ABSTRACT
During late stages of cystic fibrosis pulmonary infections, Pseudomonas aeruginosa often overproduces the exopolysaccharide alginate, protecting the bacterial community from host immunity and antimicrobials. The transcription of the alginate biosynthesis operon is under tight control by a number of factors, including AmrZ, the focus of this study. Interestingly, multiple transcription factors interact with the far-upstream region of this promoter (PalgD), in which one AmrZ binding site has been identified previously. The mechanisms of AmrZ binding and subsequent activation remain unclear and require more-detailed investigation. In this study, in-depth examinations elucidated four AmrZ binding sites, and their disruption eliminated AmrZ binding and promoter activation. Furthermore, our in vitro fluorescence resonance energy transfer experiments suggest that AmrZ holds together multiple binding sites in PalgD and thereafter induces the formation of higher-order DNA-AmrZ complexes. To determine the importance of interactions between those AmrZ oligomers in the cell, a DNA phasing experiment was performed. PalgD transcription was significantly impaired when the relative phase between AmrZ binding sites was reversed (5 bp), while a full-DNA-turn insertion (10 bp) restored promoter activity. Taken together, the investigations presented here provide a deeper mechanistic understanding of AmrZ-mediated binding to PalgD.
IMPORTANCE Overproduction of the exopolysaccharide alginate provides protection to Pseudomonas aeruginosa against antimicrobial treatments and is associated with chronic P. aeruginosa infections in the lungs of cystic fibrosis patients. In this study, we combined a variety of microbiological, genetic, biochemical, and biophysical approaches to investigate the activation of the alginate biosynthesis operon promoter by a key transcription factor named AmrZ. This study has provided important new information on the mechanism of activation of this extremely complex promoter.
INTRODUCTION
In cystic fibrosis (CF) patients, the Gram-negative bacterium Pseudomonas aeruginosa is a dominant pathogen. Infection with P. aeruginosa, especially with mucoid variants that overproduce the exopolysaccharide alginate, results in a poor prognosis for these patients (1). During CF lung infections, P. aeruginosa initially colonizes as a nonmucoid form with minimal alginate production. However, at late stages, as many as 70% of P. aeruginosa isolates are mucoid due to alginate overproduction (2–6). Alginate-dominated biofilms form a thick protective layer against host immune attacks and antimicrobials (3, 7). However, overproduction of this exopolysaccharide is an energy-exhausting process, requiring a number of enzymes and precursor substrates. Therefore, alginate production is under tight regulation involving multiple regulatory factors (8). Previous studies have shown that the sigma factor AlgT (also referred to as AlgU/σ22) is necessary for the transcription of genes in the alginate biosynthesis operon (9–11). In mucoid variants, or in previously nonmucoid strains under certain stress conditions (12), AlgT is freed from sequestration by the anti-sigma factor MucA and initiates the transcription of genes encoding the transcription factors (TFs) AmrZ, AlgB, and AlgR (13, 14). These three TFs and at least four other TFs bind to the promoter of the alginate biosynthesis operon (PalgD) and initiate transcription (15–18). Interestingly, a region >100 bp upstream of the transcription start site contains at least seven TF binding sites, and it is not known how these TFs mediate activation from such a long distance. Although a DNA loop model has been proposed, there is little supporting evidence (19).
Remote regulatory elements are commonly seen in bacterial promoters, but these elements are often coupled with proximal elements (20). Regulation by remotely bound proteins typically requires interactions with proximal proteins, which rely on their proper relative conformation. These contacts, however, can be disrupted by interference with DNA phasing (21–23). In certain regulatory systems, protein-protein interactions result in DNA looping, which has been observed in multiple transcription regulators, such as the lac repressor, the deo repressor, and AraC (24–26). DNA looping plays important roles during transcription regulation, such as allowing remotely bound proteins to execute regulation, recruiting RNA polymerase (RNAP) to the promoter, or blocking RNAP.
As a global regulator, AmrZ functions as both an activator and a repressor of multiple processes. Well-studied targets include those involved in exopolysaccharide production (alginate, Psl, and Pel), flagella, twitching motility, and the metabolism of bis-(3′,5′)-cyclic diguanylate (4, 13, 27–31). AmrZ is composed of a flexible N terminus (residues 1 to 11), a DNA binding ribbon-helix-helix domain (residues 12 to 66), and a C-terminal tetramerization domain (CTD) (residues 67 to 108) (32, 33). The ribbon-helix-helix domain is conserved in other Arc superfamily proteins, such as Arc, CopG, and MetJ (33–36). AmrZ tetramerizes in solution, and the AmrZ construct comprising residues 1 to 66 (AmrZ1-66) binds to DNA as a dimer of dimers, suggesting that AmrZ interacts with its DNA targets as oligomers, likely tetramers (32, 33, 37).
This study sought to better understand how AmrZ binds to PalgD. We identified four AmrZ binding sites (ZBSs) in PalgD, each of which is required for full promoter activation. In addition, AmrZ induces the formation of higher-order DNA-AmrZ complexes when bound to a PalgD fragment in vitro, and correct phasing is important for PalgD activation in vivo.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and culture conditions.
All bacterial strains, plasmids, and DNA oligonucleotides are listed in Table S1 in the supplemental material. Strains of Escherichia coli and Pseudomonas aeruginosa were incubated as described previously (30). All E. coli strains were cultured in LB (1 liter LB contains 10 g tryptone, 5 g yeast extract, and 5 g sodium chloride) or on LA (LB with 1.5% [wt/vol] agar). Where necessary, 15 μg · ml−1 tetracycline was used to maintain plasmids in E. coli. All P. aeruginosa strains were cultured in LBNS or on LANS (LB or LA without sodium chloride, respectively). For P. aeruginosa, 100 μg · ml−1 tetracycline was used if needed. All strains were grown at 37°C unless stated otherwise. All oligonucleotides were ordered desalted from Sigma-Aldrich with the exception of DNA primers for fluorescence resonance energy transfer (FRET) experiments, which were ordered with PAGE purification from Midland Certified Reagent Company.
Nucleotide manipulations.
PalgD variants with mutations or truncations were generated by overlap extension PCR as described previously (38). For nucleotide sequence scrambling, CLC Sequence Viewer software was used to randomize the order of nucleotide sequences. In the DNA phasing experiments, the sequences of 5-bp and 10-bp insertions are CATGC and CATGCATGCA, respectively.
Transcriptional reporter assays.
All transcriptional reporters were constructed in the mini-CTX-lacZ plasmid (39). PalgD fragments (bp −633 to +368 relative to the transcription start site) were cloned into the mini-CTX-lacZ plasmid after digestion by HindIII and BamHI (New England BioLabs). Transcriptional reporters were then transferred and integrated into the chromosomes of P. aeruginosa strains. A 96-well plate reader (SpectraMax M2; Molecular Devices) was used to measure absorbances at 420 nm and 550 nm, and β-galactosidase activities were determined as described previously (32).
DNA binding studies.
Electrophoretic mobility shift assays (EMSA) were performed as described previously (32). 5′-6-Carboxyfluorescein (6FAM)-labeled DNA used for EMSA was amplified using Quick-Load Taq 2× master mix (New England BioLabs), 6FAM-labeled forward primers, and nonlabeled reverse primers, with PAO1 genomic DNA as the template. Each EMSA reaction mixture contained 20 mM Bis-Tris (pH 6.5), 4 mM MgCl2, 200 μg/ml acetylated bovine serum albumin (BSA), 130 mM NaCl, 1 mM dithiothreitol (DTT), 5% glycerol, 150 ng/μl poly[d(I-C)] (Roche), 10 nM 6FAM-labeled DNA, and a specific concentration of recombinant AmrZ or variants as indicated for each experiment. Protein-DNA binding was equilibrated at room temperature (25°C) in the dark for 20 min after the addition of all reagents. A 10-μl volume of each reaction mixture was loaded onto a cold preequilibrated 4% nondenaturing acrylamide gel. Electrophoresis was conducted for 25 min at 200 V in 0.5% Tris-borate-EDTA (TBE) on ice in the dark. A Typhoon scanner (GE Healthcare Life Sciences) (settings: fluorescence; photomultiplier tube [PMT], 800 V; 520-nm bandpass [520 BP 40] emission filter; dyes, Cy2 [blue] and 6FAM; pixel size, 100 μm) was used to detect 6FAM fluorescence within gels.
FRET.
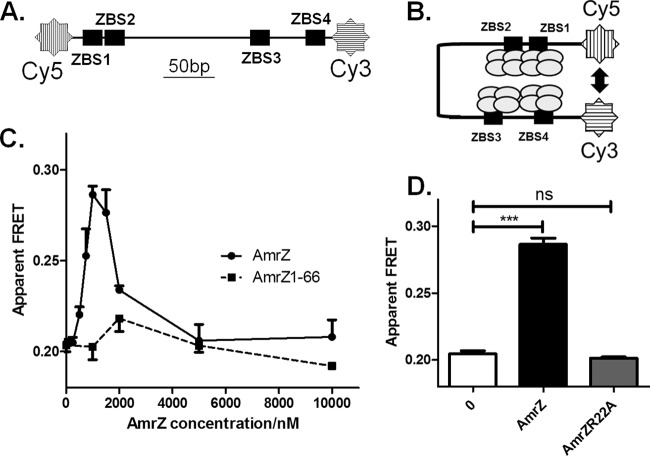
For DNA preparation, 5′ amino-modified termini of oligonucleotides algD157 and algD160 (Midland Certified Reagent Company) were conjugated with cyanine 5 N-hydroxysuccinimide (NHS) ester and cyanine 3 NHS ester (Lumiprobe), respectively, as recommended by the manufacturer. Labeled oligonucleotides were purified by high-performance liquid chromatography (HPLC) using a Poroshell 120 (50- by 4.6-mm) reversed-phase (RP) column with an EC-C18 stationary phase (Agilent). Samples were dissolved in 0.1 M triethylammonium acetate (TEAA), pH 7.0, and were eluted with a 3%-to-30% acetonitrile (ACN) gradient at 1% ACN/min. Labeled fractions were collected and ACN removed by rotary vacuum. Oligonucleotides were concentrated and buffer exchanged to TE with Amicon Ultra-4 centrifugal filter units (nominal molecular size limit of membrane, 3 kDa). Oligonucleotides were used as primers in a PCR using pBX1 as the template to generate the DNA fragment (Quick-Load Taq 2× master mix; New England BioLabs). PCR products were PAGE purified on a 4% acrylamide gel and were concentrated using Amicon Ultra-4 centrifugal filter units (nominal molecular size limit of membrane, 10 kDa). The composition of each final FRET reaction mixture was the same as that for EMSA, except for a different glycerol concentration (2%), as well as the addition of 1.5 mM Trolox and 4% polyethylene glycol 8000 (PEG 8000). Trolox and PEG 8000 were added as a triplet state quencher and the crowding agent, respectively (40, 41). The mixture was placed in a quartz cuvette (16.12F-Q-1.5/Z8.5; Starna) and was measured for fluorescent response with AmrZ concentrations up to 10 μM. Fluorescence measurements were performed on a FluoroMax-4 system (Horiba Jobin Yvon) with an attached circulating water bath set to 25°C. Samples were excited at 548 nm (Cy3) or 646 nm (Cy5), and emission spectra were scanned from 560 nm to 700 nm. Apparent FRET values were calculated by dividing the emission intensity at 666 nm by the combined intensities at 562 nm and 666 nm.
RESULTS
Identification of four AmrZ binding sites in PalgD.
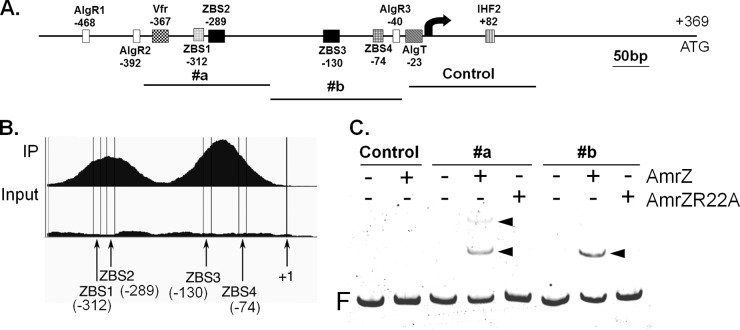
A previous study identified AmrZ as an activator of alginate production with a binding site centered at bp −289 relative to the transcription start site of the alginate biosynthesis operon (13). More recently, we utilized chromatin immunoprecipitation-DNA sequencing (ChIP-seq) to uncover AmrZ binding sites throughout the P. aeruginosa genome and discovered a second AmrZ binding region in PalgD (Fig. 1B) (29). Two ZBSs are therefore anticipated in this promoter; they are designated ZBS2 (bp −289 relative to the transcription start site) (previously identified) and ZBS3 (bp −130) (newly identified) (Fig. 1A). The rationale for this nomenclature will be described below. Electrophoretic mobility shift assays (EMSA) were then used to evaluate the binding of AmrZ to ZBS3. Three 6FAM-labeled DNA fragments were amplified such that they contained either ZBS2 (fragment #a), ZBS3 (fragment #b), or neither (control fragment), respectively. AmrZ bound to each fragment containing a ZBS but showed no binding to the negative-control DNA (DNA with neither ZBS) (Fig. 1C). The DNA binding-deficient variant AmrZR22A was also included in this assay and did not exhibit binding to any DNA fragment (Fig. 1C). Taken together, these results demonstrate that AmrZ specifically recognizes both AmrZ binding sites within PalgD.
FIG 1.
Identification of a second AmrZ binding site in PalgD. (A) Schematic of the alginate biosynthesis operon promoter region (PalgD) and most transcription factor binding sites. Note: the AlgB binding site (approximately bp −274 to bp −224), IHF1 (bp −94 to bp −74), the CysB binding site (approximately bp +90 to bp +116), and two Hp-1 (AlgP) binding sites (approximately bp −432 to bp −332 and approximately bp −116 to bp +111) are not shown here (19). Control, #a, and #b designate 6FAM-labeled algD fragments used in panel C. Numbers indicate the distance from the transcription start site (bent arrow). (B) Representative ChIP-seq image showing two AmrZ binding regions in PalgD. “IP” and “Input” refer to DNA samples after and before immunoprecipitation with AmrZ, respectively (29). +1, transcription start site. (C) EMSA were performed to determine the binding of AmrZ to DNA fragments (control, #a, and #b). Minus signs above the gel indicate that no protein was added, and plus signs indicate the addition of 50 nM AmrZ or a DNA-binding-deficient variant, AmrZR22A. Arrowheads point to AmrZ-induced mobility shifts. F, free DNA.
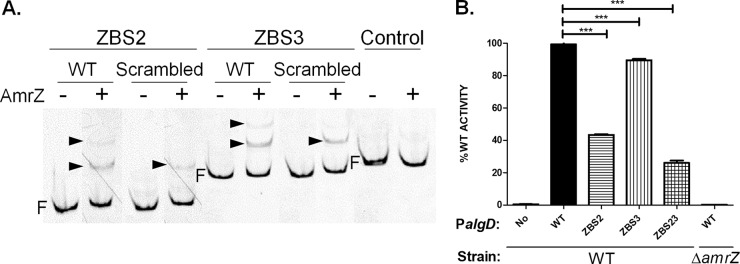
We next sought to understand the significance of both ZBSs during PalgD activation. One or both ZBSs were disrupted by scrambling their nucleotide sequences (for details, see the descriptions of plasmids pBX16, pBX35, and pBX37 in Table S1 in the supplemental material), and their effects on AmrZ binding and PalgD activation were determined. EMSA showed that disruption of either ZBS impaired, but did not eliminate, AmrZ binding to the DNA (Fig. 2A). To study their effects on algD transcription in vivo, four algD-lacZ transcriptional fusion constructs were made, including wild-type (WT) PalgD and PalgD with scrambled ZBS2, scrambled ZBS3, or both, respectively. These fusion constructs were integrated into the chromosome of the parental FRD1 strain (mucoid) or its isogenic ΔamrZ mutant (nonmucoid). In FRD1, ZBS2 scrambling caused a significant reduction of PalgD activity (57%) from that in the WT, while the loss of intact ZBS3 had only a modest effect (11% reduction). Mutations of both ZBSs resulted in further reduction (74%). However, PalgD activity was completely eliminated in the FRD1 ΔamrZ strain (Fig. 2B), validating the notion that amrZ is absolutely required for algD transcriptional activation (13). Since the disruption of AmrZ binding to both ZBSs did not recapitulate the results seen in the ΔamrZ strain, we explored additional mechanisms of regulation.
FIG 2.
Scrambling of ZBS2 and ZBS3 resulted in reduced DNA binding and impaired PalgD activation. (A) Sequences of ZBS2 and ZBS3 were randomly scrambled, and the resulting affinities to AmrZ were measured by EMSA. −, lanes with no protein; +, lanes containing 40 nM AmrZ each. Arrowheads point to DNA-AmrZ complexes. F, free DNA. (B) Scrambling of ZBS2 and ZBS3 led to impaired PalgD activities. No, promoterless lacZ (used as the negative control); WT, wild-type PalgD. ZBS2, ZBS3, and ZBS23 refer to corresponding AmrZ binding sites, which were randomly scrambled in the algD-lacZ transcriptional fusion constructs. The activity of WT PalgD in the ΔamrZ strain was also measured. PalgD activity, expressed as a percentage of WT PalgD activity, is shown along the y axis. The data in the graph are mean values and standard errors of the means. Asterisks indicate significant differences (***, P < 0.001) by one-way analysis of variance with Tukey posttest analyses of three independent experiments.
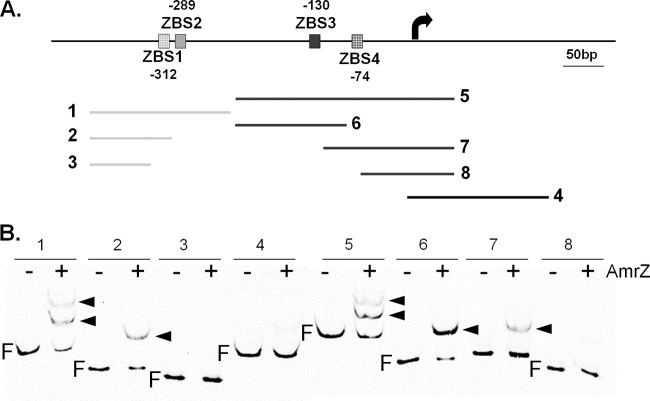
We propose two explanations to account for the data shown in Fig. 2B. (i) AmrZ activates PalgD indirectly, by regulating the production of other proteins that are required for PalgD activation. (ii) AmrZ retains residual binding to PalgD even with both ZBSs scrambled. The first scenario is unlikely, since neither RNA sequencing (RNA-seq) nor DNA microarray studies revealed an AmrZ-dependent alginate regulator (29, 42). Therefore, we reasoned that ineffective scrambling or an additional ZBS(s) in the DNA fragments might explain the phenotype observed. To test if residual AmrZ binding is due to ineffective scrambling, we determined the binding of AmrZ to a ZBS2-containing, ZBS3-deficient DNA fragment after ZBS2 deletion or replacement by a sequence known not to bind to AmrZ (4). Surprisingly, AmrZ retained binding to this fragment after ZBS2 deletion or replacement (see Fig. S1 in the supplemental material), suggesting the presence of an additional ZBS(s) in this DNA fragment. ChIP-seq analyses indicate that an additional ZBS(s) is not likely to be outside the two regions identified (Fig. 1B). Therefore, we reasoned that within each AmrZ binding region (∼100 bp), there is more than one ZBS. Taking into account the length of a ZBS (∼18 bp) and the consensus AmrZ binding sequence (29, 37), we proposed two additional ZBSs (ZBS1 and ZBS4), bringing the total number of ZBSs in PalgD to four (Fig. 3A). This hypothesis was tested via EMSA (Fig. 3). The DNA fragments containing ZBS1 or ZBS4 alone (fragments 2 and 7, respectively) retained AmrZ binding, while no binding was seen with any ZBS-free DNA fragment (fragments 3, 4, and 8) (Fig. 3B). Therefore, our DNA binding studies demonstrate four AmrZ binding sites within PalgD.
FIG 3.
Identification of two additional ZBSs in PalgD. (A) Proposal of two additional ZBSs (ZBS1 and ZBS4) and design of DNA fragments used for EMSA in panel B. DNA fragment 4 was used as the negative control. Fragments 2 and 7 contain ZBS1 and ZBS4, respectively, while fragments 3 and 8 are ZBS free. The proposed positions of ZBS1 through ZBS4 relative to the transcription start site are shown. (B) EMSA to verify the presence of two additional ZBSs. Arrowheads point to DNA shifts. Plus signs indicate the addition of 50 nM AmrZ. F, free DNA.
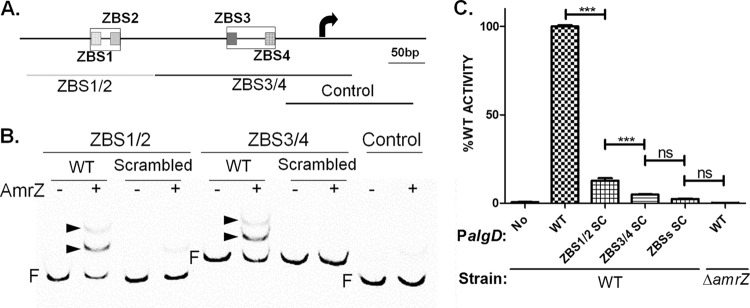
In order to evaluate the significance of these ZBSs during algD transcription, we again scrambled these ZBSs and measured the effects on AmrZ binding and PalgD activation. Since the effects of ZBS2 and ZBS3 on PalgD activation have been determined (Fig. 2), we scrambled ZBSs in pairs (ZBS1 and ZBS2 [ZBS1/2] and ZBS3/4) (Fig. 4A). Scrambling of ZBS1/2 or ZBS3/4 eliminated the binding of AmrZ to the respective DNA fragment (Fig. 4B). Importantly, PalgD activity suffered dramatic reduction from the disruption of either ZBS pair, while the elimination of all four ZBSs phenocopied the results observed with the amrZ deletion (Fig. 4C). Taken together, these results validate the hypothesis that these four ZBSs fully account for AmrZ-mediated transcriptional control of PalgD.
FIG 4.
Scrambling of all ZBSs abolished PalgD activation. (A) ZBSs were randomly scrambled in pairs as shown by outlining. The design of DNA fragments used for the EMSA for which results are given in panel B is also shown. (B) AmrZ binding affinities of WT or scrambled DNA fragments were determined by EMSA. Each DNA fragment was tested for its binding to 50 nM AmrZ. Arrowheads point to DNA shifts. F, free DNA. (C) PalgD activities were determined after the scrambling (SC) of two or four ZBSs. No, promoterless lacZ (used as the negative control). PalgD activities, expressed as percentages of WT PalgD activity, are shown along the y axis. The data in the graph are mean values and standard errors of the means. Significance was determined (***, P < 0.001; ns, not significant) using one-way analysis of variance with Tukey posttest analyses of three independent experiments.
AmrZ binding induces higher-order DNA-AmrZ complex formation.
We next investigated the mechanism of AmrZ-mediated activation of the algD promoter when AmrZ binds to four ZBSs. Since AmrZ forms tetramers (32, 33), we hypothesize that when binding to all four ZBSs AmrZ oligomers may interact with each other through bent DNA, resulting in close proximity between two DNA ends (ZBS1 and ZBS4) (Fig. 5B). Fluorescence resonance energy transfer (FRET) is widely used to confirm proximity (a distance of <10 nm) during protein-protein and protein-DNA interactions (43, 44) and was therefore used to test this hypothesis. The DNA fragment used for FRET experiments was amplified from PalgD with ZBS1 and ZBS4 at either end and was labeled with two fluorophores (Cy3 and Cy5) at opposing 5′ termini (Fig. 5A). In an AmrZ binding titration experiment, FRET efficiency peaked at 1 μM AmrZ, while excessive AmrZ concentrations inhibited FRET (Fig. 5C), a point addressed in the Discussion. In order to test if AmrZ tetramerization and DNA binding activity are necessary for inducing FRET, both the C-terminal truncation mutant protein AmrZ1-66, which does not form tetramers, and the DNA-binding-deficient variant AmrZR22A (32, 33) were used. However, neither variant was able to cause FRET, suggesting that both oligomerization and interactions with DNA are essential for the formation of higher-order AmrZ-DNA complexes (Fig. 5C and D). These FRET observations therefore indicate that AmrZ-AmrZ and AmrZ-DNA interactions enhance the proximity between the Cy3 and Cy5 fluorophores.
FIG 5.
AmrZ binding results in the formation of higher-order DNA-AmrZ complexes in vitro. (A) The PalgD DNA fragment designed for FRET experiments contains the Cy5 and Cy3 fluorophores, one at each end. (B) One type of higher-order AmrZ-DNA complex that explains the FRET observations. Circles represent AmrZ monomers, and the up-down arrow indicates close proximity between Cy3 and Cy5. (C) Titration of AmrZ or AmrZ1-66 during FRET measurements. (D) AmrZR22A-induced FRET was compared to AmrZ-induced FRET. Each FRET reaction mixture contained 1 μM protein (AmrZ or AmrZR22A). The graph shows mean values and standard errors of the means. Significance was determined (ns, not significant; ***, P < 0.001) by one-way analysis of variance with Tukey posttest analyses of three independent experiments.
PalgD activation requires a sufficient length of intervening DNA and correct phasing.
The in vitro FRET observations revealed the formation of higher-order AmrZ-DNA complexes for PalgD. The in vivo significance of these observations was then tested after removal of the intervening DNA or an alteration of DNA phasing. In most DNA looping examples, the distances between two operator sites are typically >100 bp (45, 46). Additionally, DNA duplexes of ∼50 bp are best modeled as rigid rod-like structures, and the force required to bend such a substrate greatly exceeds the capacity of a protein-protein interaction (47). We therefore constructed a PalgD DNA fragment with a 105-bp (10 DNA turns) truncation (bp −250 to bp −146) (Fig. 6A), in which the intervening DNA is shortened to ∼47 bp. The resulting intervening DNA is likely to be too short to bend, preventing complex formation. When this 105-bp-truncation construct was cloned into the lacZ transcriptional fusion construct and integrated into the WT P. aeruginosa chromosome, it abolished PalgD activation. This indicates that the intervening DNA is absolutely required for promoter activation (Fig. 6B). A second test was performed to determine if a specific DNA phasing is necessary for PalgD activation. The rationale is that in the context of transcriptional regulation, changing the phasing between TF binding sites may disrupt the ability of TFs to interact and may thus subsequently reduce promoter activation. Similar DNA phasing experiments have been performed in other transcriptional regulation systems, such as the AraC and lac repressor systems (25, 48, 49). We therefore inserted either 5 bp (half a DNA turn) or 10 bp (a full DNA turn) between ZBS2 and ZBS3 (Fig. 6A), cloned these promoter variants into the lacZ transcriptional fusion construct, and integrated them into the WT P. aeruginosa chromosome. algD promoter activity was reduced significantly (70%) with the 5-bp insertion (Fig. 6B), but when DNA phasing was restored with the 10-bp insertion, PalgD activation was recovered (Fig. 6B). This DNA phase dependence suggests that transcriptional activation of PalgD requires interactions between upstream and downstream factors. Taken together, these studies demonstrate the importance of both intervening DNA and correct phasing for PalgD activation in the cell.
FIG 6.
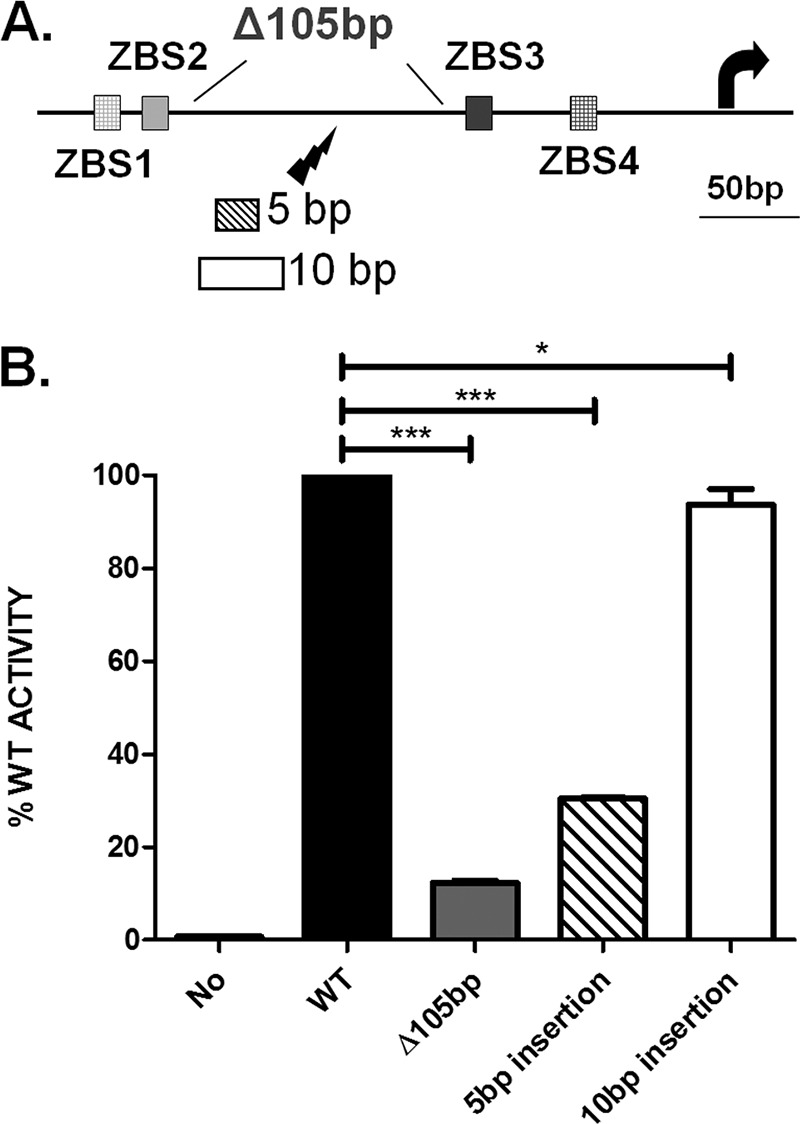
PalgD activation requires both intervening DNA and correct DNA phasing in vivo. (A) The lightning symbol indicates the insertion position (between bp −185 and bp −184 relative to the transcription start site), and the sizes of the inserts (5 bp or 10 bp) are given. The 105-bp truncation (bp −250 to bp −146) is also shown. (B) PalgD activities were measured after the 105-bp truncation or after the 5-bp or 10-bp insertion. “No” represents the lacZ fusion construct without a promoter. The graph shows mean values and standard errors of the means. Significance was determined (***, P < 0.001; ns, not significant) by one-way analysis of variance with Tukey posttests based on data from three independent experiments.
DISCUSSION
Alginate production in P. aeruginosa is under tight control by a number of regulators. In this study, we identified four AmrZ binding sites in PalgD and showed that they are important for transcription activation. Our results also reveal the formation of higher-order DNA-AmrZ complexes in vitro and highlight the significance of intervening DNA and proper phasing between ZBSs in vivo.
Alginate contributes significantly to P. aeruginosa persistence during CF lung infections. Activation of alginate production is most frequently observed during late stages of CF infections, when the gain of fitness exceeds the cost of alginate production, providing an excellent example of bacterial pathoadaptability. Alginate production requires at least 13 biosynthesis enzymes and transporter proteins, the majority of which are encoded within the alginate biosynthesis operon. The promoter of this operon is particularly complex and is under tight control by multiple regulators (4). So far, PalgD is known to contain binding sites for 7 different TFs, and strikingly, many of these sites are ≥250 bp upstream of the transcription start site (Fig. 1A). In this study, we discovered three additional AmrZ binding sites (ZBS1, ZBS3, and ZBS4) besides the previously identified ZBS2 within this promoter. It is interesting that ZBS4 overlaps with an integration host factor (IHF) binding site (IHF1.2) (Fig. 1A) (50). However, since IHF is dispensable for alginate production under our growth conditions (51), the observed effect of scrambling ZBS3/4 is likely due solely to the loss of AmrZ binding (Fig. 4C).
TFs frequently interact with target promoters at two or more positions, and ∼75% of negatively and ∼25% of positively regulated E. coli promoters have at least two binding sites for a TF (20). This arrangement confers diverse functions in different transcription control systems. For instance, low and high concentrations of E. coli Fis (factor for inverse stimulation) have opposite regulatory outcomes. The P1 promoter of topA contains two Fis binding sites with distinct affinities. At a low Fis concentration, only the higher-affinity site is occupied, resulting in activation; Fis binding to both sites however, represses transcription (52). TFs may also bind cooperatively to multiple sites. The arginine biosynthesis regulator ArgR interacts with the ARG boxes of its target promoters, and each ARG box contains two ArgR binding sites separated by 3 bp. The removal of one site results in 100-fold reduction of affinity to the other site, indicative of cooperative binding between two sites (53).
The presence of multiple binding sites may also allow interactions between DNA-bound proteins, which usually require their oligomerization. We recently showed that in solution, AmrZ exists primarily as tetramers and that oligomerization is important for AmrZ functions (32). Additionally, since multiple AmrZ-induced shifts have been seen consistently in EMSA (4, 13, 30), we asked if oligomerization allows the stacking of multiple AmrZ tetramers onto a single ZBS. A DNA fragment containing only ZBS3 was incubated with various amounts of AmrZ, and high concentrations of AmrZ resulted in multiple AmrZ-DNA complexes (see Fig. S2 in the supplemental material). These shifts were likely caused by the stacking of AmrZ tetramers onto ZBS3 rather than by occupation throughout the DNA molecule, because two AmrZ-induced shifts shared the same region of protection according to a previous DNA footprinting study (13). This observation therefore implies interactions between AmrZ tetramers. Nevertheless, it remains unclear how contacts between AmrZ tetramers are made, a question that requires more in-depth examination of the tetramerization CTD of AmrZ. This and other observations led us to hypothesize that AmrZ oligomers interact when the intervening PalgD is bent or looped.
We used FRET assays to probe if AmrZ by itself induces the looping of PalgD. When incubated with PalgD DNA, AmrZ induces energy transfer from Cy3 to Cy5, indicating proximity (<10 nm) between these two fluorophores and suggesting the formation of a higher-order DNA-AmrZ complex. This complex appears to be stable, since FRET efficiency did not fluctuate over time (measured for as long as 20 min) or with a rise in temperature from 25°C to 37°C (see Fig. S3 in the supplemental material). The formation of higher-order AmrZ-DNA complexes at both 25°C and 37°C suggests that this AmrZ-dependent phenomenon is likely to exist both in the natural environment and during growth in a mammalian host. It is intriguing that excessive AmrZ inhibits FRET (Fig. 5C), a phenomenon that may be explained by saturated binding of all DNA-bound AmrZ by free AmrZ in solution.
This in vitro FRET observation can be explained by the formation of two types of higher-order DNA-AmrZ complexes: cis (within the same DNA molecule) and trans (between two separate DNA molecules) complexes. The cis complex (shown in Fig. 5B) is formed when AmrZ binding induces DNA looping; in this case, ZBSs from two ends of the same DNA molecule are close (<10 nm). On the other hand, the formation of trans complexes (see Fig. S4A in the supplemental material) suggests close proximity between two double-stranded DNA (dsDNA) molecules when they are bound and held together by intervening AmrZ-AmrZ complexes. To distinguish between these two possibilities, two FRET experiments were conducted. The Δ105-bp PalgD fragment was labeled with Cy3 and Cy5 and was then subjected to FRET measurement when mixed with AmrZ. This 105-bp truncation is predicted to prevent cis complex formation but should not affect trans complexes. The FRET intensity was not reduced, suggesting that the complex observed was a trans complex (Fig. S4D). This result was corroborated by the experiment in which Cy5- and Cy3-monolabeled PalgD fragments were mixed (Fig. S4C and D). Therefore, these two experiments suggest that AmrZ induces the formation of the higher-order AmrZ-DNA trans complex in vitro.
Despite these findings, it should be noted that these experiments were performed in vitro and may not truly reflect events within the cell, where factors such as intracellular concentrations of proteins/DNA, as well as the effects of other TFs, need to be taken into consideration. For example, most cells contain only one copy of PalgD, while there were numerous copies in the FRET experiment, so this trans complex is unlikely to form in vivo. We acknowledge that this DNA-AmrZ trans complex may be an artifact due to the nature of the FRET experiment, but this observation still provides evidence that DNA-bound AmrZ may interact with other AmrZ molecules and form higher-order DNA-AmrZ complexes. While AmrZ itself is unable to induce the looping of PalgD in vivo, we propose that such interactions may occur if the intervening sequence between the ZBSs of PalgD is looped due to the presence of other TFs. In this regard, two DNA modifications, removal of the intervening DNA and alteration of the DNA phase, were designed to disrupt TF-TF interactions (Fig. 6A). Our results showed that each modification abolished PalgD activation (Fig. 6B). The 5-bp insertion is unlikely to affect the binding of any TF or RNA polymerase, since the insertion site is far upstream (between bp −185 and bp −184 relative to the transcription start site) and fairly distant from known TF binding sites (none were found within 20 bp upstream and 50 bp downstream). In the 105-bp-truncation experiment, however, the truncated region extends from bp −250 to bp −146, which may overlap the proposed AlgB binding site (approximately bp −274 to approximately bp −224) (54). Since the AlgB binding site was not precisely mapped, and AlgB binding to DNA was observed only at a lower pH (pH 4.5) (54), it remains unclear if this 105-bp truncation affects AlgB recognition. It should be noted that both in vivo experiments (105-bp truncation and phasing) underscore the importance of potential interactions between upstream and downstream factors. Those interactions imply, but do not provide direct evidence of, DNA looping. The results obtained in this study provide important information to aid in the understanding of PalgD activation. While the previously proposed DNA looping model (19) is supported by our data, we cannot exclude other possibilities, and more-in-depth investigations are needed to dissect this complex mechanism. In our proposed model, binding by multiple TFs (AlgR, AlgB, Vfr, IHF, AmrZ, etc.) is hypothesized to induce the looping of PalgD, which brings remote elements closer to the promoter and allows further interactions between remote TFs and RNA polymerase. Establishing these interactions is key for transcription initiation. Of note, this activation mechanism allows the integration of multiple signals, and the loss of any activating signal often prevents transcription initiation. This is important, since alginate production is an energy-exhausting process. In this model, a high AmrZ concentration is necessary for PalgD activation, because all four ZBSs need to be bound. Therefore, the intracellular AmrZ concentration may serve as an additional means of regulation, and it was shown previously that mucoid P. aeruginosa strains contain significantly more AmrZ than nonmucoid strains (30).
Overall, in this study, we have identified four AmrZ binding sites in PalgD and provided important insights into the mechanism of activation of this complex promoter. Further understanding of this promoter may allow the exploitation of this activation mechanism and the development of novel means to prevent or disrupt alginate production during chronic P. aeruginosa infections.
Supplementary Material
ACKNOWLEDGMENTS
We declare no conflict of interest.
Public Health Service awards AI097511 and NR013898 (to D.J.W.) and funds from the Public Health Preparedness for Infectious Disease (PHPID) program (phpid.osu.edu) supported this work. B.X. was partially supported by a student traineeship grant from the Cystic Fibrosis Foundation (XU13H0).
We thank Thomas Hollis for kindly providing purified AmrZ, AmrZ1-66, and AmrZR22A and Joe J. Harrison for providing the E. coli S17λpir strain. We are grateful to Sheri Dellos-Nolan and Meenu Mishra for critical reading of the manuscript and to Gayan Senavirathne for valuable scientific discussions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00259-16.
REFERENCES
- 1.Govan JRW, Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doggett RG. 1969. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol 18:936–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. 2005. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-γ-mediated macrophage killing. J Immunol 175:7512–7518. doi: 10.4049/jimmunol.175.11.7512. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey DM, Baynham PJ, Wozniak DJ. 2005. Binding of Pseudomonas aeruginosa AlgZ to sites upstream of the algZ promoter leads to repression of transcription. J Bacteriol 187:4430–4443. doi: 10.1128/JB.187.13.4430-4443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stapper APA, Narasimhan G, Ohman DE, Barakat J, Hentzer M, Molin S, Kharazmi A, Høiby N, Mathee K. 2004. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J Med Microbiol 53:679–690. doi: 10.1099/jmm.0.45539-0. [DOI] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation. 2013. Patient registry annual data report. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 7.Lovewell RR, Patankar YR, Berwin B. 2014. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsey DM, Wozniak DJ. 2005. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol Microbiol 56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 9.Wozniak DJ, Ohman DE. 1994. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J Bacteriol 176:6007–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schurr MJ, Martin DW, Mudd MH, Hibler NS, Boucher JC, Deretic V. 1993. The algD promoter: regulation of alginate production by Pseudomonas aeruginosa in cystic fibrosis. Cell Mol Biol Res 39:371–376. [PubMed] [Google Scholar]
- 11.Boucher JC, Schurr MJ, Deretic V. 2000. Dual regulation of mucoidy in Pseudomonas aeruginosa and sigma factor antagonism. Mol Microbiol 36:341–351. doi: 10.1046/j.1365-2958.2000.01846.x. [DOI] [PubMed] [Google Scholar]
- 12.Damron FH, Davis MR Jr, Withers RT, Ernst RK, Goldberg JB, Yu G, Yu HD. 2011. Vanadate and triclosan synergistically induce alginate production by Pseudomonas aeruginosa strain PAO1. Mol Microbiol 81:554–570. doi: 10.1111/j.1365-2958.2011.07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baynham PJ, Wozniak DJ. 1996. Identification and characterization of AlgZ, an AlgT-dependent DNA-binding protein required for Pseudomonas aeruginosa algD transcription. Mol Microbiol 22:97–108. doi: 10.1111/j.1365-2958.1996.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones AK, Fulcher NB, Balzer GJ, Urbanowski ML, Pritchett CL, Schurr MJ, Yahr TL, Wolfgang MC. 2010. Activation of the Pseudomonas aeruginosa AlgU regulon through mucA mutation inhibits cyclic AMP/Vfr signaling. J Bacteriol 192:5709–5717. doi: 10.1128/JB.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohr CD, Hibler NS, Deretic V. 1991. AlgR, a response regulator controlling mucoidy in Pseudomonas aeruginosa, binds to the FUS sites of the algD promoter located unusually far upstream from the mRNA start site. J Bacteriol 173:5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deretic V, Konyecsni WM. 1990. A procaryotic regulatory factor with a histone H1-like carboxy-terminal domain: clonal variation of repeats within algP, a gene involved in regulation of mucoidy in Pseudomonas aeruginosa. J Bacteriol 172:5544–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konyecsni WM, Deretic V. 1990. DNA sequence and expression analysis of algP and algQ, components of the multigene system transcriptionally regulating mucoidy in Pseudomonas aeruginosa: algP contains multiple direct repeats. J Bacteriol 172:2511–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato J, Misra TK, Chakrabarty AM. 1990. AlgR3, a protein resembling eukaryotic histone H1, regulates alginate synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 87:2887–2891. doi: 10.1073/pnas.87.8.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schurr MJ. 2013. Which bacterial biofilm exopolysaccharide is preferred, Psl or alginate? J Bacteriol 195:1623–1626. doi: 10.1128/JB.00173-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed). 1996. Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. [Google Scholar]
- 21.McLeod S, Xu J, Johnson R. 2000. Coactivation of the RpoS-dependent proP P2 promoter by Fis and cyclic AMP receptor protein. J Bacteriol 182:4180–4187. doi: 10.1128/JB.182.15.4180-4187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pul U, Lux B, Wurm R, Wagner R. 2008. Effect of upstream curvature and transcription factors H-NS and LRP on the efficiency of Escherichia coli rRNA promoters P1 and P2—a phasing analysis. Microbiology 154:2546–2558. doi: 10.1099/mic.0.2008/018408-0. [DOI] [PubMed] [Google Scholar]
- 23.Fisher RF, Long SR. 1993. Interactions of NodD at the nod box: NodD binds to two distinct sites on the same face of the helix and induces a bend in the DNA. J Mol Biol 233:336–348. doi: 10.1006/jmbi.1993.1515. [DOI] [PubMed] [Google Scholar]
- 24.Amouyal M, Mortensen L, Buc H, Hammer K. 1989. Single and double loop formation when deoR repressor binds to its natural operator sites. Cell 58:545–551. doi: 10.1016/0092-8674(89)90435-2. [DOI] [PubMed] [Google Scholar]
- 25.Krämer H, Niemöller M, Amouyal M, Revet B, von Wilcken-Bergmann B, Müller-Hill B. 1987. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J 6:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobell RB, Schleif RF. 1990. DNA looping and unlooping by AraC protein. Science 250:528–532. doi: 10.1126/science.2237403. [DOI] [PubMed] [Google Scholar]
- 27.Tart AH, Blanks MJ, Wozniak DJ. 2006. The AlgT-dependent transcriptional regulator AmrZ (AlgZ) inhibits flagellum biosynthesis in mucoid, nonmotile Pseudomonas aeruginosa cystic fibrosis isolates. J Bacteriol 188:6483–6489. doi: 10.1128/JB.00636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Granero F, Redondo-Nieto M, Vesga P, Martín M, Rivilla R. 2014. AmrZ is a global transcriptional regulator implicated in iron uptake and environmental adaption in P. fluorescens F113. BMC Genomics 15:237. doi: 10.1186/1471-2164-15-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones CJ, Newsom D, Kelly B, Irie Y, Jennings LK, Xu B, Limoli DH, Harrison JJ, Parsek MR, White P, Wozniak DJ. 2014. ChIP-Seq and RNA-Seq reveal an AmrZ-mediated mechanism for cyclic di-GMP synthesis and biofilm development by Pseudomonas aeruginosa. PLoS Pathog 10:e1003984. doi: 10.1371/journal.ppat.1003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones CJ, Ryder CR, Mann EE, Wozniak DJ. 2013. AmrZ modulates Pseudomonas aeruginosa biofilm architecture by directly repressing transcription of the psl operon. J Bacteriol 195:1637–1644. doi: 10.1128/JB.02190-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baynham PJ, Ramsey DM, Gvozdyev BV, Cordonnier EM, Wozniak DJ. 2006. The Pseudomonas aeruginosa ribbon-helix-helix DNA-binding protein AlgZ (AmrZ) controls twitching motility and biogenesis of type IV pili. J Bacteriol 188:132–140. doi: 10.1128/JB.188.1.132-140.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B, Ju Y, Soukup RJ, Ramsey DM, Fishel R, Wysocki VH, Wozniak DJ. 2016. The Pseudomonas aeruginosa AmrZ C-terminal domain mediates tetramerization and is required for its activator and repressor functions. Environ Microbiol Rep 8:85–90. doi: 10.1111/1758-2229.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waligora EA, Ramsey DM, Pryor EE Jr, Lu H, Hollis T, Sloan GP, Deora R, Wozniak DJ. 2010. AmrZ β-sheet residues are essential for DNA binding and transcriptional control of Pseudomonas aeruginosa virulence genes. J Bacteriol 192:5390–5401. doi: 10.1128/JB.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight KL, Bowie JU, Vershon AK, Kelley RD, Sauer RT. 1989. The Arc and Mnt repressors. A new class of sequence-specific DNA-binding protein. J Biol Chem 264:3639–3642. [PubMed] [Google Scholar]
- 35.Vershon AK, Youderian P, Susskind MM, Sauer RT. 1985. The bacteriophage P22 Arc and Mnt repressors. Overproduction, purification, and properties. J Biol Chem 260:12124–12129. [PubMed] [Google Scholar]
- 36.Schreiter ER, Drennan CL. 2007. Ribbon-helix-helix transcription factors: variations on a theme. Nat Rev Microbiol 5:710–720. doi: 10.1038/nrmicro1717. [DOI] [PubMed] [Google Scholar]
- 37.Pryor EE Jr, Waligora EA, Xu B, Dellos-Nolan S, Wozniak DJ, Hollis T. 2012. The transcription factor AmrZ utilizes multiple DNA binding modes to recognize activator and repressor sequences of Pseudomonas aeruginosa virulence genes. PLoS Pathog 8:e1002648. doi: 10.1371/journal.ppat.1002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heckman KL, Pease LR. 2007. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc 2:924–932. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 39.Becher A, Schweizer HP. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques 29:948–952. [DOI] [PubMed] [Google Scholar]
- 40.Paudel BP, Rueda D. 2014. Molecular crowding accelerates ribozyme docking and catalysis. J Am Chem Soc 136:16700–16703. doi: 10.1021/ja5073146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge X, Luo D, Xu J. 2011. Cell-free protein expression under macromolecular crowding conditions. PLoS One 6:e28707. doi: 10.1371/journal.pone.0028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu B, Wozniak DJ. 2015. Development of a novel method for analyzing Pseudomonas aeruginosa twitching motility and its application to define the AmrZ regulon. PLoS One 10:e0136426. doi: 10.1371/journal.pone.0136426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dragan AI, Privalov PL. 2008. Use of fluorescence resonance energy transfer (FRET) in studying protein-induced DNA bending. Methods Enzymol 450:185–199. doi: 10.1016/S0076-6879(08)03409-5. [DOI] [PubMed] [Google Scholar]
- 44.Zadran S, Standley S, Wong K, Otiniano E, Amighi A, Baudry M. 2012. Fluorescence resonance energy transfer (FRET)-based biosensors: visualizing cellular dynamics and bioenergetics. Appl Microbiol Biotechnol 96:895–902. doi: 10.1007/s00253-012-4449-6. [DOI] [PubMed] [Google Scholar]
- 45.Cournac A, Plumbridge J. 2013. DNA looping in prokaryotes: experimental and theoretical approaches. J Bacteriol 195:1109–1119. doi: 10.1128/JB.02038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allemand JF, Cocco S, Douarche N, Lia G. 2006. Loops in DNA: an overview of experimental and theoretical approaches. Eur Phys J E 19:293–302. doi: 10.1140/epje/i2005-10073-y. [DOI] [PubMed] [Google Scholar]
- 47.Frontali C, Dore E, Ferrauto A, Gratton E, Bettini A, Pozzan MR, Valdevit E. 1979. An absolute method for the determination of the persistence length of native DNA from electron micrographs. Biopolymers 18:1353–1373. doi: 10.1002/bip.1979.360180604. [DOI] [PubMed] [Google Scholar]
- 48.Han L, Garcia HG, Blumberg S, Towles KB, Beausang JF, Nelson PC, Phillips R. 2009. Concentration and length dependence of DNA looping in transcriptional regulation. PLoS One 4:e5621. doi: 10.1371/journal.pone.0005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn TM, Hahn S, Ogden S, Schleif RF. 1984. An operator at −280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A 81:5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wozniak DJ. 1994. Integration host factor and sequences downstream of the Pseudomonas aeruginosa algD transcription start site are required for expression. J Bacteriol 176:5068–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delic-Attree I, Toussaint B, Froger A, Willison JC, Vignais PM. 1996. Isolation of an IHF-deficient mutant of a Pseudomonas aeruginosa mucoid isolate and evaluation of the role of IHF in algD gene expression. Microbiology 142:2785–2793. doi: 10.1099/13500872-142-10-2785. [DOI] [PubMed] [Google Scholar]
- 52.Weinstein-Fischer D, Altuvia S. 2007. Differential regulation of Escherichia coli topoisomerase I by Fis. Mol Microbiol 63:1131–1144. doi: 10.1111/j.1365-2958.2006.05569.x. [DOI] [PubMed] [Google Scholar]
- 53.Tian G, Lim D, Carey J, Maas WK. 1992. Binding of the arginine repressor of Escherichia coli K12 to its operator sites. J Mol Biol 226:387–397. doi: 10.1016/0022-2836(92)90954-I. [DOI] [PubMed] [Google Scholar]
- 54.Leech AJ, Sprinkle A, Wood L, Wozniak DJ, Ohman DE. 2008. The NtrC family regulator AlgB, which controls alginate biosynthesis in mucoid Pseudomonas aeruginosa, binds directly to the algD promoter. J Bacteriol 190:581–589. doi: 10.1128/JB.01307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.