Abstract Abstract
We conducted an international study to evaluate practices in the diagnosis and management of pulmonary arterial hypertension (PAH) globally across different geographic regions. Between July and October 2012, PAH-treating physicians completed a 15-minute online questionnaire and provided patient record data for their 3 or 5 most recent patients with PAH. Overall, 560 physicians (Europe: 278; United States: 160; Argentina: 53; Japan: 69) completed the questionnaire and provided data for 2,618 patients. The proportion of physicians who described themselves as working in or affiliated with a specialized pulmonary hypertension center ranged from 13% in Argentina to 74% in the United States. At the time of diagnosis, patients’ New York Heart Association functional class differed significantly between regions. At the time of last assessment, functional class had improved overall, and differences between regions had largely disappeared. A large proportion of patients did not undergo right heart catheterization for the diagnosis of PAH (Europe: 7%–21%; United States: 21%; Japan: 19%; Argentina: 51%). Variations in management included greater use of phosphodiesterase 5 inhibitors in the United States than in Europe and Japan and greater use of triple or greater combination therapy in Japan than in other regions. Results from this study, which includes a global aspect of PAH care, demonstrate that there are significant differences in PAH management between regions and low adherence to guidelines recommending right heart catheterization for the diagnosis of PAH.
Keywords: pulmonary arterial hypertension, pulmonary arterial hypertension–specific therapies, diagnosis, clinical practice, guidelines
Pulmonary arterial hypertension (PAH; World Health Organization group I) is defined by a mean pulmonary artery pressure of ≥25 mmHg and increased pulmonary vascular resistance of >3 Wood units with a normal pulmonary capillary wedge pressure.1,2 PAH is a progressive disease that can ultimately lead to right heart failure and death.1,3 Despite advances in current therapies for PAH, there is still significant unmet medical need, especially in areas of the world where access to diagnostic and therapeutic options is limited. The mortality of patients with PAH remains high even in well-developed countries: 15% at 1 year and 32% at 3 years.4
The therapeutic approach to PAH is evolving; multiple classes of agents are available, and physicians from both expert pulmonary hypertension (PH) centers and the community treat patients with PAH.5,6 Data from various registries suggest that approaches to the diagnosis and treatment of PAH may differ between countries.7-11
In this international study, we sought to assess differences in the diagnosis and management of patients with PAH across different countries and regions worldwide by conducting a large, physician-based study using a quantitative online questionnaire. The objectives of the study were (1) to assess the diagnosis and management of PAH in different countries and regions by analyzing differences in referral patterns, diagnostic procedures, and use of PAH-specific drug therapies; (2) to explore PAH-treating physicians’ attitudes toward the management of PAH; and (3) to determine the accuracy of physicians’ perceptions regarding the diagnosis and management of their patients, by comparing questionnaire results with physicians’ patient medical records. The study captured responses from a variety of physicians involved in the management of PAH, including physicians practicing in specialist PH centers as well as those in non-PH centers. A parallel study evaluated the diagnosis and management of patients with chronic thromboembolic PH, with results to be reported in a separate article.
Methods
The study was conducted in five European countries (France, Germany, Italy, Spain, and the United Kingdom, between July 9 and September 30, 2012), the United States (between July 10 and August 6, 2012), Argentina (between September 4 and October 20, 2012), and Japan (between September 7 and October 22, 2012). The study was sponsored by Bayer Pharma in collaboration with Ipsos Healthcare. The study had two components: a retrospective patient chart review and a physician questionnaire. The retrospective chart review collected the current and historical data in the online patient record and was completed by the physician. Subsequent follow-up for changes in treatment or outcomes was not carried out. The physician perception questionnaire focused on physician experience and satisfaction with current PAH treatments. The same physicians were enrolled in both parts of the study and provided data from their patients’ records in addition to completing the questionnaire.
Physician selection criteria
Physicians who had previously stated that they were interested in participating in market research activities and were registered with Medefield, a market research panel provider, with an appropriate specialty (cardiology, pulmonology, rheumatology, or internal medicine with specialization in cardiology, pulmonology, or rheumatology) were invited to participate and, if they agreed, were recruited into the study if they met all the following criteria: they were actively involved in decisions for PAH-specific drug therapy in patients with PAH; they were treating at least 5 (Europe and the United States) or 3 (Argentina and Japan) PAH patients with PAH-specific drug therapy and had personally initiated PAH-specific treatment in at least 1 of these patients; and they had experience in managing PAH for at least 2 years. No more than 2 doctors from each center participated in the study. Physicians were excluded if they were employees or paid consultants of any pharmaceutical company (not including payments for clinical trial funding, advisory boards, speaker honoraria, and similar activities). Physicians recruited from the United States had to have self-reported board certification in cardiology, pulmonology, or rheumatology. Depending on the country, internal medicine physicians were also eligible to take part in the study if they specialized in cardiology, pulmonology, or rheumatology (Table 1).
Table 1.
Physician sample
| UK | FR | DE | IT | ES | US | AR | JP | |
|---|---|---|---|---|---|---|---|---|
| No. of physicians | 50 | 53 | 63 | 58 | 54 | 160 | 53 | 69 |
| Specialty, % | ||||||||
| Cardiology | 64 | 45 | 44 | 41 | 35 | 54 | 62 | 81 |
| Pulmonology | 18 | 36 | 38 | 19 | 43 | 38 | 38 | 16 |
| Rheumatology | 16 | 4 | 6 | 21 | 4 | 4 | NA | 1 |
| Internal medicinea | 2 | 15 | 11 | 19 | 19 | 4 | NA | 1 |
| Cardiology | 0 | 8 | 6 | 10 | 4 | 1 | NA | 1 |
| Pulmonology | 0 | 6 | 3 | 7 | 9 | 2 | NA | 0 |
| Rheumatology | 2 | 2 | 2 | 2 | 6 | 1 | NA | 0 |
| Setting, % | ||||||||
| Hospital | 98 | 91 | 79 | 95 | 98 | 24 | 85 | 100 |
| Office | 2 | 9 | 21 | 5 | 2 | 76 | 15 | NA |
| Affiliation, % | ||||||||
| Working in a specialized PH centerb | 24c | 38c | 32c | 12c | 30c | 51d | 13c | 30d |
| Affiliated with a PH center | 22 | NA | 17 | 19 | 28 | 23 | NA | NA |
| Not affiliated with a PH center (non-PH center) | 54 | 62 | 51 | 69 | 43 | 26 | 81 | 64 |
| Don’t know | NA | NA | NA | NA | NA | NA | 6 | 6 |
In cases where the physician type is not involved in the management of PH in their country, “not applicable” is used. AR: Argentina; DE: Germany; ES: Spain; FR: France; IT: Italy; JP: Japan; NA: not applicable; PH: pulmonary hypertension; UK: United Kingdom; US: United States.
Internal medicine physicians specializing in cardiology, pulmonology, or rheumatology.
Defined on the basis of European Society of Cardiology/European Respiratory Society 2009 guidelines;1 in Argentina and Japan, an institution with a department specialized in treating PH.
Verified recognized specialized PH center.
Self-defined specialized PH center.
Physicians were asked whether they worked in a PH center and to provide the center’s name. A PH center was defined on the basis of the 2009 European Society of Cardiology/European Respiratory Society guidelines1 as follows: manages a minimum of 50 patients with PH; receives at least 2 new patient referrals per month; performs at least 20 vasoreactivity tests per year; participates in clinical research on PH, including phase 2 and phase 3 clinical trials; contains a multidisciplinary team (including cardiologists, pulmonologists, radiologists, specialist nursing staff, and adequate on-call service); and has direct connections and quick access to other medical programs (specialists for connective-tissue disease, pulmonary endarterectomy, lung transplantation, and congenital heart disease in adults). For physicians from Europe and Argentina, verification of their PH center was performed against country lists of recognized specialist centers. Physicians from the United States and Japan were self-defined with respect to their association with PH centers. Physicians whose place of work was not affiliated with a PH center were classified as non-PH-center respondents in all analyses.
Data acquisition
Physicians were asked to provide patient records for the last 3 or 5 patients with PAH seen by the physician (3 patients for Argentina and Japan; 5 patients for Europe and the United States); the number of patient records requested for the different countries/regions was based on a prescreening questionnaire that revealed that the average caseload was higher in Europe and the United States than in Argentina and Japan. Patients had to be aged at least 18 years and currently receiving treatment with PAH-specific drug therapies. Patients were not eligible for inclusion in the study if they were participating in a clinical trial (except postmarketing clinical trials). Patient record data were collected in a deidentified manner; patient consent was therefore not required. For the physician perception questionnaire, physicians were required to complete a 15-minute online survey. In accordance with the EphMRA (European Pharmaceutical Market Research Association) Code of Conduct, no ethical approval was required from the Clinical Research Ethics Committee or Independent Review Board for this market research study with syndicated research.
Statistical analyses
A feasibility assessment was carried out to specify a realistic target physician sample size for each country (50 for Argentina, France, Germany, Italy, Spain, and the United Kingdom; 150 for the United States; and 70 for Japan), with a particular focus on recruiting pulmonologists and cardiologists. Patient record data are reported as proportions by category, according to the categorical responses to the questions listed in the figure legends. Patient record data were weighted on the physician’s self-reported caseloads of qualifying patients at a country level. No additional weighting—for example, to account for varying prevalence rates between countries—was applied to the data. Physician perception questionnaire data are reported as proportions for questions with categorical responses and as mean values ± standard error of the mean (SEM) and median values for numerical responses. Data from the perception questionnaire were collected as absolute patient numbers. Therefore, there was an implicit weighting in these data toward physicians with higher patient caseloads, and no adjustment was applied to these data.
Data are presented by country or by region. In the latter case, data from the five European countries were pooled.
Statistical significance was tested with 2-tailed t tests at the 95% confidence level. Bonferroni corrections were applied for multiple comparisons between countries for the same category. The χ2 test was used to test the distribution of New York Heart Association functional class (NYHA FC) at diagnosis and at the time of the study. Statistical testing was not performed in regions with fewer than 30 patients (termed “small base”). All statistical testing was performed as post-hoc analyses.
Results
Physician sample
A total of 560 physicians met the inclusion criteria and agreed to participate in the study. Participating physicians’ specialties are shown in Table 1. Most physicians were either cardiologists or pulmonologists, and most were practicing in a hospital-based setting in all countries except the United States, where 76% of physicians were office based. The proportion of physicians who described themselves as working in or affiliated with a specialized PH center ranged from 13% in Argentina to 74% in the United States.
Results from patients’ medical records
Patient characteristics
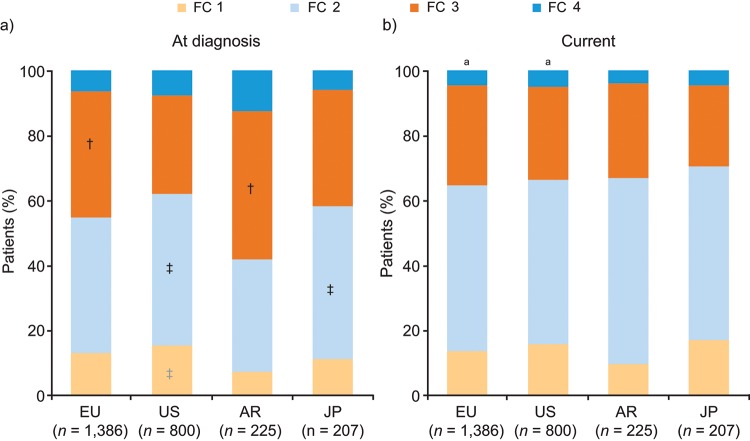
Physicians provided medical records for a total of 2,618 patients: 1,386 patients from Europe, 800 patients from the United States, 225 patients from Argentina, and 207 patients from Japan. Across the four regions (Europe, United States, Argentina, and Japan), idiopathic PAH (IPAH) and PAH associated with other conditions were the most common etiologies (Fig. 1). The most common form of associated PAH was PAH associated with connective-tissue disease (22%–29% across the four regions). Retrospective analysis of patient records showed that at diagnosis, a large proportion of patients were classified as NYHA FC 3/4, with significant variations between regions in the proportion of patients in each NYHA class (Fig. 2a). The median time from diagnosis to the time of the study was 20 months for Europe, 15 months for the United States, 19 months for Japan, and 25 months for Argentina. An improvement in NYHA FC was apparent by the time of the study (Fig. 2b). Moreover, variations between regions were markedly reduced by the time of the study, such that there were no statistically significant differences between regions. The improvement in NYHA FC between diagnosis and the time of the study was marked by shifts in the proportions of patients across the NYHA classes: in Europe and Argentina, the proportions of patients classified as NYHA FC 3 and 4 decreased over time, and the proportion classified as NYHA FC 2 increased; in Japan, the proportion of patients classified as NYHA FC 3 decreased under treatment, and the proportions classified as NYHA FC 1 and 2 both increased; and in the United States, the proportion of patients classified as NYHA FC 4 decreased over time. The improvement in NYHA FC was significant only in the European and US cohorts. In the United States and Europe, a greater proportion of patients with NYHA FC 3/4 were treated in PH centers than in non-PH-center settings, whereas in Argentina and Japan, similar proportions of patients with NYHA FC 3/4 were treated in PH and non-PH center settings (data not shown).
Figure 1.
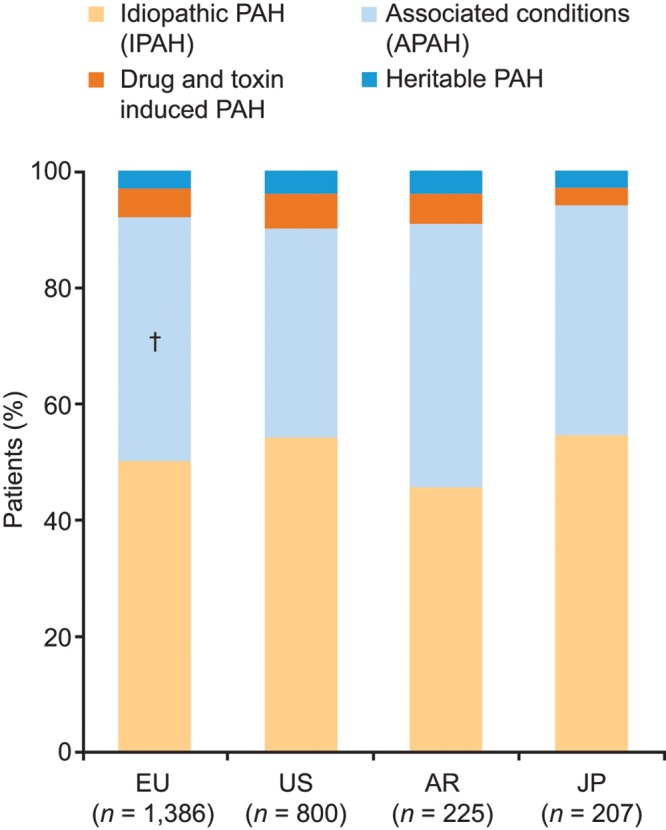
Patient characteristics (patient record data). Response to study question “What is this patient’s primary Dana Point Group 1 Classification of Pulmonary Arterial Hypertension (PAH)?” †Significantly different from the corresponding category in the US, according to t test pairwise comparisons (with Bonferroni correction). APAH: associated PAH; AR: Argentina; EU: Europe; JP: Japan; PAH: pulmonary arterial hypertension; US: United States.
Figure 2.
New York Heart Association functional class (FC) of patients at diagnosis (a) and currently (b; patient record data). Response to the study questions “What was this patient’s New York Heart Association functional class at time of diagnosis?” (a) and “What is the patient’s current New York Heart Association functional class?” (b). To compare the proportion of patients in each FC group between the different regions within the same time point (at diagnosis and at the time of the study [current]), t test pairwise comparisons (with Bonferroni correction) were performed. At diagnosis, there were significant differences in the corresponding functional class between regions (†vs. the US; ‡vs. Argentina). By contrast, there were no significant differences between regions at the time of the study. Within each country, χ2 testing was performed to compare the FC distribution at diagnosis and at the time of the study (current). aP < 0.05 indicates a significant difference between the current FC distribution and that at diagnosis. AR: Argentina; EU: Europe; JP: Japan; US: United States.
Diagnosis of PAH
The setting of PAH diagnosis varied by region: in the United States, 33% of patients received their diagnoses in a non-PH center or other setting, compared with 13% in Europe (Fig. S1). In Europe, a greater proportion of patients received diagnoses that used right heart catheterization (RHC) in PH centers than in non-PH centers, whereas in the United States, equal proportions of patients in PH centers and non-PH centers received diagnoses that used RHC (Fig. 3). In Argentina, more than 50% of patients did not undergo RHC. Reasons for not performing RHC in patients varied by country and by whether patients were being treated in PH centers or non-PH centers, with the main reasons being that other tests had indicated that the patient had PAH, that the procedure was considered too invasive for the patient, and patient refusal (Table 2).
Figure 3.
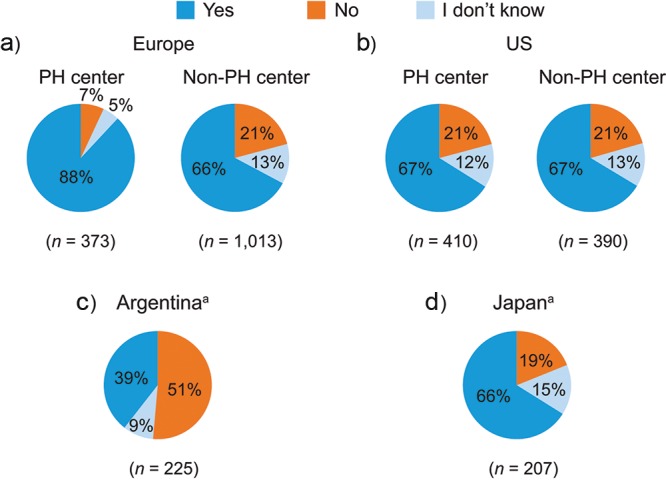
Diagnosis of PAH (patient record data). Responses to the study question “Was right heart catheterization used to diagnose this PAH patient?” aData for Argentina and Japan were not split according to PH center setting because of the small base. Combined data within each chart may not total 100% because of rounding of individual numbers. PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; US: United States.
Table 2.
Reasons for not performing right heart catheterization (patient record data)
| Europe | United States | |||||
|---|---|---|---|---|---|---|
| PH center (n = 31) |
Non-PH center (n = 208) |
PH center (n = 87) |
Non-PH center (n = 82) |
Argentina (n = 113)a |
Japan (n = 39)a |
|
| Other tests indicated that the patient had PAH | 60* | 26 | 40 | 47* | 51* | 30 |
| Too invasive for the patient | 17 | 28 | 28 | 28 | 29 | 42 |
| Patient refusal | 14 | 36† | 36† | 31 | 14 | 32 |
| Planning to perform in the future | 13 | 16 | 15 | 4 | 13 | 10 |
| Lack of capabilities in the center | 6 | 5 | 9 | 5 | 27 | … |
| Cost | 4 | 3 | 3 | 1 | 5 | 2 |
| Other | 3 | 1 | … | 4 | 7 | 3 |
Responses to the study question “Why was right heart catheterization not performed?” Data are percentage of patients; t test pairwise comparisons across rows. PAH: pulmonary arterial hypertension; PH: pulmonary hypertension.
P < 0.05 versus Europe non-PH center.
P < 0.05 versus Argentina.
Data for Argentina and Japan were not split according to PH center setting because of the small base.
Management of PAH with PAH-specific drug therapies
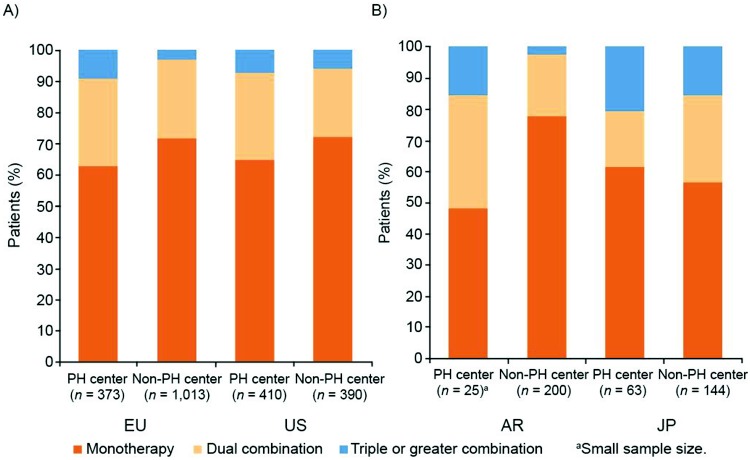
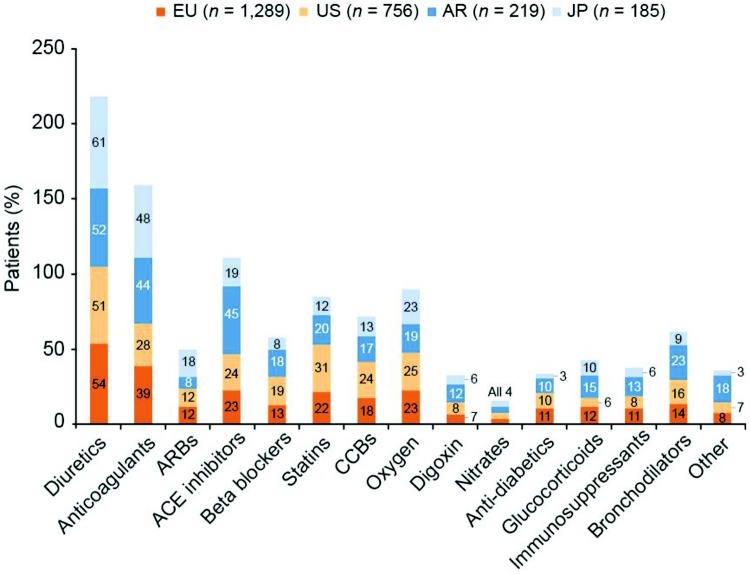
In the United States and Argentina, a majority of patients were treated with phosphodiesterase 5 (PDE-5) inhibitors, whereas in Europe, equal proportions of patients were treated with PDE-5 inhibitors and endothelin receptor antagonists (ERAs; Fig. 4a). Approximately one-third of patients in Europe, the United States, and Argentina received combination therapy with PAH-specific drugs; in Japan, 42% of patients were treated with combination therapy. However, a significantly greater proportion of patients in Japan received triple or greater combination therapy, compared with the other regions (Fig. 4b). A breakdown of the proportions of patients receiving combination therapy in PH centers and non-PH centers was remarkably similar for Europe and the United States, with more combination therapy in PH centers than in non-PH centers (statistical testing not performed; Fig. S2). Concomitant medication besides PAH-specific drug therapy is shown by region in Figure S3.
Figure 4.
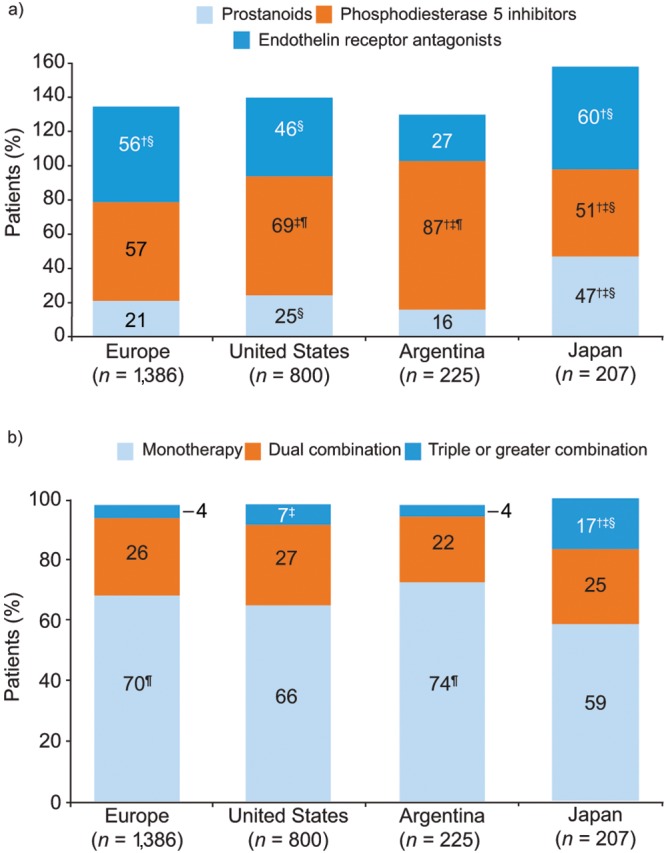
Use of PAH-specific therapies (patient record data). Responses to the study question “Please select from the options provided which PAH-specific drugs this patient is currently receiving.” a, Drugs classified as prostanoids, phosphodiesterase 5 inhibitors, or endothelin A receptor antagonists; b, PAH-specific treatment classified as monotherapy, dual combination therapy, or triple or greater combination therapy. Symbols indicate significant differences versus the corresponding category in †the United States, ‡Europe, §Argentina, and ¶Japan, from t test pairwise comparisons (with Bonferroni correction). PAH: pulmonary arterial hypertension.
Results from physician questionnaire
Diagnosis and management of PAH
The mean ± SEM number of patients with PAH personally managed by each responding physician ranged from 7.1 ± 2.3 in Argentina to 57.9 ± 16.2 in the United States, and the proportion of patients currently receiving PAH-specific therapy ranged from 76% in the United Kingdom to 89% in France (Table S1). The reasons most commonly reported by physicians for modifying first-line oral monotherapy treatment were lack of improvement or worsening of FC status and 6-minute walking distance (Fig. 5).
Figure 5.
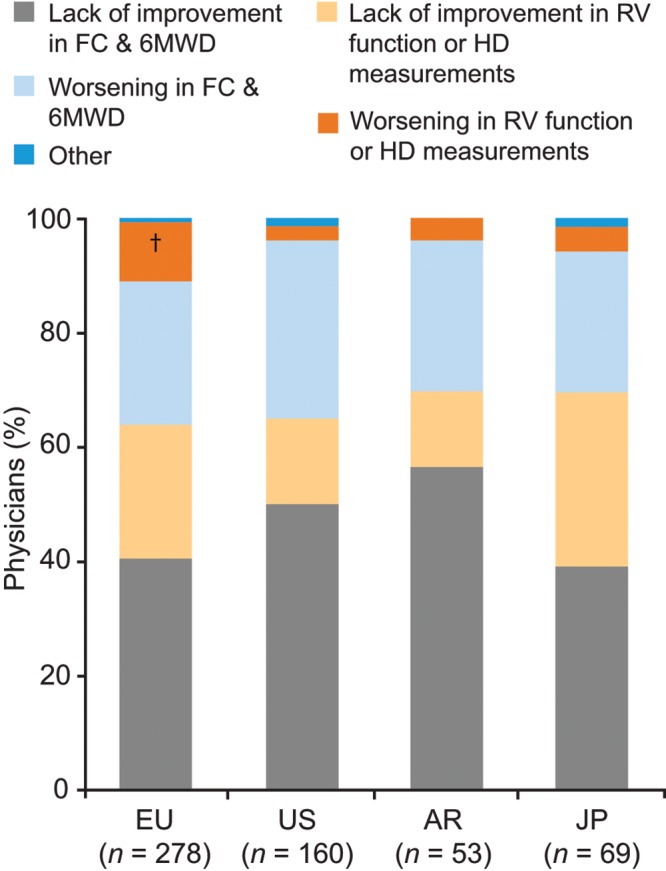
Reasons for modifying first-line oral therapy in patients with PAH (physician perception data). Responses to the study question “Please consider a PAH patient who was previously treatment naïve and was recently initiated on an oral monotherapy (e.g., ERA). Which of the provided reasons is the primary reason that would cause you to modify this patient’s therapy?” †Significantly different from the corresponding category in the US, according to t test pairwise comparisons (with Bonferroni correction). 6MWD: 6-minute walking distance; AR: Argentina; ERA: endothelin receptor antagonist; EU: Europe; FC: functional class; HD: hemodynamics; JP: Japan; PAH: pulmonary arterial hypertension; RV: right ventricular; US: United States.
Physician experience and satisfaction with PAH-specific drug therapies
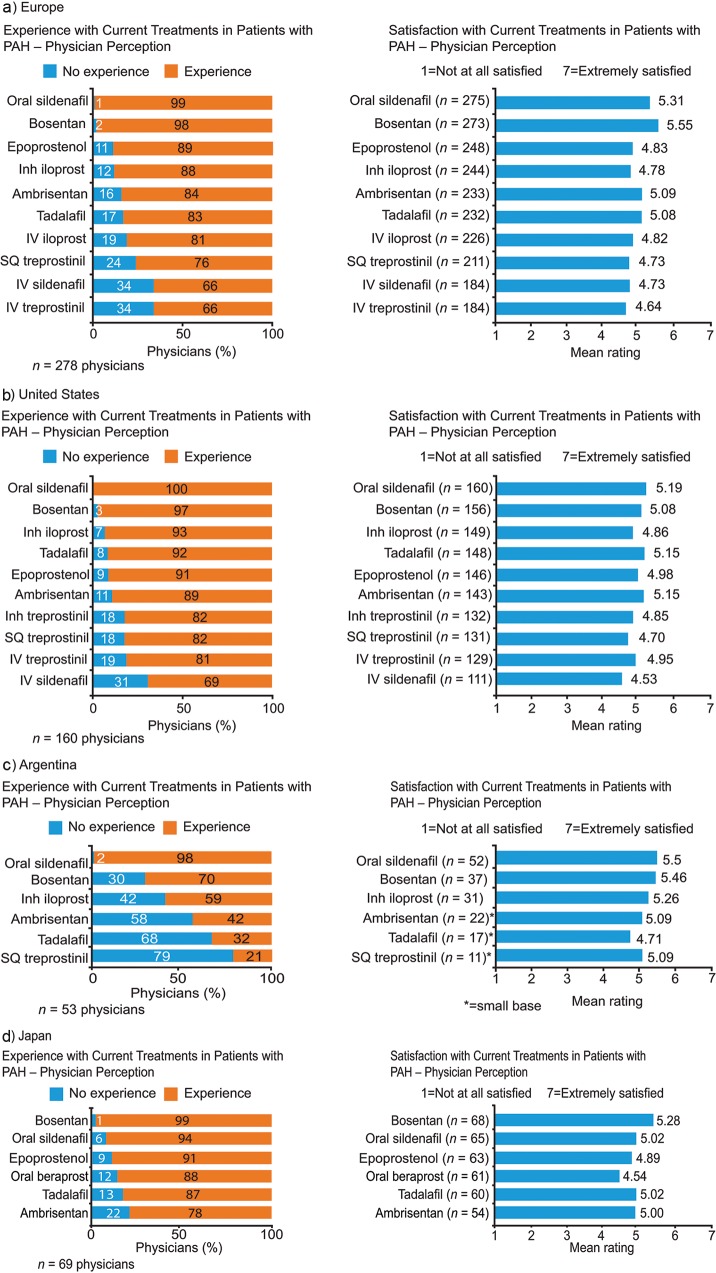
Physicians reported that the majority of their PAH patients were receiving PAH-specific therapies (76%–89%; Table S1). Physicians’ experience in using currently available PAH treatments varied between regions, with physicians from Argentina having the least experience across a range of PAH-specific therapies (Fig. 6). On a Likert scale of 1–7 (1: not at all satisfied; 7: extremely satisfied), mean satisfaction was above the midpoint (4) for all currently available medical treatment options assessed (Fig. 6).
Figure 6.
Physician experience and satisfaction with current treatments (physician perception data). Responses to the study question “From the provided list of drugs used to treat your PAH patients, please rate each in terms of your overall satisfaction on a scale of 1 to 7 where ‘1’ is Not At All Satisfied and ‘7’ is Extremely Satisfied. Please answer based on your overall satisfaction from your own experience.” If physicians did not have experience with a particular drug, there was the option to indicate this. Inh: inhaled; IV: intravenous; PAH: pulmonary arterial hypertension; SQ: subcutaneous.
Discussion
To our knowledge, this physician-based patient record and perception study is the first to introduce a global aspect of PAH care and provides valuable insights into similarities and differences in the diagnosis and management of PAH across the globe. A key feature of this study was the inclusion of physicians from a wide range of clinical settings. While many countries have designated specialist centers, it is clear from this survey that PAH is also managed outside these centers. For example, while the United Kingdom has eight PH centers (four of which are in London),11 25 UK regions are represented in this study (data not shown). This is likely a reflection of real-life practice, with some patients with PAH being followed outside of specialist PH centers, even in a centralized system like the United Kingdom’s. The similarities in patient management between specialist PH centers and nonspecialist settings might reflect areas where consensus has been achieved, while differences between these settings might reflect, among other things, lack of consensus or differential availability of resources.
In our study, 51% of patients had IPAH (54% in the United States and 50% in Europe), which is similar to the proportion of patients with IPAH in the US REVEAL registry (47%)12 but greater than that observed in the French PH registry and the UK audit (34% and 32%, respectively).7,11 Whether this difference reflects differences in methodology between the studies or temporal/geographic shifts in patterns of diagnosis is open to question.
The distribution of NYHA FC at diagnosis differed between countries, possibly reflecting underlying differences in prevalence, etiology, diagnostic process, and/or other factors. Notably, the differences in distribution of NYHA FC at diagnosis had largely disappeared by the time of the study, once patients had commenced treatment. The proportion of patients with significant disability (NYHA FC 3/4) in our study, both at diagnosis and at the time of the study, was slightly lower than that seen in the US REVEAL registry of PAH (54%),12 the French PH registry (75% at diagnosis),7 and the Swiss PH registry (88% at diagnosis).9 Nevertheless, our study still shows that significant impairment at the time of diagnosis is a global issue.
It is particularly worrying that across the regions studied, a large proportion of patients (around one-fifth in most regions and as high as one-half in Argentina) did not undergo RHC for the diagnosis of PAH, with the most commonly invoked reasons being that other tests had indicated that the patient had PAH, that the procedure was considered too invasive for the patient, and patient refusal. This practice is in contravention of guidelines that state that RHC is required to confirm the diagnosis of PAH as well as for the initiation of PAH-specific therapies, and it may therefore lead to incorrect diagnoses and inappropriate therapy for patients.1 Our finding is not surprising, however, as the lack of adherence to guidelines in the diagnosis of PAH has been previously described in other registries,13 and it further highlights a potential low level of awareness among practicing physicians of the diagnostic guidelines for PAH. This lack of adherence to the guidelines seen across different countries and registries is of particular concern because the diagnosis of PAH cannot be made without determining mean pulmonary artery pressure, pulmonary capillary wedge pressure, and pulmonary vascular resistance, all of which are obtained by RHC.
Patient record data demonstrated that there were significant differences in the use of various PAH-specific therapies between countries and that combination therapy usage also varied worldwide. Variations in the use of PAH-specific therapies are not unexpected, given the differences between countries in the availability and coverage of different treatments; in addition to differences in product licenses between countries, it can be speculated that disparities in financial aspects of health care between countries may also influence the availability of approved therapies. The US data in our study are substantially different from the US REVEAL data from 2009: we report the use of prostanoids in 25% and of PDE-5 inhibitors in 69% of patients, while in REVEAL, their use was reported in 42% and 50% of patients, respectively.12 The use of ERAs was similar between the studies (46% and 47% in our study and REVEAL, respectively). The reasons for these variations are unclear but are likely to relate to methodological differences between the studies and differences in availability of therapies at the time.
Although a substantial proportion of patients were experiencing considerable disability, physicians were generally satisfied with the current treatments available. This suggests that physicians accept the limitations of current PAH therapies.
Limitations of the study include retrospective reporting of patient information and potential bias in physician selection; practicing physicians were selected solely from the Medefield physician panel. This could also have resulted in potential center selection bias, as only centers with physicians included in the Medefield panel were assessed. It should also be noted that while a proportion of physicians in the United States and Japan indicated in their survey responses that they practiced at “specialist PH centers” or “PH referral centers,” these could not be verified and, therefore, may not equate to certified PH centers. Awareness of the diagnostic guidelines for PAH, and in particular the requirement for RHC, appeared to be low among a substantial proportion of the physicians in this study, which may affect the findings, as patients who did not undergo RHC as part of their diagnostic assessment may have been incorrectly classified as having PAH. Finally, there may be differences between respondents in the interpretation of survey questions.
In conclusion, this multinational study highlights significant differences in PAH diagnosis and management between regions and illustrates physicians’ perceptions of treating this condition. A major finding is the low occurrence of confirmatory RHC for the diagnosis of PAH across the regions studied, reflecting poor adherence to the current guidelines. Where differences are present, the use of treatment algorithms may be useful to guide optimal treatment strategy.14 Although it is yet to be demonstrated whether the current guidelines affect outcomes in PAH, the presence of such differences indicates areas where education may be useful to ensure the most appropriate management of patients with PAH.
Appendix.
Figure S1.
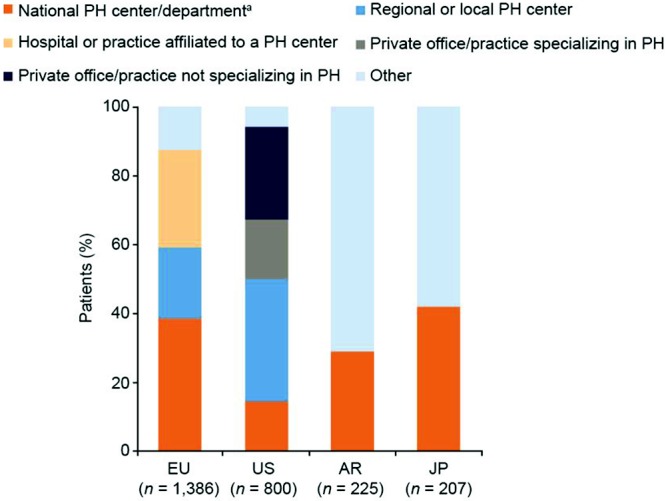
Setting of PAH diagnosis (patient record data). Responses to the study question “Which of the provided facility types best describes where this patient received his/her PAH diagnosis?” aIn Argentina or Japan, an institution with a department specialized in PH treatment. AR: Argentina; EU: Europe; JP: Japan; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; US: United States.
Figure S2.
Combination therapies by setting (patient record data). Based on responses to the study questions “Do you personally work within a PH center of excellence (EU/US)/in an institution with a department specialized in PH treatment (AR/JP)?” and “Please select from the options provided which PAH-specific drugs this patient is currently receiving.” AR: Argentina; EU: Europe; JP: Japan; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; US: United States.
Figure S3.
Concomitant medication (patient record data). Responses to the study question “Please indicate whether this patient is currently taking any concomitant medication besides PAH-specific drug therapy. Select all that apply to this patient.” ACE: angiotensin-converting enzyme; AR: Argentina; ARB: angiotensin receptor blocker; CCB: calcium channel blocker; EU: Europe; JP: Japan; PAH: pulmonary arterial hypertension; US: United States.
Table S1.
Treatment rate (physician perception data)
| UK (n = 50) | FR (n = 53) | DE (n = 63) | IT (n = 58) | ES (n = 54) | US (n = 160) | AR (n = 53) | JP (n = 69) | |
|---|---|---|---|---|---|---|---|---|
| No. of patients personally managed | 37.0 (13.8) | 37.5 (16.8) | 34.7 (11.1) | 25.9 (6.9) | 23.6 (6.4) | 57.9 (16.2) | 7.1 (2.3) | 7.5 (1.7) |
| No. of patients currently receiving PAH-specific therapy | 28.0 (13.0) | 33.6 (16.6) | 27.9 (8.9) | 21.7 (6.0) | 19.5 (5.4) | 49.3 (15.0) | 5.6 (1.5) | 6.4 (1.1) |
| Treatment rate, % | 76 | 89 | 80 | 84 | 83 | 85 | 79 | 85 |
Responses to the study questions “How many individual PH patients are you currently managing?” and “How many of the PAH patients that are currently under your direct care, are currently treated with PAH-specific drug therapy?” Unless otherwise indicated, data are mean (SEM). AR: Argentina; DE: Germany; ES: Spain; FR: France; IT: Italy; JP: Japan; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; SEM: standard error of the mean; UK: United Kingdom; US: United States.
Source of Support: The study was carried out by Ipsos Healthcare (London), supported by Bayer Pharma (Berlin). Editorial assistance was provided by Adelphi Communications (Bollington, United Kingdom), supported by Bayer Pharma.
Conflict of Interest: IRP has received grants and personal fees for services rendered (includes honoraria, royalties, or fees for consultancy, lectures, speakers bureaus, or expert testimony) from Bayer Healthcare Pharmaceuticals, Actelion, Gilead, and United Therapeutics. BH is a full-time employee of Bayer Pharma. SH declares no conflict of interest. HG reports personal fees from Actelion, AstraZeneca, Bayer Healthcare Pharmaceuticals, GlaxoSmithKline, Janssen Cilag, Lilly, Pfizer, and United Therapeutics/OMT. DJ reports personal fees from Actelion, Bayer Healthcare Pharmaceuticals, GlaxoSmithKline, and Pfizer. NHK reports personal fees from Actelion, personal fees and nonfinancial support (includes drugs/equipment, travel, writing assistance, or administrative support) from Bayer Healthcare Pharmaceuticals, and grants from Gilead, Lung Biotechnology, and United Therapeutics. IL reports grants, personal fees, and nonfinancial support from Bayer Healthcare Pharmaceuticals, Actelion, and Pfizer; grants and personal fees from AOPOrphan and United Therapeutics; and personal fees and nonfinancial support from Servier, AstraZeneca, GlaxoSmithKline, and Medtronic.
Supplements
Appendix (2.5MB, pdf)
References
- 1.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiéry JL, Barbera JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30(20):2493–2537. [DOI] [PubMed]
- 2.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D34–D41. [DOI] [PubMed]
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011;8(8):443–455. [DOI] [PMC free article] [PubMed]
- 4.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from REVEAL. Chest 2012;142(2):448–456. [DOI] [PubMed]
- 5.Low AJ, Fowler D, Manghani MK, Young I, Garsia R, Torzillo P, Youssef P, Celermajer DS. Screening and treating pulmonary arterial hypertension in a tertiary hospital-based multidisciplinary clinic: the first 200 patients. Intern Med J 2013;43(1):32–37. [DOI] [PubMed]
- 6.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013;62(25 suppl):D51–D59. [DOI] [PubMed]
- 7.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173(9):1023–1030. [DOI] [PubMed]
- 8.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev 2012;21(123):8–18. [DOI] [PMC free article] [PubMed]
- 9.Fischler M, Speich R, Dorschner L, Nicod L, Domenighetti G, Tamm M, Rochat T, Aubert JD, Ulrich S. Pulmonary hypertension in Switzerland: treatment and clinical course. Swiss Med Wkly 2008;138(25–26):371–378. [DOI] [PubMed]
- 10.Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW, Wang H, Wang ZW, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007;132(2):373–379. [DOI] [PubMed]
- 11.National Health Service Information Centre. Second annual report: key findings from the National Audit of Pulmonary Hypertension for the United Kingdom, Channel Islands, Gibraltar and Isle of Man: report for the audit period April 2010 to March 2011. Published 2011. Accessed March 26, 2014. http://www.hscic.gov.uk/catalogue/PUB04619/nati-pulm-hype-audi-2011-rep.pdf.
- 12.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122(2):164–172. [DOI] [PubMed]
- 13.McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, Tapson VF, Channick RN, Rubin LJ. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest 2013;143(2):324–332. [DOI] [PubMed]
- 14.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62(25 suppl):D60–D72. [DOI] [PubMed]