Abstract
Objectives
To estimate the associations of moderate and vigorous intensity exercise during pregnancy with the rate of gestational weight gain (GWG) from gestational diabetes (GDM) diagnosis to delivery, overall and stratified by prepregnancy overweight/obesity.
Methods
Prospective cohort study with physical activity reported shortly after the GDM diagnosis and prepregnancy weight and post-diagnosis GWG obtained from electronic medical records (n= 1,055). Multinomial logistic regression models in the full cohort and stratified by prepregnancy overweight/obesity estimated associations of moderate and vigorous intensity exercise with GWG below and above the Institute of Medicine’s (IOM) prepregnancy BMI-specific recommended ranges for weekly rate of GWG in the second and third trimesters.
Results
In the full cohort, any participation in vigorous intensity exercise was associated with decreased odds of GWG above recommended ranges as compared to no participation [Odds Ratio (95% Confidence Interval): 0.63 (0.40, 0.99)], with a significant trend for decreasing odds of excess GWG with increasing level of vigorous intensity exercise. Upon stratification by prepregnancy overweight/obesity, significant associations were only observed for BMI ≥ 25.0 kg/m2: any vigorous intensity exercise, as compared to none, was associated with 54% decreased odds of excess GWG [0.46 (0.27, 0.79)] and significant trends were detected for decreasing odds of GWG both below and above the IOM’s recommended ranges with increasing level of vigorous exercise (both P ≤ 0.03). No associations were observed for moderate intensity exercise.
Conclusions
In women with GDM, particularly overweight and obese women, vigorous intensity exercise during pregnancy may reduce the odds of excess GWG.
Keywords: Gestational diabetes mellitus, physical activity during pregnancy, moderate intensity exercise, vigorous intensity exercise, gestational weight gain
Introduction
Women with gestational diabetes mellitus (GDM), defined as diabetes with first onset or recognition in pregnancy (3), are at high risk for developing type 2 diabetes in the postpartum (2). Pregnancies affected by GDM face an increased risk of perinatal complications, including preeclampsia, excessive fetal growth and related birth injuries; children of GDM affected pregnancies are also at increased risk of obesity and type 2 diabetes (3). Excessive gestational weight gain (GWG) in a pregnancy complicated by GDM increases the risk of postpartum weight retention (5), thereby increasing the risk of recurrent GDM (6) and the long-term risk of overweight/obesity (7, 8) and type 2 diabetes (9, 10). In 2009, the Institute of Medicine (IOM) released prepregnancy body mass index (BMI) specific recommendations for appropriate GWG to assist pregnant women in attaining healthier pregnancies through healthy diet and physical activity (11).
The role of exercise in GDM prevention remains controversial and few studies have examined the relationship between exercise and GWG specifically in women with GDM (15). A 2012 Cochrane review found no significant differences in the frequency of GDM between pregnant women who participated in exercise interventions and those who did not (12). A 2015 meta-analysis concluded that regular participation in moderate-intensity exercise during pregnancy was associated with a lower incidence of GDM and reduced maternal weight gain (14). A 2015 Cochrane review of randomized trials conducted among pregnant women with and without GDM concluded that both exercise and diet interventions, as well as weight management interventions consisting of both components, reduce excessive GWG, and stated that the supporting evidence was high-quality (13).
The 2009 American College of Obstetricians and Gynecologists (ACOG) guidelines (16) and the 2008 Physical Activity Guidelines for Americans (PAG) (17) recommended that healthy pregnant women achieve ≥30 minutes/day moderate-intensity exercise, most days of the week. Citing insufficient data on the effects of vigorous-intensity exercise (16, 17), ACOG and PAG advise that women who habitually participate in vigorous-intensity exercise can continue to do so during pregnancy, as long as they remain healthy and receive medical care.
To help fill the gaps in evidence, this observational cohort study examines the association of moderate- and vigorous-intensity sports and exercise, self-reported soon after the diagnosis of GDM, with rate of GWG from GDM diagnosis to the end of pregnancy, classified in accordance with the 2009 IOM recommendations (11). Since the IOM recommendations are prepregnancy BMI dependent (11) and more overweight and obese women exceed the IOM recommendations for GWG (18), we examine associations both overall and stratified by prepregnancy overweight/obesity.
Methods
The study setting is Kaiser Permanente Northern California (KPNC), a large group practice health plan providing care to over 3 million members. KPNC’s membership includes ~30% of the area served and is similar demographically, except that the extremes of income and education are under-represented (19). This prospective cohort study is a secondary analysis of baseline (i.e., pregnancy) data collected for the GDM’s Effects on Moms (GEM) trial, a cluster randomized clinical trial (with randomization at the medical facility level) examining the comparative effectiveness of postpartum diabetes prevention strategies for women with GDM.
All GEM women received telephone counseling on pregnancy glucose control in conjunction with clinical care, which included advising moderate-intensity exercise, specifically walking after meals. All women also received mailed recommendations on diabetes prevention postpartum (i.e., usual care). Women in the intervention arm additionally received a letter on appropriate gestational weight gain (sent shortly after the GDM diagnosis) and were offered a Diabetes Prevention Program-derived lifestyle program consisting of 13 telephone sessions with lifestyle coaches between 6 weeks and 6 months postpartum (20). GEM was approved by the KPNC institutional review board and registered at Clinical Trials.gov (NCT01344278).
From March 2011 to March 2012, all women with a diagnosis of GDM according to the Carpenter and Coustan criteria, as recommended by ACOG during the study period (21, 22), who were 18 years of age or older were identified in KPNC’s electronic health record (EHR), comprising the GEM cohort. In this setting, 97.5% of pregnancies are screened for GDM (23). All GEM women were contacted shortly after the diagnosis of GDM [median 4.9 weeks (IQR 3.7–7.0)] and invited to participate in the baseline (pregnancy) survey, in English or Spanish, which included a modified Pregnancy Physical Activity Questionnaire (PPAQ) (20). The PPAQ asked women to report time spent in a variety of population-specific activities over the past three months. GEM added questions on yoga/Pilates, cardiovascular exercise machines, aerobic exercise classes, weight lifting/resistance exercises and team sports (24). [See Appendix for questionnaire.]
Activities assessed, by domain, included: household/caregiving (13 items), occupational (5 items), sports and exercise (12 items), transportation (3 items) and inactivity (3 items). Participants selected one of six categorical response options for the amount of time spent in each type of activity. The mid-point of the category selected (i.e., duration) was multiplied by the metabolic equivalent of task (MET) assigned to that activity (i.e., intensity) to arrive at an estimate of volume of physical activity [i.e., (MET ∙ hours) per week]; this proxy for energy expenditure is comparable between individuals of different body weight. MET values for walking and light to moderate-intensity household tasks came from field-based measurements among pregnant women (25); Compendium-based MET values (26) were used for all other activities.
This study focuses on the sports and exercise domain, specifically moderate-intensity sports and exercise (3–6 MET; from 10 items) and vigorous-intensity sports and exercise (> 6 MET; from 2 items: ‘walking quickly up hills for fun or exercise’ and ‘jogging’). They were examined separately and are hereafter referred to as moderate exercise and vigorous exercise. This domain was selected because the activities included are performed intentionally for health, wellness, or to increase fitness and result in energy expenditure beyond the demands of everyday living. Volume of moderate exercise was examined by tertile (i.e., ≤ 5.80, 5.81–13.80, and ≥13.81 MET hours per week), since over 95% of the cohort participated in moderate exercise to some degree. Less than a third of the cohort reported any participation in vigorous exercise, therefore it was examined as any versus none, and as none, low, and high to examine potential dose-response; the median volume among those reporting any participation in vigorous exercise (1.75 MET hours per week) differentiated low from high for the dose-response analyses. Total volume of physical activity was calculated from activities ≥ 2 MET (i.e., 32 items).
The IOM provides prepregnancy BMI-specific recommended ranges for total GWG as well as ranges for weekly rate weight gain in the second and third trimesters (11). For example, the IOM recommends that normal weight women (prepregnancy BMI 18.5–24.9) gain 0.35–0.50 kg per week and overweight women (prepregnancy BMI 25–29.9) gain 0.23–0.33 kg per week in the second and third trimesters. For women in the GEM trial, weight at the GDM diagnosis and the last pregnancy weight (within 2 weeks of delivery), as measured by KPNC clinical staff and recorded in the EHR, were used to calculate average rate of weight gain per week from GDM diagnosis to delivery. Each woman’s average rate of weekly weight gain from GDM diagnosis to delivery was classified as ‘below’ if beneath the lower limit of the BMI-specific range for rate of GWG recommended by the IOM, ‘within’ if within the bounds of the recommended range, or ‘above’ if greater than the upper limit of the recommended range. The EHR provided data on measured height and prepregnancy weight; prepregnancy BMI was calculated as the prepregnancy weight (kg) divided by the height (m), squared.
Data on potential confounders obtained from the GEM survey included age, parity, race-ethnicity, education, and household income. A semi-quantitative food frequency questionnaire, the Block 2005 (27), estimated average daily intake of food energy (kcals) in the preceding three months.
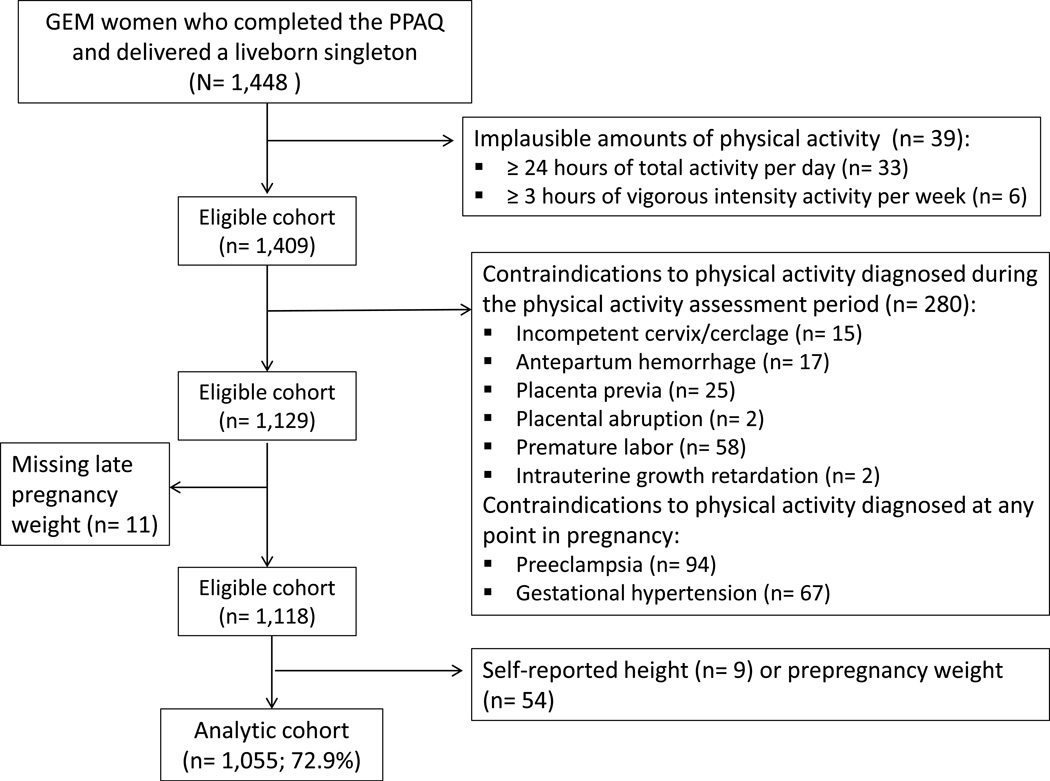
A total of 1,448 GEM participants completed the PPAQ and delivered liveborn, singletons. We excluded 39 women reporting implausible amounts of activity, specifically 33 women reporting over 24 hours of total activity per day (including sedentary and light intensity activity) and 6 women reporting over 3 hours of vigorous-intensity activity per week. We searched the EHR to exclude women with contraindications to physical activity (16) diagnosed prior to the completion of the PPAQ (n= 119), as well as women diagnosed with preeclampsia (n= 94) and pregnancy-induced hypertension (n= 67) at any point during pregnancy, given that these conditions are contraindications to physical activity during pregnancy (16) and may affect GWG (i.e., water retention). An additional 11 women missing late pregnancy weight measurements (i.e., within 2 weeks of delivery) in the EHR, who were therefore missing data on GWG, were then excluded. Lastly, 9 women missing height and 54 missing prepregnancy weight in the EHR were excluded, leaving 1,055 women (72.9%) in the analytic cohort (Figure 1).
Figure 1.
Assembly of the analytic cohort.
Statistical Analyses
Bivariate associations between exercise participation and categorical variables were assessed with chi-square tests or Fisher’s exact test for small cell sizes. For comparisons of continuous variables that were not normally distributed, medians [interquartile ranges (IQR)] were calculated and compared, and differences assessed with Kruskal-Wallis tests. Means and standard deviations (SD) were computed for normally distributed continuous variables and differences assessed with ANOVA or T tests.
Multinomial logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) estimating the associations between moderate (by tertile, lowest tertile as the reference) and vigorous exercise [some versus none (reference), and high, low and none (reference)] and the odds of GWG below and above the prepregnancy-BMI specific ranges for rate of GWG recommended by the IOM (rate of GWG ‘within’ the recommended range as the reference). Levels of moderate and vigorous exercise were modeled as continuous variables to test for trend.
Potential confounders, including age (continuous), prepregnancy BMI (continuous), parity (nulliparous vs. multiparous), race-ethnicity (White, Hispanic, Asian, African American or Multiracial), education (less than 4 years of college, college degree, or post graduate degree), annual household income (<$80,000 vs. ≥$80,000 per year), food energy intake (continuous), GEM trial treatment group (usual care vs. lifestyle intervention) and the number of weeks between the weight measurements at GDM diagnosis and before delivery (continuous), were entered individually into separate models for moderate and vigorous exercise. Variables were included in the fully adjusted model of that exercise intensity if the Wald Chi-square test indicated that it contributed meaningfully (P ≤ 0.05) or if significant differences were observed by exercise participation (Table 1).
Table 1.
Cohort characteristics by participation in moderate- and vigorous-intensity sports and exercise during pregnancy
| Tertile 1 moderate- intensity sports and exercise* (N= 345) |
Tertile 2 moderate- intensity sports and exercise* (N= 358) |
Tertile 3 moderate- intensity sports and exercise* (N= 352) |
No vigorous- intensity sports and exercise* (N= 760) |
Any vigorous- intensity sports and exercise* (N= 295) |
|||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | Pa | n (%) | n (%) | Pa | |
| Age, years | 0.08 | 0.40 | |||||
| 18–29 | 100 (29.0) | 102 (28.5) | 92 (26.1) | 219 (28.8) | 75 (25.4) | ||
| 30–34 | 129 (37.4) | 121 (33.8) | 154 (43.8) | 292 (38.4) | 112 (38.0) | ||
| 35–46 | 116 (33.6) | 135 (37.7) | 106 (30.1) | 249 (32.8) | 108 (36.6) | ||
| Prepregnancy BMI, kg/m2 | 0.16 | 0.23 | |||||
| 16–24 | 109 (31.6) | 125 (34.9) | 135 (38.4) | 254 (33.4) | 115 (39.0) | ||
| 25–29 | 110 (31.9) | 95 (26.5) | 106 (30.1) | 228 (30.0) | 83 (28.1) | ||
| 30–56 | 126 (36.5) | 138 (38.6) | 111 (31.5) | 278 (36.6) | 97 (32.9) | ||
| Race-ethnicity | 0.69 | 0.86 | |||||
| White | 69 (20.0) | 87 (24.3) | 88 (25.0) | 174 (22.9) | 70 (23.7) | ||
| Hispanic | 65 (18.8) | 61 (17.0) | 73 (20.7) | 144 (19.0) | 55 (18.6) | ||
| Asian | 151 (43.8) | 159 (44.4) | 147 (41.8) | 324 (42.6) | 133 (45.1) | ||
| African American | 13 (3.8) | 11 (3.1) | 9 (2.6) | 24 (3.2) | 9 (3.1) | ||
| Multiracial | 39 (11.3) | 33 (9.2) | 31 (8.8) | 79 (10.4) | 24 (8.1) | ||
| Other | 8 (2.3) | 7 (2.0) | 4 (1.1) | 15 (2.0) | 4 (1.4) | ||
| Parity | 0.0002 | 0.09 | |||||
| Nulliparous | 117 (33.9) | 153 (42.7) | 173 (49.2) | 307 (40.4) | 136 (46.1) | ||
| Multiparous | 228 (66.1) | 205 (57.3) | 179 (50.9) | 453 (59.6) | 159 (53.9) | ||
| Education | 0.007 | 0.96 | |||||
| Less than 4-yrs of college | 182 (52.8) | 152 (42.5) | 146 (41.5) | 344 (45.3) | 136 (46.1) | ||
| 4-yr college graduate | 110 (31.9) | 125 (34.9) | 118 (33.5) | 255 (33.6) | 98 (33.2) | ||
| Postgraduate degree | 53 (15.4) | 80 (22.4) | 86 (24.4) | 159 (20.9) | 60 (20.3) | ||
| Missing | 0 (0) | 1 (0.3) | 2 (0.6) | 2 (0.3) | 1 (0.3) | ||
| Household Income | 0.45 | 0.17 | |||||
| <$80,000 | 170 (49.3) | 166 (46.4) | 161 (45.7) | 349 (45.9) | 148 (50.2) | ||
| ≥$80,000 | 152 (44.1) | 172 (48.0) | 174 (49.4) | 369 (48.6) | 129 (43.7) | ||
| Missing | 23 (6.7) | 20 (5.6) | 17 (4.8) | 42 (5.5) | 18 (6.1) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | P | Mean (SD) | Mean (SD) | P | |
| Age, years | 31.9 (4.9) | 32.1 (5.0) | 31.8 (4.7) | 0.73 | 30.3 (6.0) | 30.2 (6.5) | 0.86 |
|
Median (IQR) |
Median (IQR) |
Median (IQR) |
Pa |
Median (IQR) |
Median (IQR) |
Pa | |
| Prepregnancy BMI, kg/m2 | 27.5 (23.8–33.5) |
26.9 (23.5–32.0) |
26.6 (23.2–31.6) |
0.14 | 27.1 (23.6–32.4) |
26.4 (23.1–31.5) |
0.12 |
| Number of weeks between weight measurements at GDM diagnosis and before delivery |
12.3 (10.0–15.1) |
12.6 (10.6–15.8) |
12.8 (10.7–16.1) |
0.15 | 12.6 (10.4–15.5) |
12.6 (10.6–16.0) |
0.74 |
| Energy intake, kcal (n= 957) |
1618 (1252–2074) |
1644 (1281–2049) |
1734 (1374–2139) |
0.03 | 1673 (1293–2054) |
1693 (1309–2188) |
0.27 |
| Total volume of physical activity, MET∙ hours per week |
146 (87–205) |
162 (114–242) |
196 (130–279) |
<0.0001 | 153 (101–224) |
199 (137–290) |
<0.0001 |
Median (IQR) MET hours per week by category of exercise participation: 2.40 (1.50–3.80) for first tertile of moderate, 9.30 (6.98–11.45) for second tertile of moderate, 23.26 (17.41–30.66) for third tertile of moderate, and 1.75 (1.63–4.88) for any vigorous
P for group differences exclude missing
GDM= Gestational diabetes
IQR= Interquartile range
For moderate exercise, the fully adjusted model included age, prepregnancy BMI, race-ethnicity, education, income, parity, energy intake, and the number of weeks between the weight measurements at GDM diagnosis and before delivery. For vigorous exercise, the fully adjusted model included age, prepregnancy BMI, race-ethnicity, education, income, and the number of weeks between the weight measurements at GDM diagnosis and before delivery. To arrive at associations that were independent of increases in the volume of physical activity, total volume of physical activity (continuous) was added to both fully adjusted models (28, 29).
Potential effect modification by GEM treatment group (usual care vs. lifestyle intervention) and prepregnancy BMI (<25 kg/m2 vs. ≥25 kg/m2) were explored through the addition of interaction terms to fully adjusted models and stratified analyses.
Women missing covariate data (16%) were compared to those with complete covariate data with chi square tests; they did not differ by participation in moderate exercise or vigorous exercise, or by rate of GWG classified by the IOM recommendations (all P > 0.05).
All analyses were conducted in SAS version 9.3 (Cary, North Carolina, U.S.A.).
Results
The 1,055 eligible women included in this study completed the PPAQ at a median of 31.9 weeks gestation (IQR 28.7–34.3), reporting a median 9.30 MET hours per week (IQR 3.90–17.43) of moderate exercise (e.g., approximately 2 hours per week of ‘walking quickly for fun or exercise’). There were 295 women (28%) participating in vigorous exercise, reporting a median 1.75 MET hours per week (e.g., approximately 15 minutes per week of jogging; IQR 1.63–4.88). The full cohort gained a median 0.22 kg per week (IQR 0.07–0.37) after the diagnosis of GDM; 56% (n= 588) of the full cohort gained below the recommended ranges for rate of GWG following the diagnosis of GDM, 18% (n= 193) gained within and 26% (n= 274) above. For total GWG, 32% of the cohort gained below the IOM recommendations, 34% met the recommendations and 34% exceeded the recommendations.
Cohort characteristics, by participation in moderate and vigorous exercise, are presented in Table 1. Women in the lowest tertile of moderate exercise were significantly more likely to be multiparous, attained lower levels of education, had lower energy intake, reported a lower total volume of physical activity and were less likely to concurrently participate in vigorous exercise (P < 0.0001). Women participating in vigorous exercise were similar to those not participating in vigorous exercise, with the exceptions of total volume of physical activity and volume of concurrent moderate exercise, both of which were significantly higher among those participating in vigorous exercise (P < 0.0001). Participation in moderate (i.e., tertile) and vigorous (i.e., any versus none) exercise did not differ by GEM trial treatment group (P= 0.62 and P= 0.14, respectively) and the interaction terms for treatment group and moderate and vigorous exercise did not achieve statistical significance (P= 0.63 and P= 0.59, respectively).
The interaction term for prepregnancy BMI and moderate exercise attained statistical significance (P= 0.03), but there was no statistically significant association between moderate exercise and GWG below and above the IOM recommendations in the full cohort or across strata of prepregnancy overweight/obesity (Tables 2).
Table 2.
Odds Ratios for the association of moderate-intensity sports and exercise with rate of gestational weight gaina below and above the Institute of Medicine’s prepregnancy BMI-specific recommended ranges.
| Gestational weight gain below recommended rates (n= 505) |
Gestational weight gain above recommended rates (n= 219) |
||
|---|---|---|---|
| N | ORb (95% CI) | ORb (95% CI) | |
| Full cohort (n= 888) | |||
| First tertilec | 277 | 1.00 | 1.00 |
| Second tertilec | 308 | 1.05 (0.67, 1.64) | 1.06 (0.64, 1.77) |
| Third tertilec | 303 | 1.18 (0.73, 1.90) | 0.84 (0.48, 1.46) |
| P for trend | 0.82 | 0.33 | |
|
Prepregnancy BMI < 25 kg/m2 (n= 319)d |
|||
| First tertilee | 90 | 1.00 | 1.00 |
| Second tertilee | 113 | 1.22 (0.59, 2.54) | 0.76 (0.28, 2.07) |
| Third tertilee | 116 | 1.40 (0.65, 3.02) | 0.72 (0.25, 2.02) |
| P for trend | 0.39 | 0.54 | |
|
Prepregnancy BMI ≥ 25 kg/m2 (n= 567) |
|||
| First tertilef | 186 | 1.00 | 1.00 |
| Second tertilef | 195 | 0.86 (0.48, 1.55) | 1.04 (0.56, 1.93) |
| Third tertilef | 186 | 0.99 (0.53, 1.85) | 0.79 (0.40, 1.57) |
| P for trend | 0.97 | 0.51 |
Average weekly rate of gestational weight gain from gestational diabetes diagnosis to end of pregnancy.
Multinomial logistic regression model adjusted for age, prepregnancy BMI, race-ethnicity, education, income, parity, energy intake, number of weeks between weight measurements at gestational diabetes diagnosis and before delivery, and total volume of physical activity; rate of gestational weight gain ‘within’ the range recommended by the IOM as the reference.
Median moderate-intensity sports and exercise (MET hours per week) in the full cohort, by tertile: 2.4 (first tertile), 9.3 (second tertile), and 23.3 (third tertile).
African American women excluded from analyses of prepregnancy BMI < 25 kg/m2 due to small cell sizes (n= 2).
Using cut points from the full cohort, median moderate-intensity sports and exercise (MET hours per week) in women with prepregnancy BMI < 25 kg/m2, by tertile: 3.3 (first tertile), 9.3 (second tertile), and 22.3 (third tertile).
Using cut points from the full cohort, median moderate-intensity sports and exercise (MET hours per week) in women with prepregnancy BMI ≥ 25 kg/m2, by tertile: 2.4 (first tertile), 9.3 (second tertile), and 23.4 (third tertile).
BMI= Body Mass Index.
Table 3 presents the results of multinomial logistic regression models for the association of vigorous exercise and GWG, adjusted for age, prepregnancy BMI, race-ethnicity, education, income, the number of weeks between the weight measurements at GDM diagnosis and before delivery, and total volume of physical activity. As compared to no vigorous exercise, any participation in vigorous exercise was associated with 37% decreased odds of GWG above the IOM’s recommended ranges [OR= 0.63, 95% CI (0.40, 0.99)] in the full cohort. The highest level of vigorous exercise was associated with a 52% decreased odds of excess GWG [OR= 0.48, 95% CI (0.27, 0.87)], as compared to no vigorous exercise, and there was a significant trend for decreasing odds of excess GWG with increasing level of vigorous exercise in the full cohort (P = 0.02).
Table 3.
Odds Ratios for the association of vigorous-intensity sports and exercise with rate of gestational weight gaina below and above the Institute of Medicine’s prepregnancy BMI-specific recommended ranges.
| Gestational weight gain below recommended rates (n= 548) |
Gestational weight gain above recommended rates (n= 251) |
||
|---|---|---|---|
| N | ORb (95% CI) | ORb (95% CI) | |
| Full cohort (n= 977) | |||
| None | 704 | 1.00 | 1.00 |
| Anyc | 273 | 0.94 (0.64, 1.38) | 0.63 (0.40, 0.99) |
| None | 704 | 1.00 | 1.00 |
| Lowd | 138 | 1.20 (0.71, 2.04) | 0.84 (0.50, 1.53) |
| Highd | 135 | 0.75 (0.46, 1.21) | 0.48 (0.27, 0.87) |
| P for trend | 0.39 | 0.02 | |
|
Prepregnancy BMI < 25 kg/m2 (n= 349)e |
|||
| None | 243 | 1.00 | 1.00 |
| Anyf | 106 | 1.66 (0.86, 3.19) | 1.24 (0.52, 2.94) |
| None | 243 | 1.00 | 1.00 |
| Lowd | 53 | 2.08 (0.80, 5.37) | 2.65 (0.87, 8.03) |
| Highd | 53 | 1.40 (0.63, 3.13) | 0.39 (0.10, 1.59) |
| P for trend | 0.23 | 0.64 | |
|
Prepregnancy BMI ≥ 25 kg/m2 (n= 626) |
|||
| None | 460 | 1.00 | 1.00 |
| Anyg | 166 | 0.65 (0.39, 1.06) | 0.46 (0.27, 0.79) |
| None | 460 | 1.00 | 1.00 |
| Lowd | 85 | 0.86 (0.45, 1.65) | 0.50 (0.24, 1.03) |
| Highd | 81 | 0.49 (0.26, 0.91) | 0.43 (0.22, 0.92) |
| P for trend | 0.03 | 0.005 |
Average weekly rate of gestational weight gain from gestational diabetes diagnosis to end of pregnancy.
Multinomial logistic regression model adjusted for age, prepregnancy BMI, race-ethnicity, education, income, number of weeks between weight measurements at gestational diabetes diagnosis and before delivery, and total volume of physical activity; rate of gestational weight gain ‘within’ the range recommended by the IOM as the reference.
Range of vigorous-intensity sports and exercise (MET hours per week) among all women reporting any participation in vigorous-intensity sports and exercise: 1.6–21.0
‘Low’ versus ‘high’ cut point (1.75 MET hours per week) is the median vigorous-intensity sports and exercise among all women reporting some participation in vigorous-intensity sports and exercise.
African American women excluded from analyses of prepregnancy BMI < 25 kg/m2 due to small cell sizes (n= 2).
Range of vigorous-intensity sports and exercise (MET hours per week) among women with prepregnancy BMI < 25 kg/m2 reporting some participation in vigorous-intensity sports and exercise: 1.6–21.0.
Range of vigorous-intensity sports and exercise (MET hours per week) among women with prepregnancy BMI ≥ 25 kg/m2 reporting some participation in vigorous-intensity sports and exercise: 1.6–19.5.
BMI= Body Mass Index.
The interaction term between prepregnancy BMI and vigorous exercise (any versus none) achieved statistical significance (P = 0.02), Stratification by pregravid BMI revealed no associations among women with prepregnancy BMI < 25.0 kg/m2 (Table 3). In women with pregravid BMI ≥ 25.0 kg/m2, any participation in vigorous exercise was associated with 35% decreased odds of GWG below the recommended ranges [0.65, (0.39, 1.06)], although not statistically significant, and 54% decreased odds of GWG above the recommended ranges [0.46, (0.27, 0.79)] compared to no vigorous exercise. In women with prepregnancy BMI ≥ 25.0 kg/m2, the highest level of vigorous exercise was associated with 51% decreased odds of GWG below the recommended ranges [0.49, (0.26, 0.91)] and 57% decreased odds of GWG above the recommended ranges [0.43, (0.23, 0.92)] compared to no vigorous exercise; significant trends for decreasing odds of GWG below and above the IOM’s recommended ranges with increasing level of vigorous exercise were also observed in women with prepregnancy BMI ≥ 25.0 kg/m2 (P= 0.03 and P= 0.005, respectively).
Discussion
In a large, diverse cohort of women with GDM, this study examined the impact of moderate and vigorous exercise on the average amount of weight gained per week from the GDM diagnosis to the end of pregnancy. Moderate exercise was not found to be associated with GWG below or above the ranges for rate of GWG recommended by the IOM. In the full cohort, vigorous exercise was associated with decreased odds of GWG above the recommended rate ranges as compared to no vigorous exercise during pregnancy. However, stratification by prepregnancy overweight/obesity revealed that this association was driven those who were overweight or obese prior to pregnancy and that in this sub-group, high levels of vigorous exercise was associated with decreases in the odds of GWG both below and above the rates ranges recommended by the IOM.
Women participating in vigorous exercise also reported higher total volume of physical activity, yet significant associations were observed between vigorous exercise and GWG independent of total volume of physical activity. This suggests that the benefits of vigorous exercise in regards to second and third trimester GWG may extend beyond contributions to overall energy expenditure to other components of energy balance (30, 31), such as increases in lipolysis, resting metabolic rate and post-exercise energy metabolism.
In non-pregnant adults, vigorous exercise increases post-exercise energy metabolism and reduces fat deposition (31), visceral adipose tissue accumulation in particular (35). It is therefore plausible that vigorous exercise during pregnancy may impact GWG by increasing post-exercise energy metabolism and reducing the accumulation of adipose tissue (30). As pregnancy progresses, adipose tissue is preferentially deposited as visceral adipose tissue and there is some evidence to suggest that the process differs between normal weight and overweight/obese women (37). Future research examining the impact of vigorous exercise during pregnancy on maternal metabolism, weight gain and fat accretion is needed, specifically among overweight and obese women with GDM.
The scientific literature on exercise and GWG in women with GDM is sparse. We identified one quasi-experimental study in which treatment group was self-selected by 96 obese women with GDM (15). Diet was compared to diet and exercise for pregnancy weight restriction and weight gain per week was significantly lower with diet and exercise as compared to diet alone (15), suggesting that, contrary to the current study, moderate exercise may play a role in reducing excessive GWG in obese women with GDM. Multiple trials have investigated the effects of exercise, primarily moderate intensity exercise, on GWG among healthy pregnant women. A 2015 Cochrane review of 49 randomized controlled trials comparing exercise interventions (mostly moderate intensity exercise interventions), diet interventions or weight management interventions consisting of both components concluded that there was high-quality evidence to suggest that all three intervention strategies reduced excessive GWG (13). A 2015 meta-analysis also concluded participation in structured moderate-intensity exercise programs, particularly when the exercise was performed throughout pregnancy, was associated with less weight gain (14).
Consistent with the findings of the current study, observational studies of healthy pregnant women suggest that vigorous exercise may be associated with less excessive GWG (32, 33). In a cohort of 1,388 women, 30 minutes per day of vigorous activity in mid-pregnancy was significantly associated with reduced odds of excess GWG; associations with walking, moderate activity and total activity were non-significant upon adjustment for confounding factors (32). A cohort study of 467 Norwegian women reported that those participating in vigorous-intensity recreational activity for a minimum of 20 minutes, once a week in the third trimester had lower GWG than inactive women, with no associations found for occupational or household activity (33). Conversely, in a cohort of 1,276 Hispanic women, no type or intensity of physical activity, including vigorous exercise, performed in early, mid or late pregnancy was associated with GWG (34). However, studies varied greatly in the types of activities assessed (i.e., domain and intensity), the survey or questions used to assess participation, and when in pregnancy the activity was performed.
A limitation to the current study is the availability of a single physical activity assessment; we lack data on change in physical activity prompted by the diagnosis and clinical management of GDM, as well as reductions in physical activity with advancing gestation. However, all women included in these analyses reported physical activity soon after the GDM diagnosis and the cohort uniformly received counseling on glucose control for GDM management (20), which included recommendations for moderate exercise, particularly walking after meals. Therefore, acknowledging that the diagnosis and management of GDM leads to changes in exercise and diet that in turn impact GWG, our outcome is specific to the rate of GWG following the diagnosis of GDM and thus misclassification of the exposure is likely to be non-differential.
Common challenges related to the measurement of physical activity also apply to the current study. Physical activity was based on an absolute measure of activity intensity; absolute measures, unlike relative measures such as perceived level of exertion, do not account for individual differences in factors impacting energy expenditure (i.e., fitness level and body weight). Our modifications to the PPAQ may have impacted the validity, but the PPAQ has been modified by others and continued to demonstrate expected associations, suggesting indirect validity. Women with similar activity habits may also have quantified their activity differently on the questionnaire and social desirability bias may have led women to over-report physical activity. We lacked data on physical activity prior to pregnancy and could not assess potential effect modification by initiation of vigorous exercise during pregnancy versus the continuation of a prepregnancy exercise regime. It should also be emphasized that all women in this study had GDM and our findings may not be generalizable to pregnant women free of this complication.
Strengths of the current study include its prospective design, as the assessment of and reference period for the exercise exposure preceded the GWG outcome. The analytic cohort was large and racially and ethnically diverse. The PPAQ included a wide variety of population-specific activities, such as prenatal exercise classes and carrying children. The use of clinically measured prepregnancy and pregnancy weights in the EHR for the calculation and classification of GWG following the GDM diagnosis additionally strengthen our analyses.
In conclusion, the results of this study suggest that for women with GDM, particularly those who were overweight or obese prior to pregnancy, participation in vigorous exercise during pregnancy may reduce excessive GWG in the second and third trimesters, as defined by the prepregnancy BMI-specific rate ranges recommended by the IOM. Current physical activity recommendations for pregnant women offer no specific guidance on participation in vigorous exercise (16, 17). Additional studies, particularly accelerometry studies, are needed to validate these findings and inform clinical recommendations for the duration and frequency of vigorous exercise during pregnancy to ensure safety and promote the health of pregnant women with GDM and their children.
Supplementary Material
Acknowledgments
This work was supported by grant R01 HS019367 from the Agency for Healthcare Research and Quality to Dr. Ferrara, who was also supported by grant P30 DK092924 from the National Institute of Diabetes Digestive and Kidney Diseases. Dr. Ehrlich is supported by K01 DK105106 from the National Institute of Diabetes Digestive and Kidney Diseases.
ABBRVIATIONS
- GWG
Gestational weight gain
- GDM
Gestational diabetes mellitus
Footnotes
Data were presented at the American Diabetes Association’s 73rd Scientific Sessions. SFE analyzed the data and drafted the manuscript; CPQ and AF analyzed the data and revised the manuscript; BS, AEK, MMH, SDB, AM, and LCT revised the manuscript.
The authors have no relevant conflict of interest to disclose.
Reference List
- 1.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286(20):2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009 May 23;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Buchanan TA, Coustan DR, de LA, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007 Jul;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011 Jul;60(7):1849–1855. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abebe DS, Von ST, Von HA, Zerwas SC, Torgersen L, Bulik CM. Developmental Trajectories of Postpartum Weight 3 Years After Birth: Norwegian Mother and Child Cohort Study. Matern Child Health J. 2014 Aug 1; doi: 10.1007/s10995-014-1593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011 Jun;117(6):1323–1330. doi: 10.1097/AOG.0b013e31821aa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stage E, Ronneby H, Damm P. Lifestyle change after gestational diabetes. Diabetes Res Clin Pract. 2004 Jan;63(1):67–72. doi: 10.1016/j.diabres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Mamun AA, Kinarivala M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr. 2010 May;91(5):1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 9.Al MA, Mannan M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Association between gestational weight gain and postpartum diabetes: evidence from a community based large cohort study. PLoS One. 2013;8(12):e75679. doi: 10.1371/journal.pone.0075679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet. 1996;347:227–230. doi: 10.1016/s0140-6736(96)90405-5. [DOI] [PubMed] [Google Scholar]
- 11.IOM (Institutes of Medicine) Weight Gain During Pregnancy: Reexamining the Guidelines. Washington DC: The National Academic Press; 2009. [PubMed] [Google Scholar]
- 12.Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7:CD009021. doi: 10.1002/14651858.CD009021.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015 Jun 11;6:CD007145. doi: 10.1002/14651858.CD007145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanabria-Martinez G, Garcia-Hermoso A, Poyatos-Leon R, Alvarez-Bueno C, Sanchez-Lopez M, Martinez-Vizcaino V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG. 2015 Jun 3; doi: 10.1111/1471-0528.13429. [DOI] [PubMed] [Google Scholar]
- 15.Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Appl Physiol Nutr Metab. 2007 Jun;32(3):596–601. doi: 10.1139/H07-024. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Obstetric Practice. ACOG Committee Opinion: Exercise During Pregnancy and the Postpartum Period. 2009 [Google Scholar]
- 17.Physical Activity Guidelines Committee. Physical Activity Guidelines Advisory Committee Report. Washington DC: Department of Health and Human Services; 2008. [Google Scholar]
- 18.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015 Apr;125(4):773–781. doi: 10.1097/AOG.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2007 California Health Interview Survey. Kaiser Permanente Northern California Division of Research. 2012 Jan 4; [Google Scholar]
- 20.Ferrara A, Hedderson MM, Albright CL, Brown SD, Ehrlich SF, Caan BJ, Sternfeld B, Gordon NP, Schmittdiel JA, Gunderson EP, Mevi AA, Tsai AL, Ching J, Crites Y, Quesenberry CP., Jr A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rational of the Gestational Diabetes' Effects on Moms (GEM) study. BMC Pregnancy Childbirth. 2014 Jan 15;14(1):21. doi: 10.1186/1471-2393-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Committee opinion no. 504: screening and diagnosis of gestational diabetes mellitus. Obstet Gynecol. 2011 Sep;118(3):751–753. doi: 10.1097/AOG.0b013e3182310cc3. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich SF, Crites YM, Hedderson MM, Darbinian JA, Ferrara A. The risk of large for gestational age across increasing categories of pregnancy glycemia. Am J Obstet Gynecol. 2011 Mar;204(3) doi: 10.1016/j.ajog.2010.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc. 2004 Oct;36(10):1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 25.Roberts DE, Fragala MS, Pober D, Chasan-Taber L, Freedson PS. Energy Cost of Physical Activities During Pregnancy. Med Sci. Sports Exerc. 2002;34(S124) [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000 Sep;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986 Sep;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 28.Tremblay A, Despres JP, Leblanc C, Craig CL, Ferris B, Stephens T, Bouchard C. Effect of intensity of physical activity on body fatness and fat distribution. Am J Clin Nutr. 1990 Feb;51(2):153–157. doi: 10.1093/ajcn/51.2.153. [DOI] [PubMed] [Google Scholar]
- 29.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001 Jun;33(6 Suppl):S459–S471. doi: 10.1097/00005768-200106001-00016. [DOI] [PubMed] [Google Scholar]
- 30.Clapp JF, III, Little KD. Effect of recreational exercise on pregnancy weight gain and subcutaneous fat deposition. Med Sci Sports Exerc. 1995 Feb;27(2):170–177. [PubMed] [Google Scholar]
- 31.Yoshioka M, Doucet E, St-Pierre S, Almeras N, Richard D, Labrie A, Despres JP, Bouchard C, Tremblay A. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord. 2001 Mar;25(3):332–339. doi: 10.1038/sj.ijo.0801554. [DOI] [PubMed] [Google Scholar]
- 32.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009 Jul;201(1):58. doi: 10.1016/j.ajog.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haakstad LA, Voldner N, Henriksen T, Bo K. Physical activity level and weight gain in a cohort of pregnant Norwegian women. Acta Obstet Gynecol Scand. 2007;86(5):559–564. doi: 10.1080/00016340601185301. [DOI] [PubMed] [Google Scholar]
- 34.Chasan-Taber L, Silveira M, Lynch KE, Pekow P, Solomon CG, Markenson G. Physical activity and gestational weight gain in Hispanic women. Obesity (Silver Spring) 2013 Jun 26; doi: 10.1002/oby.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goedecke JH, Micklesfield LK. The effect of exercise on obesity, body fat distribution and risk for type 2 diabetes. Med Sport Sci. 2014;60:82–93. doi: 10.1159/000357338. [DOI] [PubMed] [Google Scholar]
- 36.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004 Jun;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 37.Straughen JK, Trudeau S, Misra VK. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutr Diabetes. 2013;3:e84. doi: 10.1038/nutd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61(2):115–118. doi: 10.1159/000089456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.