Abstract
Background
Perforation of the GI tract during endoscopy can result in significant morbidity and mortality. Early recognition and immediate management of endoscopic perforation are essential to optimize outcome. Larger perforations, defects with complex geometry, and those complicated by leakage of luminal contents have traditionally required surgical management.
Objective
To assess the feasibility of a new method for managing complex perforations that incorporates abdominal exploration and endoscopic sutured closure.
Design
Case series.
Setting
Tertiary care center.
Patients
Two patients with large, complicated perforations and peritoneal contamination.
Interventions
Endoscopic exploration of abdomen with angiocatheter placement under direct visualization, management of leaked luminal contents, and full-thickness sutured defect closure.
Results
Endoscopic abdominal exploration through the perforation site allowed safe placement of an angiocatheter for management of pneumoperitoneum, inspection for injury that may warrant surgical management, and removal of leaked luminal contents. Endoscopic sutured closure allowed safe and robust perforation management. Repair of gastrojejunal anastomotic perforation required 2 sutures and 63 minutes. Repair of gastric perforation required 4 sutures and 48 minutes. Patients had successful endoscopic defect closure confirmed by an upper GI series and were discharged 1 day later.
Limitations
Report of a new method in 2 patients performed at tertiary care center.
Conclusions
We demonstrate successful management of complex perforations with peritoneal contamination by incorporating endoscopic exploration and sutured closure with standard treatment measures. Traditional practice would have directed these patients to surgical management, which introduces additional morbidity and cost. A means for safe and broad implementation of these techniques should be evaluated.
Endoscopic procedures are associated with a low risk of adverse events and mortality.1,2 One serious adverse event is perforation. Although rare during diagnostic upper endoscopy, with a reported incidence of 0.01% to 0.05%, perforation can occur more frequently during device-assisted enteroscopy, in patients with surgically altered anatomy, or during therapeutic procedures such as endoscopic submucosal dissection and necrosectomy.3-6 One series reported a 0.4% perforation rate for double-balloon enteroscopy, but this was significantly higher (3%) in patients with altered surgical anatomy.4 Perforation can occur via many mechanisms, including pressure applied to the GI wall by the endoscope shaft, normal pressure applied to weakened tissue such as neoplasia and anastomosis, at the site of intervention such as polyp resection and submucosal dissection, barotraumas, and incomplete closure after transmural intervention such as cystogastrostomy and percutaneous gastrostomy placement.
Perforation of the GI tract can result in significant morbidity and mortality. Early recognition and immediate management of perforation is essential to optimize outcome. Some perforations can be managed endoscopically using hemostatic clips, over-the-scope clips, and endoloops.7,8 Larger perforations, defects with complicated geometry, and those complicated by leakage of luminal contents are traditionally referred for surgical management.9
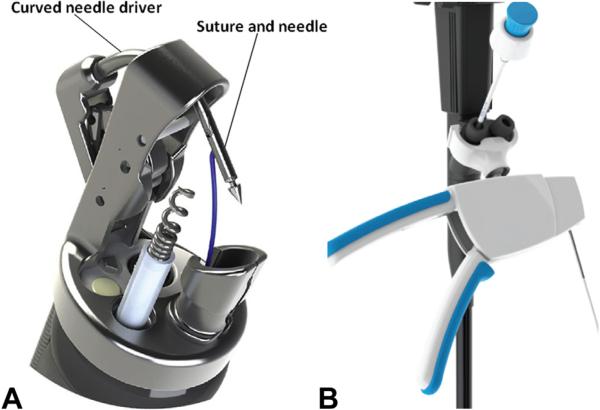
Novel endoscopic approaches and technologies are supplanting traditional surgical procedures for several conditions. The OverStitch (Apollo Endosurgery, Austin, Tex) (Fig. 1) is an endoscopic suturing device that allows full-thickness tissue apposition.10 The device attaches to a double-channel endoscope. It advances a curved needle driver and needle through tissue and has the ability to release and recapture the needle, allowing placement of a variety of suture patterns. After suture placement, the needle is released to serve as a tissue anchor. This device allows surgical suturing via an endoscopic platform. The limited natural orifice transluminal endoscopic surgery (NOTES) experience has expanded our comfort and expertise in endoscopic exploration of the peritoneum and closure of transluminal access sites.11-13 Integration of this experience with emerging technology has created an opportunity to manage larger perforations endoscopically at the time of the index procedure.
Figure 1.
A, Apollo OverStitch needle and tissue helix. B, Device control handle (modified from Abu Dayyeh et al18).
PATIENTS AND METHODS
Case 1: Perforation of gastrojejunal anastomosis during single-balloon enteroscopy for ERCP
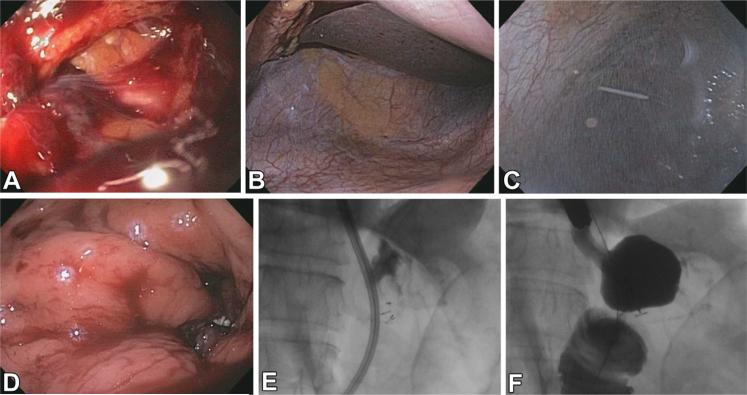
A 69-year-old woman who underwent Roux-en-Y gastric bypass 1 year earlier with multiple comorbidities presented with abdominal pain, dilated common bile duct, and elevated bilirubin level. She underwent ERCP via device-assisted enteroscopy, while under general anesthesia and carbon dioxide insufflation, in which a single-balloon enteroscope (Olympus, Center Valley, Pa) was used. The gastrojejunal anastomosis and Roux limb appeared normal. The afferent limb was intubated, and the major papilla was reached. Papillary stenosis was discovered, and a small biliary sphincterotomy was performed, followed by balloon sphincteroplasty with a controlled radial expansion (CRE) balloon (Boston Scientific, Natick, Mass) expanded to 15 mm. On withdrawal of the endoscope, a blood clot and defect were found on the jejunal side of the gastrojejunostomy (Fig. 2A). The gastric pouch and proximal Roux limb could not be fully insufflated. The defect was found to be a full-thickness, irregular perforation more than 20 mm in diameter. The abdomen was distended and firm on palpation.
Figure 2.
A, Defect at gastrojejunal anastomosis. B, Peritoneal exploration. C, Insertion of angiocatheter. D, Successful perforation closure. E, Suture tags visible during repair. F, Contrast study during repair.
Case 2: perforation of the stomach during endoscopic necrosectomy
A 58-year-old woman with history of gallstone pancreatitis and pancreatic necrosis later presented with nausea and abdominal pain. A 92 × 68-mm collection, suspicious for walled-off pancreatic necrosis, was found on imaging. She was referred for EUS with possible necrosectomy. The procedure was carried out with the patient under general anesthesia with carbon dioxide insufflation. A 70-mm hypoechoic collection was identified in the peripancreatic region abutting the gastric wall. Diagnostic needle aspiration with a 19-gauge FNA needle was performed via a transgastric approach, and fluid was removed for culture. Contrast was then instilled into the cavity under fluoroscopic guidance until the cyst was fully expanded. No communication with the pancreatic duct was seen on fluoroscopy. A 0.035-inch guidewire (Dreamwire; Boston Scientific) was coiled within the cavity. The gastrostomy tract was dilated to 20 mm with a CRE balloon (Boston Scientific). No dilation-associated bleeding was noted. On removal of the CRE balloon, omentum entered the stomach. A full-thickness defect was found (Fig. 3A). On fluoroscopy, a gap was noted between the collection and the gastric wall, and contrast was seen leaking into the abdominal cavity.
Figure 3.
A, Defect after cystogastrostomy. B, Peritoneal exploration. C, Insertion of guidewire. D, Successful perforation closure.
RESULTS
Case 1: perforation of gastrojejunal anastomosis during single-balloon enteroscopy
Ciprofloxacin 500 mg and metronidazole 500 mg were administered intravenously. An endoscope was then advanced into the peritoneum; although luminal fluid was thought to enter the abdominal cavity, no solid debris was seen on exploration (Fig. 2B). A small amount of blood was noted adjacent to the liver. No active bleeding was seen in the peritoneal cavity, and this blood was thought to be from the area of the perforation. To manage the pneumoperitoneum, a 14-gauge angiocatheter was advanced into the peritoneal cavity under direct endoscopic visualization (Fig. 2C). The patient was then prepared for endoscopic suturing. An overtube (Guardus; US Endoscopy, Mentor, Ohio) was placed into the esophagus, and the mucosa at the rim of the perforation was ablated with argon plasma coagulation. The suturing device was then used to place a figure-of-8 stitch with the intention of inverting the margins of the perforation to achieve serosa-to-serosa closure (Figs. 2D and 4). One reinforcing running suture was then placed over the closure site. Full luminal distention was restored, and carbon dioxide was no longer seen to escape from the angiocatheter on a bubble test. Dilute contrast was injected into the gastric pouch until it filled the pouch completely and extended into the Roux limb; no extravasation was seen on fluoroscopy (Fig. 2E,F). Residual peritoneal gas was removed via an angiocatheter and abdominal palpation. The angiocatheter was removed. A 0.035-inch guidewire (Dreamwire; Boston Scientific) was advanced into the small bowel, and the endoscope was withdrawn over the guidewire. A naso-oral transfer tube was used to pass the wire through the nares. A nasojejunal tube was passed over the guide-wire into the small bowel under fluoroscopic guidance. Durable closure was achieved with 2 sutures. Time from discovery of the perforation to confirmation of closure was 63 minutes. An upper GI series the next day confirmed endoscopic closure of the defect. The patient appeared clinically well the following day, and the nasojejunal tube was removed. She was discharged from the hospital on daily proton pump inhibitor therapy.
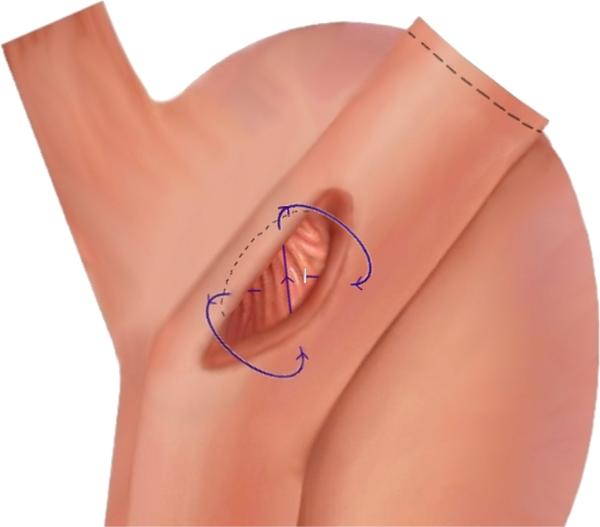
Figure 4.
Gastrojejunal anastomosis pulled back from the original configuration (dashed line) to demonstrate the figure-of-8 stitch pattern used to create serosa-to-serosa apposition.
Case 2: perforation of stomach during endoscopic necrosectomy
Ciprofloxacin 500 mg and metronidazole 500 mg were administered intravenously. The echoendoscope was exchanged for an upper endoscope, which was advanced through the perforation into the abdominal cavity (Fig. 3B). To manage the pneumoperitoneum, a 14-gauge angiocatheter was advanced into the peritoneal cavity under endoscopic visualization. The abdominal cavity was endoscopically explored for trauma and bleeding, and the contrast that had leaked into the peritoneal cavity was aspirated under fluoroscopic guidance. Additionally, the abdominal cavity was endoscopically lavaged with a dilute bacitracin solution. To mark the perforation site, a 0.035-inch guidewire (Dreamwire; Boston Scientific) was advanced into the abdominal cavity under endoscopic and fluoroscopic guidance (Fig. 3C). To assist in visualization during suturing, the mucosa at the rim of the perforation was ablated with argon plasma coagulation. The endoscope was removed. An overtube (Guardus; US Endoscopy) was placed. The OverStitch was then used to place 4 sets of interrupted stitches into the tissue adjacent to the perfo-ration, and these were secured, closing the defect (Fig. 3D). Carbon dioxide was no longer seen to escape from the angiocatheter on bubble test. Peritoneal carbon dioxide was withdrawn via an angiocatheter and abdominal palpation, and the angiocatheter was removed. Durable closure was achieved with 4 sutures. The time from discovery of the perforation to confirmation of closure was 48 minutes.
After perforation closure, the echoendoscope was advanced into the stomach and the collection was visualized. One pass was made with the 19-gauge FNA needle by using a transgastric approach. Fluid was then aspirated until cyst collapse. A nasogastric tube was placed with endoscopic and fluoroscopic guidance. Ciprofloxacin and metronidazole were given intravenously, and a 2-week oral course was initiated. An upper GI series the next day confirmed defect closure. The patient had resolution of presenting symptoms with collection decompression and appeared clinically well and was discharged from the hospital the next day. The patient had recurrent symptoms and underwent successful endoscopic necrosectomy 4 months later.
DISCUSSION
Perforation during endoscopy is a dreaded adverse event. Endoscopic closure of perforation during the index procedure may avert transfer to an operating suite for surgical repair and related morbidity.
Although standard endoscopic clips are familiar and readily available, application for perforation closure can be cumbersome and yield inconsistent results in larger perforations or perforations with irregular margins. Additionally, clips achieve mucosal apposition rather than durable full-thickness serosal apposition.14 A novel method using endoloops anchored by endoclips has been reported in case series for closure of defects after endoscopic full-thickness resection; however, the procedure is arduous and may be difficult to apply in perforations with complex tissue geometry.15 The over-the-scope clip (Ovesco, Tubingen, Germany) may allow serosa-to-serosa apposition; however, it is only approved by the U.S. Food and Drug Administration to close defects smaller than 20 mm in size, and complex geometries could yield less-consistent results.9 Suturing devices are versatile platforms with multiple applications. The Eagle Claw (Olympus, Tokyo, Japan) and Purse String Suturing Device (LSI Solutions, Victor, NY) have been investigated in animal models for closure of NOTES access sites but have not been reported for clinical closure of acute endoscopic perforation.16,17 The OverStitch has demonstrated safety and efficacy in a variety of applications, including transoral outlet reduction, endoscopic sleeve gastroplasty, fixation of luminal stents to prevent migration, and oversewing of refractory ulceration; however, acute perforation closure has not been reported.10,18-20
As described earlier, the patient's immediate condition should be addressed first. Insufflation should be changed to carbon dioxide immediately when the perforation is recognized, and intravenous antibiotics should be administered without delay. General anesthesia with proper analgesia and vital status monitoring is essential during the procedure.
Endoscopic abdominal exploration through the site of perforation is important for 3 reasons. First, it allows safe placement of an angiocatheter under direct endoscopic visualization, preventing potential visceral injury. Angiocatheter insertion is essential for management of insufflation during perforation closure. This allows unhurried and thorough suturing of the defect and evacuation of gas once the perforation has been closed. Nevertheless, the abdomen must be carefully monitored throughout the procedure to ensure that it remains soft and that tension pneumoperitoneum does not develop. Second, abdominal exploration allows inspection for injury, such as intra-abdominal hemorrhage, that may warrant surgical management. Third, although technically challenging, exploration with a flexible endoscope may allow removal of leaked luminal contents and thorough lavage.
Sutured closure should be performed with full-thickness tissue purchase and the goal of serosa-to-serosa apposition. The figure-of-8 stitch pattern requires 2 stitch placements on each side of the perforation to invert the margins of the defect (Fig. 4). In the repair of the gastrojejunal perforation, for example, the first tissue purchase was taken on the gastric side of the defect. After reloading the needle onto the needle driver, the needle driver was advanced through the defect and returned through the jejunal wall, reapposing the jejunum to the gastric pouch. A third tissue purchase was taken on the gastric side of the perforation. The fourth tissue purchase was taken by advancing the needle driver through the remaining defect and returning through the jejunal wall. The needle was dropped, and the suture was pulled tight and cinched, apposing the gastric pouch to the jejunum with the edges inverted. A running suture was then placed over the closed perforation, with tissue purchase alternating from the gastric pouch to the jejunum, to reinforce the repair.
Postprocedure admission is advised and should include monitoring of vital status, laboratory monitoring for leukocytosis and bleeding, serial clinical examination, and a course of antibiotics. Although the patients in this report were admitted for 1 day after the procedure, postprocedure observation should be longer if infection, bleeding, or medical instability is suspected. Additionally, placement of a nasogastric or nasojejunal tube and its maintenance is appropriate until the patient is deemed ready to begin a trial of oral intake.9
It is currently advisable that endoscopic closure of perforation be undertaken only when the perforation is recognized during the procedure and when closure is likely to be successful. The site of perforation affects the likelihood of successful closure. Perforation at the site of the tumor or surgical anastomosis, significant inflammation, or poor tissue integrity may be less amenable to successful endoscopic closure. Operator expertise and experience in endoscopic suturing is requisite. Given these requirements, this therapeutic option may initially be confined to centers with the necessary equipment and expertise.
The patients in this report had large defects with spillage of luminal contents and would have been less amenable to simple clip closure. Previous recommendations would have directed these patients to surgical management. However, we have demonstrated successful management of both large complex perforations and peritoneal contamination with the use of endoscopic exploration and sutured closure. Additionally, both procedures were performed in a single session in the endoscopy suite.
Our experience suggests that endoscopic abdominal exploration with sutured closure can yield expedient and definitive perforation management. Additionally, these techniques may allow the endoscopist to better address larger and more complex intraprocedural perforations. As more endoscopists gain experience with suturing, endoscopic closure may offer the prospect of decreased morbidity and lower health care costs.
Take-home Message.
Endoscopic management of complex luminal perforation with peritoneal contamination may be feasible.
Endoscopic exploration of the peritoneal cavity may allow safe angiocatheter placement under direct visualization for the management of pneumoperitoneum as well as inspection for visceral injury and removal of leaked luminal contents. Sutured full-thickness closure of large complex defects (> 20 mm, irregular margins) may allow definitive management of perforation by the endoscopist during the index procedure.
Abbreviations
- CRE
controlled radial expansion
- NOTES
natural orifice transluminal endoscopic surgery
Footnotes
DISCLOSURE: The following author disclosed a financial relationship relevant to this publication: Dr. Thompson is a consultant for Apollo Endosurgery. The other author disclosed no financial relationships relevant to this publication.
REFERENCES
- 1.Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardio-pulmonary unplanned events after GI endoscopy. Gastrointest Endosc. 2007;66:27–34. doi: 10.1016/j.gie.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Frieling T, Heise J, Kreysel C, et al. Sedation-associated complications in endoscopy–prospective multicentre survey of 191142 patients. Z Gastroenterol. 2013;51:568–72. doi: 10.1055/s-0032-1330441. [DOI] [PubMed] [Google Scholar]
- 3.Quine MA, Bell GD, McCloy RF, et al. Prospective audit of perforation rates following upper gastrointestinal endoscopy in two regions of England. Br J Surg. 1995;82:530–3. doi: 10.1002/bjs.1800820430. [DOI] [PubMed] [Google Scholar]
- 4.Gerson LB, Tokar J, Chiorean M, et al. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177–82. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Gardner TB, Coelho-Prabhu N, Gordon SR, et al. Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73:718–26. doi: 10.1016/j.gie.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000–8. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samarasena JB, Nakai Y, Park DH, et al. Endoscopic closure of an iatrogenic duodenal perforation: a novel technique using endoclips, endoloop, and fibrin glue. Endoscopy. 2012;44(Suppl 2 UCTN):E424–5. doi: 10.1055/s-0032-1325738. [DOI] [PubMed] [Google Scholar]
- 8.Sandmann M, Heike M, Faehndrich M. Application of the OTSC system for the closure of fistulas, anastomosal leakages and perforations within the gastrointestinal tract. Z Gastroenterol. 2011;49:981–5. doi: 10.1055/s-0029-1245972. [DOI] [PubMed] [Google Scholar]
- 9.Baron TH, Wong Kee Song LM, Zielinski MD, et al. A comprehensive approach to the management of acute endoscopic perforations (with videos). Gastrointest Endosc. 2012;76:838–59. doi: 10.1016/j.gie.2012.04.476. [DOI] [PubMed] [Google Scholar]
- 10.Jirapinyo P, Slattery J, Ryan MB, et al. Evaluation of an endoscopic suturing device for transoral outlet reduction in patients with weight regain following Roux-en-Y gastric bypass. Endoscopy. 2013;45:532–6. doi: 10.1055/s-0032-1326638. [DOI] [PubMed] [Google Scholar]
- 11.Ryou M, Fong DG, Pai RD, et al. Dual-port distal pancreatectomy using a prototype endoscope and endoscopic stapler: a natural orifice transluminal endoscopic surgery (NOTES) survival study in a porcine model. Endoscopy. 2007;39:881–7. doi: 10.1055/s-2007-966908. [DOI] [PubMed] [Google Scholar]
- 12.Fong DG, Pai RD, Thompson CC. Transcolonic endoscopic abdominal exploration: a NOTES survival study in a porcine model. Gastrointest Endosc. 2007;65:312–8. doi: 10.1016/j.gie.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Pai RD, Fong DG, Bundga ME, et al. Transcolonic endoscopic cholecystectomy: a NOTES survival study in a porcine model. Gastrointest Endosc. 2006;64:428–34. doi: 10.1016/j.gie.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 14.Raju GS, Thompson C, Zwischenberger JB. Emerging endoscopic options in the management of esophageal leaks. Gastrointest Endosc. 2005;62:278–86. doi: 10.1016/s0016-5107(05)01632-9. [DOI] [PubMed] [Google Scholar]
- 15.Shi Q, Chen T, Zhong YS, et al. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329–34. doi: 10.1055/s-0032-1326214. [DOI] [PubMed] [Google Scholar]
- 16.Pham B, Raju GS, Ahmed I, et al. Immediate endoscopic closure of colon perforation by using a prototype endoscopic suturing device: feasibility and outcome in a porcine model (with video). Gastrointest Endosc. 2006;64:113–9. doi: 10.1016/j.gie.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Ryou M, Fong DG, Pai RD, et al. Evaluation of a novel access and closure device for NOTES applications: a transcolonic survival study in the porcine model (with video). Gastrointest Endosc. 2007;67:964–9. doi: 10.1016/j.gie.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530–5. doi: 10.1016/j.gie.2013.04.197. [DOI] [PubMed] [Google Scholar]
- 19.Fujii LL, Bonin EA, Baron TH, et al. Utility of an endoscopic suturing system for prevention of covered luminal stent migration in the upper GI tract. Gastrointest Endosc. 2013;78:787–93. doi: 10.1016/j.gie.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Jirapinyo P, Watson RR, Thompson CC. Use of a novel endoscopic suturing device to treat recalcitrant marginal ulceration (with video). Gastrointest Endosc. 2012;76:435–9. doi: 10.1016/j.gie.2012.03.681. [DOI] [PubMed] [Google Scholar]