Editor:
Acute alveolar hypoxia causes constriction of pulmonary arterial vessels, termed hypoxic pulmonary vasoconstriction (HPV). By this mechanism, vessel perfusion and alveolar ventilation are coordinated and pulmonary gas exchange is optimized.1,2 In contrast, chronic, generalized hypoxia, besides vasoconstriction, results in remodeling of the pulmonary vasculature; both lead to pulmonary hypertension (PH).1 PH is a life-threatening disease with poor prognosis and high mortality, characterized by an increase in pulmonary vascular resistance and pulmonary arterial pressure.3 Pulmonary arterial smooth muscle cells (PASMCs) are the main effector cells in hypoxia-induced vascular remodeling, with their proliferation and constriction leading to vessel lumen obliteration.3 HPV, as well as pulmonary vascular remodeling, has been suggested to be a consequence of redox imbalance or oxidative stress, meaning elevated formation of reactive oxygen species (ROS).4 For both HPV and chronic hypoxia–induced pulmonary hypertension, discrepant concepts argue for a decrease or an increase in ROS during hypoxia as the underlying signaling event and also about the source of ROS. The situation is complex, as acute HPV (lasting seconds to minutes), prolonged HPV (starting after ∼30 minutes and lasting for hours), and chronic hypoxia–induced PH may differ with regard to the underlying mechanisms.5,6 Early investigations have suggested a decrease in ROS derived from mitochondria as the oxygen sensor and signaling event of acute HPV.2,7,8 This was, however, challenged by investigations providing evidence for increased ROS derived from either mitochondria or nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Nox),1,9-12 with mitochondrial complex III as the ROS-releasing site.9 Favoring a mitochondrial mechanism, NADPH oxidase gp91phox (Nox2) knockout mice have unaltered HPV.13 However, NADPH oxidase p47phox knockout mice showed reduced HPV, arguing for involvement of other Nox subunits.1
With regard to pulmonary arterial hypertension, strong evidence has been provided for a mitochondrial mechanism, including a decrease of ROS,14,15 a concept that may also account for hypoxia-induced PH. However, similar to acute HPV, an increase of ROS derived from either mitochondria or NADPH oxidases has also been proposed.16 With regard to NADPH oxidases, (1) Nox2 knockout mice seem to be protected from hypoxia-induced PH,17 (2) an interaction of Nox and mitochondria has been suggested,16 and (3) we and others found that Nox4 expression is elevated in hypoxic human and rat PASMCs, in lungs and especially in the pulmonary vasculature of animal models of hypoxia-induced PH, and, most importantly, in lungs of patients suffering from idiopathic pulmonary arterial hypertension.16,18-22 Nox4 contributes to PASMC proliferation and ROS formation.21,23,24 Accordingly, Nox4 silencing attenuates ROS formation and proliferation in human and rat PASMCs.20,24 Therefore, it may be concluded that upregulation of Nox4 in hypoxia actively contributes to PH pathogenesis and pulmonary vascular remodeling in vivo. In order to test this hypothesis, we analyzed the role of Nox4 in HPV and hypoxia-induced PH in vivo, with the aid of constitutive and global inducible Nox4 knockout mice.
Membrane-bound Nox are a major source of ROS in vascular cells.25 Nox allow a transmembrane electron transfer from NADPH to molecular oxygen and thereby formation of ROS such as superoxide anions () or hydrogen peroxide (H2O2).26 Seven Nox homologs are known—Nox1–5, DUOX1, and DUOX2—differing in their cellular localization, tissue distribution, regulation, activation, and expression.26 Among these, Nox4 is the only homolog that produces H2O2 in a constitutive manner.
Methods. C57BL/6J mice were obtained from Charles River Laboratories (Sulzfeld, Germany). Constitutive (Nox4−/−) and global tamoxifen-inducible (Nox4flox/flox-ERT2-CRE/0 [Nox4*/*]) Nox4 knockout mice were described previously.27,28 Mice were subjected to either normobaric normoxia (inspiratory O2 fraction [Fio2] of 0.21) or normobaric hypoxia (Fio2 of 0.10) for 21 days to induce PH. All animal experiments were approved by the local authorities.
Anesthetized animals were intubated, placed on a homeothermic plate, and artificially ventilated (MiniVent type 845, Hugo Sachs Elektronik, March-Hugstetten, Germany). A catheter was placed in the right ventricle via the right external jugular vein to assess right ventricular systolic pressure (RVSP). Afterward, the lung was fixed for histology in 3.5%–3.7% neutral buffered formalin.
For determination of right ventricular hypertrophy, the right ventricle (RV) was separated from the left ventricle plus septum (LV+S). The ratio of RV mass to LV+S mass (RV/(LV+S)) was calculated.
Muscularization of small pulmonary arteries was assessed after staining with von Willebrand factor (Dako, Hamburg) and α-smooth-muscle actin (Sigma-Aldrich, Munich) antibodies. Vessels were categorized as fully muscularized, partially muscularized, or nonmuscularized. For each lung, 85 vessels were analyzed.
For measurement of acute and sustained HPV, explanted lungs were artificially ventilated with normoxic (21% O2) or hypoxic (1% O2) gas and perfused blood-free with 1 mL/min Krebs-Henseleit buffer. Pulmonary arterial pressure is a direct measure for pulmonary vascular resistance in this setup.
Data are presented as mean ± SEM (standard error of the mean). For comparison of two groups, a Student t test was performed. Differences between more than two groups were assessed by two-way analysis of variance (ANOVA) followed by a Tukey multiple-comparisons test. A p value of <0.05 was considered significant for all analyses.
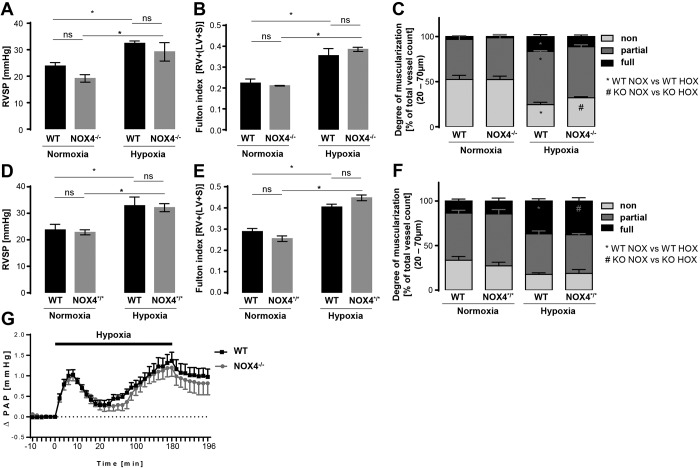
Results. Wild-type and constitutive (Nox4−/−) or global inducible (Nox4*/*) Nox4 knockout mice were exposed to normoxia (21% O2) or chronic hypoxia (10% O2) for 21 days. Tamoxifen-inducible Nox4 mice were used to exclude possible compensatory effects in constitutive Nox4−/− mice. RVSP was elevated to the same level in wild-type and Nox4−/−, as well as Nox4*/*, mice after chronic hypoxia, indicating PH development (Fig. 1A, 1D). Chronic hypoxia increased right heart hypertrophy to the same extent in wild-type, Nox4−/−, and Nox4*/* mice (Fig. 1B, 1E). In addition, PH was characterized by occurrence of pulmonary vascular remodeling in small pulmonary vessels. Chronic hypoxia–induced muscularization was not affected by the knockout of Nox4 (Fig. 1C, 1F). Eventually, Nox4 had no effect in HPV, as determined by ventilation of lungs with hypoxic gas (1% O2) for 3 hours. Nox4−/− mice developed the same biphasic vasoconstrictor response as wild-type mice (Fig. 1G).
Figure 1.
Chronic hypoxia-induced pulmonary hypertension and hypoxic pulmonary vasoconstriction in wild-type (WT), NOX4−/− (constitutive Nox4 knockout) and NOX4*/* (global tamoxifen-inducible [Nox4flox/flox-ERT2-CRE/0] Nox4 knockout) mice. A, D, Right ventricular systolic pressure (RVSP, mmHg) in normoxic (21 days at 21% O2) and chronic hypoxic (21 days at 10% O2) WT and NOX4−/− (n = 5–6; A) and NOX4*/* (n = 6–8; D) mice. *p < 0.05 (significantly different); ns: not significantly different. B, E, Fulton index, depicted by the ratio of right ventricle mass to (left ventricle + septum) mass (RV/(LV + S)), in normoxic and chronic hypoxic WT and NOX4−/− (n = 2–6; B) and NOX4*/* (n = 6–9; E) mice. *p < 0.05 (significantly different); ns: not significantly different. C, F, Vascular remodeling of normoxic and chronic hypoxic WT and NOX4−/− (n = 5–6; C) and NOX4*/* (n = 5–6; F) mice quantified by the degree of muscularization of small (outer diameter of 20–70 μm) pulmonary arterial vessels. Vessels were categorized as fully muscularized (>70% vessel media α-SMA positive), partially muscularized (>5% but ≤70% vessel media α-SMA positive), or nonmuscularized (≤5% vessel media α-SMA positive) after immunostaining against α-SMA and von Willebrand factor. One hundred vessels from each lung were analyzed. An asterisk indicates significant difference between normoxic (NOX) and hypoxic (HOX) WT mice; a pound sign indicates significant difference between NOX and HOX NOX4−/− (KO) mice (p < 0.05). G, Time course of hypoxic pulmonary vasoconstriction in isolated, buffer-perfused, and ventilated mouse lungs during 180 minutes of hypoxic (1% O2) ventilation. Changes in pulmonary arterial pressure (ΔPAP, mmHg) are depicted for WT and NOX4−/− mouse lungs (n = 8). α-SMA: α-smooth muscle actin.
Discussion. ROS regulate various cellular processes that are known to be dysregulated in pulmonary hypertension (PH).3 As described above, the sources of ROS and their specific function in the pathogenesis of PH and the regulation of HPV are not sufficiently identified yet. With regard to NADPH oxidases, Nox4 is dysregulated in PH pathogenesis and critically involved in PASMC proliferation.20,21,23 Importantly, PASMC constriction and proliferation take place in the medial layer of the pulmonary vessels, a major site of Nox4 expression.29 Accordingly, we and others have proposed a critical role of Nox4 in HPV and chronic hypoxia–induced PH. We therefore utilized constitutive and inducible Nox4 knockout mice to assess the role of Nox4 in HPV and chronic hypoxia-induced PH.
Surprisingly, neither constitutive nor acutely induced knockout of Nox4 had affected the response to acute/sustained hypoxia (HPV) or chronic hypoxia (PH). Nox4−/− mice possess a biphasic vasoconstrictor response similar to that of wild-type mice in isolated, ventilated, and buffer-perfused mouse lung experiments. This is in line with similar results obtained with another Nox4 knockout mouse line.30 Furthermore, the degree of PH quantified by RVSP, right heart hypertrophy, and vascular muscularization was not affected by Nox4 ablation. Together, those data indicate that Nox4 plays no role in the development of hypoxia-induced pathologies in the lung, such as HPV or PH. These in vivo results contrast with those of cell culture experiments depicting that Nox4 silencing or application of the Nox4 inhibitor GKT137831 negatively affected pulmonary vascular cell proliferation21,23 and ROS-induced activation (phosphorylation) of proproliferative kinases (Akt, MAPK).31 However, the in vitro situation in cell culture might not necessarily reflect the in vivo situation. Under basal conditions, Nox1 is the predominant isoform of Nox in smooth muscle cells (SMCs),32 and Nox4, in contrast to Nox1, is stress inducible, which may explain its upregulated expression in pathological conditions. In fact, it has been shown that upregulation of Nox4 in a vascular-injury model triggers a differentiation of vascular SMCs from a proliferative to a secretory phenotype.33 Nox4 further has been shown to have anti-inflammatory properties, and therefore Nox4 upregulation may serve as a protective rather than a detrimental mechanism in inflammatory diseases in vivo.34,35
Augmented production was detected in SMCs of Nox2- and Nox1-overexpressing mice, while Nox4 overexpression increased H2O2 formation.27,36,37 Nox4 is therefore incapable of scavenging NO, and its low constitutive H2O2 production might even be beneficial, while Nox1- and Nox2-derived may scavenge NO and contribute to the formation of ONOO−, which is rather detrimental and leads to vascular dysfunction. Accordingly, the type of ROS released (H2O2 vs. ) might determine whether Nox-dependent redox signaling is beneficial or detrimental. It is important to mention that Nox1 upregulation has been suggested to contribute to non-hypoxia-induced PH.38
Eventually, the cell specificity of ROS formation has an impact on its effects. Nox4 overexpression in SMCs correlates with media hypertrophy, whereas Nox4 overexpression in endothelial cells is not associated with PASMC hyperplasia.39 In other studies, Nox4 was found to be more prominently expressed in pulmonary artery fibroblasts than in PASMCs.18,40 In fact, inhibition of Nox4 by VCC588646, VCC202273, or GKT136901 reduced vascular remodeling and right heart hypertrophy in monocrotaline-treated rats.18
In conclusion, we provide evidence that neither constitutive nor acute deletion of Nox4 has any effect on hypoxia-induced PH in vivo, contrasting with results of experiments in isolated cells. At least in mice, Nox4-derived ROS, according to our data, are not the cause of hypoxia-induced PH or HPV. This, however, does not exclude a contribution of other, not yet investigated Nox isoforms to HPV or hypoxia- or non-hypoxia-induced PH.
We thank Lisa Fröhlich, Sabine Hurka, Maria Walter, and Susanne Schütz for excellent technical assistance. This work was supported by the German Research Foundation Collaborative Research Center 815 (KS), the ECCPS (NW and KS), and grant WE-1978/4–2.
References
- 1.Weissmann N, Zeller S, Schäfer RU, Turowski C, Ay M, Quanz K, Ghofrani HA, et al. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 2006;34(4):505–513. PubMed PMID: 16357364. [DOI] [PubMed]
- 2.Weir EK, López-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med 2005;353(19):2042–2055. PubMed PMID: 16282179. PubMed Central PMCID: 2803102. [DOI] [PMC free article] [PubMed]
- 3.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43(12 suppl):13S–24S. PubMed PMID: 15194174. [DOI] [PubMed]
- 4.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension 2004;44(3):248–252. PubMed PMID: 15262903. [DOI] [PubMed]
- 5.Sommer N, Strielkov I, Pak O, Weissmann N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur Respir J 2016;47(1):288–303. PubMed PMID: 26493804. [DOI] [PubMed]
- 6.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 2012;92(1):367–520. PubMed PMID: 22298659. [DOI] [PMC free article] [PubMed]
- 7.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 1993;73(6):1100–1112. PubMed PMID: 8222081. [DOI] [PubMed]
- 8.Rounds S, McMurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res 1981;48(3):393–400. PubMed PMID: 7460212. [DOI] [PubMed]
- 9.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 2013;187(4):424–432. PubMed PMID: 23328522. PubMed Central PMCID: 3603595. [DOI] [PMC free article] [PubMed]
- 10.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 2001;88(12):1259–1266. PubMed PMID: 11420302. [DOI] [PubMed]
- 11.Marshall C, Mamary AJ, Verhoeven AJ, Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol 1996;15(5):633–644. PubMed PMID: 8918370. [DOI] [PubMed]
- 12.Sommer N, Pak O, Schorner S, Derfuss T, Krug A, Gnaiger E, Ghofrani HJ, et al. Mitochondrial cytochrome redox states and respiration in acute pulmonary oxygen sensing. Eur Respir J 2010;36(5):1056–1066. PubMed PMID: 20516051. [DOI] [PubMed]
- 13.Archer SL, Reeve HL, Michelakis E, Puttagunta L, Waite R, Nelson DP, Dinauer MC, Weik EK. O2 sensing is preserved in mice lacking the gp91 phox subunit of NADPH oxidase. Proc Natl Acad Sci USA 1999;96(14):7944–7949. PubMed PMID: 10393927. PubMed Central PMCID: 22167. [DOI] [PMC free article] [PubMed]
- 14.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 2008;294(2):H570–H578. PubMed PMID: 18083891. [DOI] [PubMed]
- 15.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 2015;131(19):1691–1702. PubMed PMID: 25964279. PubMed Central PMCID: 4429908. [DOI] [PMC free article] [PubMed]
- 16.Adesina SE, Kang BY, Bijli KM, Ma J, Cheng J, Murphy TC, Hart CM, Sutliff RL. Targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension. Free Radic Biol Med 2015;87:36–47. PubMed PMID: 26073127. PubMed Central PMCID: 4615392. [DOI] [PMC free article] [PubMed]
- 17.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 2006;290(1):L2–L10. PubMed PMID: 16085672. [DOI] [PubMed]
- 18.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, et al. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol 2014;34(8):1704–1715. PubMed PMID: 24947524. PubMed Central PMCID: 4228789. [DOI] [PMC free article] [PubMed]
- 19.Lu X, Murphy TC, Nanes MS, Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol 2010;299(4):L559–L566. PubMed PMID: 20622120. PubMed Central PMCID: 2957423. [DOI] [PMC free article] [PubMed]
- 20.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, et al. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 2012;52(6):1033–1042. PubMed PMID: 22222468. [DOI] [PubMed]
- 21.Mittal M, Roth M, König P, Hofmann S, Dony E, Goyal P, Selbitz AC, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 2007;101(3):258–267. PubMed PMID: 17585072. [DOI] [PubMed]
- 22.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 2010;42(4):482–490. PubMed PMID: 19520921. PubMed Central PMCID: 2848739. [DOI] [PMC free article] [PubMed]
- 23.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 2012;47(5):718–726. PubMed PMID: 22904198. PubMed Central PMCID: 3547100. [DOI] [PMC free article] [PubMed]
- 24.Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol 2009;296(3):L489–L499. PubMed PMID: 19036873. PubMed Central PMCID: 2660216. [DOI] [PMC free article] [PubMed]
- 25.Xu S, Touyz RM. Reactive oxygen species and vascular remodelling in hypertension: still alive. Can J Cardiol 2006;22(11):947–951. PubMed PMID: 16971980. PubMed Central PMCID: 2570242. [DOI] [PMC free article] [PubMed]
- 26.Brandes RP, Weissmann N, Schröder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med 2010;49(5):687–706. PubMed PMID: 20444433. [DOI] [PubMed]
- 27.Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 2010;107(42):18121–18126. PubMed PMID: 20921387. PubMed Central PMCID: 2964252. [DOI] [PMC free article] [PubMed]
- 28.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 2012;110(9):1217–1225. PubMed PMID: 22456182. [DOI] [PubMed]
- 29.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation 2002;105(12):1429–1435. PubMed PMID: 11914250. [DOI] [PubMed]
- 30.Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 2010;8(9). doi:10.1371/journal.pbio.1000479. PubMed PMID: 20877715. PubMed Central PMCID: 2943442. [DOI] [PMC free article] [PubMed]
- 31.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 2000;20(10):2175–2183. PubMed PMID: 11031201. [DOI] [PubMed]
- 32.Schröder K. Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol 2010;10(2):122–126. PubMed PMID: 20149739. [DOI] [PubMed]
- 33.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Lassègue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 2007;27(1):42–48. PubMed PMID: 17082491. PubMed Central PMCID: 1868577. [DOI] [PMC free article] [PubMed]
- 34.Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, Calkin AC, et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler Thromb Vasc Biol 2016;36(2):295–307. PubMed PMID: 26715682. [DOI] [PubMed]
- 35.Schürmann C, Rezende F, Kruse C, Yasar Y, Löwe O, Fork C, van de Sluis B, et al. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J 2015;36(48):3447–3456. PubMed PMID: 26385958. PubMed Central PMCID: 4751217. [DOI] [PMC free article] [PubMed]
- 36.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 2007;100(7):1016–1025. PubMed PMID: 17363703. [DOI] [PubMed]
- 37.Dikalova AE, Góngora MC, Harrison DG, Lambeth JD, Dikalov S, Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 2010;299(3):H673–H679. PubMed PMID: 20639222. PubMed Central PMCID: 2944492. [DOI] [PMC free article] [PubMed]
- 38.Veit F, Pak O, Egemnazarov B, Roth M, Kosanovic D, Seimetz M, Sommer N, et al. Function of NADPH oxidase 1 in pulmonary arterial smooth muscle cells after monocrotaline-induced pulmonary vascular remodeling. Antioxid Redox Signal 2013;19(18):2213–2231. PubMed PMID: 23706097. [DOI] [PubMed]
- 39.Brandes RP, Takac I, Schröder K. No superoxide–no stress? Nox4, the good NADPH oxidase! Arterioscler Thromb Vasc Biol 2011;31(6):1255–1257. PubMed PMID: 21593458. [DOI] [PubMed]
- 40.Li S, Tabar SS, Malec V, Eul BG, Klepetko W, Weissmann N, Grimminger F, Seeger W, Rose F, Hänze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal 2008;10(10):1687–1698. PubMed PMID: 18593227. [DOI] [PubMed]