation group: Five rats survived; hesperidin + R group: Eight rats survived
Table 1.
Effect of hesperidin treatment on body weight, heart weight, and heart index of rats exposed with X-irradiation
Effect of hesperidin on the superoxide dismutase and malondialdehyde
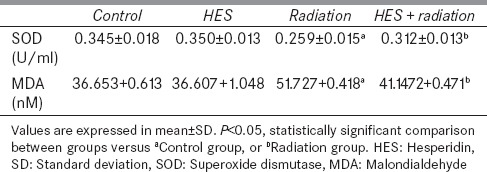
The effect of X-rays with or without HES on SOD activity and concentration of MDA in heart tissue for 24 h after irradiation are shown in Table 2. The activity of SOD in the heart tissue significantly decreased at 24 h following the irradiation as compared to control group (P < 0.001). Treatment with HES for 7 consecutive days before exposure to X-rays significantly increased the level of HES + R group to near normal, compared to radiation group (P = 0.019). MDA levels in radiation group of heart tissue showed a significant increase (P = 0.002) as compared to control group. HES-treated rats showed significantly decreased levels of MDA (P = 0.023) in HES + R group as compared to radiation group. In addition, the result analyses did not display significant difference among control, HES, and HES + R groups in the SOD and MDA levels.
Table 2.
Superoxide dismutase activities and malondialdehyde levels in control, hesperidin, radiation, and hesperidin+radiation groups (n=8)
Histopathological examination
Radiation damage and effects of hesperidin in the acute phase
The histopathological observation of the heart sections of the control, HES, radiation, and HES + R groups at 24 h postirradiation was evaluated. The descriptive factors were examined including the presence of macrophages, incidence of myocardial inflammation, myocyte necrosis, plaque, myocardial degeneration, vascular damage, and vascular leakage. According to the results, minimal myocardial inflammation was observed in the radiation and HES + R groups. However, there was no significant difference (P > 0.05) among the 24 h groups [Figure 2].
Figure 2.
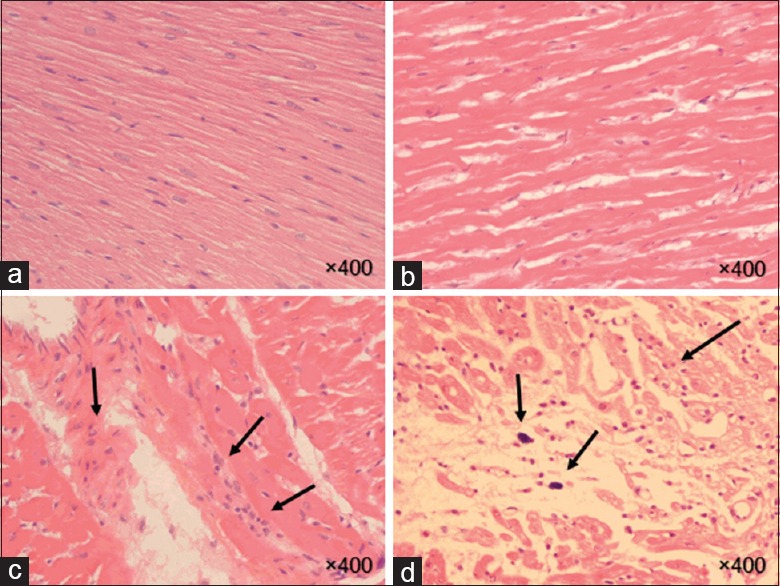
Histopathological findings of rat hearts in the acute phase (24 h). H and E stains of hearts taken at ×400 from unirradiated (a and b) and irradiated (c and d) rats reveal mild inflammation and the scattered foci of inflammatory cell in a subpopulation of the irradiated rats. The flashes indicate the accumulation of lymphocyte, macrophages, and inflammation in heart tissue. (a): Control, (b): Hesperidin, (c): Radiation, (d): Hesperidin + R
Radiation damage and effects of hesperidin in the chronic phase
To determine chronic changes in heart tissue after RT, histopathological evaluations of the heart sections were performed in the control, radiation, and HES + R groups at 8 weeks after radiation [Table 3 and Figures 3–5]. The descriptive factors examined were the presence of macrophages, incidence of myocardial inflammation, degree of fibrosis and mast cells for each layer, myocyte necrosis, plaque, thrombus, myocardial degeneration, vascular damage, and vascular leakage.
Table 3.
Effect of hesperidin treatment at 8 weeks postirradiation on histopathological factors in the heart tissue of rats
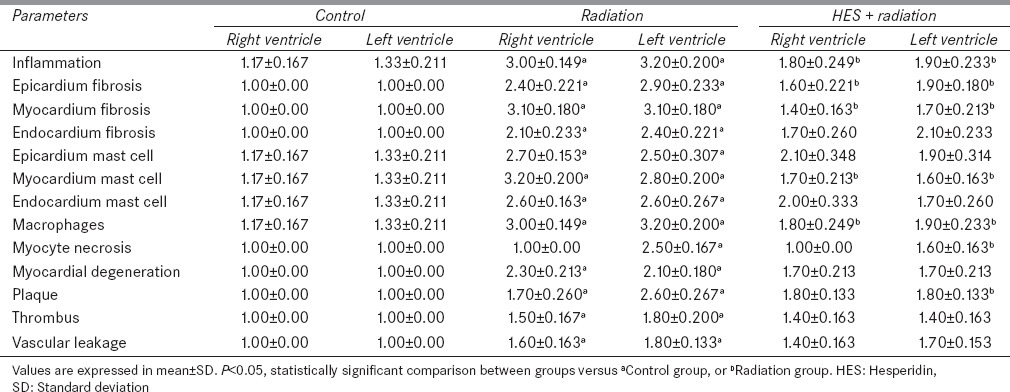
Figure 3.
Histopathological findings of rat hearts in the chronic phase (8 weeks). H and E stains of hearts were taken at x400. Myocardial tissue and vascular bed are seen normal (a and f). Acute inflammation and accumulation of inflammatory cells (b), atheroma plaque associated with intraplaque angiogenesis and macrophage infiltration (c), cardiac myocyte necrosis (d) in the myocardial tissue are observed. Mild inflammation and the decreased infiltration of inflammatory cells are also discerned (e). (a): Control, (b-d): Radiation, (e and f): Hesperidin + R
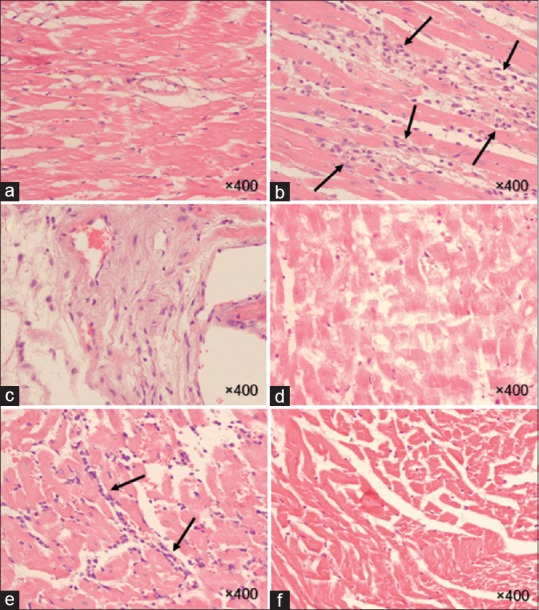
Figure 5.
Histopathological findings of rat hearts in the chronic phase (8 weeks). Acid fast stains of hearts were taken at ×400 magnification and mast cells seem dark blue points. Normal appearance is observed in the myocardium at the control group (a). Mast cell infiltration appears minimal to moderate at the epicardial (b) and endocardial (e) of the ventricle. Acute mast cells infiltration is recognized in the myocardial (d) and endocardial (c) layers of ventricle. (f) Myocardial tissue is characterized by mild infiltration of mast cells. (a): Control, (b-d): Radiation, (e and f): Hesperidin + R
Inflammation
The statistical results of the left ventricles were found to be as follows: Inflammation at the myocardial layer reached a significant difference between groups (P = 0.005). According to the results by a pair-wise comparison test, inflammation increased significantly in the radiation group when compared to control (P = 0.001) and inflammation decreased significantly in the HES + R group when compared to radiation group (P = 0.02). Furthermore, histopathological observations of the right ventricles were similar to the left ventricle with significant difference between groups (P = 0.004). Besides, the statistical results were analyzed between radiation and control groups (P = 0.001) and between HES + R and radiation groups (P = 0.02). Observationally, the accumulation of macrophages in all groups was akin to inflammation results as well as statistical results [Figure 4]. Distinctly, there was a strong, positive correlation between inflammation in the left and right ventricle of radiation and HES + R groups, which was statistically significant [Table 4].
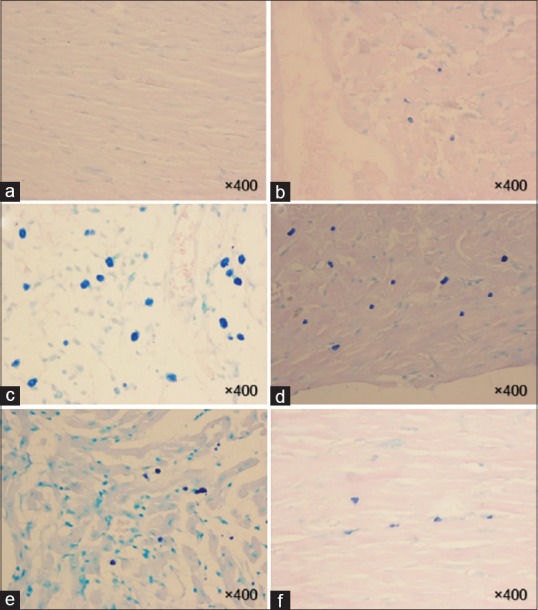
Figure 4.
Histopathological findings of rat hearts in the chronic phase (8 weeks). Masson's trichrome stains of hearts were taken at ×100 and collagen deposition as an indicator of fibrosis is seen light blue. Myocardial tissue and vascular bed are considered normal (a). Acute collagen deposition is distinguished in the epicardial (b), endocardial (c) and myocardial (d) layers of ventricle. In addition, mild collagen deposition is detected in the endocardial (e) and myocardial (f). (a): Control, (b-d): Radiation, (e and f): Hesperidin + R
Table 4.
Correlations between the descriptive factors

Fibrosis
Fibrosis in all groups is shown in Table 3 and Figure 5, according to its layers. Statistical difference was discovered between the groups in the endocardial, myocardial, and epicardial layers of left ventricle (P = 0.003, P < 0.001, and P < 0.001 respectively). A significant difference was present between radiation and control groups (P < 0.001, P < 0.001, and P = 0.001) in the three layers of left ventricle. Myocardial and epicardial fibrosis were significantly abated in the HES + R group when compared to the radiation group. In the right ventricles, observations were similar to the left ventricle with significant difference between groups in the endocardial, myocardial, and epicardial layers (P = 0.005, P < 0.001, and P = 0.002, respectively). As similar to left ventricle, myocardial and epicardial fibrosis were decreased in the HES + R group (P < 0.001 and P = 0.024) in comparison with radiation group. Furthermore, a significant difference between radiation and control groups was perceived in the three layers (P < 0.05). However, in the HES + R groups of both ventricles, endocardial fibrosis was not considered significant when compared to radiation group (P > 0.05). The left myocardial fibrosis revealed a significant correlation with right myocardial fibrosis in the radiation and HES + R groups, separately. A similar correlation was also found to be present between epicardial fibrosis in the left and right ventricles of HES + R group [Table 4].
Mast cell
According to the results, a significant difference among groups (P < 0.05) was observed in the myocardial mast cells in the left ventricles. Endocardial and epicardial mast cells were not found to be significant (P > 0.05). Myocardial mast cell infiltration was likely to be the highest in radiation group (P = 0.002 when compared to control) and the lowest in HES + R group (P = 0.001 when compared to radiation group). Endocardial and epicardial mast cell infiltrations significantly increased in the radiation group in comparison with controls, but not significantly decreased in the HES + R group. The statistical results of the right ventricles expressed a significant difference between groups in the endocardial, myocardial, and epicardial layers (P = 0.021, P = 0.001, and P = 0.023, respectively). Moreover, a significant difference was present between radiation and control groups (P = 0.001) in the three layers of right ventricle. Myocardial mast cells were significantly diminished in the HES + R group when compared to the radiation group (P = 0.001). However, endocardial and epicardial mast cells apparently decreased in the HES + R group with P > 0.05 when compared to the radiation group [Figure 5]. Correlations between the parameters are shown in Table 4.
Myocyte necrosis and myocardial degeneration
As the results indicate, necrosis appeared in the radiation and HES + R groups of left ventricles. In addition, it was unlikely to trace any necrosis in the right ventricles of heart tissues of rats [Figure 4d]. The number and severity of necrosis were observed to be high in radiation group in comparison with control group (P = 0.001). Myocyte necrosis reduced in the HES + R group with a significant difference when compared to radiation group (P = 0.003). On the other hand, myocardium degeneration was enhanced in the left and right ventricles in the radiation group when compared to controls (P = 0.001 and P = 0.002), and reduced in the HES + R group in comparison with radiation group, but no significant difference distinguished (P = 0.154 and P = 0.064). A correlation was obtained between the left and right myocardial degeneration in the radiation groups [Table 4].
Plaque and thrombus
Through group analyses, the plaque deposits were significant in the left and right ventricles (P < 0.001 and P = 0.007). In the left, plaque was the highest in the radiation group (P = 0.003 compared to control) and the lowest in the HES + R group (P = 0.009 compared to radiation) [Figure 4c]. In the right, there was a significant difference between the radiation and control groups (P = 0.047). The plaque deposits were the highest in the HES + R group contrary to the radiation group. Hence, there was no significant difference (P = 0.552). Mild thrombus was observed in the left and right ventricle of radiation and HES + R groups. However, no significant difference was recognized in the rats of HES + R and radiation groups (P > 0.05). Besides, there was a positive correlation between the left and right thrombus in the radiation group [Table 4].
Vascular leakage and vascular damage
As the results indicated, vascular leakage was distinct in the radiation and HES + R groups. On observation, mild vascular leakage was seen in the left and right ventricles of both groups [Table 3]. Statistically significant results were observed between groups in the left ventricle (P = 0.004). There was no significant difference between HES + R and radiation groups (P > 0.05) although a significant difference existed in both right and left ventricles of the radiation group in comparison with control group (P < 0.05). Unexpectedly, no vascular damage was observed in the ventricles of the rats in any groups.
Discussion
The effects of IR are mediated through direct interaction to DNA damage and the production of ROS and reactive nitrogen species in cells as a result of water radiolysis and also the reduction of endogenous scavengers such as SOD and GSH. SOD acts as an initial barrier to oxidant products and catalyzes the dismutation of the O2 °− into O2 or H2 O2. Superoxide anions have a pro-inflammatory role in many diseases.
Inflammation occurs after the accumulation of immune system cells such as lymphocyte and macrophage that result in the secretion of inflammatory mediators such as cytokines and COX products. Several studies suggest that antioxidant enzymes including SOD are the key regulators of inflammation.[33] The production of MDA, which serves as a lipid peroxidation index, is found to be increased in oxidative and inflamed cells. Lipid peroxidation is associated with upregulation of ROS and superoxides that result in the suppression of SOD activity.[34]
Oxidative cell damage and subsequent DNA damage responses provoke inflammatory responses through several pathways. Heavy cell death leads to long-term inflammatory responses in the irradiated tissues.[35] The inflammatory response of the irradiated tissues to a high-dose radiation causes the increased movement leukocytes from the blood into the irradiated tissues that contribute to the increased population of the resident macrophages, mast cells, neutrophils, and lymphocytes. Acute inflammation and oxidative stress induced by radiation may continue for a long time and lead to chronic inflammation. Chronic inflammation is associated with a long-term increase in macrophage, neutrophil, and lymphocyte populations in the irradiated tissues.[36]
The long-term upregulation of inflammatory cells in heart tissue can cause progressive and persistent oxidative stresses that lead the subsequent side effects such as fibrosis, coronary and carotid vascular injury, fatty plaque, atherosclerosis, and heart valves disease. Consequently, they lead to the elevated probability of incidence of heart failure and stroke between people who have been exposed.[9,37]
Mast cells are capable of producing a wide range of cytokines, chemokines, growth factors, proteases, and nitric oxide.[38] Several experimental studies have reported the increased numbers of mast cells involved in fibrosis, dilated cardiomyopathy, myocarditis, hypertension, and myocardial infarction.[39,40] Moreover, mast cell hyperplasia simulate the production of endothelin-1, a powerful vasoconstrictor peptide that promotes inflammation and fibrosis and cardiac hypertrophy that leads many forms of heart disease, including hypertension and vascular disease.[41] Mast cells get also involved in RIHD through changes in neuroimmune interactions and renin angiotensin system.[8]
Damage to vasculature plays an essential role in heart damages induced by radiation. Several studies have shown that the radiation induces the formation of inflammatory plaque, lipid accumulation, inflammation, and thrombosis that increase the risk of fatal heart attack or stroke.[42] Radiation can cause atherosclerosis, increase in intimal thickness, and vascular wall fibrosis in coronary and carotid arteries. These changes may bring about vascular stenosis or occlusion that leads to the disruption of blood supply to the muscle cells, necrosis, and ischemic stroke.[43] Studies have shown that IR can accelerate heart diseases induced by other heart disease risk factors.[9] On the other hand, RIHD is manifested by adjuvant chemotherapy drugs such as doxorubicin, pericardial disease, accelerated coronary artery atherosclerosis, young age at irradiation, and increasing patients age.[44,45,46]
HES is a potent antioxidant and a major flavonoid in sweet orange and lemon. Moreover, HES can exert a wide range of effects, including ROS scavenging, anti-inflammatory, and anticarcinogenic action. Pradeep et al., have shown that oral administration of HES can reduce collagen deposition, sialic acid, xanthine oxidase-induced by gamma radiation in the heart, liver, and kidney of rats. In addition, HES can increase plasma membrane integrity and reduce permeability of the tissues after exposure to g-rays.[47] Administration of HES for 3 days before exposure to 2 Gy g-radiation attenuates increase in serum asymmetric dimethyl arginine (ADMA) and decrease in nitrate/nitrite (NO) level. The elevated levels of ADMA lead to the decreased production of NO synthesis that results in endothelial dysfunction and endothelial function impairment. Moreover, treatment with HES ameliorate increased the activity of serum creatine phosphokinase (CPK) and lactate dehydrogenase (LDH) after irradiation. Treatment with HES reduced nephrotoxicity and biophysical mechanical properties of bone as well.[48] Furthermore, posttreatment with HES for 7 days after exposure to a single dose of 5 Gy g-radiation ameliorate elevated the activity of cardiac marker enzymes such as aspartate transaminases, alanine transaminases, alkaline phosphatase, LDH, and CPK.[49]
As mentioned above, oxidative damages and subsequent immune responses are counted crucial in heart damage and long-term histopathological changes followed by exposure to radiation. In this study, it is hypothesized that preadministration with HES for 7 consecutive days before exposure to a single dose of 18 Gy radiation may ameliorate oxidative damage, inflammatory responses, and long-term pathological changes after exposure to radiation.
Conclusions
The irradiation of the chest area of the rats with 18 Gy results in oxidative damage and pathological changes in the heart tissue. The early oxidative damage is associated with the increased MDA level and decreased SOD enzyme activity. Long-term changes are also associated with the decreased survival, body and heart weight, and increased heart tissue index. Besides, the irradiation of heart tissue results in inflammation in both right and left ventricles. Fibrosis and the increased number of mast cells were observed in both right and left ventricles of epicardium, myocardium, and endocardium. The increased number of macrophages, plaque, vascular leakage, and myocardial degeneration are obvious in both right and left ventricles. Moreover, myocyte necrosis is conspicuous in the left ventricles but not in the right ventricles.
The administration of HES decreases inflammation for both right and left ventricles. Fibrosis decreases in left and right ventricles of epicardium and myocardium but not in endocardium. However, mast cell numbers decrease only in left and right myocardial. The administration of HES decreases macrophage number in both left and right ventricles. The increased myocyte necrosis in left ventricle is ameliorated with HES preadministration. Plaque deposition is reduced only for left ventricle. Furthermore, HES could not ameliorate thrombus, myocardium degeneration, and vascular leakage in both left and right ventricles.
According to the results, preadministration of HES is enabled to ameliorate oxidative damages, inflammation and subsequent cell death after RT, which can cause the increased risk of heart diseases during years after radiation treatment. HES known as an antioxidant and anti-inflammatory drug may help alleviate short- and long-term side effects on patients who have undergone radiation for cancer treatment. In the end, more studies will be needed to understand the effects of HES administration on immune response signaling, different inflammation pathways, and DNA repair mechanisms.
Financial support and sponsorship
This study was financially supported by the grant number 94012 from the Vice-Chancellor of Research of Fasa University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors greatly would like to thank Vice-Chancellor of Research of Fasa University of Medical Sciences for authorizing and funding this study.
References
- 1.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 2.Madan R, Benson R, Sharma DN, Julka PK, Rath GK. Radiation induced heart disease: Pathogenesis, management and review literature. J Egypt Natl Canc Inst. 2015;27:187–93. doi: 10.1016/j.jnci.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee BK, Mariscal CS, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin's disease. J Clin Oncol. 2007;25:43–9. doi: 10.1200/JCO.2006.07.0805. [DOI] [PubMed] [Google Scholar]
- 4.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, Pierce DA, Preston DL, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, part II. Noncancer mortality: 1950-1990. Radiat Res. 1999;152:374–89. [PubMed] [Google Scholar]
- 6.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmot MG, Syme SL, Kagan A, Kato H, Cohen JB, Belsky J. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: Prevalence of coronary and hypertensive heart disease and associated risk factors. Am J Epidemiol. 1975;102:514–25. doi: 10.1093/oxfordjournals.aje.a112189. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: A clinical update. Cardiol Res Pract. 2011;2011:317659. doi: 10.4061/2011/317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boerma M, Hauer-Jensen M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract 2010. 2011:pii: 858262. doi: 10.4061/2011/858262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lett JT. Damage to cellular DNA from particulate radiations, the efficacy of its processing and the radiosensitivity of mammalian cells. Emphasis on DNA double strand breaks and chromatin breaks. Radiat Environ Biophys. 1992;31:257–77. doi: 10.1007/BF01210207. [DOI] [PubMed] [Google Scholar]
- 11.Dainiak N, Tan BJ. Utility of biological membranes as indicators for radiation exposure: Alterations in membrane structure and function over time. Stem Cells. 1995;13(Suppl 1):142–52. [PubMed] [Google Scholar]
- 12.Kaya H, Delibas N, Serteser M, Ulukaya E, Ozkaya O. The effect of melatonin on lipid peroxidation during radiotherapy in female rats. Strahlenther Onkol. 1999;175:285–8. doi: 10.1007/BF02743581. [DOI] [PubMed] [Google Scholar]
- 13.Taysi S, Uslu C, Akcay F, Sutbeyaz MY. Malondialdehyde and nitric oxide levels in the plasma of patients with advanced laryngeal cancer. Surg Today. 2003;33:651–4. doi: 10.1007/s00595-002-2562-3. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 15.Ertekin MV, Koçer I, Karslioglu I, Taysi S, Gepdiremen A, Sezen O, et al. Effects of oral Ginkgo biloba supplementation on cataract formation and oxidative stress occurring in lenses of rats exposed to total cranium radiotherapy. Jpn J Ophthalmol. 2004;48:499–502. doi: 10.1007/s10384-004-0101-z. [DOI] [PubMed] [Google Scholar]
- 16.Noaman E, Zahran AM, Kamal AM, Omran MF. Vitamin E and selenium administration as a modulator of antioxidant defense system: Biochemical assessment and modification. Biol Trace Elem Res. 2002;86:55–64. doi: 10.1385/BTER:86:1:55. [DOI] [PubMed] [Google Scholar]
- 17.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med. 1998;24:586–93. doi: 10.1016/s0891-5849(97)00291-8. [DOI] [PubMed] [Google Scholar]
- 19.Polat MF, Taysi S, Gul M, Cikman O, Yilmaz I, Bakan E, et al. Oxidant/antioxidant status in blood of patients with malignant breast tumour and benign breast disease. Cell Biochem Funct. 2002;20:327–31. doi: 10.1002/cbf.980. [DOI] [PubMed] [Google Scholar]
- 20.Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005;53:4757–61. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 21.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–31. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 22.Kuntic V, Brboric J, Holclajtner-Antunovic I, Uskokovic-Markovic S. Evaluating the bioactive effects of flavonoid hesperidin - A new literature data survey. Vojnosanit Pregl. 2014;71:60–5. doi: 10.2298/vsp1401060k. [DOI] [PubMed] [Google Scholar]
- 23.Lauk S, Kiszel Z, Buschmann J, Trott KR. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–8. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 24.Gürses I, Özeren M, Serin M, Yücel N, Erkal HS. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol Res Pract. 2014;210:863–71. doi: 10.1016/j.prp.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinimehr SJ, Nemati A. Radioprotective effects of hesperidin against gamma irradiation in mouse bone marrow cells. Br J Radiol. 2006;79:415–8. doi: 10.1259/bjr/40692384. [DOI] [PubMed] [Google Scholar]
- 26.Pradeep K, Park SH, Ko KC. Hesperidin a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in Sprague-Dawley rats. Eur J Pharmacol. 2008;587:273–80. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- 28.Gamble M. The hematoxyline and eosin. In: Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 6th ed. Philadelphia: Churchill Livingstone/Elsevier; 2008. pp. 121–34. [Google Scholar]
- 29.Sheehan D, Hrapchak B. Theory and Practice of Histotechnology. 2nd ed. Ohio: Battelle Press; 1980. pp. 189–90. [Google Scholar]
- 30.Jones ML, Bancroft JD, Gamble M. Connective tissues and stains. In: Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 6th ed. Philadelphia: Churchill Livingstone/Elsevier; 2008. pp. 135–60. [Google Scholar]
- 31.Eagle RC. Eye Pathology: An Atlas and Text. 2nd ed. Philadelphia, London: Lippincott Williams and Wilkins; 2010. p. 28. [Google Scholar]
- 32.Loew JM, Macon WR. Lymph nodes. In: Gattuso P, Reddy VB, David O, Spitz DJ, Haber MH, editors. Differential Diagnosis in Surgical Pathology. 2nd ed. Philadelphia: W.B. Saunders; 2010. p. 761. [Google Scholar]
- 33.Li C, Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res. 2011;2011:387176. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 35.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: Therapeutic implications. Curr Med Chem. 2009;16:130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 37.Stewart FA, Heeneman S, Te Poele J, Kruse J, Russell NS, Gijbels M, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–58. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 39.Levick SP, Meléndez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: The centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89:12–9. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palaniyandi Selvaraj S, Watanabe K, Ma M, Tachikawa H, Kodama M, Aizawa Y. Involvement of mast cells in the development of fibrosis in rats with postmyocarditis dilated cardiomyopathy. Biol Pharm Bull. 2005;28:2128–32. doi: 10.1248/bpb.28.2128. [DOI] [PubMed] [Google Scholar]
- 41.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: A new therapeutic target? Circulation. 2004;109:1580–9. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 42.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 43.Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol. 2010;55:1237–9. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–55. [PubMed] [Google Scholar]
- 45.Carlson RG, Mayfield WR, Normann S, Alexander JA. Radiation-associated valvular disease. Chest. 1991;99:538–45. doi: 10.1378/chest.99.3.538. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro CL, Hardenbergh PH, Gelman R, Blanks D, Hauptman P, Recht A, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol. 1998;16:3493–501. doi: 10.1200/JCO.1998.16.11.3493. [DOI] [PubMed] [Google Scholar]
- 47.Pradeep K, Ko KC, Choi MH, Kang JA, Chung YJ, Park SH. Protective effect of hesperidin, a citrus flavanoglycone, against gamma-radiation-induced tissue damage in Sprague-Dawley rats. J Med Food. 2012;15:419–27. doi: 10.1089/jmf.2011.1737. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed HM, Hussein MA, Alazonee AS. Radioprotective effect of hesperidin against gamma-irradiation-induced oxidative stress and biomechanical properties of bone in rats. Life Sci J. 2013;10:2857–65. [Google Scholar]
- 49.Park S, Pradeep K, Ko KC. Protective effect of hesperidin against g-radiation induced oxidative stress in Sprague-Dawley rats. Pharm Biol. 2009;47:940–7. [Google Scholar]


