Abstract Abstract
Total right heart function requires normal function of both the right ventricle and the right atrium. However, the degree to which right atrial (RA) function and right ventricular (RV) function each contribute to total right heart function has not been quantified. In this study, we aimed to quantify the contribution of RA function to total right heart function in a group of pulmonary arterial hypertension (PAH) patients compared to a cohort of normal controls without cardiovascular disease. The normal cohort comprised 35 subjects with normal clinical echocardiograms, while the PAH cohort included 37 patients, of whom 31 had echocardiograms before and after initiation of PAH-specific therapy. Total right heart function was measured via tricuspid annular plane excursion (TAPSE). TAPSE was broken down into two components, the excursion occurring during RA contraction (TAPSERA) and that occurring before RA contraction (TAPSERV). RA fractional area change (RA-FAC) was also compared between the two groups. In the PAH cohort, more than half of the total TAPSE occurred during atrial systole, compared to less than one-third in the normal cohort (51.0% vs. 32.1%; P < 0.0001). There was a significant correlation between RA-FAC and TAPSE in the PAH cohort but not in the normal cohort. TAPSE improved significantly in the posttreatment cohort (1.7 vs. 2.1 cm), but TAPSERA continued to account for about half of the total TAPSE after treatment. RA function accounts for a significantly greater proportion of total right heart function in patients with PAH than in normal subjects.
Keywords: echocardiography, tricuspid annular plane systolic excursion, right ventricular failure
Pulmonary arterial hypertension (PAH) is characterized by marked increases in right ventricular (RV) afterload, which if left untreated leads to progressive RV dysfunction, exercise intolerance, and potentially death.1-3 As recently emphasized by the International Right Heart Foundation Working Group, the right ventricle (also RV) is only one part of the total right heart system, and the other components, including the right atrium (RA), contribute importantly to total right heart function.4
It has been recognized that in the setting of RV failure, preserved right atrial (also RA) function is essential to maintain adequate total right heart function. After RV infarction, for example, preserved RA function has been shown to improve RV filling and performance, whereas diminished RA function compromises cardiac output.5,6 Loss of atrial function, in the form of supraventricular arrhythmias with resulting atrioventricular dyssynchrony, is also known to be poorly tolerated among patients with PAH and chronic thromboembolic pulmonary hypertension (CTEPH).7,8 Moreover, animal models have shown that RA systolic function is, in fact, augmented in the setting of RV ischemia and that if there is a subsequent ischemic insult to the RA, RV function is significantly reduced.7 Taken together, these studies suggest that RA function must not only be maintained but at times augmented, in order to optimize total right heart function in the setting of RV compromise.
Given the importance of right heart function in patients with PAH, we sought to determine the relative contributions of both RA systolic function and RV systolic function to total right heart function in a cohort of patients with PAH and a cohort of normal subjects. We also studied how PAH-specific therapy affected the RA and RV components of right heart function.
Methods
Subject selection
Normal subjects
All clinically indicated echocardiograms obtained at the Hospital of the University of Pennsylvania over a 2-month period in 2006 were screened. Those deemed normal by the clinical reviewer were selected and analyzed retrospectively by the research investigator. Inclusion criteria were designed to capture normal adult echocardiograms and have been described by our group.9
PAH patients
Patients with World Health Organization group I pulmonary hypertension (PH), PAH, were identified through the Pulmonary Hypertension Clinic at the Hospital of the University of Pennsylvania. The diagnosis of PAH was made in the standard manner, with invasive hemodynamic evidence of PAH in the absence of chronic left-sided heart disease, valvular disease, respiratory disease, or prior pulmonary emboli.1 Those patients aged 18 years or older with clinically indicated echocardiograms obtained before the initiation of PAH-specific therapy were included. The post–PAH-specific therapy analysis was performed on a subset of these patients who were followed at the University of Pennsylvania after initiation of therapy. Patients with abnormal left ventricular (LV) function or whose images were technically inadequate were excluded. Patients in our PAH clinic were reviewed over the time period of January 2007–February 2012. Our research protocol was reviewed and approved by the University of Pennsylvania’s institutional review board (protocol 812254).
Echocardiographic techniques
All studies were digitized and analyzed with the use of a commercially available off-line quantification system (Kinet Dx WS3000, ver. 4.2.0, Siemens Medical Solutions USA, Mountain View, CA; and Prosolv Cardiovascular, ver. 4.0.1, Fuji Film USA, Indianapolis). Atrial and ventricular size, valvular anatomy and function, and LV systolic and diastolic function were measured in the standard fashion.10 Doppler-estimated stroke volume was calculated in both cohorts from the velocity time integral (VTI) of flow through the LV outflow tract (LVOT) and the diameter of the LVOT, with the following standard formula: stroke volume Doppler = VTI LVOT × diameter of LVOT × 0.785.11
Right-sided heart quantitation
Tricuspid annular plane excursion (TAPSE) was measured in real time by passing an M-mode cursor through the lateral portion of the tricuspid annulus in the apical 4-chamber view over 2–3 cardiac cycles. Off-line, the brightness was adjusted to maximize the contrast between the M-mode signal arising from the tricuspid annulus and the background. TAPSE was measured with digital calipers as the total displacement (base to apex) of the tricuspid valve annulus from end-diastole to end-systole, with values representing the average of 2–3 measurements.
To evaluate the contribution of RA systole to total right heart function, we examined the longitudinal motion of the right heart at the level of the tricuspid valve annulus via M-mode. As shown in Figure 1, there are two distinct phases of right heart motion. TAPSERA (the proportion of TAPSE owed to RA contraction alone) and TAPSERV (the RV displacement occurring without the influence of RA contraction) were compared between the PAH and normal cohorts, as well as between the pre- and posttreatment groups.
Figure 1.
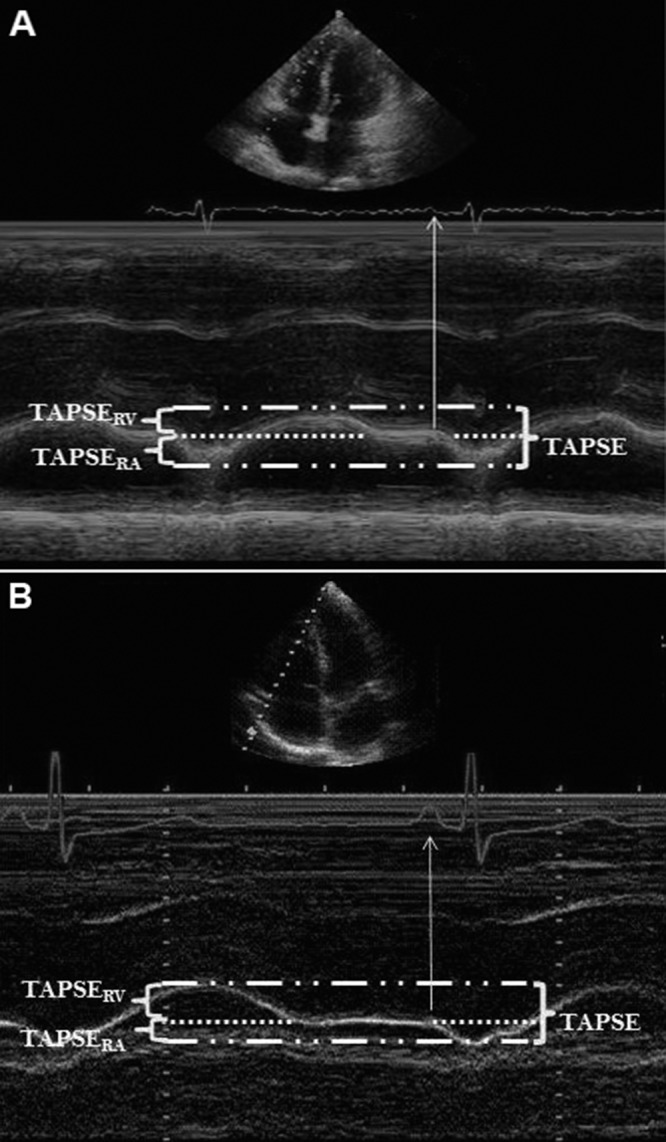
M-mode tracing of the tricuspid annulus in a pulmonary arterial hypertension patient (A) and a subject with a normal echocardiogram (B). TAPSERA accounts for the proportion of TAPSE (tricuspid annular plane excursion) owed to right atrial (RA) function alone; TAPSERV accounts for right ventricular (RV) shortening without the influence of RA contraction. Note the significantly larger contribution of TAPSERA to the total TAPSE in the PAH patient (A), compared to the normal subject (B).
RA area and RA volume measurements were performed at three points in the cardiac cycle: (1) end–ventricular systole, corresponding to early atrial diastole, was identified as the frame just before tricuspid valve opening; (2) mid-diastole, identified as the frame before the p-wave and atrial contraction; and (3) end–ventricular diastole, corresponding to end–atrial systole and identified as the point before the QRS complex and ventricular contraction. These three points in diastole were labeled RAmax (maximum RA volume in the cycle), RAp (RA volume before RA systole), and RAmin (minimum RA volume in the cycle), respectively, as described previously.12 RA area and volume measurements were made in the apical 4-chamber view with digital calipers, by digitally tracing the RA endocardium (defined as the border between the echocardiographic signal of the RA endocardium [white] and the RV cavity [black]) at the corresponding points in 2 consecutive cardiac cycles (see Fig. 2) and averaging the values. Calculation of RA fractional area change (RA-FAC) was made in the standard manner. RA volumes were estimated at the corresponding points in the cardiac cycle by the method of disks, and from the RA volumes we derived RA ejection fraction (RA-EF). Active RA emptying volume was defined as RAp − RAmin, and passive RA emptying volume as RAmax − RAp. We also examined the amount of total cardiac output accounted for by active RA emptying in the two groups. By dividing the active RA emptying volume by total cardiac stroke volume, we sought to estimate the percentage of stroke volume accounted for by active RA emptying in a given cardiac cycle.
Figure 2.
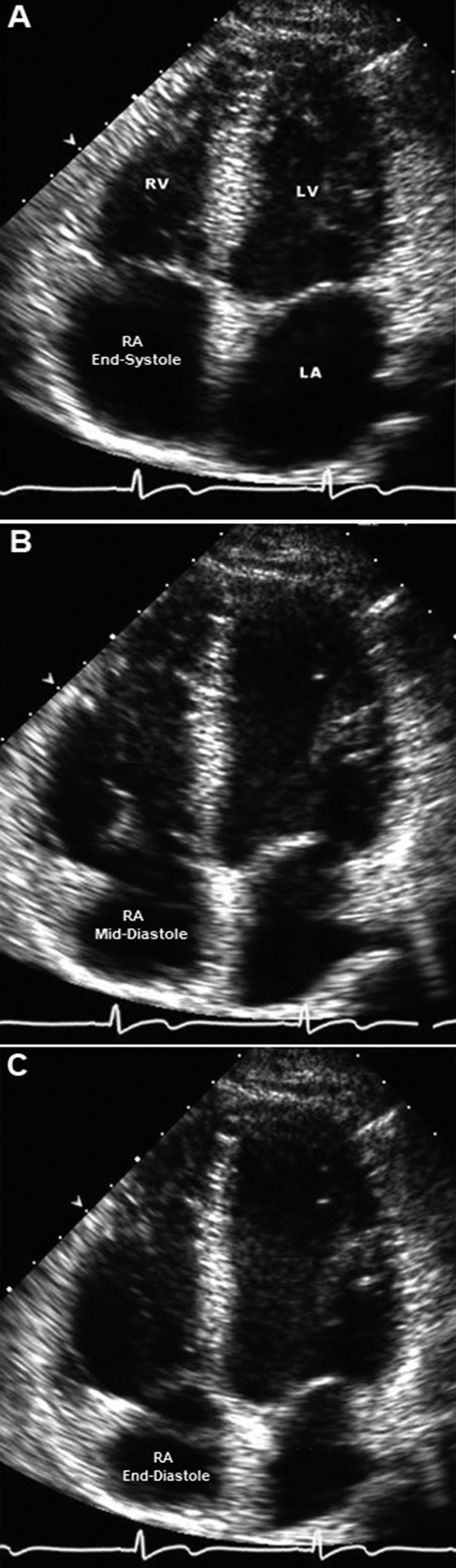
Four-chamber views of a representative echocardiogram in a normal subject. In end-systole (A), the right atrium (RA) is at its largest volume, and the right ventricle (RV) is at its smallest volume. During early diastole (B), the tricuspid valve opens, and the right atrium has emptied a significant amount of its volume. At end-diastole (C), the atria are contracting, and the tricuspid annulus is being pulled further toward the right atrium, resulting in additional volume displacement from the right atrium. LA: left atrium; LV: left ventricle.
Statistical analysis
Continuous variables were summarized as mean ± SD or median (interquartile range) where appropriate. Differences were detected by a 2-tailed, unpaired Student t test for data between groups and by paired t testing for data within groups before and after PH-specific therapy. A P value of <0.05 was considered significant. Correlations between variables of interest were determined by linear regression. All continuous data reported here were normally distributed.
Results
We identified 35 subjects with normal echocardiograms and 37 patients with PAH whose baseline echocardiograms met the criteria for inclusion in this study. Thirty-one of the PAH patients had baseline and follow-up echocardiograms after initiation of PH therapy and thus formed the pre- and post-PH therapy cohort. The causes of PAH included connective-tissue disease (scleroderma in 9 patients, lupus in 2 patients), idiopathic PAH (18 patients), portopulmonary hypertension (3 patients), atrial septal defect–related disease (2 patients), HIV (1 patient), and inoperable CTEPH (2 patients).
The baseline differences in age, sex, heart rate, right heart function, left atrial size, and LV-EF between normal and PAH cohorts are summarized in Table 1. TAPSE was significantly lower in the PAH cohort, accounted for primarily by a 50% lower TAPSERV in the PAH cohort. Notably, the absolute TAPSERA contribution in normal subjects and PAH subjects was equivalent (0.8 vs. 0.9 cm, P = 0.31). As illustrated in Figure 3, TAPSERA accounted for roughly 50% of total TAPSE in the PAH group but significantly less in the normal group. The median TAPSE in our PAH cohort was 1.6 cm, and the contribution of TAPSERA to total TAPSE was equal (50%) in the groups falling above (TAPSERA = 1.0 cm, TAPSERV = 1.0 cm) and below (TAPSERA = 0.7 cm, TAPSERV = 0.7 cm) the median.
Table 1.
Baseline characteristics and echo measurements
| Characteristic | Normals (n = 35) | PAH (n = 37) | P |
|---|---|---|---|
| Women, % | 63 | 81 | 0.09 |
| Age, years | 41.1 ± 14 | 51.6 ± 17 | 0.005 |
| Heart rate, bpm | 70 ± 10 | 78 ± 14 | 0.003 |
| TAPSE, cm | 2.5 ± 0.4 | 1.7 ± 0.4 | <0.0001 |
| TAPSERV, cm | 1.7 ± 0.4 | 0.8 ± 0.4 | <0.0001 |
| TAPSERA, cm | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.31 |
| TAPSE due to RA systole, % | 32.1 ± 8.6 | 51.0 ± 15.2 | <0.0001 |
| RA-FAC, % | 46.1 ± 12.8 | 28.6 ± 12.7 | <0.0001 |
| RAmax, mL | 30.6 ± 10.7 | 80.3 ± 43.9 | <0.0001 |
| RAp, mL | 19.7 ± 7.2 | 70.3 ± 49.7 | <0.0001 |
| RAmin, mL | 12.5 ± 5.6 | 50.0 ± 32.5 | <0.0001 |
| RA-EF, % | 59.4 ± 10.7 | 39.8 ± 16.4 | <0.0001 |
| Passive emptying volume (RAmax − RAp), mL | 10.9 ± 5.2 | 10.5 ± 8.3 | 0.77 |
| Passive emptying, % | 60.0 ± 18.6 | 34.7 ± 23.6 | <0.0001 |
| Active emptying volume (RAp − RAmin), mL | 7.2 ± 4.1 | 20.4 ± 12.5 | <0.0001 |
| Active emptying, % | 40.3 ± 18.6 | 65.3 ± 23.6 | <0.0001 |
| Total stroke volume, mL | 69.6 ± 19.8 | 50.7 ± 24.3 | 0.0010 |
| Active RA emptying∶total stroke volume, % | 10.9 ± 6.8 | 39.7 ± 25.2 | <0.0001 |
| Cardiac output, L | 4.8 ± 1.4 | 3.9 ± 1.5 | 0.01 |
| TR grade (scale: 0–4) | 0.73 ± 0.6 | 2.3 ±1.0 | <0.0001 |
| LA diameter, cm | 3.2 ± 0.4 | 3.6 ± 0.6 | 0.02 |
| LV-EF, % | 64 ± 3 | 64 ± 12 |
Except in the first row, data are presented as mean ± SD. LA: left atrial; LV-EF: left ventricular ejection fraction; PAH: pulmonary arterial hypertension; RA: right atrial; RA-EF: RA ejection fraction; RA-FAC: RA fractional area change; RAmax: maximum RA volume in the cardiac cycle; RAmin: minimum RA volume in the cardiac cycle; RAp: RA volume before RA systole; TAPSE: tricuspid annular plane excursion; TAPSERA: TAPSE during RA contraction; TAPSERV: TAPSE before RA contraction; TR: tricuspid regurgitation.
Figure 3.
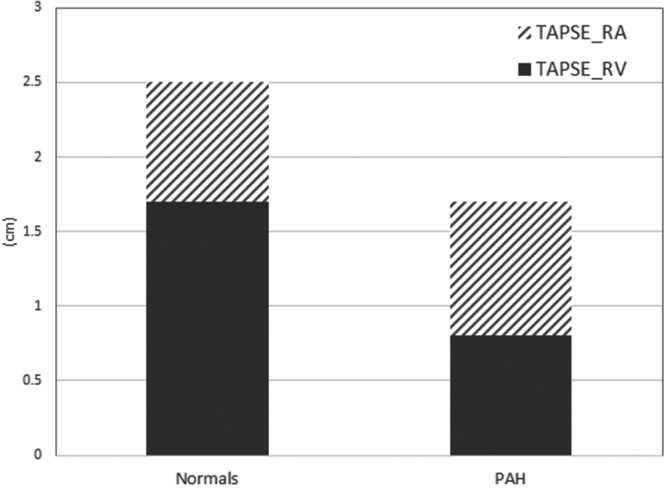
Tricuspid annular plane excursion (TAPSE, in cm) in both normal and pulmonary arterial hypertension (PAH) cohorts, separated into TAPSERA (during right atrial contraction) and TAPSERV (before right atrial contraction) components.
As expected, the RAmax and RAmin were significantly larger in the PAH cohort than in the normal cohort, resulting in a significantly lower RA-EF in the PAH cohort. In the PAH cohort, passive emptying accounted for only 35% of the total RA emptying, while active emptying made up 65% of total RA emptying. In contrast, passive emptying accounted for 60% of the total RA emptying in the normal cohort. The active RA emptying in the PAH group also played a much greater role in contributing to cardiac output, as shown in Figure 4.
Figure 4.
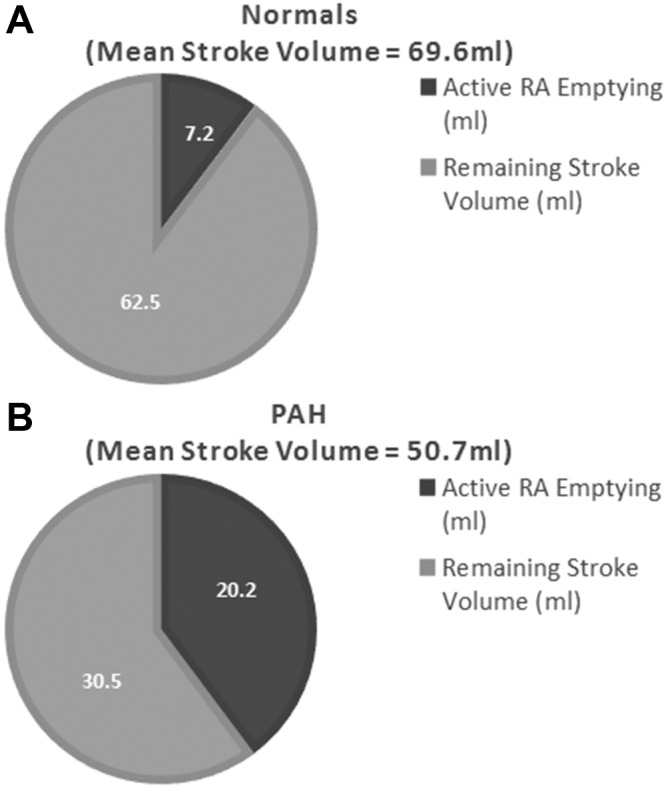
Left ventricular stroke volume in the normal (A) and pulmonary arterial hypertension (PAH; B) cohorts, with the contribution of active right atrial (RA) emptying volume highlighted.
In the PAH cohort, RA function, as indicated by RA-FAC, showed a significant direct correlation with TAPSE (r = 0.64, P < 0.001). However, RA-FAC was not significantly associated with total right heart function in the normal cohort (r = 0.05, P = 0.76).
Of the 37 patients evaluated in the PAH cohort, 31 had adequate follow-up imaging after initiation of PAH-specific therapy. The most common therapy was an oral phosphodiesterase type 5 inhibitor (80%) and/or endothelin receptor antagonist (65%). Additional therapies included inhaled iloprost (32%) and parenteral prostacyclins (4%). On average, the patients were evaluated 334 days after initiation of PAH-specific therapy. As seen in Table 2, TAPSE and its RA and RV components were significantly increased in the posttreatment group.
Table 2.
Comparison of total TAPSE, TAPSERA, and TAPSERV in the PAH cohort before and after treatment
| PAH pretreatment group (N = 31) | PAH posttreatment group (N = 31) | ||||
|---|---|---|---|---|---|
| Mean ± SD | Interquartile range | Mean ± SD | Interquartile range | P | |
| TAPSE, cm | 1.7 ± 0.4 | 1.3–2.0 | 2.1 ± 0.4 | 1.9–2.4 | <0.0001 |
| TAPSERA, cm | 0.9 ± 0.3 | 0.7–1.1 | 1.1 ± 0.4 | 0.8–1.3 | 0.03 |
| TAPSERV, cm | 0.8 ± 0.3 | 0.6–1.0 | 1.1 ± 0.3 | 0.9–1.3 | 0.0003 |
| TAPSE due to RA systole, % | 51.2 ± 13.0 | 42.1–56.3 | 49.0 ± 12.8 | 40.0–54.5 | 0.43 |
PAH: pulmonary arterial hypertension; RA: right atrial; TAPSE: tricuspid annular plane excursion; TAPSERA: TAPSE during RA contraction; TAPSERV: TAPSE before RA contraction.
Discussion
TAPSE is a measure of longitudinal right heart function, representing the displacement of the RV from base to apex during systole. TAPSE has been shown to predict morbidity and mortality in patients with PAH and has become a recommended method for measuring the efficacy of PH medical therapy in PAH.11,13,14 Previous work has shown that longitudinal RV shortening accounts for approximately 80% of global RV function in normal subjects and that longitudinal function represents the afterload-responsive element of right heart function in subjects with PAH treated with PH medical therapy.9
Until now, TAPSE has been thought of as a method specific to RV function, with the direct or indirect role of RA function not known. In this study, our data show that in normal subjects, RA function accounts for approximately 32% of tricuspid annular displacement, or TAPSE. RA systolic function, or atrial “kick,” serves to increase the distance between the RV base and the RV apex at end-diastole—thereby increasing the potential contraction length of the RV once systole begins. This is evident on an M-mode tracing of TAPSE as a “dip” in the M-mode signal at end-diastole, producing a rapid and significant increase in the distance between the RV base and apex (Fig. 1).
In the PAH patients, RA function accounted for an impressive 51% of TAPSE. The contribution of RA function to total TAPSE was similar in patients with preserved TAPSE and those with diminished TAPSE. In the overall PAH cohort, our data indicate that the mean TAPSE value of our PAH patients in the absence of RA systolic function would have been as low as 0.8 cm, instead of 1.7 cm. In relative terms, this is a marked difference in TAPSE and well below the cutoff of 1.5 cm that has been shown to be predictive of very high mortality rates in subjects with PAH.11,15 Compare this to the result from the normal cohort, who on average had an annular excursion of 1.7 cm without atrial contraction (TAPSERV)—equal to the total TAPSE in the PAH group. This difference helps to characterize and quantify the importance of maintained RA function to total right heart function in patients with PAH.
Additional analysis was performed to further characterize the increased dependence on RA function in the PAH group. As has been shown previously,12 the majority of RA emptying occurred during RA systole in the PAH group, whereas in the normal group most of the RA emptying occurred passively, before RA systole. Active RA emptying accounted for a much (nearly 3-fold) larger percentage of the total cardiac stroke volume, and therefore cardiac output, in the PAH group than in normals. Finally, there was a significant correlation between the RA-FAC and total TAPSE in the PAH group, but not in the normals.
These data clearly show the critical role of RA systolic function in terms of right heart function preservation and compensation, particularly in the setting of PAH. This observation provides quantitative imaging evidence and physiologic rationale for why atrial arrhythmias are so poorly tolerated in the setting of significant PH.5,16,17 In addition, these findings lend to prior work showing that increased RA size is a powerful predictor of mortality in PAH and suggest that increased RA size may be more than simply a marker of RV dysfunction.18-20 Instead, increased RA size may speak to RA dysfunction and a greater burden of total right heart dysfunction seen in those with an enlarged RA. Future work should specifically assess RA and RV systolic function in PAH patients, both with and without normal sinus rhythm, to gain further insight into the importance of each chamber leading to right heart failure.
These data also support prior work showing that TAPSE does indeed increase in response to PH medical therapy.9 However, we did find it interesting that the increase in TAPSE observed in our cohort was accounted for by significant and balanced increases in both TAPSERA and TAPSERV. Thus, at least in terms of longitudinal right heart function assessment, both RA and RV function improve in response to PH-specific therapy.
Limitations
Our retrospective study has several limitations. The measurement of the RA systolic contribution to TAPSE occurs at end-diastole, so it does not directly affect systolic excursion of the tricuspid annular plane. However, since the total displacement of the right heart in systole is in part dictated by the distance between the RV base and apex at the onset of systole, it is reasonable to assume that the loss of this contribution would negatively affect right heart function. TAPSE was used in this study, as opposed to another method of global RV function such as RV-FAC or RV-EF, because TAPSE is a reliable, reproducible, and well-validated method of right heart function in PAH that is strongly linked to patient outcomes and correlates well with invasively derived stroke volume index and cardiac index in patients with PH.11,15,21,22 In addition, M-mode tracings of TAPSE clearly show the delineation between atrial and nonatrial events due to the high temporal resolution inherent to M-mode. RV strain analysis is another emerging modality to evaluate right heart function in PAH, and it has been shown to predict clinical outcome and response to therapy in this cohort.23-25 Future work may incorporate RV strain analysis to further establish the role of RA function in PAH.
Conclusions
RA function accounts for a significantly greater proportion of total right heart function in patients with PAH than in normal subjects. A balanced improvement in RA and RV function was observed after PH-specific medical therapy. These findings support the importance of RA function in PAH and shed further insight into how the right heart adapts in the presence of changing afterload.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53(17):1573–1619. [DOI] [PubMed]
- 2.Galiè N, Manes A, Palazzini M, Negro L, Romanazzi S, Branzi A. Pharmacological impact on right ventricular remodelling in pulmonary arterial hypertension. Eur Heart J Suppl 2007;9(H):H68–H74.
- 3.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 4.Mehra MR, Park MH, Landzberg MJ, Lala A, Waxman AB. Right heart failure: toward a common language. J Heart Lung Transplant 2014;33(2):123–126. [DOI] [PubMed]
- 5.Goldstein JA, Harada A, Yagi Y, Barzilai B, Cox JL. Hemodynamic importance of systolic ventricular interaction, augmented right atrial contractility and atrioventricular synchrony in acute right ventricular dysfunction. J Am Coll Cardiol 1990;16(1):181–189. [DOI] [PubMed]
- 6.Cresci SG, Goldstein JA. Hemodynamic manifestations of ischemic right heart dysfunction. Catheter Cardiovasc Diagn 1992;27(1):28–33; discussion 33–34. [DOI] [PubMed]
- 7.Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev 2008;4(1):49–59. [DOI] [PMC free article] [PubMed]
- 8.Tongers J, Schwerdtfeger B, Klein G, Kempf T, Schaefer A, Knapp JM, Niehaus M, Korte T, Hoeper MM. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J 2007;153(1):127–132. [DOI] [PubMed]
- 9.Brown SB, Raina A, Katz D, Szerlip M, Wiegers SE, Forfia PR. Longitudinal shortening accounts for the majority of right ventricular contraction and improves after pulmonary vasodilator therapy in normal subjects and patients with pulmonary arterial hypertension. Chest 2011;140(1):27–33. [DOI] [PubMed]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–1463. [DOI] [PubMed]
- 11.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174(9):1034–1041. [DOI] [PubMed]
- 12.Willens HJ, Fertel DP, Qin J, Labrador E, Lowery MH. Effects of age and pulmonary arterial hypertension on the different phases of right atrial function. Int J Cardiovasc Imaging 2008;24(7):703–710. [DOI] [PubMed]
- 13.Lee CY, Chang SM, Hsiao SH, Tseng JC, Lin SK, Liu CP. Right heart function and scleroderma: insights from tricuspid annular plane systolic excursion. Echocardiography 2007;24(2):118–125. [DOI] [PubMed]
- 14.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiéry JL, Barberà JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30(20):2493–2537. [DOI] [PubMed]
- 15.Ghio S, Klersy C, Magrini G, D’Armini AM, Scelsi L, Raineri C, Pasotti M, Serio A, Campana C, Viganò M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol 2010;140(3):272–278. [DOI] [PubMed]
- 16.Baur LHB. Right atrial function: still underestimated in clinical cardiology. Int J Cardiovasc Imaging 2008;24(7):711–712. [DOI] [PMC free article] [PubMed]
- 17.Olsson KM, Nickel NP, Tongers J, Hoeper MM. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol 2013;167(5):2300–2305. [DOI] [PubMed]
- 18.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002;39(7):1214–1219. [DOI] [PubMed]
- 19.Fukuda Y, Tanaka H, Motoji Y, Ryo K, Sawa T, Imanishi J, Miyoshi T, et al. Utility of combining assessment of right ventricular function and right atrial remodeling as a prognostic factor for patients with pulmonary hypertension. Int J Cardiovasc Imaging 2014;30(7):1269–1277. [DOI] [PubMed]
- 20.Grapsa J, Gibbs JSR, Cabrita IZ, Watson GF, Pavlopoulos H, Dawson D, Gin-Sing W, Howard LS, Nihoyannopoulos P. The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: study with real-time three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012;13(8):666–672. [DOI] [PubMed]
- 21.Rajagopalan N, Saxena N, Simon MA, Edelman K, Mathier MA, López-Candales A. Correlation of tricuspid annular velocities with invasive hemodynamics in pulmonary hypertension. Congest Heart Fail 2007;13(4):200–204. [DOI] [PubMed]
- 22.Urheim S, Cauduro S, Frantz R, McGoon M, Belohlavek M, Green T, Miller F, et al. Relation of tissue displacement and strain to invasively determined right ventricular stroke volume. Am J Cardiol 2005;96(8):1173–1178. [DOI] [PubMed]
- 23.Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS, Hsiao JF, et al. Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol 2013;111(1):143–148. [DOI] [PubMed]
- 24.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013;6(5):711–721. [DOI] [PubMed]
- 25.Rajagopal S, Forsha DE, Risum N, Hornik CP, Poms AD, Fortin TA, Tapson VF, Velazquez EJ, Kisslo J, Samad Z. Comprehensive assessment of right ventricular function in patients with pulmonary hypertension with global longitudinal peak systolic strain derived from multiple right ventricular views. J Am Soc Echocardiogr 2014;27(6):657–665.e3. [DOI] [PubMed]


