Abstract Abstract
It is well described that patients with group 1 forms of pulmonary arterial hypertension have a high risk of mortality during pregnancy and in the early postpartum period. However, to the authors’ knowledge, the diagnosis and management of group 4 pulmonary hypertension due to chronic thromboembolic pulmonary hypertension (CTEPH) during pregnancy with early postpartum pulmonary endarterectomy (PEA) has not been previously reported. We report the case of a 28-year-old woman who received a diagnosis of CTEPH during her pregnancy, was managed as an inpatient by a multidisciplinary team throughout the pregnancy and early postpartum period, and underwent PEA 6 weeks after delivery. While the management of acute pulmonary embolus in pregnancy is well described, this unique case of CTEPH diagnosed during pregnancy illustrates several challenging management issues.
Keywords: ECMO, pulmonary hypertension, chronic thromboembolic pulmonary hypertension (CTEPH), pulmonary endarterectomy (PEA), pregnancy
Patients with World Health Organization (WHO) group 1 forms of pulmonary arterial hypertension (PAH) have a high risk of mortality during pregnancy and in the early postpartum period.1-3 The authors report the case of a 28-year-old woman who received a diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH) during pregnancy. Our medical-surgical team was faced with many difficult management decisions that highlight the challenges of pregnancy in patients with PAH and, in this particular case, CTEPH with early postpartum pulmonary endarterectomy (PEA), which to our knowledge has not been previously described in the literature.
Case Description
This 28-year-old patient was previously healthy aside from a history of right arm deep vein thrombosis (DVT) in 2007, which was treated with warfarin for several months. She then developed a left popliteal vein DVT in October 2013. Warfarin therapy was restarted at that time and was continued until the patient learned that she was pregnant 1 month later; warfarin therapy was then discontinued due to teratogenicity concerns. The patient initiated therapy with enoxaparin at a dosage of 120 mg administered subcutaneously once daily at an outside hospital when it was confirmed that she had bilateral lower-extremity DVTs.
At 15 weeks gestation, the patient presented to a local emergency department with acute shortness of breath and mild hemoptysis. At that time, she declined chest computed tomography (CT) with angiography because of her pregnancy. Instead, the enoxaparin dosage was escalated to 80 mg administered subcutaneously every 12 hours, and the patient was discharged home. The patient had progressive dyspnea and cough over the next 4 weeks and presented again at 19 weeks gestation with dyspnea, cough, and hypoxia. Her oxygen saturation level was 92% on 4 L nasal cannula. In retrospect, the patient reported worsening dyspnea throughout her pregnancy. An echocardiogram was obtained at the outside hospital, and findings demonstrated severe pulmonary hypertension. A chest CT with angiography was also performed and had findings consistent with CTEPH. At 19 weeks gestation, the patient was transferred to Columbia University Medical Center–New York Presbyterian for specialized management of her CTEPH and high-risk pregnancy.
At presentation to our institution (at 19 weeks gestation), the patient reported being unable to walk 1 city block or up stairs without stopping on each flight. She had a nonproductive cough. On physical examination, she was afebrile and had a heart rate of 93 bpm, blood pressure of 117/73 mmHg, respiratory rate of 18 breaths/min, room air systemic arterial oxygen saturation of 97%, and weight of 77.5 kg. She was notably dyspneic with speaking. Her cardiac examination findings were significant for a hyperdynamic precordium and prominent S2 with a continuous murmur over her right scapula. Her abdomen was gravid, and she had mild bilateral pedal edema. Lower-extremity Doppler ultrasounds were repeated at the time of presentation, and findings were negative for lower-extremity DVT. A transthoracic echocardiogram demonstrated right atrial enlargement with mild to moderate enlargement of the RV and moderately decreased RV systolic function. The right ventricular systolic pressure was estimated at 60–65 mmHg; there was mild tricuspid insufficiency with calcification of the tricuspid valve. There was borderline left ventricular hypertrophy with normal systolic function (ejection fraction: 55%–60%). The patient underwent a ventilation/perfusion scan as part of our CTEPH protocol, demonstrating absent perfusion to the right upper lung, small subsegmental perfusion deficits to right lower lung, and possible small nonsegmental perfusion defect in the lingula. Hypercoagulability work-up was performed, and the patient was found to have a JAK2 mutation (V617F JAK2; Table 1), which, although not previously described with CTEPH, is associated with myeloproliferative disorders and venous thromboembolic events.4,5
Table 1.
Hypercoagulability work-up
| Test | Result |
|---|---|
| Erythrocyte sedimentation rate, mm/h | 12.3 |
| Anti–factor Xa, IU/mL | 0.5–0.6 |
| Anticardiolipin antibodies | Negative |
| Double-stranded DNA/antinuclear antibody | Negative |
| Protein C, % | 121 |
| Protein S, % | 28 |
| JAK2 (V617F JAK2) | Positive |
| Factor V Leiden | Negative |
| Prothrombin 20210 | Negative |
| Antithrombin | Negative |
After many counseling sessions concerning the high risk of pregnancy and pulmonary hypertension, especially CTEPH, the patient decided to proceed with the pregnancy. A multidisciplinary team that included experts from high-risk obstetrics, pulmonary hypertension, extracorporeal membrane oxygenation (ECMO), thoracic surgery, critical care, obstetric anesthesia, hematology, neonatology, and psychiatry was formed to manage the patient through the remainder of pregnancy and possible pulmonary thromboendarterectomy (PTE) surgery. The team avoided teratogenic medications, including recently approved riociguat, for CTEPH. The patient initiated low-dose sildenafil therapy at 20 weeks gestation, which was uptitrated to 40 mg 3 time daily 5 days later. Inhaled iloprost therapy was also started to treat persistent dyspnea and findings of severe pulmonary hypertension by echocardiogram. The patient developed severe hemoptysis 1 day later and underwent embolization of bronchial artery collaterals.
Iloprost therapy was discontinued, and on the following day, continuous intravenous epoprostenol therapy was initiated with slow uptitration (maximum dosage: 8 ng/kg/min). The patient then underwent a right heart cardiac catheterization at 22 weeks gestation, which demonstrated mean right atrial pressure (RAPm) of 16 mmHg, right ventricle pressure of 72/4 mmHg, pulmonary arterial pressure (PAP) of 78/35/49 mmHg, mean pulmonary arterial wedge pressure (PAWPm) of 20 mmHg, pulmonary arterial saturation of 58%, cardiac output of 5.3 L/min, and a cardiac index of 2.8 L/min/m2. The patient was medically managed with furosemide, sildenafil, and intravenous epoprostenol at 8 ng/kg/min. She still had dyspnea with exertion but felt better when receiving intravenous epoprostenol and was given supplemental O2 and intravenous heparin for the duration of the pregnancy. The partial thromboplastin time was monitored closely to ensure that the heparin remained therapeutic given the high risk of thrombosis in pregnancy.
The patient had recurrent large-volume hemoptysis at 27 weeks gestation and underwent reembolization of bronchial artery collaterals, which were predominantly feeding the right upper lobe. Her cough was suppressed, and she continued targeted PAH therapies. There was no additional hemoptysis during the pregnancy, although there was serious concern about recurrence on the required anticoagulation. The fetus remained viable and grew well.
At this time, there were many discussions about the optimal timing of delivery in light of maternal and fetal risks, including the risk of additional maternal hemoptysis. The patient had a previous cesarean delivery, so mode of delivery was planned as repeat cesarean delivery. Additional planning for delivery included coordination of a large team of experts, determining surgical (vertical incision in the event reexploration was required) and anesthetic approach, determining how to manage the hypercoagulability in a postsurgical patient with CTEPH, and having a strategy for ECMO back up if the patient were to decompensate during delivery. The decision was made to deliver at 30 weeks due to concerns about worsening maternal status and risk of further hemoptysis.
The therapeutic heparin infusion was discontinued 3 hours before bringing the patient to the operating room. After placement of a radial arterial catheter, general anesthesia and intubation were accomplished uneventfully with a combination of ketamine 125 mg, remifentanil 100 μg, and succinylcholine. A central venous line with pulmonary arterial catheter introducer was inserted into the left internal jugular, and the thoracic surgical team inserted guide wires into the right femoral artery and right femoral vein to facilitate emergent ECMO cannulation if needed. Anesthesia was maintained with a remifentanil infusion (0.1–0.3 μg/kg/min) and low-dose sevoflurane (<0.5%). Maternal intraoperative hemodynamic and respiratory status was unremarkable. The female neonate was delivered 47 minutes after general anesthetic induction with Apgar scores of 3 at 1 minute, 6 at 5 minutes, and 8 at 10 minutes. The infant developed pulmonary interstitial emphysema and was intubated on high-frequency ventilation but soon improved and was discharged home on room air with no residual medical problems; she continues to thrive. The patient was extubated in the operating room and sent to the medical intensive care unit (MICU) to recover, where the expert ECMO teams are based, without the need for vasopressors or inotropic support. Heparin therapy was started 4 hours after surgery because of the high risk for thrombosis.
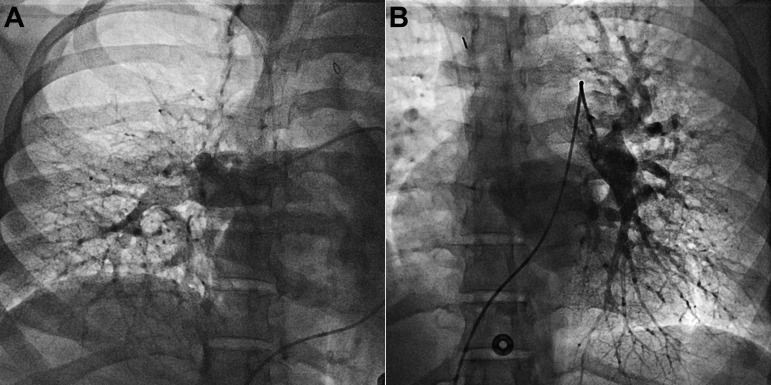
On the first postoperative day, there was an abrupt decrease in hemoglobin level and hemodynamic instability, including tachycardia and systemic hypotension with an increase in pressor requirement. The patient was taken to the operating room for abdominal exploration and wash out and was found to have slow oozing at the hysterotomy site, with 1,200 mL of clot removed. A wound vacuum-assisted closure device was placed, and the patient recovered in the MICU without any significant additional bleeding. She continued to receive oral sildenafil, and a regimen of intravenous epoprostenol and bosentan was started 10 days after delivery in an attempt to transition from intravenous epoprostenol treatment postpartum; there was limited social support at home. However, the patient remained very symptomatic with O2 use when necessary (6-minute walk distance of 37 m with O2 supplementation), and additional wean of targeted PAH therapy was not feasible. A repeat right heart catheterization with selective pulmonary angiograms was performed (Table 2). Hemodynamic characteristics confirmed severe PAH, and angiogram findings were consistent with bilateral chronic thromboembolic disease (Fig. 1).
Table 2.
Postpartum hemodynamic characteristics
| Characteristic | Result |
|---|---|
| Right atrium pressure (a/v/mean), mmHg | 22/20/16 |
| Right ventricle pressure, mmHg | 80/22 |
| Pulmonary artery pressure (systolic/diastolic/mean), mmHg | 81/31/51 |
| Pulmonary wedge pressure (a/v/mean), mmHg | 11/10/8 |
| Pulmonary artery saturation, % | 51 |
| Systemic arterial saturation, % | 90 |
| Fick cardiac output, L/min | 4.31 |
| Fick cardiac indexa | 2.35 |
| Pulmonary vascular resistance indexb | 10 |
a: a wave; v: v wave.
Calculated as liters per minute per meters squared.
Calculated as units times meters squared.
Figure 1.
Selective pulmonary artery angiograms (A, right; B, left) consistent with chronic thromboembolic disease.
The patient remained symptomatic while receiving triple PAH therapy. Unfortunately, the patient did not have the proper social support at home for continuous infusion therapy with intravenous epoprostenol. The pulmonary hypertension and thoracic surgery teams reviewed angiograms and options and decided to schedule PEA when the patient’s condition was deemed stable enough postpartum during the same hospitalization before discontinuing intravenous epoprostenol. The team’s clinical criteria for proceeding with surgery included full recovery from cesarean delivery with well-healed incision as well as optimization of fluid status, hemodynamic characteristics, and anticoagulation. The patient was taken for PEA at 6 weeks postpartum.
All targeted PAH medications were stopped during the PEA surgery. There were no reported intraoperative complications. Immediate postoperative hemodynamic characteristics included heart rate of 80 bpm, blood pressure of 104/62 mmHg, PAP of 33/14/18 mmHg, RAPm of 12 mmHg, and systemic arterial O2 saturation of 100%. The surgical specimen is shown in Figure 2.
Figure 2.
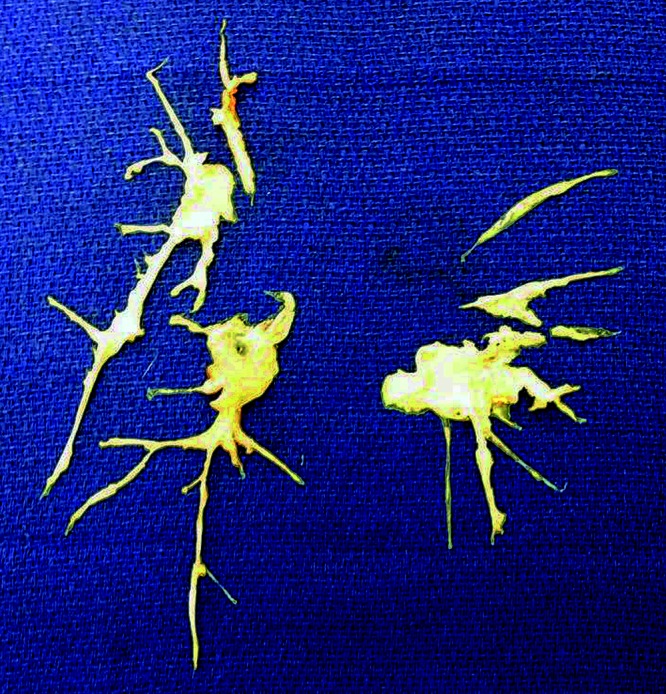
Surgical specimen obtained during postpartum pulmonary endarterectomy.
Post PEA course. The patient was extubated on postoperative day 1 and was quickly weaned from vasopressors. Her epoprostenol, bosentan, and sildenafil regimens had been discontinued at the time of PEA surgery, and she did not require use of targeted PAH therapy throughout her postoperative course. She continued to receive furosemide postoperatively. All chest tubes were removed by postoperative day 4, and enoxaparin therapy was resumed. The patient was discharged home on postoperative day 9 on room air with a regimen of enoxaparin and furosemide. The patient remains asymptomatic and continues to do well with anticoagulation therapy and no targeted PAH therapy. Her child is in excellent health.
Discussion
It is well documented that PAH carries a very high risk of mortality in pregnancy, with some series reporting a peripartum mortality of up to 50%.1-3 The risk of CTEPH with group 4 pulmonary hypertension diagnosed in pregnancy, however, is not well characterized. The authors were unable to find other reports in the literature of these conditions coexisting in the same patient with early postpartum PEA. Knowing the high risk of PAH and of hypercoagulability in pregnancy, as well as the risk to the fetus, the management team counseled the patient to consider termination of pregnancy at the time of presentation.6 However, the patient chose to continue her pregnancy, and the team had to develop a plan for the delivery and postpartum care. A recently published statement on pulmonary hypertension in pregnancy by the Pulmonary Vascular Disease Institute highlights some of the challenges in managing patients with pulmonary hypertension through pregnancy.6 Furthermore, the team had to consider all of the therapeutic options for managing the CTEPH during and after pregnancy, including anticoagulation therapy, pulmonary hypertension–targeted medical therapy, PEA, and balloon pulmonary angioplasty.7,8
There were several key decision points that arose during the course of the patient’s pregnancy and postpartum care that underscore these challenges. The team first had to address counseling the patient about the risks of pregnancy when she was already approaching 19 weeks gestation at the time of presentation. The team then had to carefully manage the pulmonary hypertension without hemodynamic data early on because of the risk of radiation exposure in early pregnancy. Thus, targeted PAH medication selection was based on previous clinical experience in PAH and pregnancy and teratogenic considerations and was very closely monitored. Endothelin receptor antagonists and riociguat were avoided because of potential teratogenicity.
In addition, the presence of hemoptysis and CTEPH posed a particularly challenging problem with regard to the anticoagulation strategy and timing of delivery and PEA surgery. Hemoptysis has been reported to occur in patients with CTEPH and is thought to be due to dilated bronchial arteries and bronchopulmonary collaterals, which are seen in CTEPH.9 Embolization of these vessels has also been reported in rare instances and, in this case, did seem to prevent the hemoptysis for several weeks. However, the recurrence of hemoptysis at 27 weeks gestation in a patient who required ongoing anticoagulation therapy posed a great challenge. Ultimately, the hemoptysis did drive the team to induce delivery early, because the patient had already had two bouts of severe hemoptysis and continued to require anticoagulation therapy for her hypercoagulable state.
The mode of delivery was established as a repeat cesearean delivery, and the multidisciplinary team prepared as it would for a pregnant patient with PAH, taking great caution to avoid pulmonary hypertension triggers and having ECMO backup if needed. Although the delivery went smoothly, the decision to anticoagulate in the early postoperative period was weighed against the risk of bleeding. Although the patient did have an intraabdominal bleed on postpartum day 1, it was surgically addressed, and there were no further venothromboembolic events following delivery.
The team also deliberated about the timing of PEA surgery and initially planned to permit the patient to fully recover and even go home before undergoing PEA. However, the patient remained quite symptomatic even while receiving triple PAH therapy, so the team decided to perform a PEA when she was deemed stable enough to undergo the procedure. There was also concern about limited social support for home intravenous prostanoid therapy. Another treatment option could have been balloon pulmonary angioplasty (BPA), which has been described for patients who are not good candidates for PEA and in unilateral disease; in this case, its use was even considered to possibly stabilize the patient earlier on during the pregnancy.10 Our team was concerned about high radiation exposure during the pregnancy, which is typically associated with BPA. BPA also often requires serial attempts, which may have been associated with reperfusion injury and not well tolerated in this patient. Therefore, the team chose PEA, which is the definitive treatment for operable CTEPH, as the appropriate surgical approach in the postpartum period to avoid the potential risks of BPA during pregnancy for questionable benefit. There was also some concern that PEA could be more challenging and less effective after BPA.
This case demonstrates the management of a rare occurrence of CTEPH presenting during pregnancy, including early postpartum PTE. Given the high-risk nature of pregnancy and pulmonary hypertension with the added risk of CTEPH and hypercoagulability, a specialized multidisciplinary team was required to map out a plan for all stages of this pregnancy, delivery, and PEA. Despite the success of this individual case, the team would still advise patients with pulmonary hypertension against pregnancy in the future.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Monagle J, Manikappa S, Ingram B, Malkoutzis V. Pulmonary hypertension and pregnancy: the experience of a tertiary institution over 15 years. Ann Card Anaesth 2015;18(2):153–160. [DOI] [PMC free article] [PubMed]
- 2.Weiss BM, Zemp L, Seifert B, Hess OM. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 1998;31(7):1650–1657. [DOI] [PubMed]
- 3.Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Euro Heart J 2009;30(3):256–265. [DOI] [PubMed]
- 4.Borowczyk M, Wojtaszewska M, Lewandowski K, Gil L, Lewandowska M, Lehmann-Kopydłowska A, Kroll-Balcerzak R, et al. The JAK2 V617F mutational status and allele burden may be related with the risk of venous thromboembolic events in patients with Philadelphia-negative myeloproliferative neoplasms. Thromb Res 2015;135(2):272–280. [DOI] [PubMed]
- 5.De T, Prabhakar P, Nagaraja D, Christopher R. Janus kinase (JAK) 2 V617F mutation in Asian Indians with cerebral venous thrombosis and without overt myeloproliferative disorders. J Neurol Sci 2012;323(1–2):178–182. [DOI] [PubMed]
- 6.Hemnes AR, Kiely DG, Cockrill BA, Safdar Z, Wilson VJ, Al Hazmi M, Preston IR, MacLean MR, Lahm T. Statement on pregnancy in pulmonary hypertension from the Pulmonary Vascular Research Institute. Pulm Circ 2015;5(3):435–465. [DOI] [PMC free article] [PubMed]
- 7.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011;124:1973–1981. [DOI] [PubMed]
- 8.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014;130:508–518. [DOI] [PubMed]
- 9.Reesink HJ, van Delden OM, Kloek JJ, et al. Embolization for hemoptysis in chronic thromboembolic pulmonary hypertension: report of two cases and a review of the literature. Cardiovasc Intervent Radiol 2007;30:136–139. [DOI] [PubMed]
- 10.Ogawa A, Matsubara H. Balloon pulmonary angioplasty: a treatment option for inoperable patients with chronic thromboembolic pulmonary hypertension. Front Cardiovasc Med 2015;2:4. [DOI] [PMC free article] [PubMed]