ABSTRACT
Nontypeable Haemophilus influenzae (NTHI), a commensal of the human nasopharynx (hNP), is a common cause of biofilm-associated diseases of the respiratory tract. However, NTHI biofilm biology at the average hNP temperature, i.e., 34°C, has not been well studied. Here we grew NTHI biofilms at 34°C and 37°C, to evaluate relative biofilm growth, expression, and function of the type IV pilus (Tfp), a critical adhesin important for NTHI biofilm formation. The kinetics and regulation of Tfp expression in NTHI biofilms are unclear, especially at 34°C. Tfp expression, as estimated by pilA promoter activity, was distributed throughout the biofilms, with a unique pattern that was dependent on temperature, time in culture, and position within the maturing biofilm. Tfp expression was required for the formation of the characteristic tower structures of NTHI biofilms and was significantly upregulated in NTHI biofilms formed at 34°C versus 37°C. This increase correlated with significantly greater twitching motility at 34°C than at 37°C. Treatment with antisera targeting the major subunit of Tfp (PilA) significantly inhibited NTHI biofilm formation at both temperatures, confirming the importance of this critical adhesin in biofilm formation. Additionally, treatment of preestablished biofilms with antisera against PilA significantly decreased biofilm biomass and mean thickness at both temperatures. These results demonstrated a pivotal role for Tfp in NTHI biofilm formation and stability at the temperature of the hNP, and they underscore the utility of PilA as a vaccine candidate for treatment and/or prevention of NTHI biofilm-associated diseases.
IMPORTANCE NTHI is an important cause of chronic respiratory tract infections, including otitis media, chronic rhinosinusitis, and exacerbations of chronic obstructive pulmonary disease and cystic fibrosis. The chronic and recurrent nature of these diseases is attributed to the presence of bacterial biofilms, which are highly resistant to antimicrobials. We characterized NTHI biofilm growth and expression of PilA, the major subunit of the Tfp, at the temperature of the hNP, which is the commensal habitat of NTHI. Our results expand the current understanding of the role of Tfp during biofilm formation and maturation at the temperature of both the hNP and the middle ear, and they strengthen support for PilA as a vaccine candidate for the prevention and treatment of NTHI biofilm-associated diseases.
INTRODUCTION
Nontypeable Haemophilus influenzae (NTHI), a Gram-negative coccobacillus, is a commensal of the human nasopharynx (hNP). When the local host immune system is compromised by an upper respiratory tract viral infection, NTHI can ascend the Eustachian tube to the middle ear (ME) space and induce otitis media (OM). OM is the most common bacterial disease of childhood (1) and a primary reason for emergency department visits (2) and pediatric antibiotic prescriptions (3) in the United States. NTHI is the most prevalent bacterial pathogen in chronic and recurrent OM and the most common bacterium isolated from patients who have experienced antibiotic treatment failure (4). The chronic and recurrent nature of these infections is associated with the ability of NTHI to form biofilms. Bacterial biofilms are present on the ME mucosae of children with OM (5), as well as in the ME space of chinchillas during experimental infection (1, 6–10). NTHI biofilm formation has also been associated with other respiratory tract infections, including chronic rhinosinusitis and exacerbations of both chronic obstructive pulmonary disease (COPD) and cystic fibrosis (11–13). Bacteria residing within biofilms are up to 1,000 times more resistant to antibiotics and innate immune effectors than their planktonic counterparts (14), which contributes to the chronic and recurrent nature of these biofilm-associated diseases. Consequently, there is a great need for novel ways to manage biofilm-associated NTHI infections.
The demonstration of type IV pilus (Tfp) expression and twitching motility for NTHI (15, 16), a bacterium long considered to be nonmotile, has greatly enhanced our understanding of NTHI biology. Like those of other human pathogens, such as Neisseria and Pseudomonas species, the Tfp of NTHI participates in multiple important biological functions, including adherence, competence, twitching motility, and biofilm formation (6, 15, 16). Although multiple protein subunits are required for Tfp formation and function in NTHI (17), the pilus expressed by NTHI is composed of multiple PilA subunits, whose amino acid sequence is highly conserved across NTHI clinical strains (15). Therefore, PilA protein is being developed by our laboratory as a potential vaccine candidate. To date, we have demonstrated that immunization with a soluble form of PilA results in the formation of antibodies that can prevent NTHI-induced OM in experimentally infected chinchillas (1, 7, 18, 19). Moreover, antisera targeting these immunogens can resolve experimental OM in the same animal model by eradicating preexisting NTHI biofilms from the ME space (1, 7, 10, 18).
We showed previously that expression of Tfp is required for long-term persistence of NTHI in the chinchilla nasopharynx (NP) after experimental challenge (6). Although we have learned a great deal regarding the role of NTHI Tfp in pathogenesis (1, 6, 15, 17), to date we have had a limited understanding of the role of Tfp in NTHI colonization and persistence as a commensal organism. The first step in the progression of NTHI biofilm-associated diseases is colonization of the NP; consequently, it is important to understand the biology of NTHI at this site. Previously we, like others, studied NTHI biofilm biology in vitro at 37°C, a temperature chosen to reflect that of the ME and distal respiratory system, typical sites of NTHI biofilm-associated infections. However, the hNP, the sole commensal habitat of NTHI, has an average temperature of 34°C (20). Studies with Streptococcus pneumoniae, another otopathogen and hNP commensal, demonstrated that small, biologically relevant, temperature differences can significantly affect bacterial phenotypes, with important consequences for biofilm biology (21, 22). We reasoned that the temperature difference between the hNP and more distal sites in the respiratory system might result in important differences in NTHI biofilm biology.
The aim of this study was to characterize NTHI biofilm growth and the expression and function of Tfp during biofilm formation at the average hNP and ME temperatures of 34°C and 37°C, respectively. Our results expand the current understanding of the role of NTHI Tfp during biofilm formation and maturation in vitro at the temperatures of both the hNP and the ME, and they strengthen the support for PilA as a vaccine candidate for the prevention and treatment of NTHI biofilm-associated diseases.
MATERIALS AND METHODS
Bacterial strains.
The NTHI strains and plasmids used in this study are listed in Table 1. To visualize pilA expression in biofilms, we utilized a reporter construct, NTHI 86-028NP:pilA-green fluorescent protein (GFP), which harbored a plasmid in which expression of gfpmut3 was driven by the promoter for pilA (1). To make NTHI 86-028NP ΔpilA/pRSM2211, pRSM2211 (23) was electroporated into NTHI 86-028NP ΔpilA (17). Colonies were selected by overnight growth on chocolate agar supplemented with 20 μg kanamycin ml−1. For comparison, we also prepared a reporter isolate in which expression of gfpmut3 was under the control of the promoter for ompP5, which allowed us to monitor the expression of an additional adhesin, the constitutively expressed outer membrane protein (OMP) P5 (24). The ompP5 promoter was excised from plasmid pKMLN-05 (25) and cloned into the BamHI and SalI sites of pRSM2169 (26). The resulting plasmid, pMDC-P1, was used to transform TOP10 Escherichia coli (Invitrogen), and transformants were selected after overnight growth on Luria-Bertani (LB) agar with 20 μg kanamycin ml−1. pMDC1-P1 isolated from E. coli was methylated with CpG methylase (NEB) and electroporated into NTHI 86-028NP (26). Colonies were selected by overnight growth on chocolate agar supplemented with 20 μg kanamycin ml−1.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Designation | Description or sequences | Reference |
|---|---|---|
| Bacterial strains | ||
| NTHI 86-028NP | Isolated from nasopharynx of child with chronic OM | 56 |
| NTHI 86-028NP ΔpilA | Nonpolar pilA mutant | 17 |
| NTHI 86-028NP ΔpilA/pPIL1 | Complemented nonpolar pilA mutant | 17 |
| NTHI 86-028NP:pilA-GFP | NTHI 86-028NP in which expression of GFP is under control of pilA promoter | 1 |
| NTHI 86-028NP/pMDC-P1 | NTHI 86-028NP in which expression of GFP is under control of ompP5 promoter | This study |
| NTHI 86-028NP/pRSM2211 | NTHI 86-028NP in which expression of GFP is under control of ompP2 promoter | 23 |
| NTHI 86-028NP ΔpilA/pRSM2211 | NTHI 86-028NP ΔpilA in which expression of GFP is under control of ompP2 promoter | This study |
| Plasmids | ||
| pRSM2169 | Promoterless derivative of Haemophilus-E. coli shuttle vector pGZRS-39A that incorporates gfpmut3 | 23 |
| pGFP-PpilA | pilA promoter driving expression of gfpmut3 within pGFP | 1 |
| MDC-P1 | ompP5 promoter driving expression of gfpmut3 within pRSM2169 | This study |
| pRSM2211 | ompP2 promoter driving expression of gfpmut3 within pRSM2169 | 23 |
| pPIL1 | Derivative of pSPEC1 containing pilA under control of its native promoter | 17 |
| Primers | ||
| ompP5 (NTHI 1332) | Forward, CCGTTTCGGTCAAGGCGAAG; reverse, GCCATAGACGCTGTCTAATGTTGC | This study |
| pilA (NTHI 0409) | Forward, GCAAAAGGCTATGTAAAATCAGTG; reverse, AAATAAAGAGGCATCCGTTCC | This study |
| ompP6 (NTHI 0501) | Forward, CGTTCATCAGTGTTACCTTCTACT; reverse, CGGTTACTCTGTTGCTGATCTT | This study |
Biofilm formation and visualization.
NTHI biofilms were grown in chambered coverglasses (Fisher Scientific, Hampton, NH), as described previously (27). Briefly, NTHI was grown overnight on chocolate agar plates (Remel, Columbus, OH). GFP-expressing strains and NTHI 86-028NP ΔpilA/pPIL1 (the complemented pilA mutant) were grown on chocolate agar that contained 20 μg kanamycin ml−1 and 200 μg spectinomycin ml−1, respectively, as the plasmids carried antibiotic resistance cassettes. Colonies were suspended at an optical density at 490 nm (OD490) of 0.65 in brain heart infusion (BHI) broth (Invitrogen, Carlsbad, CA) supplemented with 2 μg/ml heme and β-NAD (sBHI) and were grown at 37°C in air–5% CO2. After 3 h, the bacterial suspension was diluted to 2 × 105 CFU ml−1 in sBHI; 200 μl was seeded into each well of the chambered coverglass and incubated in air–5% CO2 at either 37°C or 34°C. For biofilms grown longer than 16 h, medium was replaced twice daily. For biofilms formed with two strains, bacterial suspensions of each strain at 1 × 105 CFU ml−1 were combined in equal volumes just before seeding. The final (mixed) inoculum was diluted and plated on chocolate agar with and without antibiotic, to confirm a 1:1 strain ratio. For imaging, biofilms were stained with the BacLight bacterial viability kit (Molecular Probes, Eugene, OR), according to the manufacturer's protocol, and fixed in a solution of 1.6% paraformaldehyde, 2.5% glutaraldehyde, and 4.0% acetic acid in 0.1 M phosphate buffer (28). Duplicate wells were viewed by confocal scanning laser microscopy (CSLM) with a Zeiss 510 Meta-laser microscope, and images were rendered with Zeiss Zen software. Biofilm metrics (biomass, thickness, and roughness) were calculated by Comstat2 analysis (29, 30). All assays were repeated a minimum of three times, on separate days. Data represent the mean ± standard error of the mean (SEM).
Strains listed in Table 1 were tested for any differences in planktonic growth at 34°C and 37°C. NTHI strains were grown in sBHI, and the OD490 was measured every 30 min for 24 h. We found no differences in doubling times at 34°C versus 37°C for any of the strains (data not shown).
Antibiotic sensitivity of biofilms.
To assess the sensitivity of NTHI biofilms formed at 37°C or 34°C to antibiotics typically used to treat NTHI infections, 24-h biofilms were established as described above and were incubated for 16 h with ampicillin (32.0 mg ml−1), cefdinir (250 μg ml−1), or amoxicillin (1.0 mg ml−1) plus clavulanate-lithium (0.5 mg ml−1). Each antibiotic was used at 1,000 times the planktonic MIC90 for NTHI, as bacteria within biofilms are known to be highly resistant to antibiotics (14, 31). After 16 h, the biofilms were gently washed twice with sterile saline to remove loosely adherent bacteria, and NTHI within the biofilm was recovered by repeated forceful pipetting. Bacteria were plated to determine CFU per milliliter.
Visualization of biofilms formed by GFP-expressing reporter isolates.
Biofilms were rinsed with sterile saline and then stained with FM 4-64 dye {N-(3-triethylammoniumpropyl)-4-(6-[4-(diethylamino)phenyl]hexatrienyl)pyridinium dibromide; Molecular Probes}, a fluorescent dye that labels bacterial outer membranes, according to the manufacturer's directions. After 30 min, the stain was replaced by sterile saline, and biofilms were immediately viewed by confocal microscopy. Time points of 6, 12, 24, and 48 h were chosen on the basis of preliminary experiments that showed that the GFP fluorescence of NTHI 86-028NP:pilA-GFP peaked at 12 h or 24 h for biofilms formed at 37°C or 34°C, respectively. Mean fluorescence values were calculated with Zeiss AxioVision software, and images were compiled with Zeiss Zen software. All assays were repeated a minimum of three times, on separate days. Data represent the mean ± SEM.
RNA isolation and qRT-PCR.
Biofilms grown for 12 or 15 h at 37°C or 34°C were placed on ice, washed once with saline, and collected with TRIzol reagent (Ambion; Thermo Fisher) by repeated forceful pipetting. RNA was purified with a Qiagen RNeasy kit. DNA was removed from the RNA preparation by treatment with DNase I (NEB), according to the manufacturer's instructions, for 45 min at 37°C in the presence of 20 U SUPERase In RNase inhibitor (Ambion). DNase I-treated RNA samples were purified with a Qiagen RNeasy kit. RNA quality was assessed with an Agilent 2100 bioanalyzer (Agilent Technologies).
Three sets of biofilms were collected at 37°C and 34°C for both time points, and the relative pilA and ompP5 expression levels were assessed using a SuperScript III Platinum SYBR Green One-Step quantitative reverse transcription-PCR (qRT-PCR) kit (Invitrogen), according to the manufacturer's protocol, with the primers listed in Table 1. Expression was normalized to ompP6, which is constitutively expressed in NTHI (24, 32). Results were reported as relative gene expression at 34°C, compared to that at 37°C, by the comparative (ΔΔCT) method.
Assay for twitching motility.
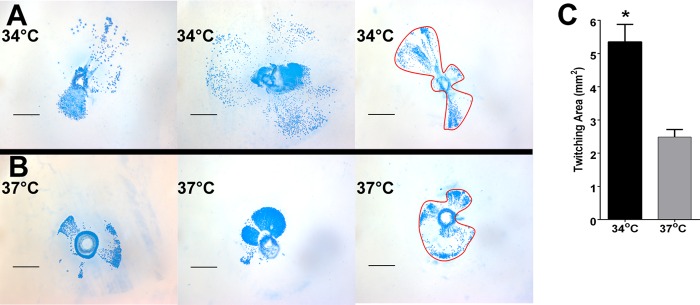
NTHI motility was assessed with a subagarose twitching assay, as described previously (1). Briefly, NTHI was inoculated into 0.3% agarose and incubated on a level surface for 24 h at 34°C or 37°C, in humidified 5% CO2–air. Agarose plugs were stained with BioSafe Coomassie blue stain (Bio-Rad), visualized with a dissecting microscope, and imaged with AxioVision software (Zeiss). The area of bacterial growth extending from the inoculation site was circumscribed and measured with Axiovision software. The mean area ± SEM is reported for ≥20 replicates from three independent assays.
Chinchilla epithelial cell culture.
The chinchilla is a well-documented animal model for OM, and chinchilla airway epithelial cells recapitulate much of the biology of human epithelial cells (33, 34). Chinchilla nasopharyngeal epithelial cells (CNPE) and chinchilla middle ear epithelial cells (CMEE) were isolated and cultured as described previously (35–39). Briefly, freshly dissected chinchilla NP and ME epithelia were finely chopped, placed on 6.5-mm Transwell permeable supports (Costar, Corning, NY), and fed with explant isolation medium. Tissue pieces were removed when epithelial cells had migrated onto the surface of the Transwell membrane. When the cells reached confluence, apical medium was removed to allow for polarization at the air-liquid interface. During polarization, cells were fed with growth medium every other day.
NTHI biofilm formation on epithelial cells.
The day before use, polarized CMEE and CNPE on Transwells were fed with antibiotic-free medium. On the day of infection, cells were fed with antibiotic-free growth medium and washed apically with 2 × 100 μl Dulbecco's phosphate-buffered saline (DPBS). NTHI 86-028NP:pilA-GFP and NTHI 86-028NP/pMDC-P1 were grown in sBHI to an OD490 of 0.65, as described above, and were diluted in DPBS to 4 × 106 CFU ml−1. Epithelial cells were inoculated with 50 μl of bacterial suspension (multiplicity of infection of 20) and incubated as described above, with protection from light. After 1 h, the apical surface was washed with 100 μl DPBS to remove nonadherent bacteria. Just before imaging, the apical surface was gently washed with DPBS, and 200 μl fresh DPBS was added. Transwells were rinsed basolaterally with DPBS and placed upright on a 2-well chambered coverglass with a drop of DPBS in the bottom. The cells and bacteria were visualized by confocal microscopy 6, 10, and 23 h after infection, times that were chosen on the basis of preliminary data showing increased GFP fluorescence at those times (data not shown).
Biofilm inhibition and disruption assays.
We tested the effects of three different antisera, targeting either Tfp or NTHI OMP P5, on NTHI biofilm formation and stability. Rabbit polyclonal antisera were generated at Spring Valley Laboratories (Woodbine, MD) and were not heat inactivated. Antiserum to recombinant soluble PilA (rsPilA) targets PilA, the major subunit of the NTHI Tfp; rsPilA is an N-terminally truncated protein that represents a modified soluble form of mature PilA derived from NTHI 86-028NP (19). Antiserum to OMP P5 targets a critical adhesin that is known to be important for NTHI adherence to epithelial cells and biofilm formation (19, 24). ChimV4 is a chimeric candidate vaccine antigen composed of protective epitopes from both OMP P5 and rsPilA; therefore, antiserum to chimV4 targets both Tfp and OMP P5 as virulence determinants (1, 19). Naive rabbit serum served as a negative control.
For biofilm inhibition assays, biofilms were seeded as described above. After 1 h, the supernatant was removed and replaced with fresh medium containing an arbitrarily selected 1:50 dilution of antiserum. After 16 h, biofilms were stained and visualized by CSLM. For biofilm disruption assays, NTHI biofilms were established for 24 h prior to treatment for 16 h with a 1:50 dilution of antiserum, as described previously (1, 28).
Statistics.
Data analyses were performed with GraphPad Prism software version 6.03. For comparison of biomass and mean thickness values in the biofilm inhibition and disruption experiments, one-way analysis of variance (ANOVA), with the Holm-Sidak correction for multiple comparisons, was used. All other comparisons were made with unpaired t tests.
RESULTS
NTHI biofilms grown at 37°C and 34°C showed differences in growth rates and architecture.
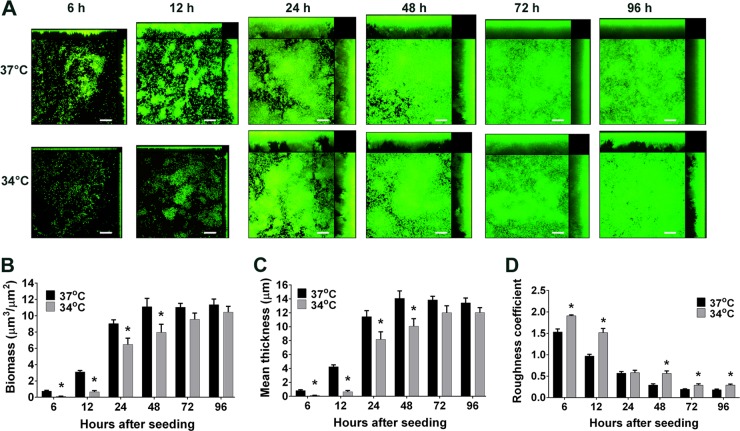
NTHI biofilms formed in vitro at 37°C developed a characteristic architecture, with compact towers (areas of horizontal consolidation and regional increases in thickness) and intervening water channels, that became apparent by 12 h (Fig. 1A) (1, 28). Biofilms formed at 34°C grew more slowly, with towers forming between 12 and 24 h of culture. Biomass was significantly greater in biofilms formed at 37°C up to 48 h of culture (P < 0.05) (Fig. 1B); this trend continued up to 96 h of culture, although the differences were not statistically significant at later time points. Similarly, the mean thickness of biofilms formed at 37°C was significantly greater than that of biofilms formed at 34°C up to 48 h of culture (P < 0.05) (Fig. 1C); this trend also continued up to 96 h of culture, although the differences were not significant at later time points. The lag in initial biofilm growth at 34°C was not simply due to a lack of bacterial growth at this temperature, since the planktonic growth rates at 34°C and 37°C were not significantly different (doublings per hour of 1.36 ± 0.08 and 1.54 ± 0.10, respectively; P = 0.22).
FIG 1.
NTHI forms a biofilm at the temperature of the hNP. NTHI biofilms were grown at 37°C or 34°C for the indicated times, stained with live/dead stain, and imaged by CSLM. (A) At both temperatures, biofilms grew to form compact towers with intervening water channels, as seen in CSLM images. Scale bars, 20 μm. (B to D) Comstat2 analysis was used to quantitate biofilm biomass (B), mean thickness (C), and roughness (D). Biofilms formed at 34°C had both significantly less biomass and reduced mean thickness, compared to their 37°C counterparts, for the first 48 h of culture. The roughness coefficient was also significantly greater at almost every time point in biofilms formed at 34°C versus 37°C, which indicated a more varied topology in biofilms formed at the cooler temperature. *, significantly different from 37°C value (P < 0.05).
Biofilms formed at the two temperatures also differed in their three-dimensional architecture. Biofilms formed at 37°C showed distinct tower structures at early time points but by 48 h, as the biofilms matured, the spaces between the towers began to fill in and the biofilms appeared more flattened (Fig. 1A). In contrast, biofilms formed at 34°C did not flatten with time but instead contained tower structures that were taller and thinner than those of biofilms formed at 37°C for up to 96 h of culture. Consistent with these observations, biofilms formed at 34°C had significantly greater roughness coefficients at almost every time point, compared with biofilms formed at 37°C (P < 0.05) (Fig. 1D). The roughness coefficient is used to measure variations in biofilm height per unit area and thus is an indicator of biofilm heterogeneity (29).
As another way to evaluate the functional and structural characteristics of these biofilms, we examined the sensitivity to antibiotic treatment of biofilms formed at 37°C versus 34°C. We established NTHI biofilms at 37°C or 34°C for 24 h and then treated them for 16 h with ampicillin, cefdinir, or amoxicillin plus clavulanic acid, antibiotics that are frequently used to treat otitis media caused by NTHI. Because biofilms are known to be highly resistant to antibiotics, we used concentrations equal to 1,000 times the planktonic MIC90 for NTHI. We found no differences in antibiotic sensitivity between biofilms formed at the two temperatures, which suggested that these biofilms were equally recalcitrant to treatment with these antimicrobials (see Fig. S1 in the supplemental material).
The distribution of pilA promoter activity within NTHI biofilms was unique.
Since expression of Tfp is important for NTHI biofilm formation both in vitro (1) and in the middle ear of chinchillas during experimental OM (6), we next examined the role of Tfp in NTHI biofilms formed at the temperature of the hNP. To estimate Tfp expression, we grew biofilms formed with the parent strain NTHI 86-028NP or with NTHI 86-028NP:pilA-GFP, in which expression of GFP is driven by the pilA promoter. We then used CSLM to visualize when and where bacteria within these biofilms expressed GFP and, by inference, Tfp.
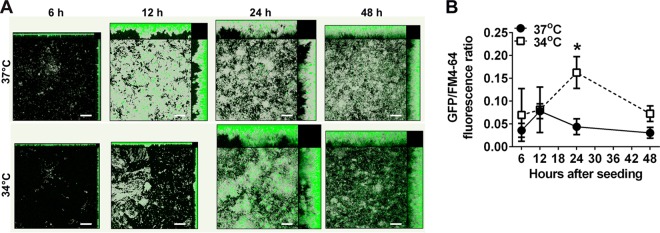
GFP expression by NTHI 86-028NP:pilA-GFP demonstrated a unique spatial distribution as the biofilm matured (Fig. 2A). In biofilms formed at 37°C, GFP fluorescence at 6 h was greatest near the substratum, consistent with the known roles of Tfp in bacterial attachment and organization via twitching motility (6, 15, 17). By 12 h, however, when towers had begun to form, discrete patches of intense fluorescence appeared both at the base and in the middle region of the biofilm. By 24 and 48 h, most of the fluorescent bacteria were located in the middle or apical regions of the biofilm. In biofilms formed at 34°C, fluorescent bacteria again appeared concentrated near the substratum at 6 and 12 h. By 24 h, however, large areas of intense fluorescence were visible throughout the biofilms and were notably predominant at the apexes of towers. This distribution of GFP fluorescence was maintained at 48 h. These data suggested that Tfp expression, as estimated by pilA promoter activity, was influenced by factors including temperature, time in culture, and position within the maturing biofilm.
FIG 2.
Expression of GFP by NTHI 86-028NP:pilA-GFP demonstrated a unique spatial distribution over time. (A) Biofilms formed with NTHI 86-028NP:pilA-GFP were imaged at 6, 12, 24, and 48 h. GFP fluorescence is shown (green). To visualize total biomass, biofilms were counterstained with FM 4-64 (gray). At 6 and 12 h, GFP fluorescence was greatest near the base of the biofilms, the site of initial bacterial attachment. As the biofilms matured, however, regions of intense fluorescence became more prevalent toward the apexes of towers. Scale bars, 20 μm. (B) Mean GFP fluorescence was normalized to total biomass (as measured by FM 4-64 fluorescence) for biofilms formed at 34°C or 37°C. *, the peak fluorescence ratio for biofilms grown at 34°C for 24 h was significantly greater than those for biofilms grown at 37°C for either 12 h (P < 0.05) or 24 h (P < 0.01).
Expression of pilA was upregulated at 34°C.
To further explore the differences in pilA promoter activity at 34°C versus 37°C, we measured the mean fluorescence intensity of biofilms formed by 86-028NP:pilA-GFP normalized to total biomass, as measured by FM 4-64 fluorescence. At both temperatures, the fluorescence ratio increased over time to a peak value and then decreased again within the 48-h period. As seen in Fig. 2, however, both the timing and the magnitude of pilA promoter activity were significantly affected by the 3°C difference in temperature. In biofilms grown at 34°C, the fluorescence ratio peaked at 24 h and was significantly greater than that in biofilms formed at 37°C at this time point (0.16 ± 0.03 versus 0.04 ± 0.02; P < 0.01) (Fig. 2B). Furthermore, this 34°C value at 24 h was significantly greater than the highest fluorescence ratio value measured in biofilms formed at 37°C, which occurred at 12 h (0.16 ± 0.03 versus 0.08 ± 0.02; P < 0.05). These results demonstrated a difference in timing and a significantly greater magnitude of pilA promoter activity (and by inference Tfp expression) in NTHI biofilms formed at the temperature of the hNP, compared to 37°C.
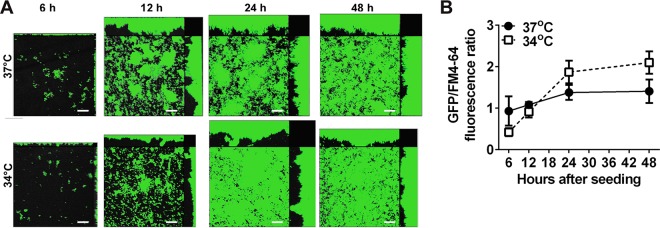
For comparison, we also grew biofilms with NTHI 86-028NP/pMDC-P1, in which GFP expression was driven by the ompP5 promoter. OMP P5, another critical adhesin of NTHI, is also important in NTHI adherence (40, 41) and is known to be constitutively expressed (24); therefore, this reporter served as a positive control in this assay system. In contrast to our results with 86-028NP:pilA-GFP, fluorescence due to ompP5 promoter activity was evenly distributed throughout NTHI 86-028NP/pMDC-P1 biofilms at all times and at both temperatures, as expected for a constitutively expressed protein (Fig. 3A). Although 86-028NP/pMDC-P1 mean fluorescence intensity increased with the time in culture, the timing of the increase was similar for the two temperatures, and the GFP fluorescence intensity levels were not significantly different at any time point (Fig. 3B). Confirming this result, we observed similar results with biofilms formed by NTHI 86-028NP/pRSM2211, in which GFP expression is driven by the ompP2 promoter (data not shown). OMP P2 is a constitutively expressed porin important for glucose uptake. These results demonstrated that ompP5 and ompP2 promoter activity (and by inference expression of OMP P5 and OMP P2, respectively) were similar in biofilms formed at 34°C versus 37°C.
FIG 3.
Expression of GFP by NTHI 86-028NP/pMDC-P1 was similar in biofilms formed at 34°C and at 37°C. (A) Biofilms formed with NTHI 86-028NP/pMDC-P1 were imaged at 6, 12, 24, and 48 h. GFP fluorescence is shown (green). To visualize total biomass, biofilms were counterstained with FM 4-64 (gray). ompP5 promoter activity was distributed evenly throughout the biofilms at all times, as expected for this constitutively expressed protein. Scale bars, 20 μm. (B) Mean GFP/FM 4-64 fluorescence ratios were determined for biofilms formed at 34°C or 37°C. Although at later time points the fluorescence ratios were slightly higher for biofilms formed at 34°C, the differences were not significant.
Because gene promoter activity is an indirect indicator of gene expression, we also measured pilA and ompP5 expression in biofilms formed at 34°C or 37°C. We used qRT-PCR to measure relative gene expression in biofilms formed for 12 or 15 h, because our microscopy and mean fluorescence data showed pilA promoter activity to be higher in biofilms formed at 37°C at 12 h but this trend had begun to reverse by 15 h (Fig. 2A and B). Consistent with these results, we found less pilA expression at 34°C than at 37°C at 12 h of culture. By 15 h of culture, however, pilA expression was greater at 34°C than at 37°C (see Fig. S2A in the supplemental material). In contrast, ompP5 expression in biofilms formed at 34°C was slightly greater than that in biofilms formed at 37°C at 12 h of culture, and levels were not different at 15 h of culture (see Fig. S2B). These data support the conclusion that pilA promoter activity is enhanced at 34°C versus 37°C, whereas ompP5 promoter activity is not significantly affected by this temperature difference.
Tfp was required for biofilm tower formation.
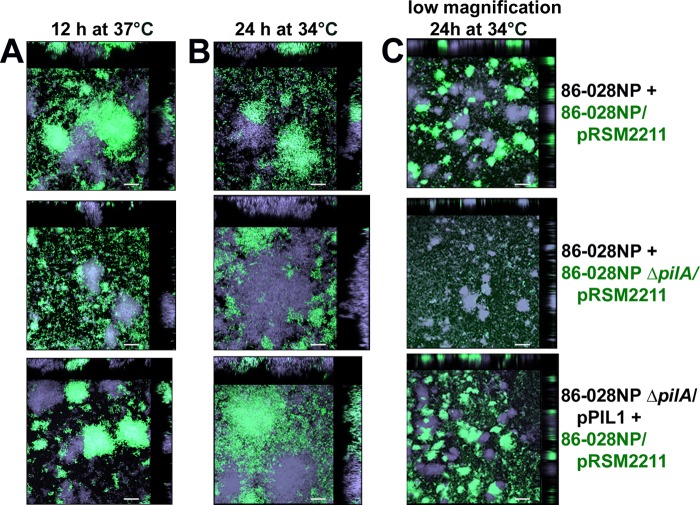
Interestingly, the times of peak pilA promoter activity seen in Fig. 2B corresponded to the times at which we first observed the presence of tower structures in the maturing biofilms at both 34°C and 37°C (Fig. 1A). This suggested a potential role for Tfp in tower formation. To test this hypothesis, we grew biofilms with a 1:1 mixture of strain pairs; one strain constitutively expressed GFP and one was nonfluorescent. Biofilms formed at 37°C were examined for tower structures at 12 h of culture (Fig. 4A), whereas biofilms formed at 34°C were examined at 24 h (Fig. 4B and C), as those were times at which tower structures were known to be present at each temperature (Fig. 1A).
FIG 4.
Tfp was required for biofilm tower formation. Biofilms were formed with equal numbers of GFP-expressing (green) or nonfluorescent (violet) NTHI strains, to visualize tower formation by each strain individually within the biofilms. Biofilms formed with 86-028NP and 86-028NP/pRSM2211, 86-028NP and 86-028NP ΔpilA/pRSM2211, or 86-028NP ΔpilA/pPIL1 and 86-028NP/pRSM2211 were imaged after 12 h at 37°C (A) or after 24 h at 34°C (B and C). Green labels, GFP-expressing strains. Times were chosen based on the earliest time of tower formation seen in biofilms formed at each temperature (Fig. 1A). Biofilm towers were formed by NTHI strains capable of Tfp formation (top) but not by 86-028NP ΔpilA/pRSM2211, which cannot express Tfp (middle); complementation of ΔpilA restored tower formation (bottom). In all images, cross sections of towers seen in orthogonal views showed that towers were composed of only one strain (either violet or green). Scale bars, 20 μm (A and B) or 100 μm (C). Images in panel C were obtained at a lower magnification to show the prevalence of violet and green towers over a larger surface area in biofilms grown for 24 h at 34°C.
Biofilms seeded with two strains capable of producing Tfp (86-028NP and 86-028NP/pRSM2211) contained towers formed by each strain, as expected (Fig. 4, top row, green and violet). In contrast, all towers in biofilms seeded with 86-028NP and 86-028NP ΔpilA/pRSM2211 were composed only of the parent strain (Fig. 4, middle row, violet), whereas the pilA mutant, which cannot express Tfp, was seen only near the substratum (Fig. 4, middle row, green). Complementation of the pilA mutant restored its ability to form towers (Fig. 4, bottom row). As the biofilms matured, towers became larger and more numerous but continued to be composed solely of strains capable of Tfp expression. Interestingly, many of the towers were uniform in color, which suggested that tower growth was clonal (Fig. 4A and B, top and bottom, orthogonal views). These data demonstrated that, for NTHI biofilms formed at either 34°C or 37°C, Tfp was required for the formation of tower structures.
Twitching motility was upregulated at 34°C.
Thus far, our results underscored the importance of Tfp in NTHI biofilms formed at both 34°C and 37°C. However, the observed increases in pilA promoter activity in biofilms formed at 34°C versus 37°C led us to wonder whether other Tfp functions differed at the two temperatures. Since twitching via Tfp is the only source of motility for NTHI and motility has been shown to be important for biofilm formation in other bacterial species (42–44), we hypothesized that twitching motility might be increased in biofilms formed at 34°C. To test this hypothesis, we used a subagarose twitching motility assay to measure the area, from the site of inoculation, traveled by NTHI via twitching over 24 h at 34°C or 37°C (Fig. 5A and B). As NTHI cells twitch away from the site of inoculation, they form shapes on the agar that resemble fan blades (1). As we hypothesized, despite the heterogeneity of fan blade sizes seen at the two temperatures, the overall distance traveled and area occupied by NTHI via twitching were significantly greater at 34°C than at 37°C (Fig. 5C). These results demonstrated that NTHI twitching motility was also enhanced at the temperature of the hNP.
FIG 5.
NTHI twitching motility was significantly greater at 34°C than at 37°C. (A and B) We used a subagarose twitching assay to measure the distance traveled and the area occupied by NTHI after 24 h at 34°C (A) or at 37°C (B). Red outlines illustrate how areas encompassed by NTHI due to twitching motility were measured. Scale bars, 1 mm. (C) Twitching areas were measured for each replicate and averaged (n = 3, with ≥20 replicates). NTHI traveled over a significantly greater area when grown at 34°C versus 37°C. *, P < 0.001.
PilA expression was upregulated in NTHI biofilms grown at 34°C on polarized epithelial cells.
Our observations that pilA promoter activity and twitching motility were increased in biofilms formed at 34°C suggested to us that these changes could represent an important difference in NTHI biofilms formed in the hNP versus those formed in warmer sites such as the ME. However, because studies with other hNP commensals have shown that biofilms grown on abiotic surfaces may have different characteristics than biofilms grown on epithelial cell surfaces (45, 46), we decided to test whether the temperature-dependent differences in pilA expression would persist in NTHI biofilms formed on the surface of the epithelia that they would normally encounter in vivo. Therefore, we grew NTHI biofilms on polarized epithelial cells from the chinchilla NP (CNPE) or ME (CMEE), at 34°C and 37°C, respectively. Enhancing the physiological relevance of our model, polarized epithelia form tight junctions that do not allow the movement of culture medium from the basolateral compartment to the apical surface. Thus, NTHI inoculated onto the polarized epithelial surface must obtain all necessary nutrients from that microenvironment.
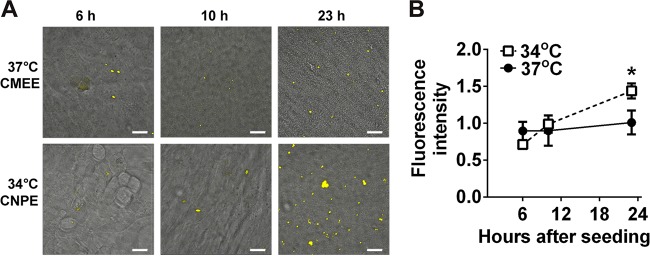
Preliminary data for NTHI 86-028NP:pilA-GFP inoculated onto CNPE at 34°C and monitored by time-lapse microscopy showed small peaks of GFP fluorescence at 6, 10, and 23 h after inoculation (data not shown). Consequently, we chose these time points for further evaluation of pilA promoter activity over time in NTHI 86-028NP:pilA-GFP biofilms formed on CMEE at 37°C or on CNPE at 34°C. NTHI 86-028NP:pilA-GFP biofilms formed on CMEE at 37°C demonstrated a low level of pilA promoter activity that remained constant over time (Fig. 6A, top). In contrast, in biofilms formed on CNPE at 34°C, 86-028NP:pilA-GFP fluorescence increased with the time in coculture (Fig. 6A, bottom). The mean GFP fluorescence intensity of 86-028NP:pilA-GFP at 23 h in coculture was significantly greater in biofilms formed on CNPE at 34°C than in biofilms formed on CMEE at 37°C (P < 0.05) (Fig. 6B). It is unlikely that this difference was due to differences in NTHI biofilm growth in general, since both biofilms formed with NTHI 86-028NP:pilA-GFP were comparable to those formed with the parent strain (Fig. 1A and 2A). Also, for comparison, we repeated these experiments with NTHI 86-028NP/pMDC-P1. Because the ompP5 promoter was constitutively active, we were able to visualize all bacteria on the epithelial cells. Biofilms formed by 86-028NP/pMDC-P1 appeared larger with time (see Fig. S3A in the supplemental material), but the mean GFP fluorescence intensity due to ompP5 expression was not significantly different in biofilms formed on CMEE at 37°C versus CNPE at 34°C (see Fig. S3B), which suggested that there were no significant differences in the numbers of bacteria colonizing CMEE versus CNPE. Taken together, these results strongly supported the hypothesis that NTHI Tfp expression occurs in vivo both in the nasopharynx and in warmer anatomical sites such as the ME and that the regulation of this expression differs between the two sites. While it is possible that differences between CNPE and CMEE and how they interact with NTHI contributed to this result, our combined results strongly supported the hypothesis that pilA expression in NTHI biofilms in vivo is likely to be significantly affected by the temperature of the anatomical environment.
FIG 6.
NTHI biofilms formed on polarized epithelial cells demonstrated enhanced pilA expression at 34°C. (A) NTHI 86-028NP:pilA-GFP was inoculated onto polarized CMEE at 37°C or CNPE at 34°C, and biofilms were imaged by CSLM after 6, 10, or 23 h in coculture. Time points were chosen based on preliminary data that showed increased fluorescence due to pilA promoter activity at those times. Images show GFP fluorescence (yellow) of NTHI expressing the pilA promoter, overlaid on differential interference contrast (DIC) images of the underlying epithelial cells. In biofilms formed at 37°C on CMEE, 86-028NP:pilA-GFP fluorescence showed little change with time over 23 h (top). In contrast, in biofilms formed at 34°C on CNPE, 86-028NP:pilA-GFP fluorescence increased with time in coculture (bottom). Scale bars, 20 μm. (B) The mean fluorescence intensity of 86-028NP:pilA-GFP at 23 h was significantly greater for biofilms formed on CNPE at 34°C than for those formed on CMEE at 37°C. *, P < 0.05.
Biofilm formation was inhibited by antibodies against Tfp or OMP P5.
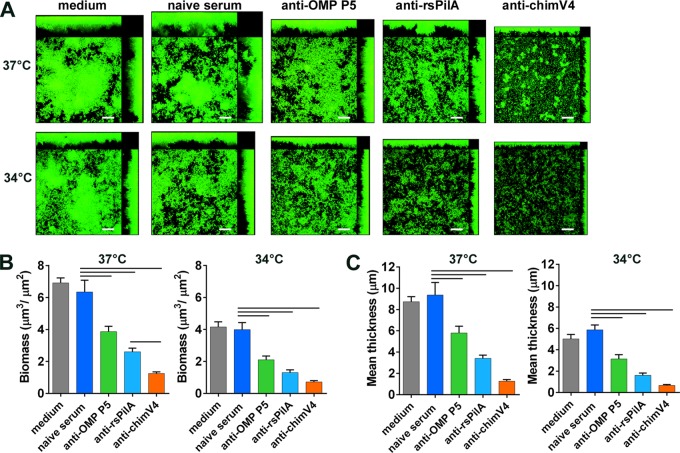
Taken together, our results so far demonstrated important differences in NTHI biofilm architecture and expression of the major pilin subunit of an important virulence factor, Tfp. We next wondered how these differences would affect the ability of biofilms formed at 34°C or 37°C to respond to antibodies designed to disrupt or to prevent biofilm formation in vivo. Since previous studies showed that both Tfp and OMP P5 play roles in NTHI adherence early in biofilm formation (6, 19, 36), we hypothesized that, at 34°C, exposure of newly seeded biofilms to antibodies targeting PilA or OMP P5 would inhibit bacterial adherence and, consequently, biofilm formation. To that end, we exposed newly seeded biofilms to medium that contained antisera directed against rsPilA, OMP P5, or the chimeric immunogen chimV4, which is composed of both PilA and OMP P5 epitopes (19). At both 34°C and 37°C, biofilms treated with either anti-rsPilA or anti-OMP P5 serum were significantly smaller than those treated with naive serum, as indicated by differences in biofilm biomass (P < 0.001) (Fig. 7A and B) and mean biofilm thickness (P < 0.001) (Fig. 7A and C). Substantial inhibition of biofilm formation was also seen with anti-chimV4 serum, and the effects of this dual-determinant-targeted antiserum on biofilms formed at 37°C were significantly greater than the effects of either anti-rsPilA or anti-OMP P5 serum alone, with respect to both biomass and mean thickness (Fig. 7B and C). Taken together, these results demonstrated that bacterial adherence, as mediated by both Tfp and OMP P5, was important for biofilm formation at the temperature of the hNP as well as at 37°C.
FIG 7.
Antibodies targeting OMP P5 or PilA inhibited NTHI biofilm formation at both 34°C and 37°C. One hour after seeding, biofilms were treated with antiserum against OMP P5, rsPilA, or chimV4. After 4 h, treatments were replaced with fresh medium and biofilms were grown for 24 h. (A) Exposure to either anti-OMP P5 or anti-rsPilA serum inhibited biofilm formation, as seen in CSLM images. Scale bars, 20 μm. (B and C) Treatment with these antisera resulted in significantly less biofilm biomass (B) and smaller biofilm mean thickness (C) than did treatment with naive serum. Antiserum to the dual antigen chimV4 inhibited biofilm formation to a greater extent than did either anti-OMP P5 or anti-rsPilA alone. These results are consistent with the roles of OMP P5 and PilA as critical adhesins at both 34°C and 37°C. Bars, significant differences versus naive serum (P < 0.001) and between anti-rsPilA and anti-chimV4 sera (P < 0.02).
Antibodies targeting Tfp disrupted established biofilms.
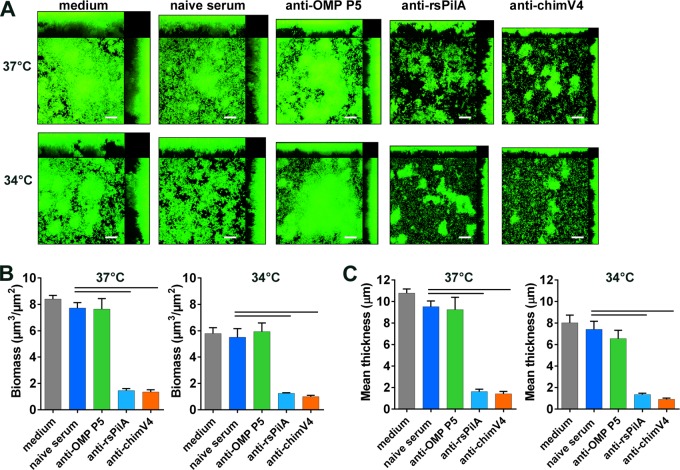
We recently showed that NTHI biofilms established at 37°C were dispersed by exposure to anti-rsPilA serum, via a mechanism involving both PilA (and by inference Tfp) and the luxS quorum-sensing system (1). Those studies revealed that, in addition to biofilm formation, Tfp is important in NTHI biofilm cohesion and dispersion (1). Since biofilms formed at 34°C expressed Tfp, which was active in biofilm formation, we wondered whether targeting Tfp with anti-rsPilA would also disrupt biofilms formed at the lower hNP temperature. Figure 8 shows biofilms that were formed at 34°C or 37°C for 24 h and then treated overnight with anti-rsPilA, anti-OMP P5, or anti-chimV4. Treatments with antisera targeting Tfp (both anti-rsPilA and anti-chimV4) significantly disrupted established biofilms and decreased biomass by >80% at 37°C and >76% at 34°C (P < 0.001) (Fig. 8B). Similarly, treatment with anti-rsPilA or anti-chimV4 serum decreased the mean biofilm thickness by >80% at both temperatures (P < 0.001) (Fig. 8C). In contrast, treatment with anti-OMP P5 had no effect at either temperature, consistent with previous reports for 37°C biofilms (1). Moreover, biofilm disruption was not the result of complement-mediated killing, since the vital dye used to stain the biofilms indicated only live bacteria (Fig. 8A; note an absence of red color in the biofilm images).
FIG 8.
Antibodies targeting PilA disrupted established NTHI biofilms at both 34°C and 37°C. Biofilms were formed for 24 h at 34°C or 37°C and then were fed with medium containing antiserum against OMP P5, rsPilA, or chimV4 for 16 h. (A) At both temperatures, established biofilms were disrupted by exposure to anti-rsPilA or anti-chimV4 but not anti-OMP P5, as seen in confocal images. Scale bars, 20 μm. (B and C) Biofilm biomass (B) and mean thickness (C) were also significantly decreased by treatment with anti-rsPilA or anti-chimV4. Results suggest an important role for Tfp in biofilm stability at both temperatures. Bars, significant differences versus naive serum (P < 0.0001).
DISCUSSION
In this study, we examined the impact of the average NP temperature, i.e., 34°C, on NTHI biofilm formation and expression of the critical adhesin and motility factor Tfp. Our results demonstrated that NTHI was capable of forming a biofilm at the cooler temperature of the hNP, both in chambered coverglasses and on polarized CNPE, as well as at 37°C on glass and on CMEE. Marks et al. (45) showed the presence of highly structured biofilms in the mouse NP after intranasal inoculation of another NP commensal, S. pneumoniae. Given the mechanical stresses due to breathing, ciliary movement, and changing compositions and movement of surface liquids, as well as the relative scarcity of nutrients in the healthy hNP, it is likely that NTHI survives and persists in this anatomical niche by forming biofilms.
To date, much progress has been made in our understanding of the role of NTHI Tfp at 37°C in vitro and in the middle ears of animals after experimental infection (17, 19, 47). We have learned that Tfp expression is regulated and responds to changes in the environment, including pH, quorum-sensing molecules such as AI-2, and the presence of cells (1, 6, 15). However, much remains to be learned about the mechanisms through which Tfp influences biofilm formation, maturation, and dispersion, especially in the hNP, the site of asymptomatic colonization. Here we demonstrated that pilA promoter activity (and by inference Tfp expression [1, 23]) was significantly greater in NTHI biofilms formed at the cooler temperature of the hNP than in biofilms formed at 37°C. We also found enhanced twitching motility at 34°C, compared to 37°C. Although our studies of biofilms in chambered coverglasses illustrated NTHI biofilm growth under nutrient-replete conditions, we also demonstrated the relevance of these findings in a more complex experimental model in which biofilms were grown directly on polarized CNPE at 34°C or CMEE at 37°C. That model, while still in vitro, more closely mimics in vivo conditions by allowing the biofilms to interact with the epithelial cells they would normally encounter, at the appropriate temperatures for the NP or ME. In addition, NTHI cells on the surface of polarized cells are restricted nutritionally to whatever they can obtain from the epithelial surface. Because of those factors, NTHI growth on epithelial cells was much less than that seen in chambered coverglasses with rich bacterial medium. Even in the more complex environment, however, NTHI biofilms continued to demonstrate greater pilA promoter activity under conditions that resembled those of the hNP rather than those of the ME. These results support the hypothesis that pilA promoter activity (and by inference Tfp expression) is important both in the asymptomatically colonized hNP and in the infected ME.
The findings of greater pilA promoter activity and twitching motility in biofilms at 34°C are intriguing, as they suggest a specific role for Tfp in NTHI adaptation to long-term existence in the hNP. Here we demonstrated that twitching motility was significantly enhanced at 34°C and likely plays an important role in the maintenance of colonization in the dynamic environment of the hNP. The ability to move to a new location and to adhere quickly would be advantageous in a dynamic environment such as the NP, where environmental conditions can change quickly and dramatically and epithelial cell turnover presents constant challenges to the maintenance of colonization. In contrast, the warmer middle ear is an enclosed space where biofilms are less likely to be physically disturbed.
Temperature regulation of twitching motility has also been described for other bacterial species. Liles et al. showed that for Legionella pneumophila, which can live in aquatic environments as well as in the mammalian lung, 5 to 10% of bacterial cells were piliated at 30°C, whereas no Tfp was observed at 37°C; the authors also showed that transcription of pilB was significantly greater at 30°C than at 37°C (48). In L. pneumophila, pilB codes for a protein similar to PilB from Pseudomonas aeruginosa, which is a component of the type II secretory pathway that is required for the assembly of Tfp (48). In another study, temperature-dependent control of biofilm formation through the expression of a single gene in a clinical strain of Pseudomonas aeruginosa was recently described by Randall et al. (49). The gene product, thermosensing diguanylate cyclase A, catalyzed the synthesis of cyclic di-GMP 35 times faster at 37°C than at 25°C. The mechanism by which NTHI senses a temperature change in the environment is currently not known but could involve both genetic and epigenetic mechanisms.
Studies of P. aeruginosa biofilm formation revealed that twitching via Tfp is required for the initial organization of bacteria on the substratum, and both Tfp and flagella are required to form the “cap” structures on the mushroom-like towers formed by this species (50). During mushroom cap formation, the flagella are required for motility, whereas Tfp binds to extracellular DNA (eDNA) that is produced by bacteria in the supporting “stalk” portion of the biofilm. Here we demonstrated that, for NTHI, biofilm tower structures can be formed only by bacteria capable of Tfp expression. eDNA is a common structural component of biofilms formed by many bacterial species, and we have demonstrated the presence of eDNA lattices in NTHI biofilms formed both in vitro and in vivo (16, 51, 52). Hence, it is likely that tower formation in NTHI biofilms requires interaction between bacteria expressing Tfp and eDNA. Our results suggest a unique pattern of Tfp expression within maturing biofilms, which progresses over time from the base of the biofilm toward the apical surface. Collectively, the results of this study are consistent with the following proposed mechanism: (i) NTHI uses its Tfp to adhere to the substrate, (ii) the community of adherent NTHI bacteria organize themselves into denser regions using twitching motility, (iii) tower formation begins with clonal growth in these dense areas, and (iv) a subset of bacteria use twitching motility to scale the sides of existing bacterial structures and attach themselves at or near the top of the nascent tower.
Although tower structures are an integral part of biofilms formed by many bacterial species (31), the role of these structures is not fully known. The architecture of towers surrounded by intermittent water channels is thought to allow for nutrient availability and the removal of metabolic waste from the biofilm. Biofilm towers can be particularly resistant to various antimicrobials, including SDS detergent and tobramycin, in P. aeruginosa (53, 54). Here we showed that NTHI biofilms are highly resistant to antibiotics when formed at either 34°C or 37°C. However, significant differences in biomass, thickness, and roughness of the biofilms formed at 34°C versus 37°C suggested that biofilms formed at the temperature of the hNP differed in important ways from those formed at the typical internal body temperature of 37°C. Biofilm towers are also highly resistant to mechanical stresses such as shearing forces (31, 55). Given that, as a commensal in the human NP, NTHI is subject to rapid fluctuations in airflow, temperature, and mucociliary clearance, it is likely that these characteristic biofilm towers contribute to its survival and persistence at this site.
Because of the central role of Tfp in NTHI biofilm biology, PilA is being developed by our laboratory as a potential traditional and therapeutic vaccine candidate. Therefore, it is of interest to know whether strategies designed to target PilA in NTHI biofilms at 37°C would also be effective against both adherent NTHI and any biofilms formed by this microbe in the cooler NP. Here we showed that antibodies to rsPilA were equally effective in preventing biofilm formation at 34°C and at 37°C, consistent with the known roles of Tfp in adherence and twitching motility. Moreover, anti-rsPilA was equally effective in disrupting biofilms at the two temperatures. We showed recently that this disruption from the apical surface of the biofilm is actually a dispersion mediated by quorum sensing via the luxS system (1). The ability of anti-rsPilA to disrupt preformed biofilms and to inhibit biofilm formation is central to the success of PilA as a vaccine candidate for both therapeutic and preventative immunization strategies for OM. Consequently, our results strongly suggest that immunization strategies that target Tfp in biofilms present within the ME may also be useful for limiting the excessive growth of NTHI in the NP that is concurrent with upper respiratory tract viral infection and is known to precede the development of OM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Neelans for help with manuscript preparation and Kenneth Brockman for help with qRT-PCR assays.
Funding Statement
The funding agency had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01022-15.
REFERENCES
- 1.Novotny LA, Jurcisek JA, Ward MO Jr, Jordan ZB, Goodman SD, Bakaletz LO. 2015. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol Microbiol 96:276–292. doi: 10.1111/mmi.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassell GH, Archer GL, Beam TR, Gilchrist MJ, Goldmann D, Hooper DC, Jones RN, Kleven SH, Lederberg J, Levy SB, Lein DH, Moellering RC, O'Brien TF, Osburn B, Osterholm M, Shlaes DM, Terry M, Tolin SA, Tomasz A. 1994. Report of the ASM task force on antibiotic resistance. American Society for Microbiology, Washington, DC. [Google Scholar]
- 3.Bakaletz LO. 2012. Bacterial biofilms in the upper airway: evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev 13:154–159. doi: 10.1016/j.prrv.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haggard M. 2008. Otitis media: prospects for prevention. Vaccine 26(Suppl 7):G20–G24. doi: 10.1016/j.vaccine.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurcisek JA, Bookwalter JE, Baker BD, Fernandez S, Novotny LA, Munson RS Jr, Bakaletz LO. 2007. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol 65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 7.Novotny LA, Clements JD, Bakaletz LO. 2013. Kinetic analysis and evaluation of the mechanisms involved in the resolution of experimental nontypeable Haemophilus influenzae-induced otitis media after transcutaneous immunization. Vaccine 31:3417–3426. doi: 10.1016/j.vaccine.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez AC, Pang B, King LB, Tan L, Murrah KA, Reimche JL, Wren JT, Richardson SH, Ghandi U, Swords WE. 2014. Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathog Dis 70:280–288. doi: 10.1111/2049-632X.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murrah KA, Pang B, Richardson S, Perez A, Reimche J, King L, Wren J, Swords WE. 2015. Nonencapsulated Streptococcus pneumoniae causes otitis media during single-species infection and during polymicrobial infection with nontypeable Haemophilus influenzae. Pathog Dis 73:ftu011. doi: 10.1093/femspd/ftu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novotny LA, Clements JD, Bakaletz LO. 2015. Therapeutic transcutaneous immunization with a band-aid vaccine resolves experimental otitis media. Clin Vaccine Immunol 22:867–874. doi: 10.1128/CVI.00090-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foreman A, Jervis-Bardy J, Wormald PJ. 2011. Do biofilms contribute to the initiation and recalcitrance of chronic rhinosinusitis? Laryngoscope 121:1085–1091. doi: 10.1002/lary.21438. [DOI] [PubMed] [Google Scholar]
- 12.Garmendia J, Viadas C, Calatayud L, Mell JC, Marti-Lliteras P, Euba B, Llobet E, Gil C, Bengoechea JA, Redfield RJ, Linares J. 2014. Characterization of nontypable Haemophilus influenzae isolates recovered from adult patients with underlying chronic lung disease reveals genotypic and phenotypic traits associated with persistent infection. PLoS One 9:e97020. doi: 10.1371/journal.pone.0097020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardines R, Giufre M, Pompilio A, Fiscarelli E, Ricciotti G, Di Bonaventura G, Cerquetti M. 2012. Haemophilus influenzae in children with cystic fibrosis: antimicrobial susceptibility, molecular epidemiology, distribution of adhesins and biofilm formation. Int J Med Microbiol 302:45–52. doi: 10.1016/j.ijmm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Nickel JC, Ruseska I, Wright JB, Costerton JW. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27:619–624. doi: 10.1128/AAC.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakaletz LO, Baker BD, Jurcisek JA, Harrison A, Novotny LA, Bookwalter JE, Mungur R, Munson RS Jr. 2005. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun 73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurcisek JA, Bakaletz LO. 2007. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol 189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carruthers MD, Tracy EN, Dickson AC, Ganser KB, Munson RS Jr, Bakaletz LO. 2012. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol 194:1927–1933. doi: 10.1128/JB.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novotny LA, Clements JD, Bakaletz LO. 2011. Transcutaneous immunization as preventative and therapeutic regimens to protect against experimental otitis media due to nontypeable Haemophilus influenzae. Mucosal Immunol 4:456–467. doi: 10.1038/mi.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novotny LA, Adams LD, Kang DR, Wiet GJ, Cai X, Sethi S, Murphy TF, Bakaletz LO. 2009. Epitope mapping immunodominant regions of the PilA protein of nontypeable Haemophilus influenzae (NTHI) to facilitate the design of two novel chimeric vaccine candidates. Vaccine 28:279–289. doi: 10.1016/j.vaccine.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb P. 1951. Air temperatures in respiratory tracts of resting subjects in cold. J Appl Physiol 4:378–382. [DOI] [PubMed] [Google Scholar]
- 21.Marks LR, Reddinger RM, Hakansson AP. 2012. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. mBio 3:e00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks LR, Davidson BA, Knight PR, Hakansson AP. 2013. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 4:e00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason KM, Munson RS Jr, Bakaletz LO. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect Immun 71:3454–3462. doi: 10.1128/IAI.71.6.3454-3462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy TF, Kirkham C. 2002. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol 2:7. doi: 10.1186/1471-2180-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novotny LA, Mason KM, Bakaletz LO. 2005. Development of a chinchilla model to allow direct, continuous, biophotonic imaging of bioluminescent nontypeable Haemophilus influenzae during experimental otitis media. Infect Immun 73:609–611. doi: 10.1128/IAI.73.1.609-611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason KM, Munson RS Jr, Bakaletz LO. 2005. A mutation in the sap operon attenuates survival of nontypeable Haemophilus influenzae in a chinchilla model of otitis media. Infect Immun 73:599–608. doi: 10.1128/IAI.73.1.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. 2011. In vitro biofilm formation in an 8-well chamber slide. J Vis Exp 47:e2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockson ME, Novotny LA, Mokrzan EM, Malhotra S, Jurcisek JA, Akbar R, Devaraj A, Goodman SD, Bakaletz LO. 2014. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol 93:1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 30.Vorregaard M. 2008. Comstat2: a modern 3D image analysis environment for biofilms. Master's thesis. Technical University of Denmark, Kongens Lyngby, Denmark. [Google Scholar]
- 31.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho C, Chande A, Gakhar L, Bakaletz LO, Jurcisek JA, Ketterer M, Shao J, Gotoh K, Foster E, Hunt J, O'Brien E, Apicella MA. 2015. Role of the nuclease of nontypeable Haemophilus influenzae in dispersal of organisms from biofilms. Infect Immun 83:950–957. doi: 10.1128/IAI.02601-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerschner JE, Khampang P, Samuels T. 2010. Extending the chinchilla middle ear epithelial model for mucin gene investigation. Int J Pediatr Otorhinolaryngol 74:980–985. doi: 10.1016/j.ijporl.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGillivary G, Ray WC, Bevins CL, Munson RS Jr, Bakaletz LO. 2007. A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media. Mol Immunol 44:2446–2458. doi: 10.1016/j.molimm.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGillivary G, Jordan ZB, Peeples ME, Bakaletz LO. 2013. Replication of respiratory syncytial virus is inhibited by the host defense molecule viperin. J Innate Immun 5:60–71. doi: 10.1159/000342473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bookwalter JE, Jurcisek JA, Gray-Owen SD, Fernandez S, McGillivary G, Bakaletz LO. 2008. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect Immun 76:48–55. doi: 10.1128/IAI.00980-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura A, DeMaria TF, Lim DJ, van Blitterswijk CA. 1991. Primary culture of chinchilla middle ear epithelium. Ann Otol Rhinol Laryngol 100:774–782. doi: 10.1177/000348949110000916. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura A, DeMaria TF, van Blitterswijk C, Lim DJ. 1992. Effect of endotoxin on cultured chinchilla middle ear epithelium. Ann Otol Rhinol Laryngol 101:607–611. doi: 10.1177/000348949210100712. [DOI] [PubMed] [Google Scholar]
- 39.McGillivary G, Bakaletz LO. 2010. The multifunctional host defense peptide SPLUNC1 is critical for homeostasis of the mammalian upper airway. PLoS One 5:e13224. doi: 10.1371/journal.pone.0013224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Z, Nagata N, Molina E, Bakaletz LO, Hawkins H, Patel JA. 1999. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect Immun 67:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakaletz LO, Tallan BM, Hoepf T, DeMaria TF, Birck HG, Lim DJ. 1988. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun 56:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol 50:61–68. doi: 10.1046/j.1365-2958.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- 43.Luke NR, Jurcisek JA, Bakaletz LO, Campagnari AA. 2007. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun 75:5559–5564. doi: 10.1128/IAI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mattick JS. 2002. Type IV pili and twitching motility. Annu Rev Microbiol 56:289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 45.Marks LR, Parameswaran GI, Hakansson AP. 2012. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect Immun 80:2744–2760. doi: 10.1128/IAI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marks LR, Mashburn-Warren L, Federle MJ, Hakansson AP. 2014. Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. J Infect Dis 210:25–34. doi: 10.1093/infdis/jiu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swords WE. 2012. Nontypeable Haemophilus influenzae biofilms: role in chronic airway infections. Front Cell Infect Microbiol 2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liles MR, Viswanathan VK, Cianciotto NP. 1998. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect Immun 66:1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randall TE, Liu F, Jennings L, Kittichotirat W, Bumgarner R, Winsor GL, Brinkman FS, Winstone TM, Singletary LA, Hynes MF, Leblanc K, Yang JJ, Good NM, Tseng B, Parsek MR, Harrison JJ. 2015. A bacterial thermosensor that modulates biofilm formation in response to human body temperature, abstr S12:3, p 42–43. Final program abstr 7th Am Soc Microbiol Conf Biofilms. American Society for Microbiology, Washington, DC: http://conferences.asm.org/images//asm%20biofilms%20web.pdf. [Google Scholar]
- 50.Barken KB, Pamp SJ, Yang L, Gjermansen M, Bertrand JJ, Klausen M, Givskov M, Whitchurch CB, Engel JN, Tolker-Nielsen T. 2008. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ Microbiol 10:2331–2343. doi: 10.1111/j.1462-2920.2008.01658.x. [DOI] [PubMed] [Google Scholar]
- 51.Jones EA, McGillivary G, Bakaletz LO. 2013. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun 5:24–38. doi: 10.1159/000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodman SD, Obergfell KP, Jurcisek JA, Novotny LA, Downey JS, Ayala EA, Tjokro N, Li B, Justice SS, Bakaletz LO. 2011. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol 4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 53.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 54.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brossard KA, Campagnari AA. 2012. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80:228–233. doi: 10.1128/IAI.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sirakova T, Kolattukudy PE, Murwin D, Billy J, Leake E, Lim D, DeMaria T, Bakaletz L. 1994. Role of fimbriae expressed by nontypeable Haemophilus influenzae in pathogenesis of and protection against otitis media and relatedness of the fimbrin subunit to outer membrane protein A. Infect Immun 62:2002–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.