Abstract Abstract
Although commonly encountered, patients with combined postcapillary and precapillary pulmonary hypertension (Cpc-PH) have poorly understood pulmonary vascular properties. The product of pulmonary vascular resistance and compliance, resistance-compliance (RC) time, is a measure of pulmonary vascular physiology. While RC time is lower in postcapillary PH than in precapillary PH, the RC time in Cpc-PH and the effect of pulmonary wedge pressure (PWP) on RC time are unknown. We tested the hypothesis that Cpc-PH has an RC time that resembles that in pulmonary arterial hypertension (PAH) more than that in isolated postcapillary PH (Ipc-PH). We analyzed the hemodynamics of 282 consecutive patients with PH referred for right heart catheterization (RHC) with a fluid challenge from 2004 to 2013 (cohort A) and 4,382 patients who underwent RHC between 1998 and 2014 for validation (cohort B). Baseline RC time in Cpc-PH was higher than that in Ipc-PH and lower than that in PAH in both cohorts (P < 0.001). In cohort A, RC time decreased after fluid challenge in patients with Ipc-PH but not in those with PAH or Cpc-PH (P < 0.001). In cohort B, the inverse relationship of pulmonary vascular compliance and resistance, as well as that of RC time and PWP, in Cpc-PH was similar to that in PAH and distinct from that in Ipc-PH. Our findings demonstrate that patients with Cpc-PH have pulmonary vascular physiology that resembles that of patients with PAH more than that of Ipc-PH patients. Further study is warranted to identify determinants of vascular remodeling and assess therapeutic response in this subset of PH.
Keywords: pulmonary vascular resistance, pulmonary vascular compliance, resistance-compliance time, pulmonary hypertension, precapillary, postcapillary
Despite strict diagnostic criteria to distinguish precapillary from postcapillary pulmonary hypertension (PH), up to 20% of patients have features of both categories after thorough evaluation.1-4 This hybrid vascular phenotype, known as combined postcapillary and precapillary PH (Cpc-PH), is variably identified by an elevated pulmonary vascular resistance (PVR), transpulmonary gradient (TPG), or diastolic pressure gradient (DPG)5-8 in the presence of left heart disease.
The 2013 World Symposium on PH recently defined Cpc-PH as a pulmonary wedge pressure (PWP) of >15 mmHg with a DPG of ≥7 mmHg.9 Since most large epidemiologic studies of pulmonary arterial hypertension (PAH) excluded subjects meeting these criteria,10-12 it is unknown whether patients with Cpc-PH have pulmonary vascular properties that more closely resemble those of PAH patients or those of patients with isolated postcapillary PH (Ipc-PH).
The resistance-compliance (RC) time has emerged as a measure of pulmonary vascular physiology that integrates the mean and pulsatile afterload of the right ventricle.13,14 Based on an electrical model of the pulmonary circulation, RC time is calculated by multiplying two inversely related hemodynamic variables, PVR and pulmonary vascular compliance (PVC). Previously considered a fixed constant,14-16 RC time is increased by pulmonary arterial remodeling17,18 and reduced by elevations in pulmonary venous pressure.19,20 While RC time in Cpc-PH has recently been shown to differ from that in Ipc-PH,21 the extent to which baseline differences and rapid changes in PWP affect RC time has not been explored.
We hypothesized that despite an elevated PWP, patients with Cpc-PH would have an RC time and PVC-PVR relationship that more closely resemble those of PAH patients than those of Ipc-PH patients, both at rest and after acute volume expansion. We tested this hypothesis in a well-phenotyped cohort of patients with PH and in a large electronic medical record (EMR)–based cohort spanning 17 years.
Methods
Patient populations
This study consisted of two retrospective cohorts seen at Vanderbilt University who underwent evaluation with a right heart catheterization (RHC). The Institutional Review Board at Vanderbilt approved the study of cohorts A and B (IRB 141151 and 140544, respectively).
Cohort A included consecutively evaluated patients seen at the Vanderbilt Center for Pulmonary Vascular Disease from 2004 to 2013 who underwent an RHC with a fluid bolus for known or suspected PH. Hemodynamic measurements were obtained at baseline and after the infusion of 0.5 L of normal saline, according to institutional protocol.1 One of the authors (ARH, IMR, or TRA) manually reviewed all RHC tracings and recorded pressures at end-expiration. Demographic data were tabulated through EMR review. Patients were excluded if their tracings were unavailable for review or if they were categorized as having World Health Organization (WHO) group 3, 4, or 5 PH.22 Final PH categorization was based on hemodynamics after fluid challenge (Table 1), according to consensus guidelines for the diagnosis of PH at rest.9,22 Briefly, PH was defined as a mean pulmonary arterial pressure of ≥25 mmHg. PAH was defined as PH with a PWP of ≤15 mmHg and a PVR of >3 Wood units. Finally, Cpc-PH and Ipc-PH were defined as PH with a PWP of >15 mmHg and a DPG of ≥7 mmHg and <7 mmHg, respectively.
Table 1.
Hemodynamic classification of PH in cohorts A and B
| Category | mPAP, mmHg | PWP, mmHg | PVR, WU | DPG, mmHg |
|---|---|---|---|---|
| No PH | <25 | … | … | … |
| PCPH | ≥25 | ≤15 | … | … |
| PAH | ≥25 | ≤15 | >3 | … |
| Ipc-PH | ≥25 | >15 | … | <7 |
| Cpc-PH | ≥25 | >15 | … | ≥7 |
Cpc-PH: combined postcapillary and precapillary pulmonary hypertension; DPG: diastolic pulmonary artery–to–pulmonary wedge pressure gradient; Ipc-PH: isolated postcapillary pulmonary hypertension; mPAP: mean pulmonary artery pressure; PAH: pulmonary arterial hypertension; PCPH: precapillary pulmonary hypertension; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; PWP: pulmonary wedge pressure; WU: Wood units.
Cohort B consisted of patients who underwent RHC from 1998 to 2014 and were in Vanderbilt’s Synthetic Derivative database,23,24 a deidentified version of the Vanderbilt EMR that contains detailed demographic, medication, laboratory, and billing data, along with clinical documentation and procedural reports, for more than 2 million unique subjects. If patients had multiple catheterizations, only the first RHC was included in this analysis. We excluded patients with insufficient data (right ventricular and pulmonary artery pressures were absent), previous cardiac (International Classification of Diseases, Ninth Revision [ICD-9] code V42.1) or lung (ICD-9 code V42.6) transplantation, acute myocardial infarction (ICD-9 code 410.*), or chronic pulmonary embolism (ICD-9 code 416.2). Furthermore, as the purpose of this study was to evaluate hemodynamics in patients who were not acutely decompensated, patients with physiologic outliers due to presumed shock, hypertensive crisis, or left-to-right shunt at the time of RHC were excluded (defined in Table S1). Structured hemodynamic data were extracted from the RHC report, and fields containing relevant structured data were identified within reports by content experts (TRA, ELB, and LND). Corresponding numeric values were subsequently parsed by means of regular expressions and pattern matching. Nonphysiologic values (e.g., arterial saturation of >100% or a negative cardiac output; thresholds are defined in Table S1) were systematically deleted in a manner similar to that used in other large EMR-based RHC cohorts.25,26 Data that were deleted (391 values [0.2%]) or missing (41,983 values [17.5%]) were imputed by means of multiple imputation with additive regression, bootstrapping, and predictive mean matching.
The remaining patients were classified according to contemporary guidelines as above. In order to isolate a true PAH cohort, we excluded patients with precapillary PH and chronic obstructive pulmonary disease (ICD-9 codes 491.*, 492.*, and 496) or interstitial lung disease (ICD-9 codes 515, 516.3*, 516.4, and 516.5). To determine the reliability of the PWP in the cohort B database,27-30 we compared the end-expiratory PWP identified manually and the computer-generated PWP from the RHC report in 116 randomly selected cohort A patients.
Calculations and statistics
RC time (seconds) is the product of PVR (mmHg × seconds × mL−1) and PVC (mL × mmHg−1). PVC was calculated as the stroke volume (mL) divided by the pulmonary artery pulse pressure (mmHg). Statistical analysis was performed in R (http://cran.r-project.org/) and Prism (ver. 6.0f, GraphPad Software). The PVC-PVR relationship was modeled with an inverse regression with offset as previously reported: PVC = A/(PVR + B).19 For cohort B, PVR and PVC were also logarithmically transformed and modeled with a linear regression: log (PVC) = A × log (PVR) + B. Unless stated otherwise, continuous variables are presented as mean ± SD, while categorical variables are presented as absolute numbers and percentages. Continuous variables were compared via the Mann-Whitney U test, the Kruskal-Wallis test, or the Wilcoxon signed-rank test, as indicated. Significance was determined as a P value of <0.05.
Results
Cohort A
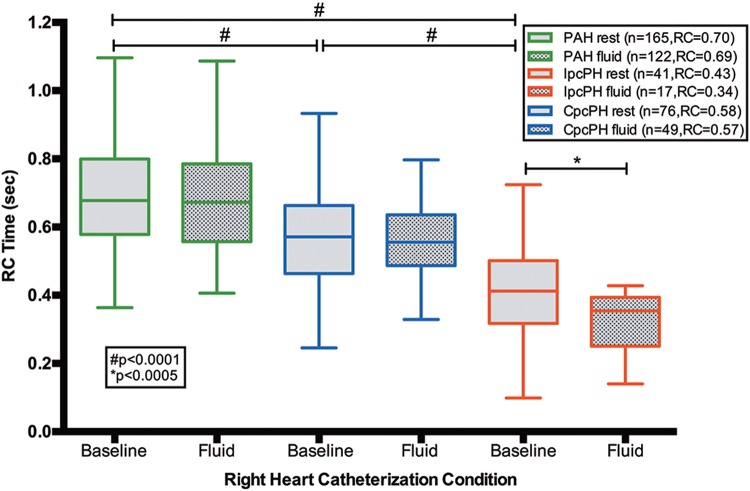
There were 22 patients without PH (Table S2) and 282 patients with PH in cohort A: 165 (59%) with PAH, 41 (14%) with Ipc-PH, and 76 (27%) with Cpc-PH. The baseline RC time in Cpc-PH patients was higher than that in Ipc-PH patients and lower than that in PAH patients (both P < 0.0001; Table 2). In Cpc-PH and PAH patients, RC time was unchanged from baseline after a fluid challenge, whereas RC time significantly decreased in Ipc-PH patients (P < 0.0005; Fig. 1).
Table 2.
Clinical and hemodynamic characteristics of patients with PH in cohorts A and B
| Cohort A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpc-PH | Ipc-PH | PAH | Cohort B | |||||||||
| Category | Total | Before FC | After FC | Total | Before FC | After FC | Total | Before FC | After FC | Cpc-PH | Ipc-PH | PAH |
| Clinical characteristics | ||||||||||||
| Patients, no. | 76 | 41 | 165 | 364 | 1,456 | 593 | ||||||
| Age, mean, years | 58 ± 11 | 65 ± 10 | 51 ± 14 | 56 ± 14 | 62 ± 14 | 55 ± 15 | ||||||
| Sex, % female | 72 | 95 | 83 | 49 | 43 | 67 | ||||||
| Hemodynamic characteristics | ||||||||||||
| Heart rate, beats/min | 76 ± 15 | 74 ± 12 | 74 ± 12 | 73 ± 11 | 77 ± 15 | 76 ± 14 | 81 ± 15 | 77 ± 16 | 78 ± 14 | |||
| Mean arterial pressure, mmHg | 97 ± 14 | 98 ± 14 | 98 ± 13 | 97 ± 14 | 94 ± 14 | 93 ± 14 | 96 ± 18 | 92 ± 26 | 94 ± 19 | |||
| Right atrial pressure, mmHg | 10 ± 5 | 13 ± 6 | 11 ± 6 | 15 ± 7 | 8 ± 5 | 10 ± 5 | 14 ± 7 | 12 ± 6 | 8 ± 5 | |||
| Systolic PAP, mmHg | 73 ± 21 | 78 ± 20 | 56 ± 19 | 62 ± 18 | 81 ± 20 | 81 ± 19 | 69 ± 20 | 53 ± 13 | 71 ± 23 | |||
| Diastolic PAP, mmHg | 31 ± 19 | 35 ± 7 | 23 ± 7 | 26 ± 5 | 34 ± 10 | 34 ± 9 | 34 ± 8 | 23 ± 6 | 28 ± 10 | |||
| Pulmonary artery pulse pressure, mmHg | 42 ± 15 | 43 ± 15 | 34 ± 17 | 37 ± 18 | 47 ± 13 | 47 ± 14 | 35 ± 16 | 29 ± 11 | 43 ± 16 | |||
| Mean PAP, mmHg | 45 ± 13 | 50 ± 11 | 34 ± 10 | 38 ± 7 | 50 ± 13 | 50 ± 12 | 47 ± 11 | 35 ± 8 | 44 ± 14 | |||
| PWP, mmHg | 15 ± 5 | 20 ± 4 | 17 ± 6 | 24 ± 5 | 9 ± 3 | 11 ± 3 | 22 ± 5 | 24 ± 6 | 10 ± 4 | |||
| TPG, mmHg | 31 ± 12 | 30 ± 10 | 17 ± 9 | 13 ± 6 | 41 ± 12 | 39 ± 12 | 26 ± 10 | 12 ± 5 | 35 ± 15 | |||
| DPG, mmHg | 16 ± 9 | 15 ± 6 | 5 ± 6 | 1 ± 3 | 25 ± 10 | 24 ± 10 | 13 ± 6 | −1 ± 5 | 19 ± 11 | |||
| SVR, Wood units | 17.2 ± 6.2 | 15.5 ± 4.2 | 16.8 ± 5.5 | 16.2 ± 6.7 | 21.2 ± 7.3 | 18.9 ± 6.6 | 17.8 ± 6.6 | 17.1 ± 6.2 | 19.8 ± 6.8 | |||
| Pulmonary artery saturation, % | 67 ± 7 | 68 ± 9 | 70 ± 6 | 73 ± 10 | 65 ± 9 | 68 ± 7 | 61 ± 10 | 63 ± 10 | 64 ± 9 | |||
| Stroke volume, mL | 74 ± 27 | 77 ± 27 | 76 ± 22 | 81 ± 22 | 60 ± 24 | 67 ± 25 | 65 ± 27 | 74 ± 32 | 65 ± 28 | |||
| Fick cardiac output, L/min | 5.5 ± 1.6 | 5.6 ± 1.5 | 5.6 ± 1.8 | 5.7 ± 1.8 | 4.4 ± 1.4 | 4.9 ± 1.6 | 5.1 ± 1.9 | 5.4 ± 2.0 | 4.8 ± 1.7 | |||
| Cardiac index, L/min/m2 | 2.8 ± 0.8 | 2.9 ± 0.7 | 3.0 ± 0.9 | 3.1 ± 0.9 | 2.4 ± 0.7 | 2.7 ± 0.9 | 2.6 ± 0.9 | 2.8 ± 1.0 | 2.5 ± 0.9 | |||
| PVR, Wood units | 6.0 ± 3.4 | 5.8 ± 2.8 | 3.4 ± 2.3 | 2.7 ± 1.7 | 10.4 ± 5.3 | 8.8 ± 4.3 | 5.7 ± 3.1 | 2.6 ± 1.6 | 8.4 ± 5.0 | |||
| RVSWI, g/m2/beat | 14.2 ± 7.3 | 12.5 ± 9.2 | 7.8 ± 4.1 | 7.1 ± 2.5 | 20.6 ± 10.9 | 17.3 ± 8.6 | 15.6 ± 8.3 | 9.9 ± 5.6 | 17.5 ± 11.2 | |||
| PVC, mL/mmHg | 2.0 ± 1.2 | 2.1 ± 1.1 | 3.0 ± 2.2 | 3.2 ± 3.5 | 1.4 ± 0.7 | 1.6 ± 0.9 | 2.4 ± 1.9 | 2.9 ± 1.9 | 1.8 ± 1.2 | |||
| RC time, seconds | 0.58 ± 0.19 | 0.57 ± 0.16 | 0.43 ± 0.18 | 0.34 ± 0.14 | 0.70 ± 0.17 | 0.69 ± 0.17 | 0.65 ± 0.35 | 0.38 ± 0.24 | 0.70 ± 0.34 | |||
Data are mean ± SD unless otherwise indicated. Cpc-PH: combined postcapillary and precapillary pulmonary hypertension; DPG: diastolic pulmonary artery pressure–to–pulmonary wedge pressure gradient; FC: fluid challenge; Ipc-PH: isolated postcapillary pulmonary hypertension; PAH: pulmonary arterial hypertension; PAP: pulmonary arterial pressure; PH: pulmonary hypertension; PVC: pulmonary vascular compliance; PVR: pulmonary vascular resistance; PWP: pulmonary wedge pressure; RC time: resistance-compliance time; RVSWI: right ventricular stroke work index; SVR: systemic vascular resistance; TPG: transpulmonary gradient.
Figure 1.
Resistance-compliance (RC) time remains fixed in combined postcapillary and precapillary pulmonary hypertension (CpcPH) and pulmonary arterial hypertension (PAH) after fluid challenge in cohort A. In cohort A, baseline RC times are different between PAH, CpcPH, and isolated postcapillary pulmonary hypertension (IpcPH; all P < 0.0001). After fluid challenge, RC time remains fixed in patients with PAH or CpcPH (P > 0.05) but is significantly reduced in patients with IpcPH (P < 0.0005). Data presented as a Tukey box plot.
The inverse-regression curves of PVC versus PVR for Cpc-PH, PAH, and Ipc-PH were not significantly different at baseline (Fig. 2A). After fluid challenge, however, the regression curves for patients with PAH and Cpc-PH were mostly unchanged, while the curve for Ipc-PH shifted down and to the left, such that the Ipc-PH curve never crossed the curves for PAH and Cpc-PH (Fig. 2B, 2C). These data indicate that in patients with Ipc-PH, a given PVR is coupled with a PVC that is significantly lower than that in a corresponding patient with PAH and Cpc-PH after a volume load.
Figure 2.
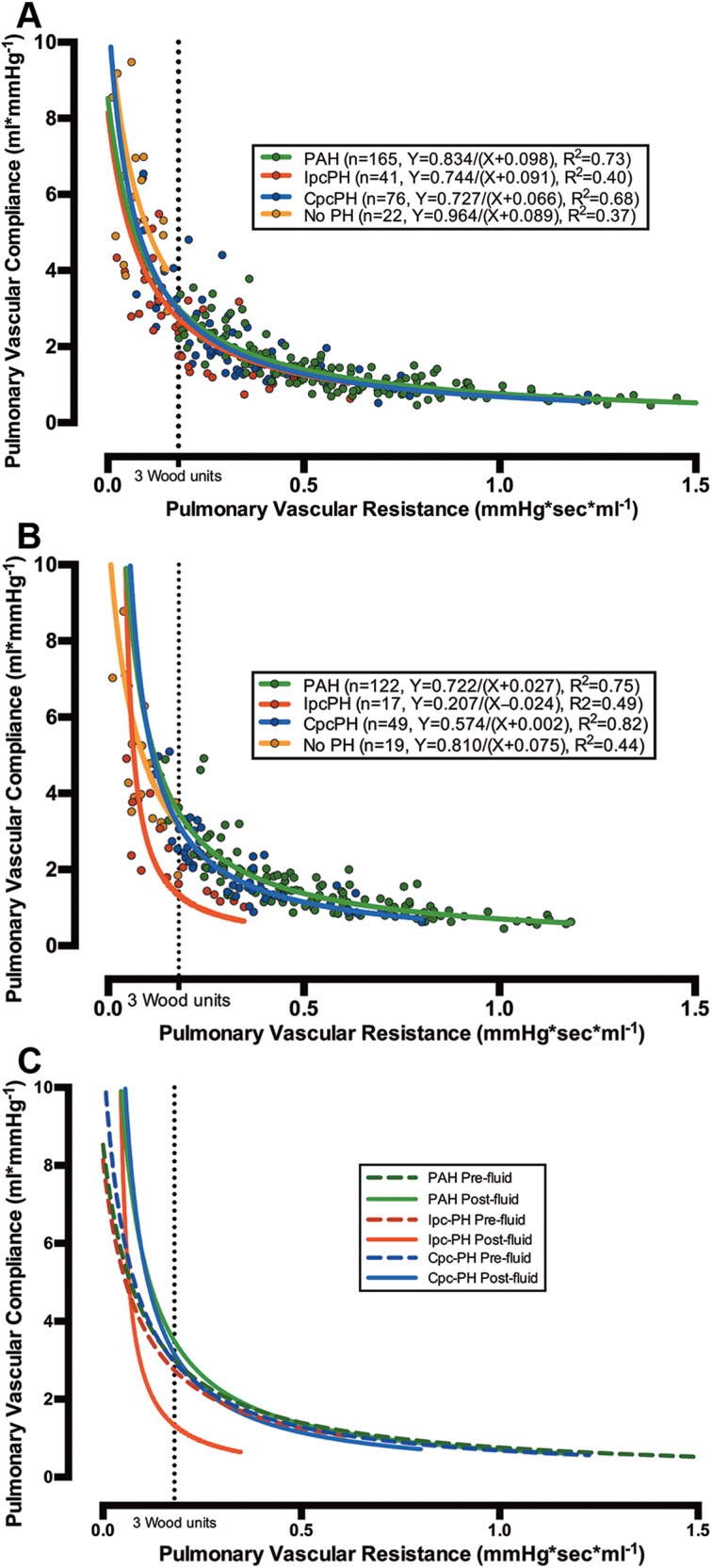
Relationship of pulmonary vascular resistance (PVR) and compliance (PVC) in combined postcapillary and precapillary pulmonary hypertension (CpcPH) and pulmonary arterial hypertension (PAH), as distinct from isolated postcapillary pulmonary hypertension (IpcPH), following fluid challenge in cohort A. The X-Y scatter plots of PVC versus PVR for patients in cohort A at rest (303 patients; A) and after fluid challenge (207 patients; B) are shown (colored circles). The data were modeled with a nonlinear inverse regression with offset (bold lines in all plots). The curve for patients with IpcPH was displaced to the left after fluid challenge and did not change for patients with PAH and CpcPH (B, C). The dotted line represents 3 Wood units.
Comparison of manual and computer-generated mean PWP
We randomly selected 116 patients from cohort A (49 with PAH, 22 with Cpc-PH, 23 with Ipc-PH, and 22 without PH) and compared the manual and computer-generated PWPs at baseline and after fluid challenge, using Bland-Altman analysis. The findings demonstrated adequate agreement in all groups of patients (Fig. S2), with a slight underestimation of the computer-generated PWP relative to the manual review at baseline (−2.1 ± 2.6 mmHg) and after fluid challenge (−2.7 ± 3.3 mmHg).
Cohort B
After all exclusions, we identified 1,969 patients without PH (Fig. S1; Table S2) and 2,817 patients with PH in cohort B: 593 (21%) with PAH (the “true PAH” cohort, as defined in “Patient populations” above), 1,456 (52%) with Ipc-PH, and 364 (13%) with Cpc-PH. RC time in PAH was higher than that in Cpc-PH (P < 0.004), both of which were higher than that in Ipc-PH (both P < 0.0001; Table 2). The inverse-regression curves of PVC versus PVR were identical in PAH and Cpc-PH, while the curve for Ipc-PH was consistently shifted to the left (Fig. 3A). The curve for patients without PH was positioned above the Ipc-PH curve, such that their PVC was nearly 1.5 times that for Ipc-PH patients at every PVR. The curve for patients without PH was positioned above the PAH and Cpc-PH curves at a PVR of >2 Wood units.
Figure 3.
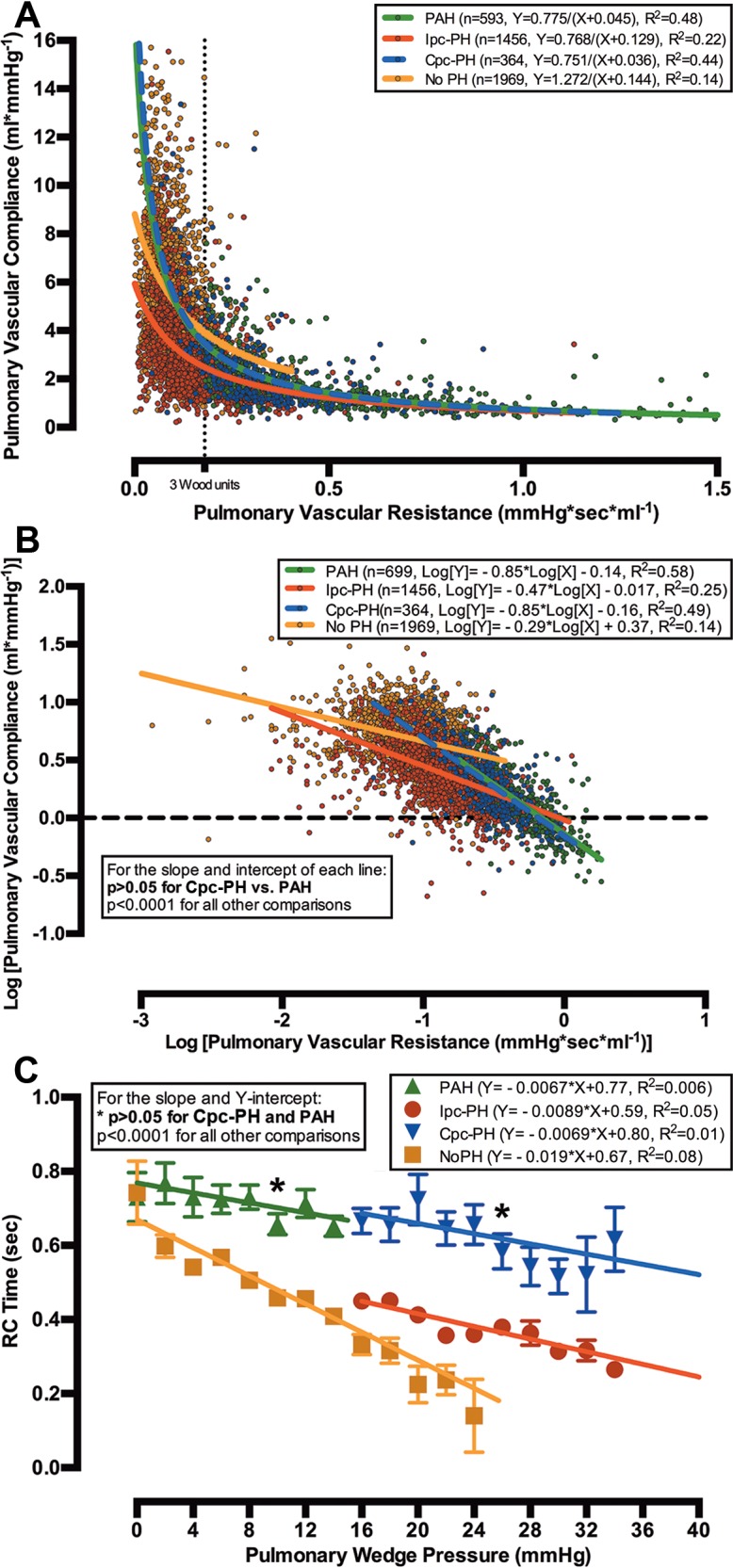
Relationships of pulmonary vascular resistance (PVR) and compliance (PVC) and of resistance-compliance (RC) time and pulmonary wedge pressure (PWP) in combined postcapillary and precapillary pulmonary hypertension (Cpc-PH) and pulmonary arterial hypertension (PAH) are similar in cohort B. The X-Y scatter plot of PVC versus PVR for all 4,382 patients in cohort B (colored circles) is modeled with a nonlinear inverse regression with offset (bold lines; A). When PVR and PVC were logarithmically transformed (colored circles), the slope and Y-intercept of the linear regression (bold lines) for patients with Cpc-PH and PAH were similar (P > 0.05), and they were different from those of patients with isolated postcapillary pulmonary hypertension (Ipc-PH) or without pulmonary hypertension (No PH; B). The relationship of RC time to PWP for Cpc-PH was identical to that for PAH (P > 0.05) and distinct from that for Ipc-PH at every PWP (P < 0.0001; C).
To compare the RC relationship for each group, the PVR and PVC data were logarithmically transformed and plotted on a linear axis (Fig. 3B). The slope of the linear regression for PAH was identical to that for Cpc-PH (P > 0.05), and both were significantly different from the Ipc-PH and no-PH regressions (all P < 0.0001). The slope for the PAH and Cpc-PH regressions approximated −1, indicating that PVR and PVC are tightly and inversely coupled in both groups of patients, such that a change in PVR is matched by a proportional, but counterdirectional, change in PVC. The slopes for the Ipc-PH and no-PH groups were less than −0.5, indicating a PVR-PVC relationship that is less interdependent. In addition, the Y-intercepts for Cpc-PH and PAH were similar and significantly higher than that for Ipc-PH (P < 0.0001), demonstrating that the PVCs in Cpc-PH and PAH would be higher than that for Ipc-PH at a PVR that approximates 0.
To quantify the effect of increased left-sided filling pressures on RC time, we analyzed the relationship of PWP and RC time in each group of patients (Fig. 3C). After plotting RC time versus PWP, we found that the slope of the linear regression was negative in all categories, although the slope did not significantly differ from 0 in the Cpc-PH and PAH groups (both P > 0.05). This indicates that elevations in PWP are associated with a significant reduction in RC time only in patients with Ipc-PH or no PH. Finally, patients with Cpc-PH or PAH had similar Y-intercepts for their linear regression, and both intercepts were significantly higher than that for Ipc-PH (both P < 0.0001). The Y-intercept represents a hypothetical condition where PWP is 0, demonstrating that Cpc-PH and PAH patients would have an identical RC time under theoretical circumstances where their PWPs were the same.
Discussion
We have demonstrated that Cpc-PH has hemodynamic properties that resemble those of PAH more than those of Ipc-PH. By rapidly increasing PWP in individual patients with a fluid bolus, we observed that RC time is not fixed in all patients: RC time was lowered in Ipc-PH patients, while it remained unchanged in Cpc-PH and PAH patients. Furthermore, the RC time and the PVC-PVR relationship in Cpc-PH were similar to those in PAH and distinct from those in Ipc-PH, after baseline differences in PWP were accounted for. These findings suggest that Cpc-PH is a distinct pulmonary vascular phenotype, and further study is warranted to identify the molecular etiology and therapeutic targets for this disease.
Several reports have previously investigated the hemodynamic profile of Cpc-PH, often defined by an elevated TPG or PVR.21,31-35 As the TPG is influenced by changes in PWP and pulmonary blood flow, it may be less accurate than the DPG as a measure of pulmonary vascular remodeling.5,36,37 Furthermore, patients with a DPG of ≥7 mmHg have histologic evidence of vascular remodeling.35 While the European Respiratory Society and the European Society of Cardiology have recommended the use of a DPG of ≥7 mmHg and/or PVR of >3 Wood units for the diagnosis of Cpc-PH in their updated 2015 guidelines,38 we chose to define Cpc-PH by an elevated DPG alone, according to consensus international guidelines.9 However, the vast majority of patients with Cpc-PH in our cohort also had a PVR of >3 Wood units, as compared to a minority of patients with Ipc-PH (85% and 32%, respectively).
We utilized RC time, the product of PVR and PVC, to infer the hemodynamic properties of each PH phenotype. RC time, which represents the diastolic pulmonary artery pressure decay in seconds,13,14 was initially considered a universal constant in the pulmonary circulation.14-16 However, several recent publications have demonstrated otherwise.17,18,21,39 While RC time remains fixed after ineffective PH therapy,16 it decreases significantly after inhaled nitric oxide in vasodilator-responsive PAH39 and after pulmonary thromboendarterectomy in proximal chronic thromboembolic PH.17 Moreover, Tedford et al.19 demonstrated a lower RC time and altered RC plots in patients with an elevated PWP, compared to those in patients with a normal PWP, and Gerges et al.21 noted a further distinction in the RC time and RC plots of patients with Cpc-PH and Ipc-PH. Changes in an individual patient’s RC time after a volume load in a large and diverse PH cohort have not been described previously.
We first sought to use changes in right ventricular loading conditions after a fluid challenge to distinguish different hemodynamic phenotypes in PH. In cohort A, the fluid challenge had no effect on the RC time in patients with Cpc-PH or PAH. In contrast, patients with Ipc-PH had a lower RC time and an RC plot that was displaced toward the left, such that PVC was lower for a given PVR. This discrepant response to a fluid challenge may be explained by differences between the pulmonary vascular properties of PAH and Cpc-PH and those of Ipc-PH. In PAH, PWP, stroke volume, and cardiac output increased without changes in pulse pressure. PVC increased and PVR decreased, suggesting that the volume load resulted in augmented cardiac contractility and pulmonary vascular recruitment. While patients with Cpc-PH had no improvement in their stroke volume or cardiac output, their PVC and PVR remained stable, possibly because their pulmonary capillary bed was maximally recruited at baseline. In both conditions, PVR and PVC remained tightly coupled, and RC time did not change. In Ipc-PH, a proportional increase in stroke volume and pulse pressure was observed, along with a reduction in the TPG. The increased pulmonary blood flow did not alter cardiac contractility or PVC, although the reduced PVR suggests increased vascular distensibility. The reduced PVR and stable PVC led to an overall reduction in RC time.
The large numbers of patients in cohort B with and without PH confirmed that all four groups of patients had different baseline RC times. The RC times in Cpc-PH and PAH were nearly the same, and both were almost twice as high as that in Ipc-PH. However, the nearly 2,000 patients without PH demonstrated that a “normal” RC time lies somewhere in between. After examining the components of RC time, we found that patients with PAH or Cpc-PH have an increased TPG (i.e., downstream gradient) relative to pulse pressure (i.e., upstream gradient), and the opposite is true in Ipc-PH. The location of these gradients suggests that PAH and Cpc-PH share pulmonary arterial remodeling, whereas Ipc-PH is due to passive venous congestion alone.
The RC plot for each group of patients in cohort B (Fig. 3A) demonstrates that patients with Ipc-PH have reduced PVC for a given PVR when compared to PAH and Cpc-PH patients, a relationship that persists until PVR is very elevated. In addition, the curve for patients without PH never converges with the Ipc-PH curve, indicating that patients with Ipc-PH have a systematic loss of PVC relative to patients without PH, as a consequence of venous distension. This is in contrast to Cpc-PH and PAH patients, as their PVC markedly improves once their PVR is lowered, a sign of pulmonary arterial disease. Increased PVC has been associated with improved mortality in patients with heart failure and PH,20,40-42 and these data suggest that reducing PVR may increase PVC more effectively in patients with Cpc-PH and PAH than in patients with Ipc-PH.
Finally, cohort B elucidated the effect of PWP on RC time, revealing that RC time is higher in patients with Cpc-PH than in those with Ipc-PH and higher in those with PAH than in those with no PH, at a given PWP. Whereas increased PWP was associated with significant reductions of RC time in patients without PH and in those with Ipc-PH, it did not significantly alter RC time in Cpc-PH and PAH patients. Finally, the linear regressions of RC time versus PWP were identical in PAH and Cpc-PH, indicating that RC time would be the same for Cpc-PH and PAH under theoretical conditions when PWP was the same in each group. These findings corroborate our findings from cohort A, suggesting the presence of pulmonary arterial remodeling, and not passive venous congestion, in Cpc-PH patients.
There are a number of limitations to our study. While RC time differs in large groups of patients with and without PH, the clinical significance of this remains unclear for individual patients. RC time is a simplified calculation derived from two other calculated variables, limited by the imprecision of its measured components and the assumptions of their relationship. The PVR calculation does not consider the viscosity of blood, recruitment and distensibility of blood vessels, or pulsatility of flow,43,44 while the PVC calculation has not been validated in large cohorts and may be unreliable in patients without pulmonary vascular disease.14 Importantly, the empiric RC time calculation fails to consider the exponential decay of the pulmonary arterial diastolic pressure.45 Therefore, RC time must be considered an incomplete measure of right ventricular afterload,46-48 with considerable variability and scatter even in hemodynamically similar patients.45 We have attempted to address these issues by the large number of patients in cohort B, but the implications of a given RC time for an individual patient are unknown at present.
Although our cohort sizes are large, both represent data from a single center. Referral bias may be present in cohort A, which includes only patients seen at the Vanderbilt Pulmonary Vascular Center who underwent a fluid challenge. While we were unable to manually review RHC tracings from cohort B, there was little difference between manual and computer-generated mean PWPs on a population level (Fig. S2). Furthermore, as we did not directly measure left ventricular end-diastolic pressure in the majority of patients in our study, the presence of precapillary PH may be overestimated in our cohort.49 Finally, it is possible that patients with mild forms of WHO group 3–5 PH are present in cohort B. Given the size of the groups and the limitations of EMR-based cohorts, chart review for each patient is not possible.
We have shown that patients with Cpc-PH have hemodynamic characteristics that resemble those of patients with PAH more than those of Ipc-PH patients. The presence of a fixed RC time in Cpc-PH (but not in Ipc-PH) after a fluid challenge is a novel finding, indicating that pulmonary vascular remodeling may affect RC time more than venous congestion does. While further study is needed to identify the underlying pathophysiology and molecular mechanisms of Cpc-PH, our study suggests that trials of therapies that target the pulmonary arteries may be warranted in the Cpc-PH population.
Appendix.
Figure S1.
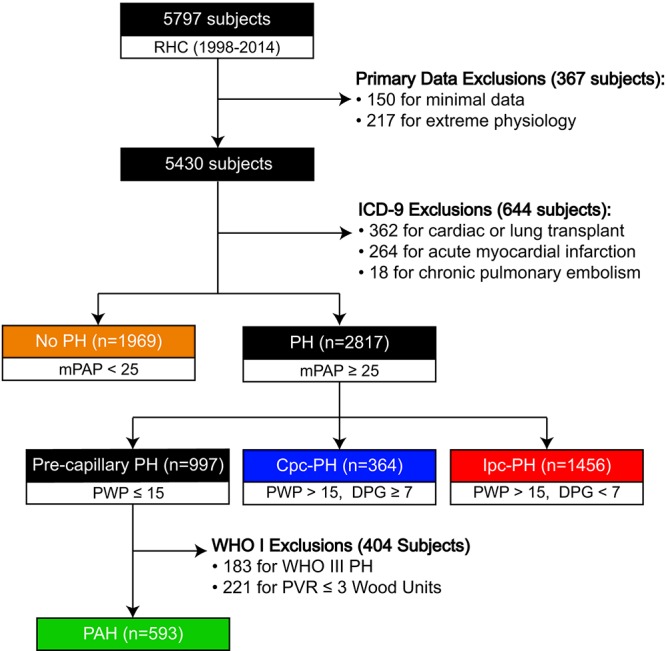
Flow diagram for cohort B. This schematic represents the initial data set and subsequent exclusions that led to our categorization of cohort B. The disease classification is based on the 2013 Nice World Health Symposium on Pulmonary Hypertension. All units are mmHg, unless stated otherwise. Cpc-PH: combined postcapillary and precapillary pulmonary hypertension; DPG: diastolic pulmonary artery–to–pulmonary wedge pressure gradient; ICD-9: International Classification of Diseases, Ninth Revision; Ipc-PH: isolated postcapillary pulmonary hypertension; mPAP: mean pulmonary artery pressure; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; PWP: pulmonary wedge pressure; RHC: right heart catheterization; WHO III PH: World Health Organization group III–V PH.
Figure S2.
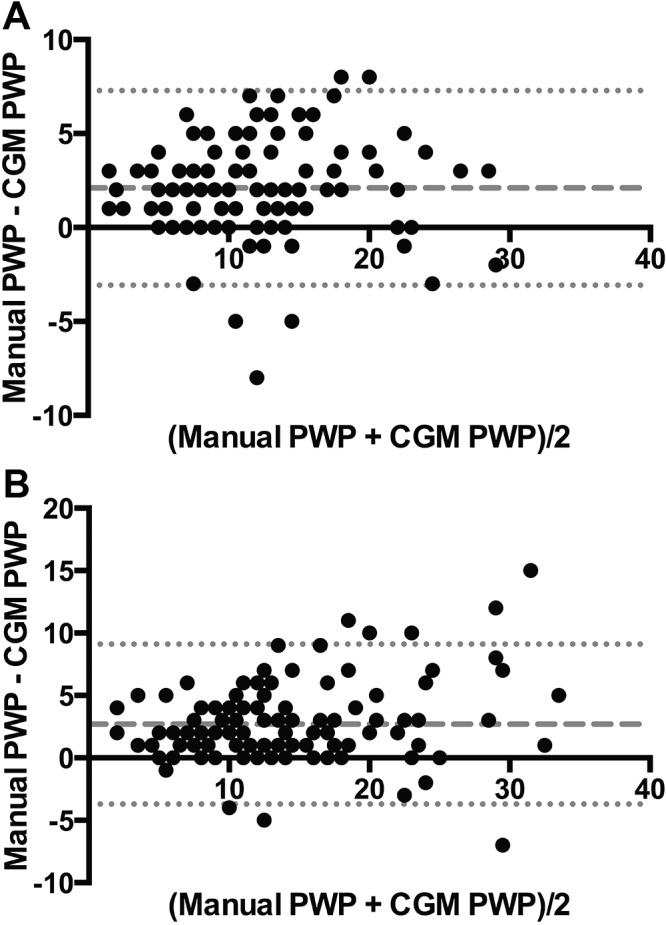
Different methods of estimating pulmonary wedge pressure (PWP) yield similar results. With Bland-Altman analysis, the manual and computer-generated mean (CGM) PWPs from 116 patients in cohort A demonstrated excellent agreement, with a slight underestimation of the CGM relative to the manual at baseline (−2.1 ± 2.6 mmHg; A) and after fluid challenge (−2.7 ± 3.3 mmHg; B).
Table S1.
Exclusion and imputation thresholds for cohort B
| Removed and imputed value thresholds | Patient exclusion thresholds | Missing values | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Low | No. | High | No. | Low | No. | High | No. | No. | Imputed, % |
| Heart rate, beats/min | <30 | 25 | >170 | 1 | <40 | 26 | >120 | 67 | 2,172 | 40.00 |
| Systolic BP, mmHga | <50 | 19 | >255 | 2 | <70 | 18 | >210 | 1 | 507 | 9.34 |
| Diastolic BP, mmHga | <30 | 35 | >145 | 0 | <36 | 16 | >120 | 0 | 584 | 10.76 |
| RA pressure, mmHgb | None | … | >42 | 2 | None | … | None | … | 424 | 7.81 |
| PA systolic pressure, mmHga | <10 | 6 | None | … | None | … | None | … | 528 | 9.72 |
| PA diastolic pressure, mmHga,b | <−2 | 0 | >80 | 3 | None | … | None | … | 524 | 9.65 |
| PA mean pressure, mmHga,b | <3 | 2 | None | … | None | … | None | … | 525 | 9.67 |
| Mean PWP, mmHg | None | … | >53 | 1 | None | … | None | … | 509 | 9.37 |
| CO, Fick, L/min | None | … | >13 by 1 method, <10 by the other | 53c | <1.2 | 0 | >13 by both methods | 3c | 991 | 18.25 |
| CO, TD, L/min | None | … | Same as for Fick | 53c | <1.2 | 2 | Same as for Fick | 3c | 910 | 16.76 |
| CI, Fick, L/min/m2 | None | … | >6.6 by 1 method, <5 by the other | 44c | <0.8 | 6 | >6.6 by both methods | 2c | 996 | 18.34 |
| CI, TD, L/min/m2 | None | … | Same as for Fick | 44c | <0.8 | 6 | Same as for Fick | 2c | 966 | 17.79 |
| SVR, dynesd | None | … | None | … | <285 | 11 | >3,476 | 7 | 3,529 | 64.99 |
| PVR, dynesd | 0 | 9 | None | … | None | … | >2,587 | 0 | 2,513 | 46.28 |
| SVR, Wood unitsd | None | … | None | … | <4 | 10 | >43 | 12 | 1,184 | 21.80 |
| PVR, Wood unitsd | ≤0 | 22 | >40 | 2 | None | … | >32 | 1 | 609 | 11.22 |
| Aortic sat, % | <70 | 11 | >100 | 7 | None | … | None | … | 1,495 | 27.53 |
| RA sat, % | <22 | 6 | None | … | None | … | None | … | 2,485 | 45.76 |
| PA sat, % | <23 | 5 | None | … | None | … | >96 | 3 | 816 | 15.03 |
| LV diastolic pressure, mmHg | None | … | >50 | 7 | None | … | >39 | 16 | 2,646 | 48.73 |
BP: blood pressure; CO: cardiac output; LV: left ventricular; PA: pulmonary artery; PVR: pulmonary vascular resistance; PWP: pulmonary wedge pressure; RA: right atrial; sat: saturation; SVR: systemic vascular resistance; TD: thermodilution.
For pressures with a systolic and a diastolic component, the systolic pressure was required to be greater than the mean value (if present), and the mean value (or systolic value, if no mean) was required to be greater than the diastolic value. If these criteria were not met, then both values were deleted and imputed (BP: 4 patients; right ventricular pressure: 7; PA pressure: 6). If right ventricular systolic pressure was greater than PA systolic pressure by >30 mmHg, the patient was considered to have a left-to-right shunt. Of these, 4 patients were imputed, given confounding results, and 10 patients were excluded as true positives (right ventricular data not shown).
Negative values were converted to 0 for RA pressure (22 patients), PA diastolic pressure (2), and mean PWP (1).
Total number of patients affected by CO or CI high threshold.
For resistance measurements in dynes and Wood units, if the number of dynes was not greater than the number of Wood units, both values were deleted and imputed (SVR: 4 patients; PVR: 2).
Table S2.
Clinical and hemodynamic characteristics of patients with no PH in cohorts A and B
| Cohort A | ||||
|---|---|---|---|---|
| Category | Total | Before fluid challenge | After fluid challenge | Cohort B |
| Clinical characteristics | ||||
| Patients, no. | 28 | 1,969 | ||
| Age, mean, years | 51 ± 15 | 60 ± 15 | ||
| Sex, % female | 89 | 47 | ||
| Hemodynamic characteristics | ||||
| Heart rate, beats/min | 77 ± 15 | 76 ± 14 | 73 ± 15 | |
| Mean arterial pressure, mmHg | 95 ± 11 | 96 ± 14 | 89 ± 22 | |
| Right atrial pressure, mmHg | 5 ± 4 | 7 ± 4 | 5 ± 4 | |
| Systolic PAP, mmHg | 29 ± 10 | 36 ± 9 | 28 ± 7 | |
| Diastolic PAP, mmHg | 12 ± 4 | 15 ± 6 | 10 ± 5 | |
| Pulmonary artery pulse pressure, mmHg | 17 ± 7 | 20 ± 6 | 18 ± 6 | |
| Mean PAP, mmHg | 17 ± 6 | 23 ± 6 | 18 ± 4 | |
| PWP, mmHg | 8 ± 3 | 12 ± 4 | 9 ± 4 | |
| TPG, mmHg | 9 ± 5 | 11 ± 6 | 8 ± 3 | |
| DPG, mmHg | 3 ± 4 | 4 ± 5 | 1 ± 4 | |
| SVR, Wood units | 14.1 ± 4.8 | 13.7 ± 4.4 | 16.6 ± 5.4 | |
| Pulmonary artery saturation, % | 75 ± 8 | 75 ± 9 | 72 ± 8 | |
| Stroke volume, mL | 93 ± 33 | 99 ± 40 | 86 ± 31 | |
| Cardiac output, L/min | 7.0 ± 1.9 | 7.1 ± 1.8 | 6.0 ± 1.9 | |
| Cardiac index, L/min/m2 | 3.6 ± 0.9 | 3.8 ± 1.0 | 3.2 ± 1.0 | |
| PVR, Wood units | 1.4 ± 1.8 | 1.6 ± 0.9 | 1.7 ± 0.9 | |
| RVSWI, g/m2/beat | 3.8 ± 1.7 | 4.5 ± 2.2 | 4.5 ± 2.3 | |
| PVC, mL/mmHg | 7.0 ± 6.2 | 5.2 ± 2.5 | 5.4 ± 2.9 | |
| RC time, seconds | 0.46 ± 0.34 | 0.44 ± 0.18 | 0.49 ± 0.29 | |
DPG: diastolic pulmonary artery pressure–to–pulmonary wedge pressure gradient; PAP: pulmonary arterial pressure; PH: pulmonary hypertension; PVC: pulmonary vascular compliance; PVR: pulmonary vascular resistance; PWP: pulmonary wedge pressure; RC time: resistance-compliance time; RVSWI: right ventricular stroke work index; SVR: systemic vascular resistance; TPG: transpulmonary gradient.
Source of Support: National Institutes of Health project U01 HL125212-01.
Conflict of Interest: None declared.
Supplements
Appendix (676.3KB, pdf)
References
- 1.Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, Fong PP, Newman JH. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014;7(1):116–122. [DOI] [PMC free article] [PubMed]
- 2.Fox BD, Shimony A, Langleben D, Hirsch A, Rudski L, Schlesinger R, Eisenberg MJ, et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J 2013;42(4):1083–1091. [DOI] [PubMed]
- 3.Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, Carrick-Ranson G, Levine BD. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation 2013;127(1):55–62. [DOI] [PMC free article] [PubMed]
- 4.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail 2015;8(1):41–48. [DOI] [PubMed]
- 5.Naeije R, Vachiéry JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013;41(1):217–223. [DOI] [PubMed]
- 6.Torres-Macho J, Delgado-Jiménez JF, Sanz-Salvo J, González-Mansilla A, Sánchez-Sánchez V, Gámez-Díez S, Escribano-Subías P, Sáenz de la Calzada C. Predictors of pulmonary hypertension in patients with end-stage heart failure. Congest Heart Fail 2012;18(4):212–216. [DOI] [PubMed]
- 7.Ohara T, Ohte N, Little WC. Pulmonary hypertension in heart failure with preserved left ventricular ejection fraction: diagnosis and management. Curr Opin Cardiol 2012;27(3):281–287. [DOI] [PubMed]
- 8.Delgado JF, Conde E, Sánchez V, López-Ríos F, Gómez-Sánchez MA, Escribano P, Sotelo T, Gómez de la Cámara A, Cortina J, Sáenz de la Calzada C. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 2005;7(6):1011–1016. [DOI] [PubMed]
- 9.Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol 2013;62(25 suppl):D100–D108. [DOI] [PubMed]
- 10.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173(9):1023–1030. [DOI] [PubMed]
- 11.McGoon MD, Krichman A, Farber HW, Barst RJ, Raskob GE, Liou TG, Miller DP, Feldkircher K, Giles S. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc 2008;83(8):923–931. [DOI] [PubMed]
- 12.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186(8):790–796. [DOI] [PubMed]
- 13.Reuben SR. Compliance of the human pulmonary arterial system in disease. Circ Res 1971;29(1):40–50. [DOI] [PubMed]
- 14.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol 2006;291(4):H1731–H1737. [DOI] [PubMed]
- 15.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof BE, Boonstra A, Postmus PE, Vonk-Noordegraaf A. RC time constant of single lung equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009;297(6):H2154–H2160. [DOI] [PubMed]
- 16.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J 2008;29(13):1688–1695. [DOI] [PubMed]
- 17.MacKenzie Ross RV, Toshner MR, Soon E, Naeije R, Pepke-Zaba J. Decreased time constant of the pulmonary circulation in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2013;305(2):H259–H264. [DOI] [PMC free article] [PubMed]
- 18.Hadinnapola C, Li Q, Su L, Pepke-Zaba J, Toshner M. The resistance-compliance product of the pulmonary circulation varies in health and pulmonary vascular disease. Physiol Rep 2015;3(4):e12363. doi:10.14814/phy2.12363. [DOI] [PMC free article] [PubMed]
- 19.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012;125(2):289–297. [DOI] [PMC free article] [PubMed]
- 20.Dragu R, Rispler S, Habib M, Sholy H, Hammerman H, Galiè N, Aronson D. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur J Heart Fail 2015;17(1):74–80. [DOI] [PubMed]
- 21.Gerges M, Gerges C, Pistritto A-M, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary hypertension in heart failure: epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 2015;192(10):1234–1246. [DOI] [PubMed]
- 22.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gómez Sánchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D34–D41. [DOI] [PubMed]
- 23.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, Masys DR. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 2008;84(3):362–369. [DOI] [PMC free article] [PubMed]
- 24.Pulley J, Clayton E, Bernard GR, Roden DM, Masys DR. Principles of human subjects protections applied in an opt-out, de-identified biobank. Clin Transl Sci 2010;3(1):42–48. [DOI] [PMC free article] [PubMed]
- 25.Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. Circulation 2016;133(13):1240–1248. [DOI] [PMC free article] [PubMed]
- 26.Brittain E, Chan S. Integration of complex data sources to provide biologic insight in pulmonary vascular disease (2015 Grover Conference Series). Pulm Circ 2016;6(3):251–260. [DOI] [PMC free article] [PubMed]
- 27.Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings: how to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med 2014;190(3):252–257. [DOI] [PubMed]
- 28.LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J 2014;44(2):425–434. [DOI] [PMC free article] [PubMed]
- 29.Boerrigter BG, Waxman AB, Westerhof N, Vonk-Noordegraaf A, Systrom DM. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J 2014;43(5):1316–1325. [DOI] [PubMed]
- 30.Ryan JJ, Rich JD, Thiruvoipati T, Swamy R, Kim GH, Rich S. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J 2012;163(4):589–594. [DOI] [PubMed]
- 31.Berger G, Hardak E, Obaid W, Shaham B, Carasso S, Kerner A, Yigla M, Azzam ZS. Characterization of pulmonary venous hypertension patients with reactive pulmonary hypertension as compared to proportional pulmonary hypertension. Respiration 2012;83(6):494–498. [DOI] [PubMed]
- 32.Adir Y, Humbert M, Sitbon O, Wolf R, Lador F, Jaïs X, Simonneau G, Amir O. Out-of-proportion pulmonary hypertension and heart failure with preserved ejection fraction. Respiration 2013;85(6):471–477. [DOI] [PubMed]
- 33.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail 2013;1(4):290–299. [DOI] [PubMed]
- 34.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, Rich S. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011;4(3):257–265. [DOI] [PubMed]
- 35.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, Maurer G, Lang IM. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013;143(3):758–766. [DOI] [PubMed]
- 36.Harvey RM, Enson Y, Ferrer MI. A reconsideration of the origins of pulmonary hypertension. Chest 1971;59(1):82–94. [DOI] [PubMed]
- 37.Naeije R. Measurement to predict survival: the case of diastolic pulmonary gradient. JACC Heart Fail 2015;3(5):425. [DOI] [PubMed]
- 38.Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46(4):903–975. [DOI] [PubMed]
- 39.Newman JH, Brittain EL, Robbins IM, Hemnes AR. Effect of acute arteriolar vasodilation on capacitance and resistance in pulmonary arterial hypertension. Chest 2015;147(4):1080–1085. [DOI] [PMC free article] [PubMed]
- 40.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 2014;145(5):1064–1070. [DOI] [PubMed]
- 41.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006;47(4):799–803. [DOI] [PubMed]
- 42.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail 2015;3(6):467–474. [DOI] [PMC free article] [PubMed]
- 43.Naeije R. Pulmonary vascular resistance. a meaningless variable? Intensive Care Med 2003;29(4):526–529. [DOI] [PubMed]
- 44.Naeije R. Physiology of the pulmonary circulation and the right heart. Curr Hypertens Rep 2013;15(6):623–631. [DOI] [PubMed]
- 45.Chemla D, Lau EM, Papelier Y, Attal P, Hervé P. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur Respir J 2015;46(4):1178–1189. [DOI] [PubMed]
- 46.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev 2010;19(117):197–203. [DOI] [PMC free article] [PubMed]
- 47.Castelain V, Hervé P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol 2001;37(4):1085–1092. [DOI] [PubMed]
- 48.Tedford RJ. Determinants of right ventricular afterload (2013 Grover Conference series). Pulm Circ 2014;4(2):211–219. [DOI] [PMC free article] [PubMed]
- 49.Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest 2009;136(1):37–43. [DOI] [PubMed]